Heat Transfer Performance Potential with a High-Temperature Phase Change Dispersion
Abstract
:1. Introduction
- They offer great stability during both storage and thermo-mechanical cycling;
- They have low degrees of supercooling (through either the addition of nucleating agents or the modification of surfactant properties);
- They have a phase change temperature that matches the desired application in which they will be thermally managing/cooling;
- They have a higher specific heat capacity (generally listed as twice that of water);
- They have a low viscosity such that they have low pressure drops and, thus, low pumping powers;
- They have high heat transfer coefficients.
Envisioned Application
2. Materials and Methods
2.1. Materials
2.2. Thermal Characterisation
2.3. Particle Size Distribution
2.4. Experimental Test Rig
2.5. Equations of Interest
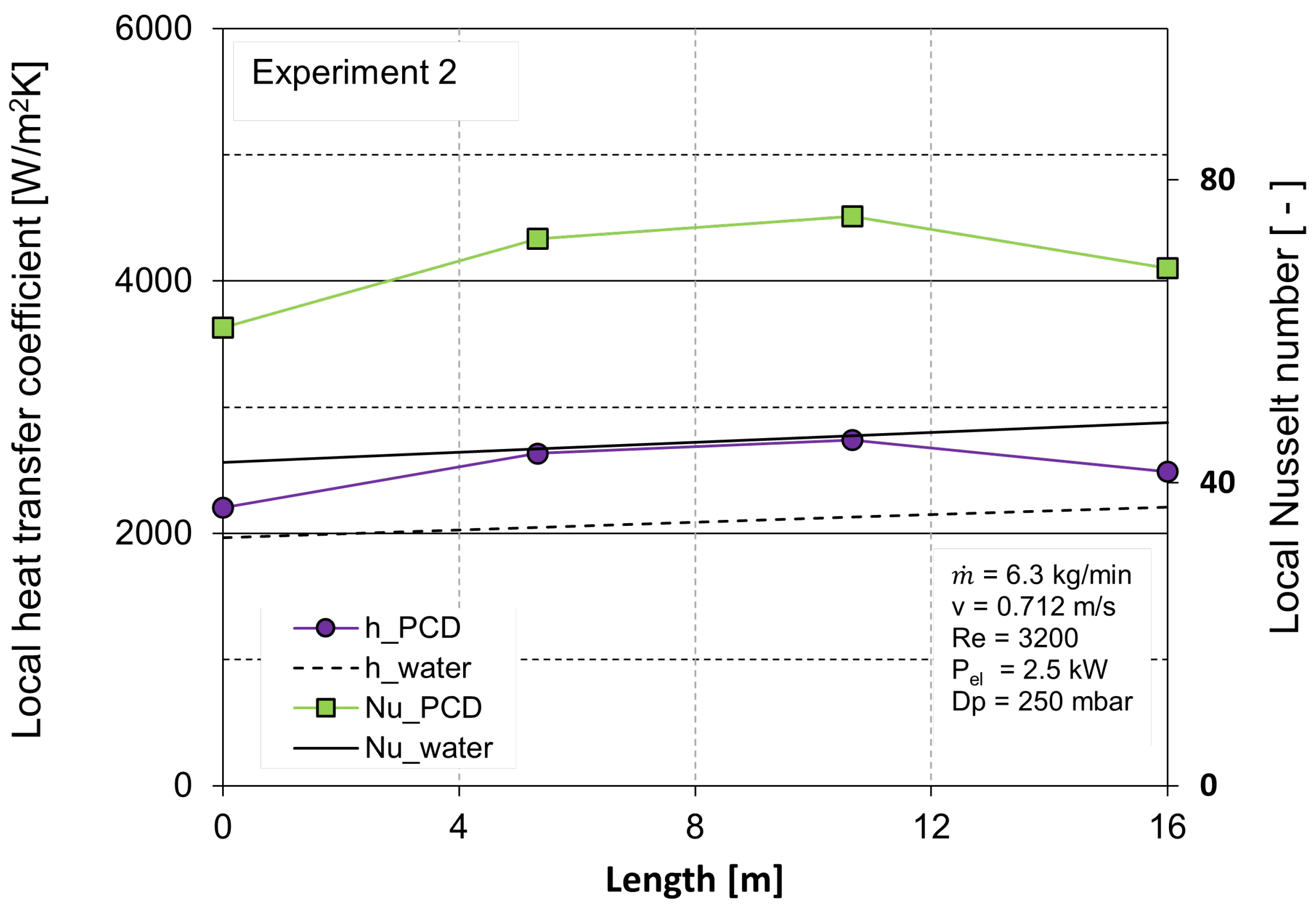
3. Results
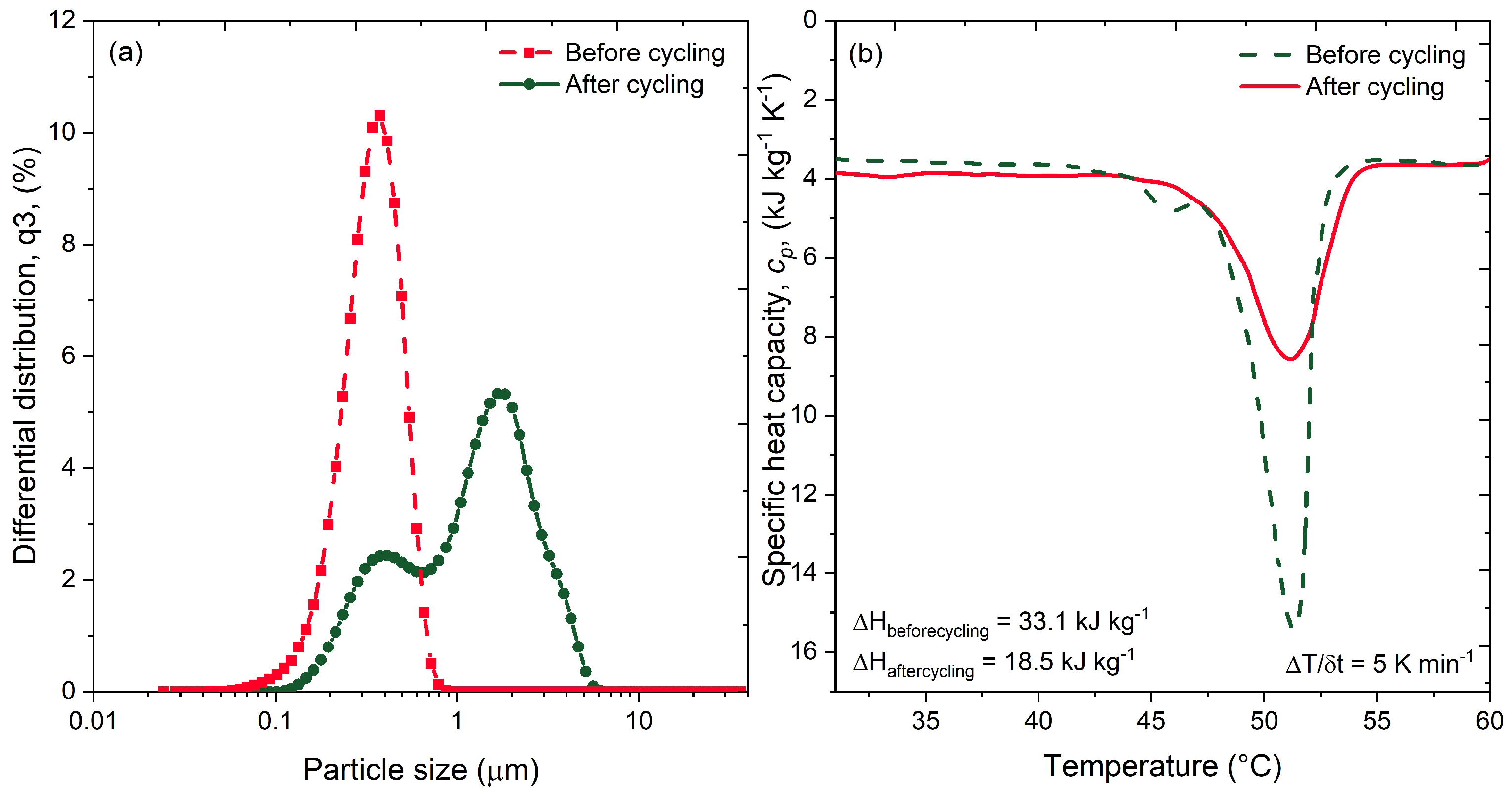
3.1. Thermophysical Characterisation
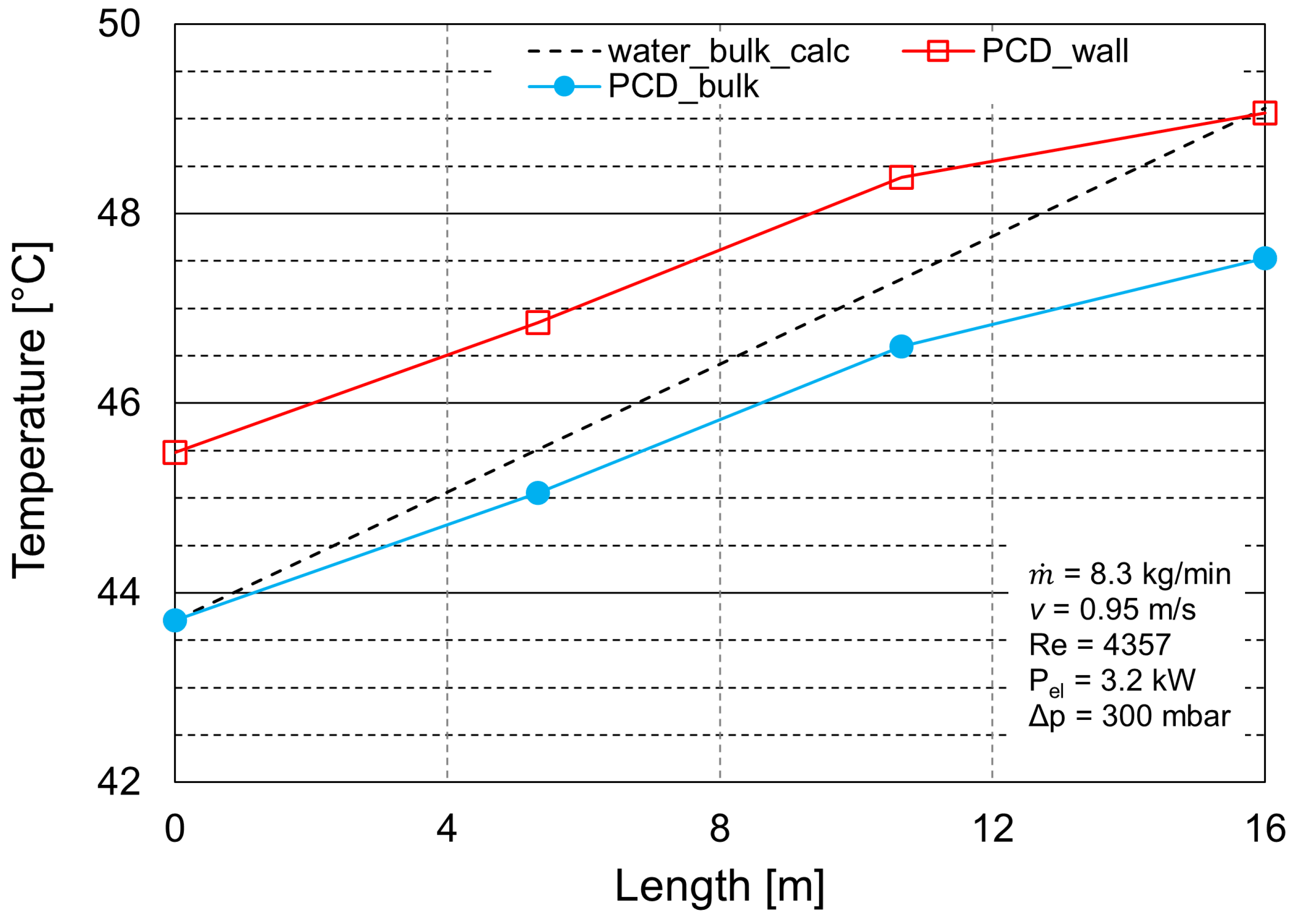
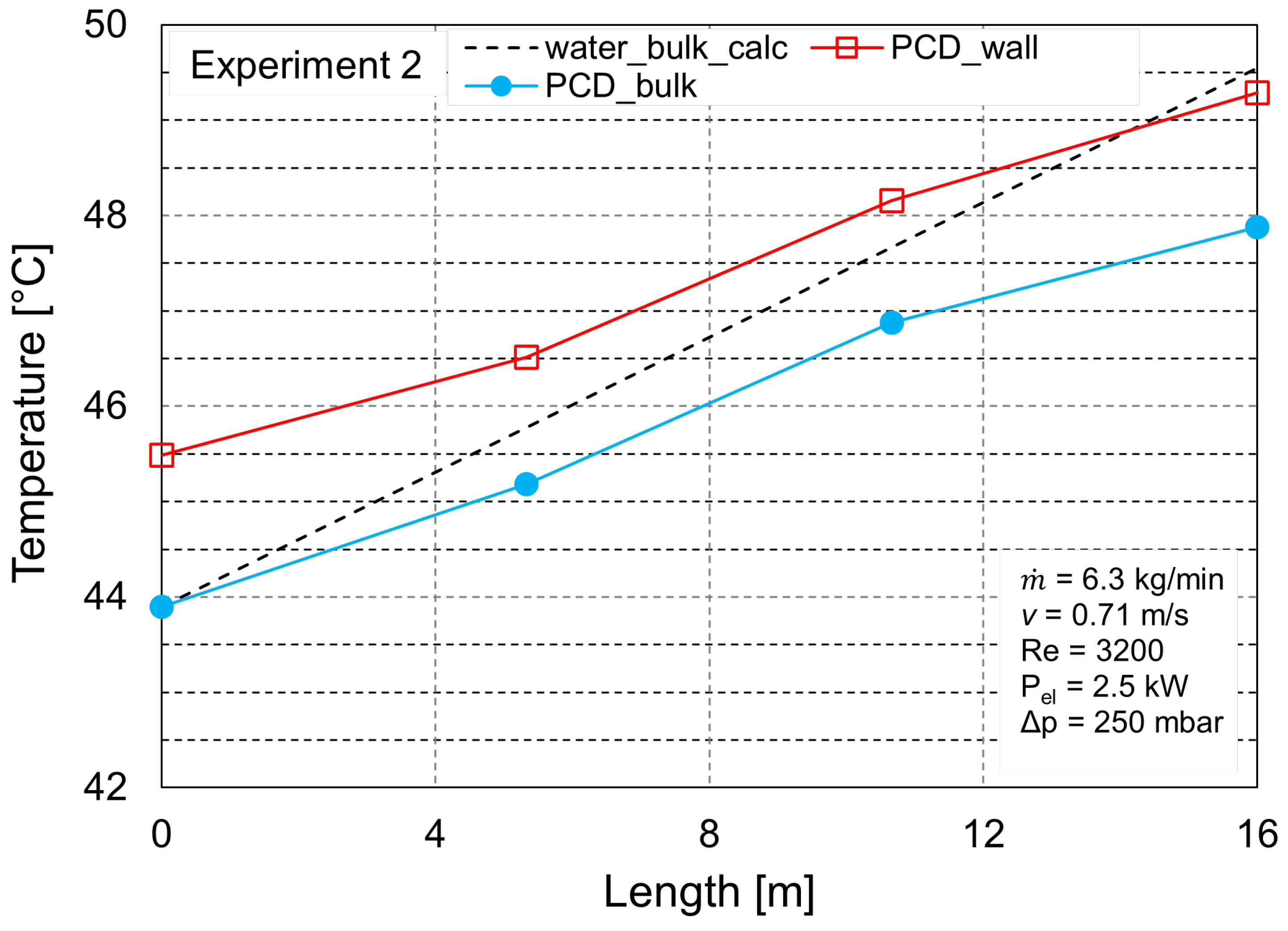
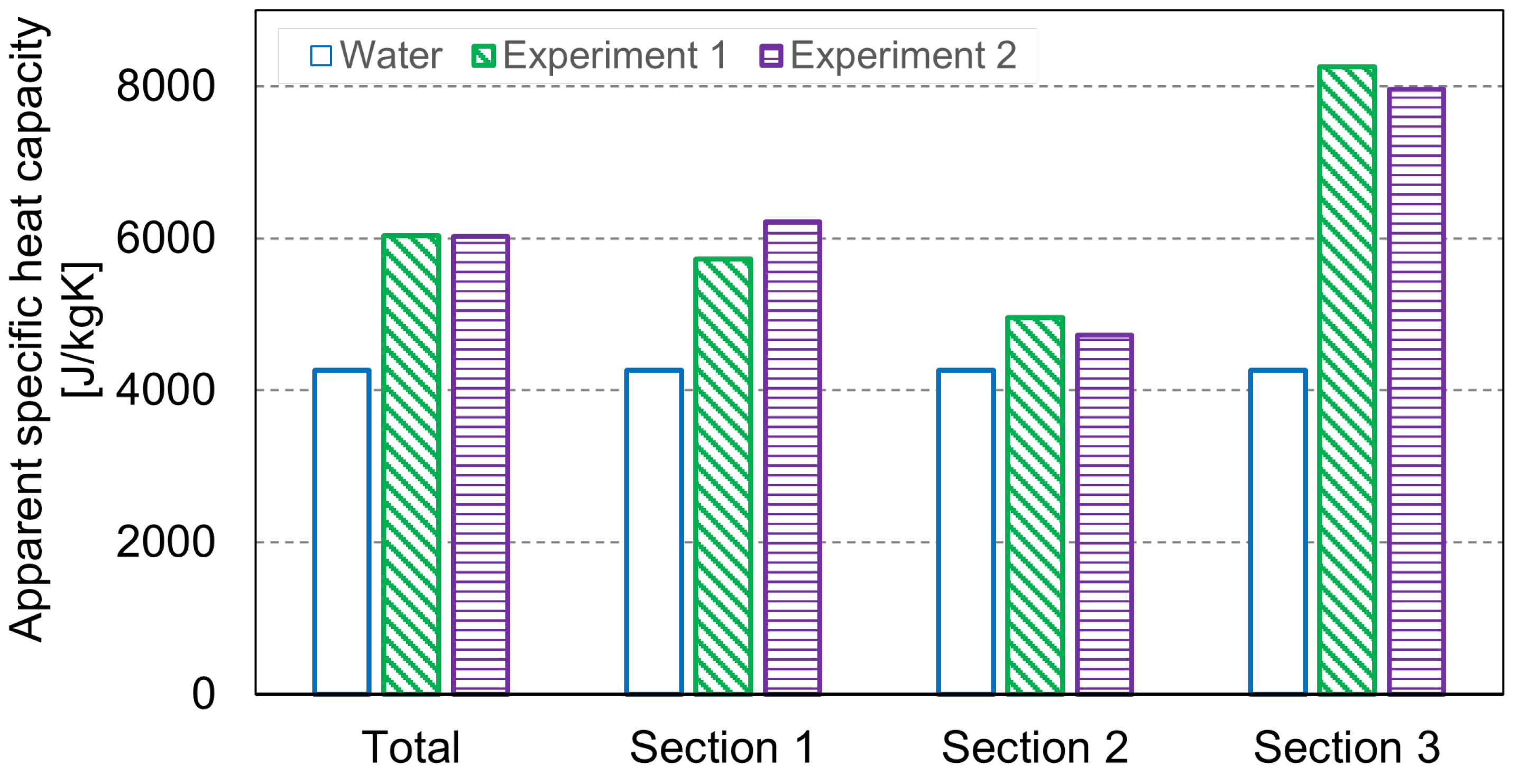
3.2. Heat Transfer Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Roman Letters | Parameter |
| a | Thermal diffusivity (m s) |
| A | Area (m) |
