A Review of Hydrothermal Liquefaction of Biomass for Biofuels Production with a Special Focus on the Effect of Process Parameters, Co-Solvents, and Extraction Solvents
Abstract
:1. Introduction
1.1. Hydrothermal Liquefaction
1.2. Major Lignocellulosic Biomass Feedstocks and HTL Mechanism
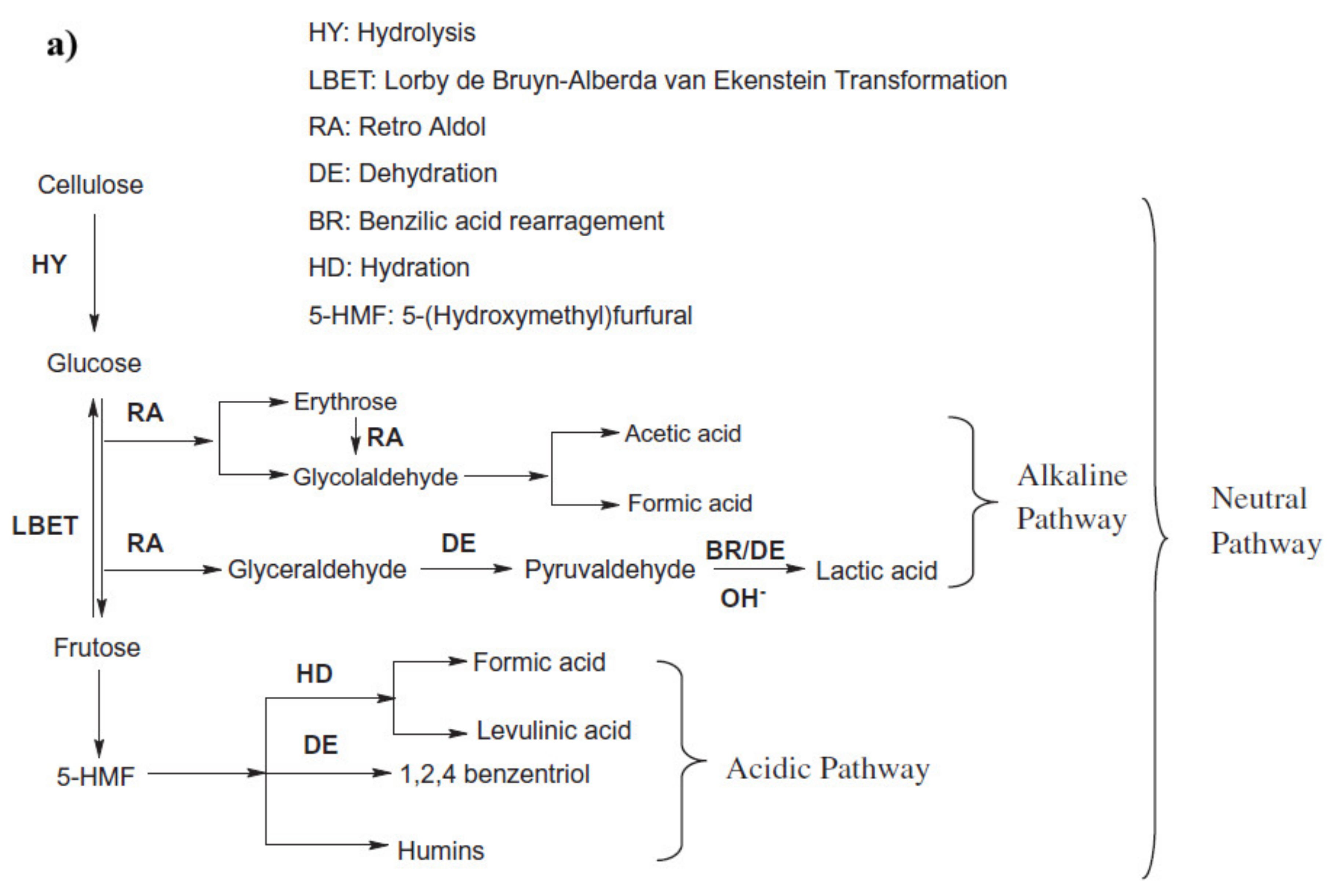
1.2.1. Cellulose Decomposition
1.2.2. Lignin Decomposition
1.3. Hydrothermal Liquefaction Process
1.3.1. Batch Process
1.3.2. Continuous Process
1.3.3. Extraction Methods and Product Calculations
2. Effect of Process Parameters
2.1. Temperature
2.2. Pressure
2.3. Retention Time
2.4. Heating Rate
2.5. Biomass-to-Solvent Ratio
3. Effect of Catalysts and Solvents
3.1. Effect of Catalysts
3.1.1. Homogeneous Catalysts
3.1.2. Heterogeneous Catalysts
3.2. Effect of Solvents and Co-Solvents
3.3. Effect of Extraction Solvent on Products
4. HTL Products and Applications
4.1. Bio-Oil
4.2. Aqueous Phase
4.3. Char
4.4. Gases
5. Conclusions
- A suitable range of temperatures for the HTL of agricultural and forest-based residues is 275–325 °C and the choice of a final temperature strongly varies with the feedstock composition. A lower final temperature results in an incomplete conversion, whereas a temperature above 350 °C promotes carbonization and cracking, leading to an increased char and gas yield.
