Cascade Membrane System for Separation of Water and Organics from Liquid By-Products of HTC of the Agricultural Digestate—Evaluation of Performance
Abstract
:1. Introduction
2. Materials and Methods
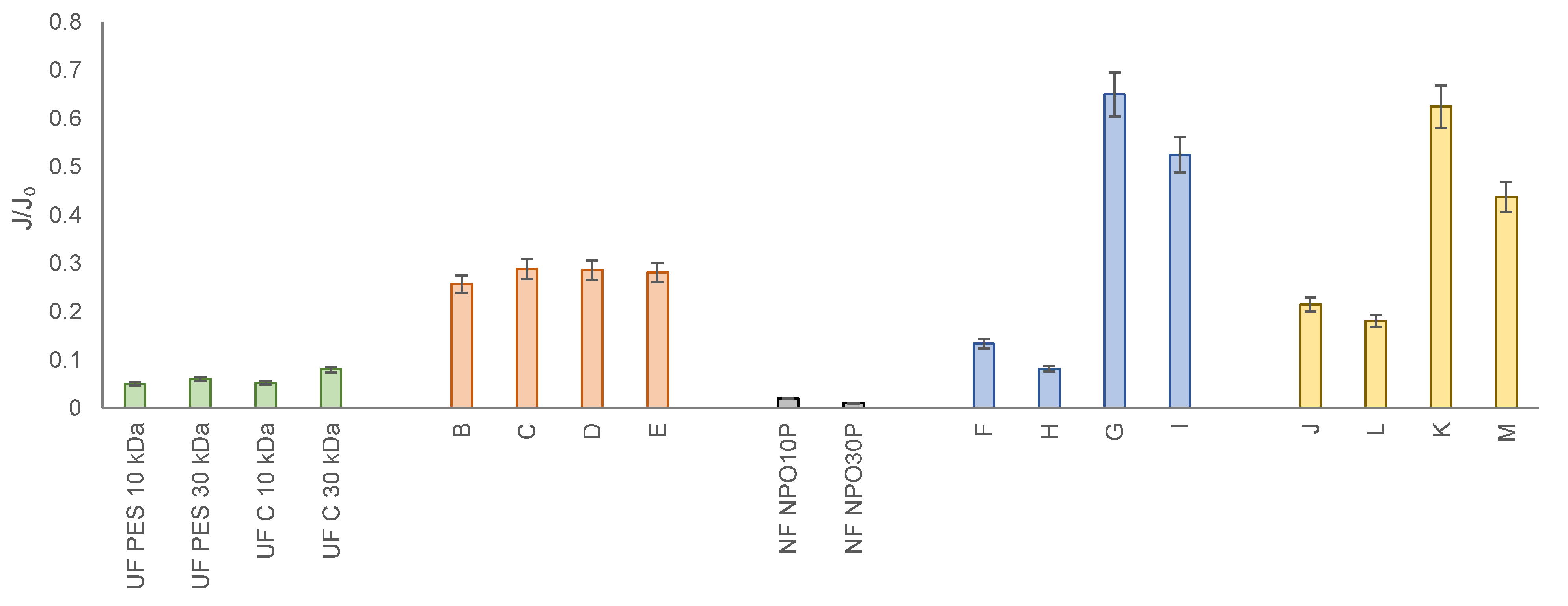
- MF membrane with a pore size of 0.2 µm made of polypropylene, 25.4 µm thick and 60% porosity (Hoechst Celgard Corporation),
- Four types of UF membranes (PES 10, PES 30, C 10, C 30) (Microdyn Nadir),
- Two types of NF membranes (NPO10P and NPO30P) (Microdyn Nadir).
- A—solution after MF membrane;
- B—solution after MF followed by UF using a PES 10 kDa membrane;
- C—solution after MF followed by UF using a PES 30 kDa membrane;
- D—solution after MF followed by UF using a C 10 kDa membrane;
- E—solution after MF followed by UF using a C 30 kDa membrane;
- F—solution after MF followed by UF using a PES 10 kDa membrane and NF using NPO10P membrane;
- G—solution after MF followed by UF using a PES 10 kDa membrane and NF using NPO30P membrane;
- H—solution after MF followed by UF using a PES 30 kDa membrane and NF using NPO10P membrane;
- I—solution after MF followed by UF using a PES 30 kDa membrane and NF using NPO30P membrane;
- J—solution after MF followed by UF using a C 10 kDa membrane and NF using NPO10P membrane;
- K—solution after MF followed by UF using a C 10 kDa membrane and NF using NPO30P membrane;
- L—solution after MF followed by UF using a C 30 kDa membrane and NF using NPO10P membrane;
- M—solution after MF followed by UF using a C 30 kDa membrane and NF using NPO30P membrane.