| Cp | Apparent specific heat capacity (J kg K) |
| d | Inner diameter of the pipe (m) |
| h | Convective heat transfer coefficient (W m K) |
| H | Phase change enthalpy (kJ kg) |
| L | Length of pipe (m) |
| Mass flow rate (kg s) | |
| P | Power (W) |
| Heat transfer rate (W) | |
| r | Radius (m) |
| t | Time (s) |
| T | Temperature (°C) |
| Dimensionless Numbers | |
| Nu | Nusselt number |
| Pr | Prandtl number |
| Re | Reynolds number |
| Ste | Stefan number |
| Subscripts | |
| b | Bulk fluid |
| w | Wall |
| el | electrical |
| Greek Letters | |
| Dynamic viscosity (mPa·s) | |
| Thermal conductivity (W m K ) | |
| Density (kg m) | |
| Abbreviations | |
| HVDC | High-voltage direct-current |
| PCM | Phase change material |
| PCD | Phase change dispersion |
| PSD | Particle size distribution |
| FAE | Fatty acid ester |
| NA | Nucleating agents |
| DSC | Differential scanning calorimeter |
| MPCMS | Microencapsulated phase change material slurry |
| HTF | Heat transfer fluid |
References
- O’Neill, P.; Fischer, L.; Revellin, R.; Bonjour, J. Phase change dispersions: A literature review on their thermo-rheological performance for cooling applications. Appl. Therm. Eng. 2021, 192, 116920. [Google Scholar] [CrossRef]