- Water enhances the decomposition of cellulose, hemicellulose, and to some extent, lignin. The organic solvents help in degradation of lignin. The synergistic effect of using an organic solvent–water mixture during HTL improves the overall liquefaction process.
- In the presence of extraction solvents, many macromolecular oligomers are dissolved, leading to the formation of new compounds and high-value chemicals.
- The catalysts are important to trigger earlier and faster biomass degradation by activating the kinetics of primary reactions, hindering secondary reactions, such as repolymerization and cracking, and reducing dependency on other parameters, such as pressure, temperature, and retention time.
- Undoubtedly, alkali metals are the most effective at easing the degradation of biomass because they disrupt the physical barrier in the biomass. More generally, K2CO3 is a first-rate choice for a catalyst.
- Pressure is important to maintain a single-phase liquid slurry of the feed material so that the reaction can proceed smoothly. In the subcritical temperature range, pressure has some effect on the bio-oil yield, whereas at supercritical conditions, even a pressure of 30–40 MPa is inadequate to affect hydrolysis and the oil yield.
- The choice of retention time is subject to parameters, such as temperature, heating rate, and catalyst. A short retention time can lead to the partial decomposition of biomass, whereas a long retention time can encourage the repolymerization and cracking of oil into char and gas.
6. Challenges and Future Scope
- Some feeds with low overall conversion and poor bio-oil yield during HTL show very promising results in the presence of another class of feedstocks. The research on co-liquefaction is sparse; thus, more can be done to examine the co-liquefaction of two or more feedstocks to improve the understanding of their synergistic effect on bio-oil yield.
- The heating value of bio-oil is low compared to that of conventional liquid fuels, which limits its applications. The low heating value is directly related to the high oxygen content. Hence, there is a need to develop new methodology and techniques to upgrade the bio-oil produced from HTL.
- Both bio-oil and char obtained from HTL contain a significant portion of heterogeneous compounds with nitrogenous impurities. A study can focus on understanding the reaction pathways of major impurities, such as nitrogen, to clarify its biodegradability and toxicity.
- Apart from the experimental section, not enough research has been conducted on the theoretical aspects of the HTL process:
- ◦
- Establishing a correlation for similar types of feedstocks by doing regression analysis.
- ◦
- Developing simulation models using computation fluid dynamics to develop more insight into the effect of the physical properties of biomass water slurry and the effect of various parameters in a continuous pilot scale process.
- ◦
- Utilizing the concept of molecular dynamic simulations to develop a more cohesive understanding on the reaction pathways; such investigations are warranted.
- ◦
- Understanding the techno-economic analysis of commercializing the HTL process and extending it from production all the way through to distillation. A life cycle assessment of the process is further suggested.