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Den Boer, J.; Obersteiner, G.; Gollnow, S.; den Boer, E.; Bodnárné Sándor, R. Enhancement of food waste management and its environmental consequences. Energies 2021, 14, 1790. [Google Scholar] [CrossRef]
- Den Boer, J.; Kobel, P.; Dyjakon, A.; Urbańska, K.; Obersteiner, G.; Hrad, M.; Schmied, E.; den Boer, E. Food waste in Central Europe—Challenges and solutions. E3S Web Conf. 2017, 22, 00019. [Google Scholar] [CrossRef] [Green Version]
- Piechota, G.; Igliński, B. Biomethane in Poland—Current Status, potential, perspective and development. Energies 2021, 14, 1517. [Google Scholar] [CrossRef]
- Schmid, C.; Horschig, T.; Pfeiffer, A.; Szarka, N.; Thrän, D. Biogas upgrading: A review of national biomethane strategies and support policies in selected countries. Energies 2019, 12, 3803. [Google Scholar] [CrossRef] [Green Version]
- Baltrėnas, P.; Kolodynskij, V.; Zagorskis, A.; Baltrėnaitė, E. Research and analysis of biogas produced from sewage sludge using a batch bioreactor. Environ. Technol. 2018, 39, 3104–3112. [Google Scholar] [CrossRef]
- Rasheed, T.; Anwar, M.T.; Ahmad, N.; Sher, F.; Khan, S.U.-D.; Ahmad, A.; Khan, R.; Wazeer, I. Valorisation and emerging perspective of biomass based waste-to-energy technologies and their socio-environmental impact: A review. J. Environ. Manag. 2021, 287, 112257. [Google Scholar] [CrossRef]
- Plana, P.V.; Noche, B. A review of the current digestate distribution models: Storage and transport. In Proceedings of the 8 International Conference on Waste Management and The Environment (WM 2016), Valencia, Spain, 7–9 June 2016; Volume 202, pp. 345–357. [Google Scholar]
- Monfet, E.; Aubry, G.; Ramirez, A.A. Nutrient removal and recovery from digestate: A review of the technology. Biofuels 2017, 9, 247–262. [Google Scholar] [CrossRef]
- Daija, L.; Selberg, A.; Rikmann, E.; Zekker, I.; Tenno, T.; Tenno, T. The influence of lower temperature, influent fluctuations and long retention time on the performance of an upflow mode laboratory-scale septic tank. Desalin. Water Treat. 2016, 57, 18679–18687. [Google Scholar] [CrossRef]
- Zekker, I.; Bhowmick, G.D.; Priks, H.; Nath, D.; Rikmann, E.; Jaagura, M.; Tenno, T.; Tämm, K.; Ghangrekar, M.M. ANAMMOX-denitrification biomass in microbial fuel cell to enhance the electricity generation and nitrogen removal efficiency. Biodegradation 2020, 31, 249–264. [Google Scholar] [CrossRef]
- Tenno, T.; Uiga, K.; Mashirin, A.; Zekker, I.; Rikmann, E. Modeling closed equilibrium systems of H2O-dissolved CO2-solid CaCO3. J. Phys. Chem. A 2017, 121, 3094–3100. [Google Scholar] [CrossRef]
- Tenno, T.; Rikmann, E.; Uiga, K.; Zekker, I.; Mashirin, A.; Tenno, T. A novel proton transfer model of the closed equilibrium system H2O-CO2-CaCO3-NHx. Proc. Est. Acad. Sci. 2018, 67, 260–270. [Google Scholar] [CrossRef]
- Zekker, I.; Artemchuk, O.; Rikmann, E.; Ohimai, K.; Dhar Bhowmick, G.; Madhao Ghangrekar, M.; Burlakovs, J.; Tenno, T. Start-Up of Anammox SBR from non-specific inoculum and process acceleration methods by hydrazine. Water 2021, 13, 350. [Google Scholar] [CrossRef]
- Zekker, I.; Rikmann, E.; Tenno, T.; Vabamäe, P.; Tomingas, M.; Menert, A.; Loorits, L.; Tenno, T. Anammox bacteria enrichment and phylogenic analysis in moving bed biofilm reactors. Environ. Eng. Sci. 2012, 29, 946–950. [Google Scholar] [CrossRef]
- Zekker, I.; Raudkivi, M.; Artemchuk, O.; Rikmann, E.; Priks, H.; Jaagura, M.; Tenno, T. Mainstream-sidestream wastewater switching promotes anammox nitrogen removal rate in organic-rich, low-temperature streams. Environ. Technol. 2020, 42, 3073–3082. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Rowe, I.; Golkowska, K.; Lebuf, V.; Vaneeckhaute, C.; Michels, E.; Meers, E.; Benetto, E.; Koster, D. Environmental assessment of digestate treatment technologies using LCA methodology. Waste Manag. 2015, 43, 442–459. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Liu, D.L.; Feng, P.; Wang, B.; Waters, C.; Shen, Y.; Qi, Y.; Bai, H.; Tang, J. Future climate change impacts on grain yield and groundwater use under different cropping systems in the North China Plain. Agric. Water Manag. 2021, 246, 106685. [Google Scholar] [CrossRef]
- Aghapour Sabbaghi, M.; Nazari, M.; Araghinejad, S.; Soufizadeh, S. Economic impacts of climate change on water resources and agriculture in Zayandehroud river basin in Iran. Agric. Water Manag. 2020, 241, 106323. [Google Scholar] [CrossRef]
- Cortignani, R.; Dell’Unto, D.; Dono, G. Paths of adaptation to climate change in major Italian agricultural areas: Effectiveness and limits in supporting the profitability of farms. Agric. Water Manag. 2021, 244, 106433. [Google Scholar] [CrossRef]
- Grusson, Y.; Wesström, I.; Svedberg, E.; Joel, A. Influence of climate change on water partitioning in agricultural watersheds: Examples from Sweden. Agric. Water Manag. 2021, 249, 106766. [Google Scholar] [CrossRef]