- Fischer, L.J.; von Arx, S.; Wechsler, U.; Züst, S.; Worlitschek, J. Phase change dispersion, potentially a new class of heat transfer fluids. J. Phys. Conf. Ser. 2016, 745, 032133. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Lin, W.; Ling, Z.; Fang, X. A comprehensive review on phase change material emulsions: Fabrication, characteristics, and heat transfer performance. Sol. Energy Mater. Sol. Cells 2019, 191, 218–234. [Google Scholar] [CrossRef]
- Shao, J.; Darkwa, J.; Kokogiannakis, G. Review of phase change emulsions (PCMEs) and their applications in HVAC systems. Energy Build. 2015, 94, 200–217. [Google Scholar] [CrossRef] [Green Version]
- Delgado, M.; Lázaro, A.; Mazo, J.; Zalba, B. Review on phase change material emulsions and microencapsulated phase change material slurries: Materials, heat transfer studies and applications. Renew. Sustain. Energy Rev. 2012, 16, 253–273. [Google Scholar] [CrossRef]
- Wang, F.; Fang, X.; Zhang, Z. Preparation of phase change material emulsions with good stability and little supercooling by using a mixed polymeric emulsifier for thermal energy storage. Sol. Energy Mater. Sol. Cells 2018, 176, 381–390. [Google Scholar] [CrossRef]