- ◦
- More robust understanding about co-processing of bio-oil can be acquired using detailed aspen models on the distillation process.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pudasainee, D.; Kurian, V.; Gupta, R. Application Status of Post-Combustion CO2 Capture; Royal Society of Chemistry (RSC): London, UK, 2018; pp. 259–289. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, C.; Chen, H.; Tsang, D.; Luo, G.; Zhang, S.; Chen, J. Hydrothermal Liquefaction of Agricultural and Forestry Wastes: State-of-the-Art Review and Future Prospects. Bioresour. Technol. 2017, 245, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Oyedun, A.O.; Kumar, A. A Review on the Current Status of Various Hydrothermal Technologies on Biomass Feedstock. Renew. Sustain. Energy Rev. 2018, 81, 1742–1770. [Google Scholar] [CrossRef]
- Basu, P. Introduction. Biomass Gasif. Pyrolysis Torrefaction 2013, 2013, 1–27. [Google Scholar] [CrossRef]
- Vaezi, M.; Kumar, A. Development of Correlations for the Flow of Agricultural Residues as Slurries in Pipes for Bio-Refining. Biosyst. Eng. 2014, 127, 144–158. [Google Scholar] [CrossRef]
- Basu, P. 3.6.2 Proximate Analysis. In Biomass Gasification, Pyrolysis and Torrefaction: Practical Design and Theory; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Xiao, L.-P.; Shi, Z.-J.; Xu, F.; Sun, R.-C. Hydrothermal Carbonization of Lignocellulosic Biomass. Bioresour. Technol. 2012, 118, 619–623. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, R.; Yan, C.; Han, L.; Lei, H.; Ruan, R.; Zhang, X. Bioresource Technology Fast Hydrothermal Co-Liquefaction of Corn Stover and Cow Manure for Biocrude and Hydrochar Production. Bioresour. Technol. 2021, 340, 125630. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Shah, A.; Toor, S.; Seehar, T.; Pedersen, T.; Rosendahl, L. Co-Hydrothermal Liquefaction of Lignocellulosic Biomass in Supercritical Water. Energies 2021, 14, 1708. [Google Scholar] [CrossRef]
- Elliott, D.; Biller, P.; Ross, A.B.; Schmidt, A.J.; Jones, S.B. Hydrothermal Liquefaction of Biomass: Developments from Batch to Continuous Process. Bioresour. Technol. 2015, 178, 147–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krylova, A.Y.; Zaitchenko, V.M. Hydrothermal Carbonization of Biomass: A Review. Solid Fuel Chem. 2018, 52, 91–103. [Google Scholar] [CrossRef]
- Jain, A.; Balasubramanian, R.; Srinivasan, M. Hydrothermal Conversion of Biomass Waste to Activated Carbon with High Porosity: A Review. Chem. Eng. J. 2016, 283, 789–805. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal Carbonization of Biomass: A Summary and Discussion of Chemical Mechanisms for Process Engineering. Biofuels Bioprod. Biorefining 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Mathanker, A. Hydrothermal Liquefaction of Lignocellulosic Biomass to Produce Biofuels. Master’s Thesis. 2020. Available online: https://era.library.ualberta.ca/items/ff551bc6-9676-4108-a098-ca315250fa24 (accessed on 1 July 2021). [CrossRef]
- Zhu, Z.; Toor, S.S.; Rosendahl, L.; Yu, D.; Chen, G. Influence of Alkali Catalyst on Product Yield and Properties via Hydrothermal Liquefaction of Barley Straw. Energy 2015, 80, 284–292. [Google Scholar] [CrossRef]
- Elliott, D.C. Catalytic Hydrothermal Gasification of Biomass. Biofuels Bioprod. Biorefining 2008, 2, 254–265. [Google Scholar] [CrossRef]
- Matsumura, Y. Hydrothermal Gasification of Biomass. Recent Adv. Chem. Convers. Biomass 2015, 2015, 251–267. [Google Scholar] [CrossRef]
- Azadi, P.; Syed, K.; Farnood, R. Catalytic Gasification of Biomass Model Compound in Near-Critical Water. Appl. Catal. A Gen. 2009, 358, 65–72. [Google Scholar] [CrossRef]
