Estimating the State of Health of Lithium-Ion Batteries with a High Discharge Rate through Impedance
Abstract
:1. Introduction
2. Modelling of the Battery Based on the Impedance Spectroscopy
3. Materials and Methods
4. Results and Analysis
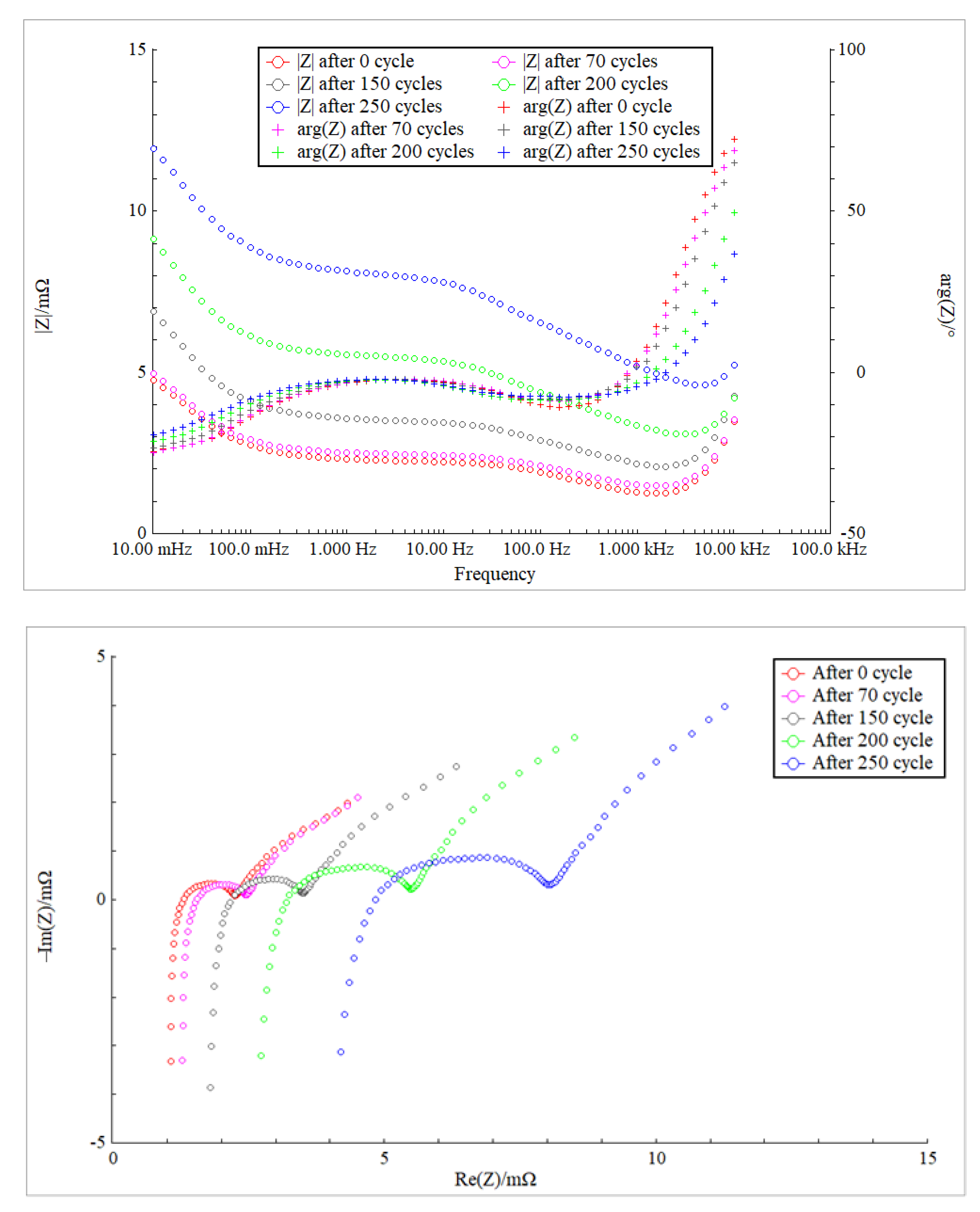
4.1. Impedance Analysis
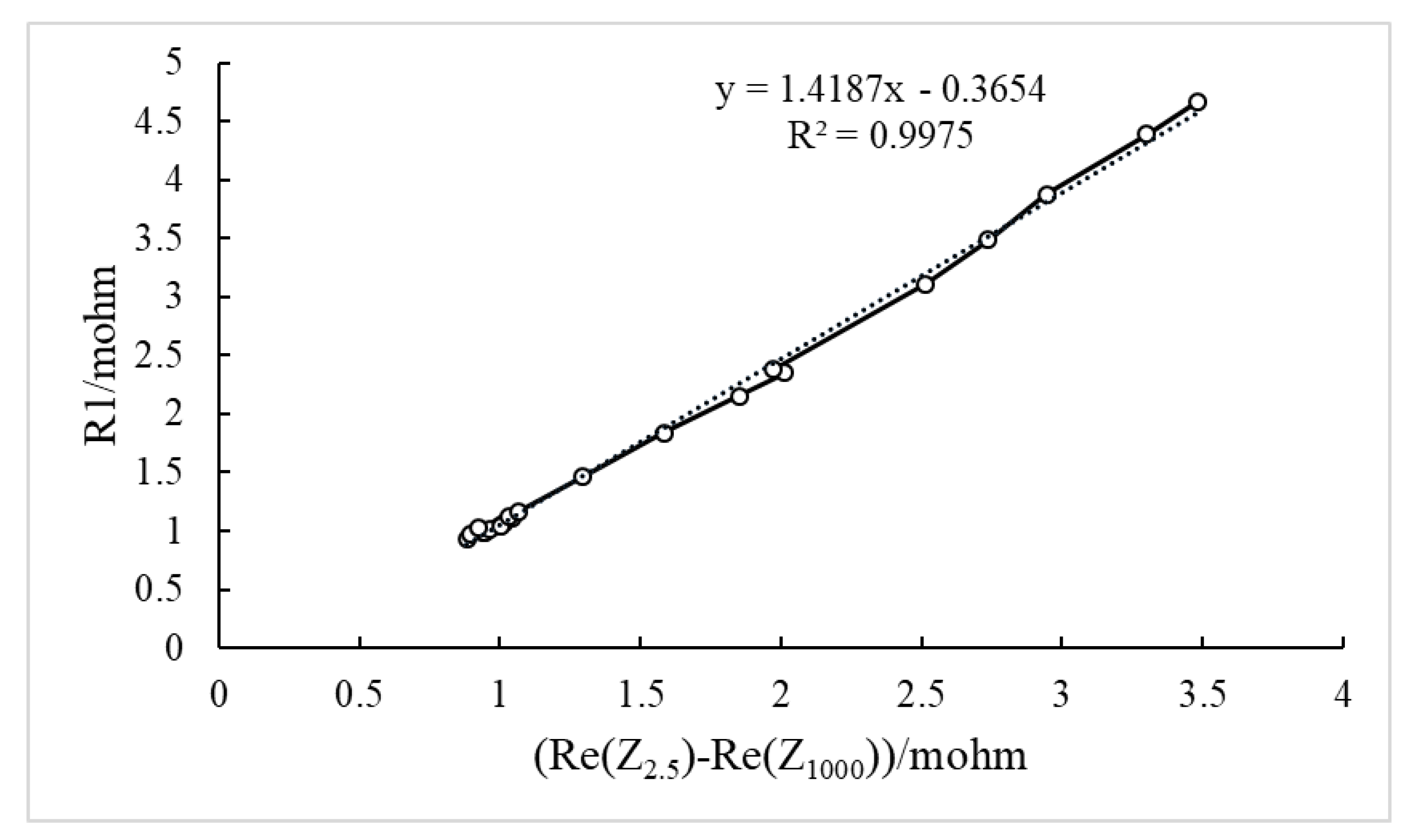
4.2. SOH Estimation Based on EIS
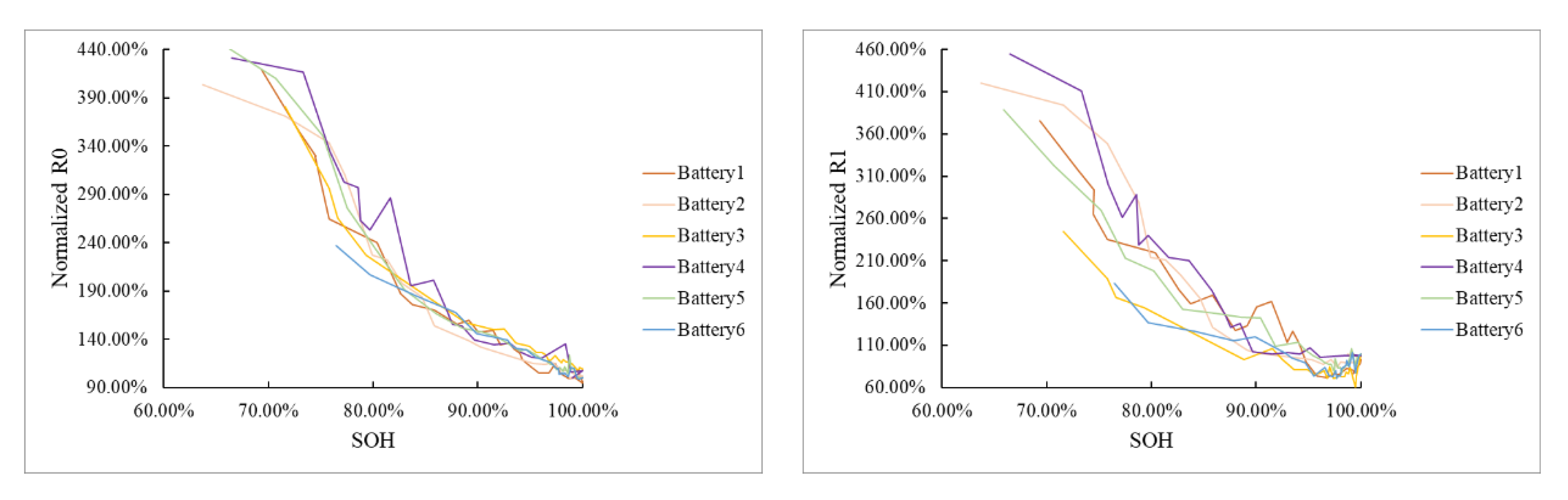
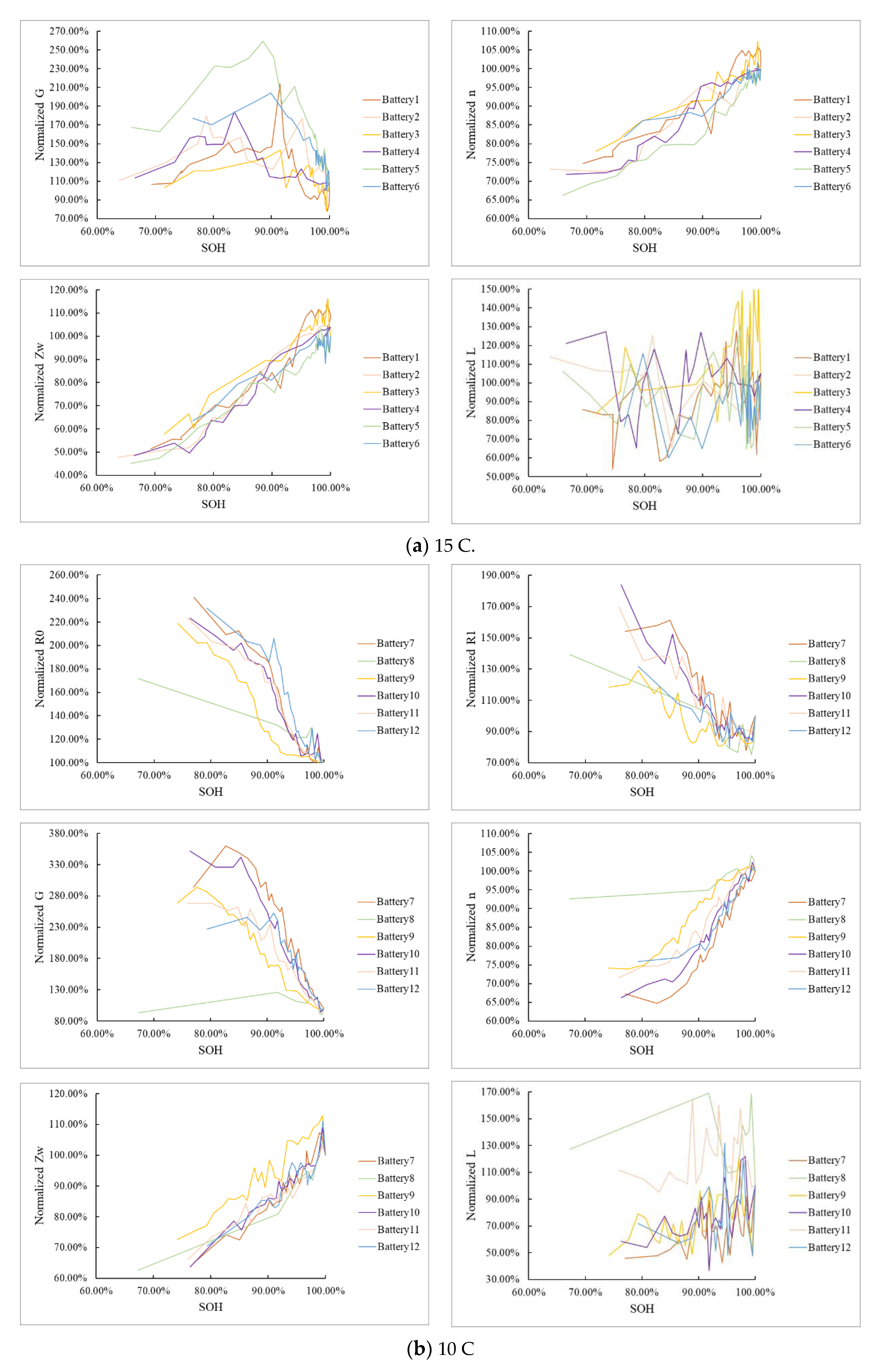
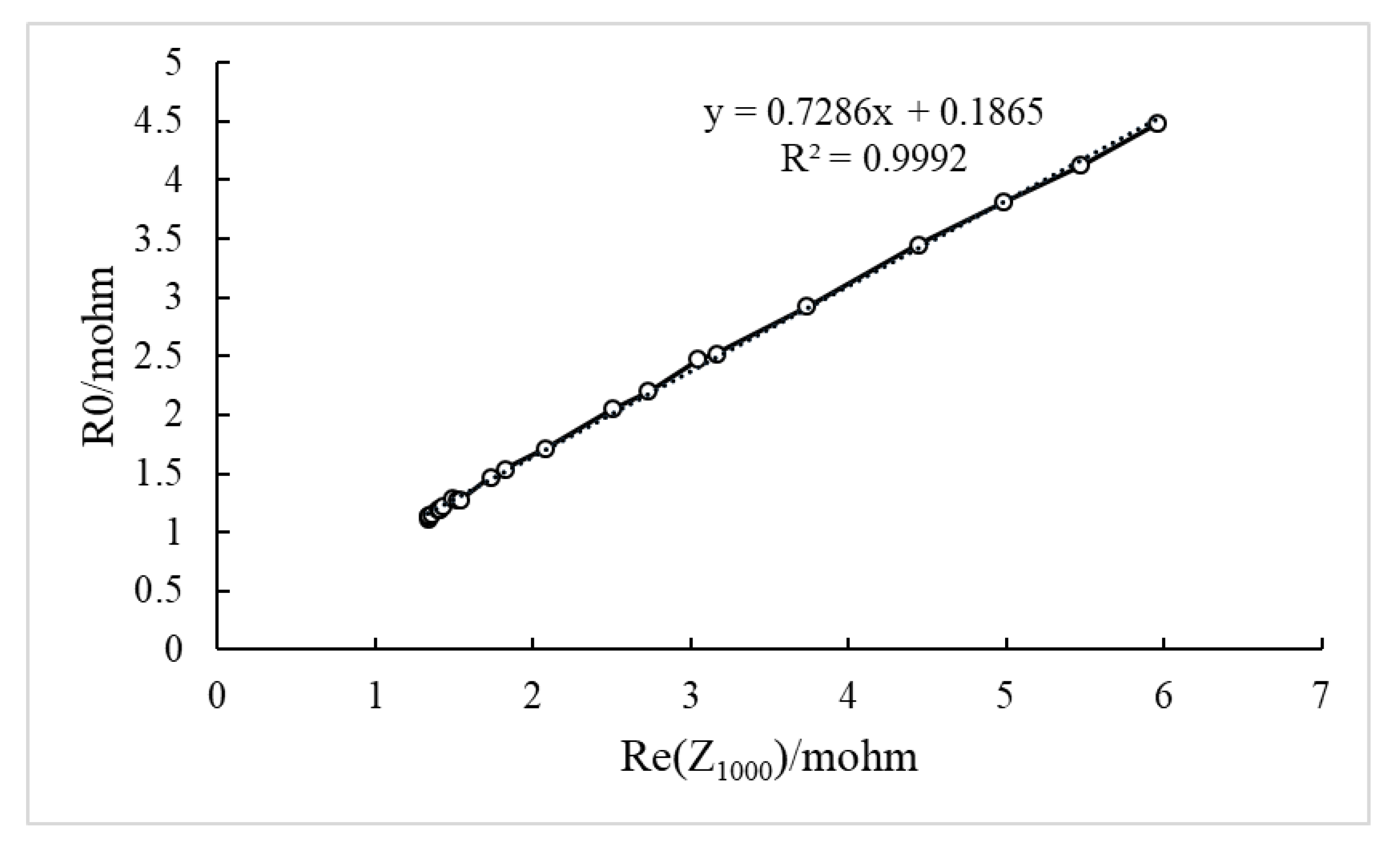
4.3. Determination of Characteristic Frequencies
4.3.1. Determination of the Frequency Related to R0
4.3.2. Determination of Frequency Related to R1
4.3.3. Determination of Frequency Related to n
4.4. SOH Estimation Based on Impedance at Characteristic Frequencies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

| Regression Function: y = a0 + a1x1 + a2x2 +… | ||||||||
|---|---|---|---|---|---|---|---|---|
| Regression Coefficients | a0 | a1 | a2 | a3 | a4 | a5 | a6 | |
| 6-PARM model (15 C) | Value | 0.1553 | −0.0420 | 0.0320 | 0.7185 | 0.1383 | 0.0243 | 0.0720 |
| t statistic | 0.9112 | −3.7802 | 2.6188 | 3.2765 | 3.8469 | 3.5903 | 0.3623 | |
| p value | 0.3653 | 0.0003 | 0.0108 | 0.0016 | 0.0003 | 0.0006 | 0.7182 | |
| 4-PARM model (15 C) | Value | 0.8177 | −0.0525 | 0.0139 | −0.2325 | 0.0003 | ||
| t statistic | 10.3665 | −5.2303 | 1.2157 | 0.5001 | 5.1017 | |||
| p value | 0.0000 | 0.0000 | 0.2280 | 0.6185 | 0.0000 | |||
| 3-PARM model (15 C) | Value | 0.5532 | −0.0729 | 0.0234 | 0.6169 | |||
| t statistic | 7.8030 | −6.1914 | 1.8603 | 7.4872 | ||||
| p value | 0.0000 | 0.0000 | 0.0668 | 0.0000 | ||||
| 6-PARM model (10 C) | Value | −1.5530 | −0.0565 | 0.1277 | 2.4500 | 0.1817 | 0.0677 | 0.5931 |