- Morimoto, T.; Sugiyama, M.; Kumano, H. Experimental study of heat transfer characteristics of phase change material emulsions in a horizontal circular tube. Appl. Therm. Eng. 2021, 188, 116634. [Google Scholar] [CrossRef]
- Delgado, M.; Lázaro, A.; Mazo, J.; Peñalosa, C.; Dolado, P.; Zalba, B. Experimental analysis of a low cost phase change material emulsion for its use as thermal storage system. Energy Convers. Manag. 2015, 106, 201–212. [Google Scholar] [CrossRef]
- Fischer, L.; Mura, E.; O’Neill, P.; von Arx, S.; Worlitschek, J.; Qiao, G.; Li, Q.; Ding, Y. Thermophysical properties of a phase change dispersion for cooling around 50 °C. Int. J. Refrig. 2020, 119, 410–419. [Google Scholar] [CrossRef]
- Puupponen, S.; Seppälä, A.; Vartia, O.; Saari, K.; Ala-Nissilä, T. Preparation of paraffin and fatty acid phase changing nanoemulsions for heat transfer. Thermochim. Acta 2015, 601, 33–38. [Google Scholar] [CrossRef]
- Zhang, Z.; Yuan, Y.; Zhang, N.; Cao, X. Experimental investigation on thermophysical properties of capric acid–lauric acid phase change slurries for thermal storage system. Energy 2015, 90, 359–368. [Google Scholar] [CrossRef]
- Zhang, Z.; Yuan, Y.; Zhang, N.; Cao, X. Thermophysical properties of some fatty acids/surfactants as phase change slurries for thermal energy storage. J. Chem. Eng. Data 2015, 60, 2495–2501. [Google Scholar] [CrossRef]