- Angelidaki, I.; Ahring, B.K. Methods for increasing the biogas potential from the recalcitrant organic matter contained in manure. Water Sci. Technol. 2000, 41, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Żubrowska-Sudoł, M.; Podedworna, J.; Bisak, A.; Sytek-Szmeichel, K.; Krawczyk, P.; Garlicka, A. Intensification of anaerobic digestion efficiency with use of mechanical excess sludge disintegration in the context of increased energy production in wastewater treatment plants. E3S Web Conf. 2017, 22, 00208. [Google Scholar] [CrossRef] [Green Version]
- Szatyłowicz, E.; Garlicka, A.; Żubrowska-Sudoł, M. The Effectiveness of the organic compounds released due to the hydrodynamic disintegration of sewage sludge. Inżynieria Ekol. 2017, 18, 47–55. [Google Scholar] [CrossRef]
- Azman, S.; Milh, H.; Somers, M.H.; Zhang, H.; Huybrechts, I.; Meers, E.; Meesschaert, B.; Dewil, R.; Appels, L. Ultrasound-assisted digestate treatment of manure digestate for increased biogas production in small pilot scale anaerobic digesters. Renew. Energy 2020, 152, 664–673. [Google Scholar] [CrossRef]
- Lippert, T.; Bandelin, J.; Xu, Y.; Liu, Y.C.; Robles, G.H.; Drewes, J.E.; Koch, K. From pre-treatment to co-treatment—How successful is ultrasonication of digested sewage sludge in continuously operated anaerobic digesters? Renew. Energy 2020, 166, 56–65. [Google Scholar] [CrossRef]
- Villamil, J.A.; Mohedano, A.F.; Rodriguez, J.J.; De la Rubia, M.A. Anaerobic co-digestion of the aqueous phase from hydrothermally treated waste activated sludge with primary sewage sludge. A kinetic study. J. Environ. Manag. 2019, 231, 726–733. [Google Scholar] [CrossRef]
- Seruga, P.; Krzywonos, M.; Seruga, A.; Niedzwiecki, L.; Pawlak-Kruczek, H.; Urbanowska, A. Anaerobic digestion performance: Separate collected vs. mechanical segregated organic fractions of municipal solid waste as feedstock. Energies 2020, 13, 3768. [Google Scholar] [CrossRef]
- Seruga, P.; Krzywonos, M.; Pyżanowska, J.; Pawlak-Kruczek, H.; Urbanowska, A.; Niedzwiecki, L. Removal of ammonia from the municipal waste treatment effluents using natural minerals. Molecules 2019, 24, 3633. [Google Scholar] [CrossRef] [Green Version]
- Pawlak-Kruczek, H.; Urbanowska, A.; Yang, W.; Brem, G.; Magdziarz, A.; Seruga, P.; Niedzwiecki, L.; Pozarlik, A.; Mlonka-Medrala, A.; Kabsch-Korbutowicz, M.; et al. Industrial process description for the recovery of agricultural water from digestate. J. Energy Resour. Technol. 2020, 147, 070917. [Google Scholar] [CrossRef]
- Wilk, M.; Magdziarz, A.; Jayaraman, K.; Szymańska-Chargot, M.; Gökalp, I. Hydrothermal carbonization characteristics of sewage sludge and lignocellulosic biomass. A comparative study. Biomass Bioenergy 2019, 120, 166–175. [Google Scholar] [CrossRef]
- Magdziarz, A.; Wilk, M.; Wądrzyk, M. Pyrolysis of hydrochar derived from biomass—Experimental investigation. Fuel 2020, 267, 117246. [Google Scholar] [CrossRef]
- Smith, A.M.; Ekpo, U.; Ross, A.B. The influence of pH on the combustion properties of bio-coal following hydrothermal treatment of swine manure. Energies 2020, 13, 331. [Google Scholar] [CrossRef] [Green Version]
- Sieradzka, M.; Rajca, P.; Zajemska, M.; Mlonka-Mędrala, A.; Magdziarz, A. Prediction of gaseous products from refuse derived fuel pyrolysis using chemical modelling software—Ansys Chemkin-Pro. J. Clean. Prod. 2020, 248, 119277. [Google Scholar] [CrossRef]
- Aragón-Briceño, C.I.; Ross, A.B.; Camargo-Valero, M.A. Mass and energy integration study of hydrothermal carbonization with anaerobic digestion of sewage sludge. Renew. Energy 2021, 167, 473–483. [Google Scholar] [CrossRef]
- Mihajlović, M.; Petrović, J.; Maletić, S.; Isakovski, M.K.; Stojanović, M.; Lopičić, Z.; Trifunović, S. Hydrothermal carbonization of Miscanthus × giganteus: Structural and fuel properties of hydrochars and organic profile with the ecotoxicological assessment of the liquid phase. Energy Convers. Manag. 2018, 159, 254–263. [Google Scholar] [CrossRef]
- Gao, N.; Li, Z.; Quan, C.; Miskolczi, N.; Egedy, A. A new method combining hydrothermal carbonization and mechanical compression in-situ for sewage sludge dewatering: Bench-scale verification. J. Anal. Appl. Pyrolysis 2019, 139, 187–195. [Google Scholar] [CrossRef]
- Jackowski, M.; Semba, D.; Trusek, A.; Wnukowski, M.; Niedzwiecki, L.; Baranowski, M.; Krochmalny, K.; Pawlak-Kruczek, H. Hydrothermal carbonization of brewery’s spent grains for the production of solid biofuels. Beverages 2019, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Persson, H.; Yang, W.; Jönsson, P.G. Pyrolysis study of hydrothermal carbonization-treated digested sewage sludge using a Py-GC/MS and a bench-scale pyrolyzer. Fuel 2020, 262, 116335. [Google Scholar] [CrossRef]