| t statistic | −1.9177 | −1.1560 | 4.0218 | 3.1012 | 2.5257 | 2.1798 | 1.1241 | |
| p value | 0.0599 | 0.2523 | 0.0002 | 0.0029 | 0.0142 | 0.0332 | 0.2655 | |
| 4-PARM model (10 C) | Value | 0.7217 | −0.0560 | 0.0134 | 0.0650 | 0.1840 | ||
| t statistic | 3.3933 | −3.6346 | 3.0468 | −1.3009 | 3.8290 | |||
| p value | 0.0012 | 0.0006 | 0.0034 | 0.1981 | 0.0003 | |||
| 3-PARM model (10 C) | Value | 1.1990 | −0.1922 | 0.0555 | −0.1140 | |||
| t statistic | 5.2254 | −4.3484 | 1.6565 | −0.4884 | ||||
| p value | 0.0000 | 0.0001 | 0.1026 | 0.6270 | ||||
| Regression Function: y = a0 + a1x1 + a2x2 + a3x3 | |||||
|---|---|---|---|---|---|
| Regression Coefficients | a0 | a1 | a2 | a3 | |
| 15 C | Value | 1.0660 | −0.0895 | −0.0974 | 0.0210 |
| Standard error | 89.6795 | −2.8865 | −0.8607 | 0.7507 | |
| p value | 0.0000 | 0.0051 | 0.3922 | 0.4552 | |
| 10 C | Value | 1.1120 | −0.3384 | −0.4210 | 0.1831 |
| t statistic | 20.084 | −3.145 | −1.778 | 2.226 | |
| p value | 0.000 | 0.0025 | 0.0803 | 0.0296 | |
References
- Li, Y.; Liu, K.; Foley, A.M.; Zülke, A.; Berecibar, M.; Nanini-Maury, E.; Van Mierlo, J.; Hoster, H.E. Data-Driven Health Estimation and Lifetime Prediction of Lithium-Ion Batteries: A Review. Renew. Sustain. Energy Rev. 2019, 113, 109254. [Google Scholar] [CrossRef]
- Ge, M.-F.; Liu, Y.; Jiang, X.; Liu, J. A Review on State of Health Estimations and Remaining Useful Life Prognostics of Lithium-Ion Batteries. Measurement 2021, 174, 109057. [Google Scholar] [CrossRef]