- Li, Q.; Qiao, G.; Mura, E.; Li, C.; Fischer, L.; Ding, Y. Experimental and numerical studies of a fatty acid based phase change dispersion for enhancing cooling of high voltage electrical devices. Energy 2020, 198, 117280. [Google Scholar] [CrossRef]
- Li, Q.; Fischer, L.; Qiao, G.; Mura, E.; Li, C.; Ding, Y. High performance cooling of a HVDC converter using a fatty acid ester-based phase change dispersion in a heat sink with double-layer oblique-crossed ribs. Int. J. Energy Res. 2020, 44, 5819–5840. [Google Scholar] [CrossRef]
- Kang, S.S. Advanced cooling for power electronics. In Proceedings of the 2012 7th International Conference on Integrated Power Electronics Systems (Cips), Nuremberg, Germany, 6–8 March 2012; pp. 1–8. [Google Scholar]
- Wang, F.; Cao, J.; Ling, Z.; Zhang, Z.; Fang, X. Experimental and simulative investigations on a phase change material nano-emulsion-based liquid cooling thermal management system for a lithium-ion battery pack. Energy 2020, 207, 118215. [Google Scholar] [CrossRef]
- Kabalyk, Y. Determination of energy loss in power voltage inverters for power supply of locomotive traction motors. Procedia Eng. 2016, 165, 1437–1443. [Google Scholar] [CrossRef]