- Gollakota, A.; Kishore, N.; Gu, S. A Review on Hydrothermal Liquefaction of Biomass. Renew. Sustain. Energy Rev. 2018, 81, 1378–1392. [Google Scholar] [CrossRef]
- De Jong, W.; Van Ommen, J.R. Biomass As a Sustainable Energy Source for the Future. In Biomass as a Sustainable Energy Source for the Future; Wiley: Hoboken, NJ, USA, 2014; Volume 9781118304, pp. 1–582. [Google Scholar] [CrossRef]
- Tursi, A. A Review on Biomass: Importance, Chemistry, Classification, and Conversion. Biofuel Res. J. 2019, 6, 962–979. [Google Scholar] [CrossRef]
- Rowell, R.M.; Peterssen, R.; Han, J.S. Cell Wall Chemistry. In Handbook of Wood Chemistry and Wood Composites; Routledge: Oxfordshire, UK, 2005. [Google Scholar]
- Tian, Y.; Wang, F.; Djandja, J.O.; Zhang, S.-L.; Xu, Y.-P.; Duan, P.-G. Hydrothermal Liquefaction of Crop Straws: Effect of Feedstock Composition. Fuel 2020, 265, 116946. [Google Scholar] [CrossRef]
- Mathanker, A.; Pudasainee, D.; Kumar, A.; Gupta, R. Hydrothermal Liquefaction of Lignocellulosic Biomass Feedstock to Produce Biofuels: Parametric Study and Products Characterization. Fuel 2020, 271, 117534. [Google Scholar] [CrossRef]
- Patel, M.; Oyedun, A.O.; Kumar, A.; Gupta, R. What Is the Production Cost of Renewable Diesel from Woody Biomass and Agricultural Residue Based on Experimentation? A Comparative Assessment. Fuel Process. Technol. 2019, 191, 79–92. [Google Scholar] [CrossRef]
- Younas, R.; Hao, S.; Zhang, L.; Zhang, S. Hydrothermal Liquefaction of Rice Straw with NiO Nanocatalyst for Bio-Oil Production. Renew. Energy 2017, 113, 532–545. [Google Scholar] [CrossRef]
- Zhu, Z.; Rosendahl, L.; Toor, S.S.; Yu, D.; Chen, G. Hydrothermal Liquefaction of Barley Straw to Bio-Crude Oil: Effects of Reaction Temperature and Aqueous Phase Recirculation. Appl. Energy 2015, 137, 183–192. [Google Scholar] [CrossRef]
- Kaur, R.; Gera, P.; Jha, M.K.; Bhaskar, T. Optimization of Process Parameters for Hydrothermal Conversion of Castor Residue. Sci. Total Environ. 2019, 686, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Bi, Z.; Zhang, J.; Peterson, E.; Zhu, Z.; Xia, C.; Liang, Y.; Wiltowski, T. Biocrude from Pretreated Sorghum Bagasse through Catalytic Hydrothermal Liquefaction. Fuel 2017, 188, 112–120. [Google Scholar] [CrossRef]
- De Caprariis, B.; De Filippis, P.; Petrullo, A.; Scarsella, M. Hydrothermal Liquefaction of Biomass: Influence of Temperature and Biomass Composition on the Bio-Oil Production. Fuel 2017, 208, 618–625. [Google Scholar] [CrossRef]
- De Caprariis, B.; Bavasso, I.; Bracciale, M.P.; Damizia, M.; De Filippis, P.; Scarsella, M. Enhanced Bio-Crude Yield and Quality by Reductive Hydrothermal Liquefaction of Oak Wood Biomass: Effect of Iron Addition. J. Anal. Appl. Pyrolysis 2019, 139, 123–130. [Google Scholar] [CrossRef]
- Chan, Y.H.; Yusup, S.; Quitain, A.T.; Uemura, Y.; Sasaki, M. Bio-Oil Production from Oil Palm Biomass via Subcritical and Supercritical Hydrothermal Liquefaction. J. Supercrit. Fluids 2014, 95, 407–412. [Google Scholar] [CrossRef]
- Wu, X.-F.; Zhou, Q.; Li, M.-F.; Li, S.-X.; Bian, J.; Peng, F. Conversion of Poplar into Bio-Oil via Subcritical Hydrothermal Liquefaction: Structure and Antioxidant Capacity. Bioresour. Technol. 2018, 270, 216–222. [Google Scholar] [CrossRef]
- Malins, K. Production of Bio-Oil via Hydrothermal Liquefaction of Birch Sawdust. Energy Convers. Manag. 2017, 144, 243–251. [Google Scholar] [CrossRef]
- Jasiūnas, L.; Pedersen, T.H.; Toor, S.S.; Rosendahl, L.A. Biocrude Production via Supercritical Hydrothermal Co-Liquefaction of Spent Mushroom Compost and Aspen Wood Sawdust. Renew. Energy 2017, 111, 392–398. [Google Scholar] [CrossRef]