- Wong, D.N.; Wetz, D.A.; Heinzel, J.M.; Mansour, A.N. Characterizing Rapid Capacity Fade and Impedance Evolution in High Rate Pulsed Discharged Lithium Iron Phosphate Cells for Complex, High Power Loads. J. Power Sources 2016, 328, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.M.; Zhong, X.; Sun, Z.; Xiong, H.; Hu, W. Real-Time Frequency Regulation Using Aggregated Electric Vehicles in Smart Grid. Comput. Ind. Eng. 2019, 134, 11–26. [Google Scholar] [CrossRef]
- Spotnitz, R. Simulation of Capacity Fade in Lithium-Ion Batteries. J. Power Sources 2003, 113, 72–80. [Google Scholar] [CrossRef]
- Arora, P.; White, R.; Doyle, M. Capacity Fade Mechanisms and Side Reactions in Lithium-Ion Batteries. J. Electrochem. Soc. 1998, 145, 3647–3667. [Google Scholar] [CrossRef] [Green Version]
- van Schalkwijk, W.; Scrosati, B. Advances in Lithium-Ion Batteries, 1st ed.; Springer: New York, NY, USA, 2002; ISBN 9780306475085. [Google Scholar]
- Broussely, M.; Herreyre, S.; Biensan, P.; Kasztejna, P.; Nechev, K.; Staniewicz, R.J. Aging Mechanism in Li Ion Cells and Calendar Life Predictions. J. Power Sources 2001, 97–98, 13–21. [Google Scholar] [CrossRef]
- Markovsky, B.; Rodkin, A.; Cohen, Y.S.; Palchik, O.; Levi, E.; Aurbach, D.; Kim, H.-J.; Schmidt, M. The Study of Capacity Fading Processes of Li-Ion Batteries: Major Factors That Play a Role. J. Power Sources 2003, 119–121, 504–510. [Google Scholar] [CrossRef]
- Berecibar, M.; Gandiaga, I.; Villarreal, I.; Omar, N.; Van Mierlo, J.; Van den Bossche, P. Critical Review of State of Health Estimation Methods of Li-Ion Batteries for Real Applications. Renew. Sustain. Energy Rev. 2016, 56, 572–587. [Google Scholar] [CrossRef]
- Pastor-Fernández, C.; Yu, T.F.; Widanage, W.D.; Marco, J. Critical Review of Non-Invasive Diagnosis Techniques for Quantification of Degradation Modes in Lithium-Ion Batteries. Renew. Sustain. Energy Rev. 2019, 109, 138–159. [Google Scholar] [CrossRef]