- Fischer, L.; Maranda, S.; Stamatiou, A.; von Arx, S.; Worlitschek, J. Experimental investigation on heat transfer with a phase change dispersion. Appl. Therm. Eng. 2019, 147, 61–73. [Google Scholar] [CrossRef]
- Morimoto, T.; Kumano, H. Flow and heat transfer characteristics of phase change emulsions in a circular tube: Part 2. Turbulent flow. Int. J. Heat Mass Transf. 2018, 117, 903–911. [Google Scholar] [CrossRef]


| Material of the tubes | Stainless steel 1.401/304L |
| Inner diameter of the pipe (d) | 14 mm |
| Wall thickness of the pipe | 3 mm |
| Total length of the heated part of the pipe | 15.966 m |
| Total length of the measuring section | 16.05 m |
| Material of the flange | Stainless steel 1.4404/316L |
| Flange thickness | 14 mm |
| Length of the inlet section | 140 mm |
| Material of the spring | Stainless steel |
| Diameter of the spring | 3 mm |
| Pitch of the spring | 6.36 mm |
| Thickness of insulation | 50 mm |
| Sensor | Manufacturer | Type | Range | Uncertainty |
|---|---|---|---|---|
| T (T101/03/05/07) | Roth+Co AG | PT100 4 wire, A | −65–200 °C | 0.03 °C |
| T (T102/04/06/08) | Roth+Co AG | PT100 4 wire, A | −65–200 °C | 0.03 °C |
| Coriolis, | Endress+Hauser | F83, Type 4x | 0–6500 | 0.004 |
| Electrical power, P | Jumo GmbH | JUMO TYA 201 | 0–4 kW | 0.002 P |