- Arauzo, P.; Olszewski, M.; Kruse, A. Hydrothermal carbonization Brewer’s spent grains with the focus on improving the degradation of the feedstock. Energies 2018, 11, 3226. [Google Scholar] [CrossRef] [Green Version]
- Reza, M.T.; Yan, W.; Uddin, M.H.; Lynam, J.G.; Hoekman, S.K.; Coronella, C.J.; Vásquez, V.R. Reaction kinetics of hydrothermal carbonization of loblolly pine. Bioresour. Technol. 2013, 139, 161–169. [Google Scholar] [CrossRef]
- Román, S.; Libra, J.; Berge, N.; Sabio, E.; Ro, K.; Li, L.; Ledesma, B.; Alvarez, A.; Bae, S. Hydrothermal carbonization: Modeling, final properties design and applications: A review. Energies 2018, 11, 216. [Google Scholar] [CrossRef] [Green Version]
- Kruse, A.; Zevaco, T.A. Properties of hydrochar as function of feedstock, reaction conditions and post-treatment. Energies 2018, 11, 674. [Google Scholar] [CrossRef] [Green Version]
- Picone, A.; Volpe, M.; Messineo, A. Process water recirculation during hydrothermal carbonization of waste biomass: Current knowledge and challenges. Energies 2021, 14, 2962. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal carbonisation of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod. Biorefin. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Reza, M.T.; Andert, J.; Wirth, B.; Busch, D.; Pielert, J.; Lynam, J.G.; Mumme, J. Hydrothermal carbonization of biomass for energy and crop production. Appl. Bioenergy 2014, 1, 11–29. [Google Scholar] [CrossRef]
- Ameen, M.; Zamri, N.M.; May, S.T.; Azizan, M.T.; Aqsha, A.; Sabzoi, N.; Sher, F. Effect of acid catalysts on hydrothermal carbonization of Malaysian oil palm residues (leaves, fronds, and shells) for hydrochar production. Biomass Convers. Biorefin. 2021, 1–12. [Google Scholar] [CrossRef]
- Maniscalco, M.P.; Volpe, M.; Messineo, A. Hydrothermal carbonization as a valuable tool for energy and environmental applications: A review. Energies 2020, 13, 4098. [Google Scholar] [CrossRef]
- Pauline, A.L.; Joseph, K. Hydrothermal carbonization of organic wastes to carbonaceous solid fuel—A review of mechanisms and process parameters. Fuel 2020, 279, 118472. [Google Scholar] [CrossRef]
- Lucian, M.; Volpe, M.; Gao, L.; Piro, G.; Goldfarb, J.L.; Fiori, L. Impact of hydrothermal carbonization conditions on the formation of hydrochars and secondary chars from the organic fraction of municipal solid waste. Fuel 2018, 233, 257–268. [Google Scholar] [CrossRef]
- Shafie, S.A.; Al-attab, K.A.; Zainal, Z.A. Effect of hydrothermal and vapothermal carbonization of wet biomass waste on bound moisture removal and combustion characteristics. Appl. Therm. Eng. 2018, 139, 187–195. [Google Scholar] [CrossRef]
- Acharjee, T.C.; Coronella, C.J.; Vasquez, V.R. Effect of thermal pretreatment on equilibrium moisture content of lignocellulosic biomass. Bioresour. Technol. 2011, 102, 4849–4854. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Andreottola, G.; Elagroudy, S.; Negm, M.S.; Fiori, L. Coupling hydrothermal carbonization and anaerobic digestion for sewage digestate management: Influence of hydrothermal treatment time on dewaterability and bio-methane production. J. Environ. Manag. 2021, 281, 111910. [Google Scholar] [CrossRef]
- Szufa, S.; Piersa, P.; Adrian, Ł.; Czerwińska, J.; Lewandowski, A.; Lewandowska, W.; Sielski, J.; Dzikuć, M.; Wróbel, M.; Jewiarz, M.; et al. Sustainable drying and torrefaction processes of miscanthus for use as a pelletized solid biofuel and biocarbon-carrier for fertilizers. Molecules 2021, 26, 1014. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Li, A. Hydrothermal treatment coupled with mechanical expression at increased temperature for excess sludge dewatering: Influence of operating conditions and the process energetics. Water Res. 2014, 65, 85–97. [Google Scholar] [CrossRef]
- Wang, L.F.; Qian, C.; Jiang, J.K.; Ye, X.D.; Yu, H.Q. Response of extracellular polymeric substances to thermal treatment in sludge dewatering process. Environ. Pollut. 2017, 231, 1388–1392. [Google Scholar] [CrossRef]
- Louwes, A.C.; Halfwerk, R.B.; Bramer, E.A.; Brem, G. Experimental study on fast pyrolysis of raw and torrefied woody Biomass. Energy Technol. 2020, 8, 1900799. [Google Scholar] [CrossRef] [Green Version]
- Louwes, A.C.; Basile, L.; Yukananto, R.; Bhagwandas, J.C.; Bramer, E.A.; Brem, G. Torrefied biomass as feed for fast pyrolysis: An experimental study and chain analysis. Biomass Bioenergy 2017, 105, 116–126. [Google Scholar] [CrossRef]
- Kaczor, Z.; Buliński, Z.; Werle, S. Modelling approaches to waste biomass pyrolysis: A review. Renew. Energy 2020, 159, 427–443. [Google Scholar] [CrossRef]
- Sobek, S.; Werle, S. Solar pyrolysis of waste biomass: Part 1 reactor design. Renew. Energy 2019, 143, 1939–1948. [Google Scholar] [CrossRef]
- Kantorek, M.; Jesionek, K.; Polesek-Karczewska, S.; Ziółkowski, P.; Stajnke, M.; Badur, J. Thermal utilization of meat-and-bone meal using the rotary kiln pyrolyzer and the fluidized bed boiler—The performance of pilot-scale installation. Renew. Energy 2021, 164, 1447–1456. [Google Scholar] [CrossRef]