- Hu, C.; Youn, B.D.; Chung, J. A Multiscale Framework with Extended Kalman Filter for Lithium-Ion Battery SOC and Capacity Estimation. Appl. Energy 2012, 92, 694–704. [Google Scholar] [CrossRef]
- Fang, L.; Li, J.; Peng, B. Online Estimation and Error Analysis of Both SOC and SOH of Lithium-Ion Battery Based on DEKF Method. Energy Procedia 2019, 158, 3008–3013. [Google Scholar] [CrossRef]
- Mawonou, K.S.R.; Eddahech, A.; Dumur, D.; Beauvois, D.; Godoy, E. Improved State of Charge Estimation for Li-Ion Batteries Using Fractional Order Extended Kalman Filter. J. Power Sources 2019, 435, 226710. [Google Scholar] [CrossRef]
- Li, Y.; Abdel-Monem, M.; Gopalakrishnan, R.; Berecibar, M.; Nanini-Maury, E.; Omar, N.; van den Bossche, P.; Van Mierlo, J. A Quick On-Line State of Health Estimation Method for Li-Ion Battery with Incremental Capacity Curves Processed by Gaussian Filter. J. Power Sources 2018, 373, 40–53. [Google Scholar] [CrossRef]
- Xing, Y.; Ma, E.W.M.; Tsui, K.-L.; Pecht, M. An Ensemble Model for Predicting the Remaining Useful Performance of Lithium-Ion Batteries. Microelectron. Reliab. 2013, 53, 811–820. [Google Scholar] [CrossRef]
- Waag, W.; Käbitz, S.; Sauer, D.U. Experimental Investigation of the Lithium-Ion Battery Impedance Characteristic at Various Conditions and Aging States and Its Influence on the Application. Appl. Energy 2013, 102, 885–897. [Google Scholar] [CrossRef]
- Wang, L.; Pan, C.; Liu, L.; Cheng, Y.; Zhao, X. On-Board State of Health Estimation of LiFePO4 Battery Pack through Differential Voltage Analysis. Appl. Energy 2016, 168, 465–472. [Google Scholar] [CrossRef]