| Experiment Number | (kg h) | Reynolds Number (-) | T (°C) | Electrical Heat Input (kW) |
|---|---|---|---|---|
| 1 | 500 | 4357 | 43.7 | 3.2 |
| 2 | 375 | 3200 | 43.9 | 2.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fischer, L.; Mura, E.; O’Neill, P.; von Arx, S.; Worlitschek, J.; Qiao, G.; Li, Q.; Ding, Y. Heat Transfer Performance Potential with a High-Temperature Phase Change Dispersion. Energies 2021, 14, 4899. https://doi.org/10.3390/en14164899
Fischer L, Mura E, O’Neill P, von Arx S, Worlitschek J, Qiao G, Li Q, Ding Y. Heat Transfer Performance Potential with a High-Temperature Phase Change Dispersion. Energies. 2021; 14(16):4899. https://doi.org/10.3390/en14164899
Chicago/Turabian StyleFischer, Ludger, Ernesto Mura, Poppy O’Neill, Silvan von Arx, Jörg Worlitschek, Geng Qiao, Qi Li, and Yulong Ding. 2021. "Heat Transfer Performance Potential with a High-Temperature Phase Change Dispersion" Energies 14, no. 16: 4899. https://doi.org/10.3390/en14164899
APA StyleFischer, L., Mura, E., O’Neill, P., von Arx, S., Worlitschek, J., Qiao, G., Li, Q., & Ding, Y. (2021). Heat Transfer Performance Potential with a High-Temperature Phase Change Dispersion. Energies, 14(16), 4899. https://doi.org/10.3390/en14164899