- Volpe, M.; Wüst, D.; Merzari, F.; Lucian, M.; Andreottola, G.; Kruse, A.; Fiori, L. One stage olive mill waste streams valorisation via hydrothermal carbonisation. Waste Manag. 2018, 80, 224–234. [Google Scholar] [CrossRef]
- Procentese, A.; Russo, M.E.; Di Somma, I.; Marzocchella, A. Kinetic characterization of enzymatic hydrolysis of apple pomace as feedstock for a sugar-based biorefinery. Energies 2020, 13, 1051. [Google Scholar] [CrossRef] [Green Version]
- Steinbach, D.; Kruse, A.; Sauer, J.; Vetter, P. Sucrose is a promising feedstock for the synthesis of the platform chemical hydroxymethylfurfural. Energies 2018, 11, 645. [Google Scholar] [CrossRef] [Green Version]
- Lühmann, T.; Wirth, B. Sewage sludge valorization via hydrothermal carbonization: Optimizing dewaterability and phosphorus release. Energies 2020, 13, 4417. [Google Scholar] [CrossRef]
- Meisel, K.; Clemens, A.; Fühner, C.; Breulmann, M.; Majer, S.; Thrän, D. Comparative life cycle assessment of HTC concepts valorizing sewage sludge for energetic and agricultural use. Energies 2019, 12, 786. [Google Scholar] [CrossRef] [Green Version]
- Paul, S.; Dutta, A.; Defersha, F. Mechanical and alkaline hydrothermal treated corn residue conversion in to bioenergy and biofertilizer: A resource recovery concept. Energies 2018, 11, 516. [Google Scholar] [CrossRef] [Green Version]
- Aragon-Briceño, C.I.; Pozarlik, A.K.; Bramer, E.A.; Niedzwiecki, L.; Pawlak-Kruczek, H.; Brem, G. Hydrothermal carbonization of wet biomass from nitrogen and phosphorus approach: A review. Renew. Energy 2021, 171, 401–415. [Google Scholar] [CrossRef]
- Ismail, H.Y.; Shirazian, S.; Skoretska, I.; Mynko, O.; Ghanim, B.; Leahy, J.J.; Walker, G.M.; Kwapinski, W. ANN-Kriging hybrid model for predicting carbon and inorganic phosphorus recovery in hydrothermal carbonization. Waste Manag. 2019, 85, 242–252. [Google Scholar] [CrossRef]
- Wilk, M.; Magdziarz, A.; Kalemba-Rec, I.; Szymańska-Chargot, M. Upgrading of green waste into carbon-rich solid biofuel by hydrothermal carbonization: The effect of process parameters on hydrochar derived from acacia. Energy 2020, 202, 117717. [Google Scholar] [CrossRef]
- Atallah, E.; Zeaiter, J.; Ahmad, M.N.; Kwapinska, M.; Leahy, J.J.; Kwapinski, W. The effect of temperature, residence time, and water-sludge ratio on hydrothermal carbonization of DAF dairy sludge. J. Environ. Chem. Eng. 2020, 8, 103599. [Google Scholar] [CrossRef]
- Atallah, E.; Kwapinski, W.; Ahmad, M.N.; Leahy, J.J.; Zeaiter, J. Effect of water-sludge ratio and reaction time on the hydrothermal carbonization of olive oil mill wastewater treatment: Hydrochar characterization. J. Water Process. Eng. 2019, 31, 100813. [Google Scholar] [CrossRef]
- Ghanim, B.M.; Kwapinski, W.; Leahy, J.J. Hydrothermal carbonisation of poultry litter: Effects of initial pH on yields and chemical properties of hydrochars. Bioresour. Technol. 2017, 238, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Aragón-Briceño, C.I.; Grasham, O.; Ross, A.B.; Dupont, V.; Camargo-Valero, M.A. Hydrothermal carbonization of sewage digestate at wastewater treatment works: Influence of solid loading on characteristics of hydrochar, process water and plant energetics. Renew. Energy 2020, 157, 959–973. [Google Scholar] [CrossRef]
- Śliz, M.; Wilk, M. A comprehensive investigation of hydrothermal carbonization: Energy potential of hydrochar derived from Virginia mallow. Renew. Energy 2020, 156, 942–950. [Google Scholar] [CrossRef]
- Surup, G.R.; Leahy, J.J.; Timko, M.T.; Trubetskaya, A. Hydrothermal carbonization of olive wastes to produce renewable, binder-free pellets for use as metallurgical reducing agents. Renew. Energy 2020, 155, 347–357. [Google Scholar] [CrossRef]
- Ghanim, B.M.; Pandey, D.S.; Kwapinski, W.; Leahy, J.J. Hydrothermal carbonisation of poultry litter: Effects of treatment temperature and residence time on yields and chemical properties of hydrochars. Bioresour. Technol. 2016, 216, 373–380. [Google Scholar] [CrossRef]
- Shrestha, A.; Acharya, B.; Farooque, A.A. Study of hydrochar and process water from hydrothermal carbonization of sea lettuce. Renew. Energy 2021, 163, 589–598. [Google Scholar] [CrossRef]
- Atallah, E.; Zeaiter, J.; Ahmad, M.N.; Leahy, J.J.; Kwapinski, W. Hydrothermal carbonization of spent mushroom compost waste compared against torrefaction and pyrolysis. Fuel Process. Technol. 2021, 216, 106795. [Google Scholar] [CrossRef]
- Atallah, E.; Kwapinski, W.; Ahmad, M.N.; Leahy, J.J.; Al-Muhtaseb, A.H.; Zeaiter, J. Hydrothermal carbonization of olive mill wastewater: Liquid phase product analysis. J. Environ. Chem. Eng. 2019, 7, 102833. [Google Scholar] [CrossRef]