- Galeotti, M.; Cinà, L.; Giammanco, C.; Cordiner, S.; Di Carlo, A. Performance Analysis and SOH (State of Health) Evaluation of Lithium Polymer Batteries through Electrochemical Impedance Spectroscopy. Energy 2015, 89, 678–686. [Google Scholar] [CrossRef]
- Murariu, T.; Morari, C. Time-Dependent Analysis of the State-of-Health for Lead-Acid Batteries: An EIS Study. J. Energy Storage 2019, 21, 87–93. [Google Scholar] [CrossRef]
- Stevanatto, L.C.; Brusamarello, V.J.; Tairov, S. Parameter Identification and Analysis of Uncertainties in Measurements of Lead–Acid Batteries. IEEE Trans. Instrum. Meas. 2014, 63, 761–768. [Google Scholar] [CrossRef]
- Haiying, W.; Long, H.; Jianhua, S.; Shuanquan, L.; Feng, W. Study on Correlation with SOH and EIS Model of Li-Ion Battery. In Proceedings of the 2011 6th International Forum on Strategic Technology, Harbin, China, 22–24 August 2011; Volume 1, pp. 261–264. [Google Scholar]
- Mingant, R.; Bernard, J.; Sauvant-Moynot, V.; Delaille, A.; Mailley, S.; Hognon, J.-L.; Huet, F. EIS Measurements for Determining the SoC and SoH of Li-ion Batteries. In Battery/Energy Technology (General)—218th Ecs Meeting; Dudney, N., Johnson, C., Yakovleva, M., Eds.; Electrochemical Society, Inc.: Pennington, NJ, USA, 2011; Volume 33, pp. 41–53. ISBN 9781607682493. [Google Scholar]
- Lin, Q.; Wang, J.; Xiong, R.; Shen, W.; He, H. Towards a Smarter Battery Management System: A Critical Review on Optimal Charging Methods of Lithium Ion Batteries. Energy 2019, 183, 220–234. [Google Scholar] [CrossRef]
- Meng, J.; Cai, L.; Luo, G.; Stroe, D.-I.; Teodorescu, R. Lithium-Ion Battery State of Health Estimation with Short-Term Current Pulse Test and Support Vector Machine. Microelectron. Reliab. 2018, 88–90, 1216–1220. [Google Scholar] [CrossRef]