- Durak, H.; Aysu, T. Structural Analysis of Bio-Oils from Subcritical and Supercritical Hydrothermal Liquefaction of Datura stramonium L. J. Supercrit. Fluids 2016, 108, 123–135. [Google Scholar] [CrossRef]
- Tekin, K.; Akalin, M.K.; Karagöz, S. The Effects of Water Tolerant Lewis Acids on the Hydrothermal Liquefaction of Lignocellulosic Biomass. J. Energy Inst. 2016, 89, 627–635. [Google Scholar] [CrossRef]
- Jindal, M.K.; Jha, M.K. Effect of Process Parameters on Hydrothermal Liquefaction of Waste Furniture Sawdust for Bio-Oil Production. RSC Adv. 2016, 6, 41772–41780. [Google Scholar] [CrossRef]
- Liu, H.-M.; Wang, F.-Y.; Liu, Y.-L. Alkaline Pretreatment and Hydrothermal Liquefaction of Cypress for High Yield Bio-Oil Production. J. Anal. Appl. Pyrolysis 2014, 108, 136–142. [Google Scholar] [CrossRef]
- Zhao, Y.-P.; Zhu, W.-W.; Wei, X.-Y.; Fan, X.; Cao, J.-P.; Dou, Y.-Q.; Zong, Z.-M.; Zhao, W. Synergic Effect of Methanol and Water on Pine Liquefaction. Bioresour. Technol. 2013, 142, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Heng, M.; Sun, S.; Chen, J. Direct Liquefaction of Paulownia in Hot Compressed Water: Influence of Catalysts. Energy 2010, 35, 5421–5429. [Google Scholar] [CrossRef]
- Mazaheri, H.; Lee, K.T.; Mohamed, A.R. Influence of Temperature on Liquid Products Yield of Oil Palm Shell via Subcritical Water Liquefaction in the Presence of Alkali Catalyst. Fuel Process. Technol. 2013, 110, 197–205. [Google Scholar] [CrossRef]
- Tekin, K.; Karagöz, S.; Bektaş, S. Hydrothermal Liquefaction of Beech Wood Using a Natural Calcium Borate Mineral. J. Supercrit. Fluids 2012, 72, 134–139. [Google Scholar] [CrossRef]
- Tekin, K.; Karagöz, S.; Bektaş, S. Effect of Sodium Perborate Monohydrate Concentrations on Product Distributions from the Hydrothermal Liquefaction of Scotch Pine Wood. Fuel Process. Technol. 2013, 110, 17–23. [Google Scholar] [CrossRef]
- Yin, S.; Tan, Z. Hydrothermal Liquefaction of Cellulose to Bio-Oil under Acidic, Neutral and Alkaline Conditions. Appl. Energy 2012, 92, 234–239. [Google Scholar] [CrossRef]
- Joksimovic, G.; Markovic, Z. Investigation of the Mechanism of Acidic Hydrolysis of Cellulose. Acta Agric. Serbica 2007, 12, 51–57. [Google Scholar]
- Jin, F.; Wang, Y.; Zeng, X.; Shen, Z.; Yao, G. Water Under High Temperature and Pressure Conditions and Its Applications to Develop Green Technologies for Biomass Conversion. In Application of Hydrothermal Reactions to Biomass Conversion. Green Chemistry and Sustainable Technology; Jin, F., Ed.; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Hirano, Y.; Miyataab, Y.; Taniguchia, M.; Funakoshia, N.; Yamazakia, Y.; Oginoa, C.; Kitaa, Y. Fe-Assisted Hydrothermal Liquefaction of Cellulose: Effects of Hydrogenation Catalyst Addition on Properties of Water-Soluble Fraction. J. Anal. Appl. Pyrolysis 2020, 145, 104719. [Google Scholar] [CrossRef]
- Li, Q.; Liu, D.; Hou, X.; Wu, P.; Song, L.; Yan, Z. Hydro-Liquefaction of Microcrystalline Cellulose, Xylan and Industrial Lignin in Different Supercritical Solvents. Bioresour. Technol. 2016, 219, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, X.-H.; Yang, H.-P.; Chen, H.-P. Characterization of Products from Hydrothermal Treatments of Cellulose. Energy 2012, 42, 457–465. [Google Scholar] [CrossRef]
- Miyata, Y.; Sagata, K.; Hirose, M.; Yamazaki, Y.; Nishimura, A.; Okuda, N.; Arita, Y.; Hirano, Y.; Kita, Y. Fe-Assisted Hydrothermal Liquefaction of Lignocellulosic Biomass for Producing High-Grade Bio-Oil. ACS Sustain. Chem. Eng. 2017, 5, 3562–3569. [Google Scholar] [CrossRef]