- Parmar, K.R.; Ross, A.B. Integration of hydrothermal carbonisation with anaerobic digestion; Opportunities for valorisation of digestate. Energies 2019, 12, 1586. [Google Scholar] [CrossRef] [Green Version]
- Ferrentino, R.; Merzari, F.; Fiori, L.; Andreottola, G. Coupling hydrothermal carbonization with anaerobic digestion for sewage sludge treatment: Influence of HTC liquor and hydrochar on biomethane production. Energies 2020, 13, 6262. [Google Scholar] [CrossRef]
- Musa, B.; Arauzo, P.J.; Olszewski, M.P.; Kruse, A. Electricity generation in microbial fuel cell from wet torrefaction wastewater and locally developed corncob electrodes. Fuel Cells 2021, 21, 182–194. [Google Scholar] [CrossRef]
- Román, F.; Adolph, J.; Hensel, O. Hydrothermal treatment of biogas digestate as a pretreatment to reduce fouling in membrane filtration. Bioresour. Technol. Rep. 2021, 13, 100638. [Google Scholar] [CrossRef]
- Urbanowska, A.; Kabsch-Korbutowicz, M.; Wnukowski, M.; Seruga, P.; Baranowski, M.; Pawlak-Kruczek, H.; Serafin-Tkaczuk, M.; Krochmalny, K.; Niedzwiecki, L. Treatment of liquid by-products of hydrothermal carbonization (HTC) of agricultural digestate using membrane separation. Energies 2020, 13, 262. [Google Scholar] [CrossRef] [Green Version]
- Cao, Z.; Hülsemann, B.; Wüst, D.; Illi, L.; Oechsner, H.; Kruse, A. Valorization of maize silage digestate from two-stage anaerobic digestion by hydrothermal carbonization. Energy Convers. Manag. 2020, 222, 113218. [Google Scholar] [CrossRef]
- Cao, Z.; Jung, D.; Olszewski, M.P.; Arauzo, P.J.; Kruse, A. Hydrothermal carbonization of biogas digestate: Effect of digestate origin and process conditions. Waste Manag. 2019, 100, 138–150. [Google Scholar] [CrossRef] [PubMed]
- MICRODYN-NADIR. Flat Sheet Membrane Data Sheets—MICRODYN-NADIR. Available online: https://www.microdyn-nadir.com/flat-sheet-membrane-data-sheets/ (accessed on 15 February 2021).
- Kabsch-Korbutowicz, M. Ultrafiltration as a method of separation of natural organic matter from water. Mater. Sci. Pol. 2008, 26, 459–467. [Google Scholar]
- Rice, E.W.; Baird, R.B.; Eaton, A.D. (Eds.) Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA; American Water Works Association: Denver, CO, USA; Water Environment Federation: Alexandria, VA, USA, 2017. [Google Scholar]
- Kovacs, Z.; Samhaber, W. Characterization of nanofiltration membranes with uncharged solutes. Membrantechnika 2008, 12, 22–36. [Google Scholar]
- Klein, K.; Kattel, E.; Goi, A.; Kivi, A.; Dulova, N.; Saluste, A.; Zekker, I.; Trapido, M.; Tenno, T. Combined treatment of pyrogenic wastewater from oil shale retorting. Oil Shale 2017, 34, 82–96. [Google Scholar] [CrossRef] [Green Version]
- Lucian, M.; Volpe, M.; Merzari, F.; Wüst, D.; Kruse, A.; Andreottola, G.; Fiori, L. Hydrothermal carbonization coupled with anaerobic digestion for the valorization of the organic fraction of municipal solid waste. Bioresour. Technol. 2020, 314, 123734. [Google Scholar] [CrossRef]
- Córdova, A.; Astudillo, C.; Giorno, L.; Guerrero, C.; Conidi, C.; Illanes, A.; Cassano, A. Nanofiltration potential for the purification of highly concentrated enzymatically produced oligosaccharides. Food Bioprod. Process. 2016, 98, 50–61. [Google Scholar] [CrossRef]
- Reza, M.T.; Werner, M.; Pohl, M.; Mumme, J. Evaluation of Integrated Anaerobic Digestion and Hydrothermal Carbonization for Bioenergy Production. J. Vis. Exp. 2014, 88, e51734. [Google Scholar] [CrossRef] [Green Version]
- Kozłowski, K.; Dach, J.; Lewicki, A.; Malińska, K.; Do Carmo, I.E.P.; Czekała, W. Potential of biogas production from animal manure in Poland. Arch. Environ. Prot. 2019, 45, 99–108. [Google Scholar] [CrossRef]
- Seruga, P.; Krzywonos, M.; Wilk, M. Thermophilic co-digestion of the organic fraction of municipal solid wastes—The influence of food industry wastes addition on biogas production in full-scale operation. Molecules 2018, 23, 3146. [Google Scholar] [CrossRef] [Green Version]
- Krier, J. S69 | LUXPEST | Pesticide Screening List for Luxembourg. Zenodo 2020, 23, 3146. [Google Scholar] [CrossRef]
- Substance Information ECHA. 2,5-Dimethylpyrazine. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.004.200 (accessed on 19 February 2021).
- Moran, E.J.; Easterday, O.D.; Oser, B.L. Acute oral toxicity of selected flavor chemicals. Drug Chem. Toxicol. 1980, 3, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Nishie, K.; Waiss, A.C.; Keyl, A.C. Pharmacology of alkyl and hydroxyalkylpyrazines. Toxicol. Appl. Pharmacol. 1970, 17, 244–249. [Google Scholar] [CrossRef]
- Substance Information ECHA. Pyrazine. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.005.480 (accessed on 19 February 2021).
- PubChem. 2-Ethylpyrazine | C6H8N2. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/26331 (accessed on 19 February 2021).
- FEMA. 2-Ethylpyrazine. Available online: https://www.femaflavor.org/flavor-library/2-ethylpyrazine (accessed on 19 February 2021).