- Mingant, R.; Bernard, J.; Sauvant-Moynot, V. Novel State-of-Health Diagnostic Method for Li-Ion Battery in Service. Appl. Energy 2016, 183, 390–398. [Google Scholar] [CrossRef] [Green Version]
- Mingant, R.; Bernard, J.; Sauvant-Moynot, V. Towards Onboard Li-Ion Battery State-of-Health Diagnosis by a Virtual Sensor. World Electr. Veh. J. 2012, 5, 405–411. [Google Scholar] [CrossRef] [Green Version]
- Ning, G.; Haran, B.; Popov, B.N. Capacity Fade Study of Lithium-Ion Batteries Cycled at High Discharge Rates. J. Power Sources 2003, 117, 160–169. [Google Scholar] [CrossRef]
- Sun, S.; Guan, T.; Cheng, X.; Zuo, P.; Gao, Y.; Du, C.; Yin, G. Accelerated Aging and Degradation Mechanism of LiFePO4/Graphite Batteries Cycled at High Discharge Rates. RSC Adv. 2018, 8, 25695–25703. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Wang, C.; Miao, C.; Gao, Y.; Sun, Y. Aging Characteristics of Lithium Titanate Batteries under Ultrahigh Discharge Rate. In Proceedings of the 2021 International Conference on Power Grid System and Green Energy (PGSGE 2021), Wuhan, China, 26–28 March 2021; Volume 252. [Google Scholar] [CrossRef]
- Iurilli, P.; Brivio, C.; Wood, V. On the Use of Electrochemical Impedance Spectroscopy to Characterize and Model the Aging Phenomena of Lithium-Ion Batteries: A Critical Review. J. Power Sources 2021, 505, 229860. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.-Y. Cycle-Life Characterization of Automotive Lithium-Ion Batteries with LiNiO2 Cathode. J. Electrochem. Soc. 2009, 156, A527–A535. [Google Scholar] [CrossRef]
- Eddahech, A.; Briat, O.; Henry, H.; Delétage, J.-Y.; Woirgard, E.; Vinassa, J.-M. Ageing Monitoring of Lithium-Ion Cell during Power Cycling Tests. Microelectron. Reliab. 2011, 51, 1968–1971. [Google Scholar] [CrossRef]
- Wong, D.; Shrestha, B.; Wetz, D.A.; Heinzel, J.M. Impact of High Rate Discharge on the Aging of Lithium Nickel Cobalt Aluminum Oxide Batteries. J. Power Sources 2015, 280, 363–372. [Google Scholar] [CrossRef]
- Chen, L.; Lü, Z.; Lin, W.; Li, J.; Pan, H. A New State-of-Health Estimation Method for Lithium-Ion Batteries through the Intrinsic Relationship between Ohmic Internal Resistance and Capacity. Measurement 2018, 116, 586–595. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, X.; Du, Z.; Liao, Q. Aging Performance Characterization and State-of-Health Assessment of Retired Lithium-Ion Battery Modules. J. Energy Storage 2021, 40, 102743. [Google Scholar] [CrossRef]
- Remmlinger, J.; Buchholz, M.; Meiler, M.; Bernreuter, P.; Dietmayer, K. State-of-Health Monitoring of Lithium-Ion Batteries in Electric Vehicles by on-Board Internal Resistance Estimation. J. Power Sources 2011, 196, 5357–5363. [Google Scholar] [CrossRef]
- Oldenburger, M.; Bedürftig, B.; Gruhle, A.; Grimsmann, F.; Richter, E.; Findeisen, R.; Hintennach, A. Investigation of the Low Frequency Warburg Impedance of Li-Ion Cells by Frequency Domain Measurements. J. Energy Storage 2019, 21, 272–280. [Google Scholar] [CrossRef]
- Xiong, R.; Pan, Y.; Shen, W.; Li, H.; Sun, F. Lithium-Ion Battery Aging Mechanisms and Diagnosis Method for Automotive Applications: Recent Advances and Perspectives. Renew. Sustain. Energy Rev. 2020, 131, 110048. [Google Scholar] [CrossRef]
- Pastor-Fernández, C.; Dhammika Widanage, W.; Marco, J.; Gama-Valdez, M.-Á.; Chouchelamane, G.H. Identification and Quantification of Ageing Mechanisms in Lithium-Ion Batteries Using the EIS Technique. In Proceedings of the 2016 IEEE Transportation Electrification Conference and Expo (ITEC), Dearborn, MI, USA, 27–29 June 2016; pp. 1–6. [Google Scholar]