- Amoore, J.E.; Hautala, E. Odor as an ald to chemical safety: Odor thresholds compared with threshold limit values and volatilities for 214 industrial chemicals in air and water dilution. J. Appl. Toxicol. 1983, 3, 272–290. [Google Scholar] [CrossRef]
- PubChem. 3-Phenylpropionic acid C9H10O2. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/107 (accessed on 19 February 2021).
- Substance Information ECHA. Cyclohexanecarboxylic Acid. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.002.465 (accessed on 19 February 2021).
- Substance Information ECHA. Acetamide. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.000.430 (accessed on 19 February 2021).
- Székely, G.; Fritz, E.; Bandarra, J.; Heggie, W.; Sellergren, B. Removal of potentially genotoxic acetamide and arylsulfonate impurities from crude drugs by molecular imprinting. J. Chromatogr. A 2012, 1240, 52–58. [Google Scholar] [CrossRef] [PubMed]



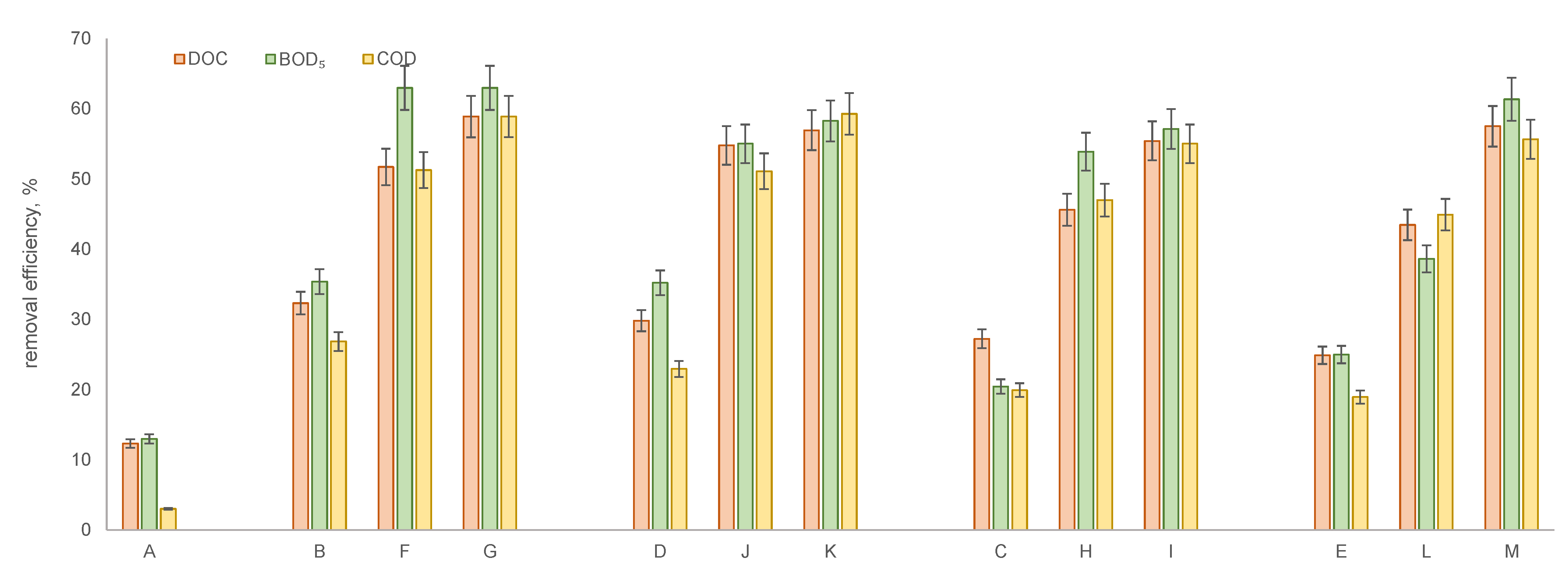

| Index | Value |
|---|---|
| pH | 7.2 |
| Conductivity, mS/cm | 14.95 |
| Total suspended solids, mg/dm3 | 3950 |
| Chemical oxygen demand (COD), mg O2/dm3 | 38.595 |
| 5-day biochemical oxygen demand (BOD5), mg O2/dm3 | 12.320 |
| Dissolved organic carbon (DOC), mg C/dm3 | 23.070 |
| Na, mg/dm3 | 521.3 |
| K, mg/dm3 | 1966.5 |
| Ca, mg/dm3 | 104.7 |
| Mg, mg/dm3 | 101.9 |
| Fe, mg/dm3 | 15.9 |
| Mn, mg/dm3 | 1.5 |
| Cu, mg/dm3 | 0.545 |
| Zn, mg/dm3 | 3.977 |
| Hg, mg/dm3 | 0.0029 |
| Co, mg/dm3 | 0.069 |
| Ni, mg/dm3 | 0.147 |
| Membrane Symbol | Membrane Material | Cut-Off, kDa [87] | Mean Pore Radius, nm [88] | Contact Angle, ° * | Polarity, % [88] | H2O Flux, m3/m2d (0.4 MPa) * |
|---|---|---|---|---|---|---|
| PES 10 | Polyethersulphone | 10 | 2.04 | 52.0 | 44.27 | 6.6 |
| PES 30 | 30 | 8.38 | 13.3 | |||
| C 10 | Regenerated cellulose | 10 | 5.01 | 37.8 | 49.92 | 2.7 |
| C 30 | 30 | 12.55 | 21.5 |
| Membrane Type | Membrane Material | Na2SO4 Retention [87] | Cut-Off, kDa [87] | Contact Angle, ° * | H2O Flux, m3/m2d (0.4 MPa) * |
|---|---|---|---|---|---|
| NP010P | Polyethersulfone | 25–40% | 1040–1400 | 57.5 | 1.3 |
| NP030P | 80–95% | 520–700 | 58.6 | 0.25 |
| Compound | Acetic Acid (MW 60.05 g/mol) | Pyrazine (MW 80.09 g/mol) | Pyrazine, 2,5-Dimethyl- (MW 108.14 g/mol) | Pyrazine, Ethyl- (MW 108.14 g/mol) | Propionic Acid (MW 74.08 g/mol) | Acetamide (MW 59.07 g/mol) | Cyclohexanecarboxylic Acid (MW 128.171 g/mol) | Hydrocinnamic Acid (MW 207.23 g/mol) |
|---|---|---|---|---|---|---|---|---|
| Sample/Unit | mg/dm3 | |||||||
| HTC effluent | 1080 ± 340 | 48 ± 9 | 31 ± 4 | 32 ± 3 | 110 ± 42 | 81 ± 14 | 18 ± 6 | 4 ± 3 |
| (A) MF 0.2 µm | 1210 ± 340 | 40 ± 6 | 36 ± 4 | 36 ± 3 | 91 ± 43 | 89 ± 14 | 24 ± 5 | 2 ± 3 |
| (B) MF 0.2 µm → UF PES 10 kDa | 960 ± 360 | 38 ± 6 | 34 ± 4 | 33 ± 3 | 155 ± 44 | 89 ± 14 | 20 ± 5 | 0.3 ± 3 |
| (C) MF 0.2 µm → UF PES 30 kDa | 1480 ± 350 | 42 ± 6 | 37 ± 4 | 38 ± 3 | 104 ± 45 | 94 ± 14 | 28 ± 5 | 2 ± 3 |
| (D) MF 0.2 µm → UF C 10 kDa | 1050 ± 350 | 38 ± 6 | 34 ± 4 | 34 ± 3 | 165 ± 45 | 87 ± 14 | 20 ± 5 | 4 ± 3 |
| (E) MF 0.2 µm → UF C 30 kDa | 1150 ± 340 | 40 ± 6 | 37 ± 4 | 37 ± 3 | 75 ± 42 | 90 ± 14 | 24 ± 5 | 1 ± 3 |
| (F) MF 0.2 µm → UF PES 10 kDa → NF NPO10P | 560 ± 340 | 40 ± 6 | 37 ± 4 | 33 ± 3 | 77 ± 42 | 97 ± 14 | 16 ± 5 | n.d. |
| (G) MF 0.2 µm → UF PES 10 kDa → NF NPO30P | 510 ± 340 | 37 ± 6 | 29 ± 4 | 30 ± 3 | 110 ± 42 | 86 ± 14 | 14 ± 5 | n.d. |
| (H) MF 0.2 µm → UF PES 30 kDa → NF NPO10P | 1650 ± 390 | 41 ± 7 | 33 ± 6 | 31 ± 6 | 104 ± 43 | 108 ± 15 | 28 ± 5 | n.d. |
| (I) MF 0.2 µm → UF PES 30 kDa → NF NPO30P | n.d. | 45 ± 6 | 36 ± 4 | 35 ± 3 | 15 ± 42 | 106 ± 14 | 12 ± 5 | n.d. |
| (J) MF 0.2 µm → UF C 10 kDa → NF NPO10P | 340 ± 350 | 40 ± 6 | 34 ± 4 | 33 ± 3 | 61 ± 43 | 96 ± 15 | 13 ± 6 | n.d. |
| (K) MF 0.2 µm → UF C 10 kDa → NF NPO30P | 590 ± 340 | 38 ± 6 | 31 ± 4 | 30 ± 3 | 128 ± 42 | 88 ± 14 | 15 ± 5 | n.d. |
| (L) MF 0.2 µm → UF C 30 kDa → NF NPO10P | 1500 ± 360 | 42 ± 6 | 37 ± 4 | 36 ± 3 | 94 ± 42 | 98 ± 14 | 25 ± 5 | n.d. |
| (M) MF 0.2 µm → UF C 30 kDa → NF NPO30P | n.d. | 40 ± 6 | 31 ± 4 | 30 ± 4 | 16 ± 42 | 103 ± 15 | 10 ± 5 | n.d. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbanowska, A.; Kabsch-Korbutowicz, M.; Aragon-Briceño, C.; Wnukowski, M.; Pożarlik, A.; Niedzwiecki, L.; Baranowski, M.; Czerep, M.; Seruga, P.; Pawlak-Kruczek, H.; et al. Cascade Membrane System for Separation of Water and Organics from Liquid By-Products of HTC of the Agricultural Digestate—Evaluation of Performance. Energies 2021, 14, 4752. https://doi.org/10.3390/en14164752
Urbanowska A, Kabsch-Korbutowicz M, Aragon-Briceño C, Wnukowski M, Pożarlik A, Niedzwiecki L, Baranowski M, Czerep M, Seruga P, Pawlak-Kruczek H, et al. Cascade Membrane System for Separation of Water and Organics from Liquid By-Products of HTC of the Agricultural Digestate—Evaluation of Performance. Energies. 2021; 14(16):4752. https://doi.org/10.3390/en14164752
Chicago/Turabian StyleUrbanowska, Agnieszka, Małgorzata Kabsch-Korbutowicz, Christian Aragon-Briceño, Mateusz Wnukowski, Artur Pożarlik, Lukasz Niedzwiecki, Marcin Baranowski, Michał Czerep, Przemysław Seruga, Halina Pawlak-Kruczek, and et al. 2021. "Cascade Membrane System for Separation of Water and Organics from Liquid By-Products of HTC of the Agricultural Digestate—Evaluation of Performance" Energies 14, no. 16: 4752. https://doi.org/10.3390/en14164752