| Item | Manufacturer | Characteristics |
|---|---|---|
| High Discharge Li-PO Battery | GREPOW | Capacity: 5000 mAh |
| Voltage: 3.7 V | ||
| Continuous discharge current: 200 A | ||
| Recommended charge current: 10 A |
| Symbols | y | x1 | x2 | x3 | x4 | x5 | x6 |
|---|---|---|---|---|---|---|---|
| Meanings | SOH/1 | R0/mohm | R1/mohm | n/1 | Zw/(103 × S × s0.5) | G/(S × sn) | L/μH |
| Results | Model with Six Parameters | Model with Four Parameters | Model with Three Parameters | ||||
| Regression Function (15 C) | |||||||
| RMS of relative error | 2.76% | 2.51% | 2.78% | ||||
| Regression Function (10 C) | |||||||
| RMS of relative error | 4.19% | 3.1% | 3.41% | ||||
| Symbols | y | x1 | x2 | x3 |
|---|---|---|---|---|
| Meanings | SOH/1 | Re(Z1000)/mohm | –Im(Z100)/mohm | Re(Z2.5)/mohm |
| Regression Function (at 15 C) | ||||
| RMS of relative error | 4.25% | |||
| Regression Function (at 10 C) | ||||
| RMS of relative error | 3.11% | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, S.; Song, Z. Estimating the State of Health of Lithium-Ion Batteries with a High Discharge Rate through Impedance. Energies 2021, 14, 4833. https://doi.org/10.3390/en14164833
Jiang S, Song Z. Estimating the State of Health of Lithium-Ion Batteries with a High Discharge Rate through Impedance. Energies. 2021; 14(16):4833. https://doi.org/10.3390/en14164833
Chicago/Turabian StyleJiang, Shida, and Zhengxiang Song. 2021. "Estimating the State of Health of Lithium-Ion Batteries with a High Discharge Rate through Impedance" Energies 14, no. 16: 4833. https://doi.org/10.3390/en14164833