- Kang, S.; Li, X.; Fan, J.; Chang, J. Classified Separation of Lignin Hydrothermal Liquefied Products. Ind. Eng. Chem. Res. 2011, 50, 11288–11296. [Google Scholar] [CrossRef]
- Arturi, K.R.; Strandgaard, M.; Nielsen, R.P.; Søgaard, E.G.; Maschietti, M. Hydrothermal Liquefaction of Lignin in Near-Critical Water in a New Batch Reactor: Influence of Phenol and Temperature. J. Supercrit. Fluids 2017, 123, 28–39. [Google Scholar] [CrossRef]
- Barbier, J.; Charon, N.; Dupassieux, N.; Loppinet-Serani, A.; Mahé, L.; Ponthus, J.; Courtiade, M.; Ducrozet, A.; Quoineaud, A.-A.; Cansell, F. Hydrothermal Conversion of Lignin Compounds. A Detailed Study of Fragmentation and Condensation Reaction Pathways. Biomass Bioenergy 2012, 46, 479–491. [Google Scholar] [CrossRef]
- Kang, S.; Li, X.; Fan, J.; Chang, J. Hydrothermal Conversion of Lignin: A Review. Renew. Sustain. Energy Rev. 2013, 27, 546–558. [Google Scholar] [CrossRef]
- Nguyen, T.D.H.; Maschietti, M.; Åmand, L.-E.; Vamling, L.; Olausson, L.; Andersson, S.-I.; Theliander, H. The Effect of Temperature on the Catalytic Conversion of Kraft Lignin Using Near-Critical Water. Bioresour. Technol. 2014, 170, 196–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Chang, Z.; Duan, P.; Yan, W.; Xu, Y.; Zhang, L.; Miao, J.; Fan, Y. Hydrothermal Liquefaction of Litsea cubeba Seed to Produce Bio-Oils. Bioresour. Technol. 2013, 149, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Brand, S.; Hardi, F.; Kim, J.; Suh, D.J. Effect of Heating Rate on Biomass Liquefaction: Differences Between Subcritical Water and Supercritical Ethanol. Energy 2014, 68, 420–427. [Google Scholar] [CrossRef]
- Toor, S.S.; Rosendahl, L.; Nielsen, M.P.; Glasius, M.; Rudolf, A.; Iversen, S.B. Continuous Production of Bio-Oil by Catalytic Liquefaction from Wet distiller’s Grain with Solubles (WDGS) from Bio-Ethanol Production. Biomass Bioenergy 2012, 36, 327–332. [Google Scholar] [CrossRef]
- Mørup, A.J.; Christensen, P.R.; Aarup, D.F.; Dithmer, L.; Mamakhel, A.; Glasius, M.; Iversen, B.B. Hydrothermal Liquefaction of Dried Distillers Grains with Solubles: A Reaction Temperature Study. Energy Fuels 2012, 26, 5944–5953. [Google Scholar] [CrossRef]
- Anastasakis, K.; Biller, P.; Madsen, R.B.; Glasius, M.; Johannsen, I. Continuous Hydrothermal Liquefaction of Biomass in a Novel Pilot Plant with Heat Recovery and Hydraulic Oscillation. Energies 2018, 11, 2695. [Google Scholar] [CrossRef] [Green Version]
- Castello, D.; Pedersen, T.H.; Rosendahl, L.A. Continuous Hydrothermal Liquefaction of Biomass: A Critical Review. Energies 2018, 11, 3165. [Google Scholar] [CrossRef] [Green Version]
- Tran, K.-Q. Fast Hydrothermal Liquefaction for Production of Chemicals and Biofuels from Wet Biomass—The Need to Develop a Plug-Flow Reactor. Bioresour. Technol. 2016, 213, 327–332. [Google Scholar] [CrossRef] [Green Version]
- Sun, P.; Heng, M.; Sun, S.-H.; Chen, J. Analysis of Liquid and Solid Products from Liquefaction of Paulownia in Hot-Compressed Water. Energy Convers. Manag. 2011, 52, 924–933. [Google Scholar] [CrossRef]
- Gan, J.; Yuan, W.; Johnson, L.; Wang, D.; Nelson, R.; Zhang, K. Hydrothermal Conversion of Big Bluestem for Bio-Oil Production: The Effect of Ecotype and Planting Location. Bioresour. Technol. 2012, 116, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Nazari, L.; Yuan, Z.; Souzanchi, S.; Ray, M.B.; Xu, C.C. Hydrothermal Liquefaction of Woody Biomass in Hot-Compressed Water: Catalyst Screening and Comprehensive Characterization of Bio-Crude Oils. Fuel 2015, 162, 74–83. [Google Scholar] [CrossRef]
- Yan, Y.; Xu, J.; Li, T.; Ren, Z. Liquefaction of Sawdust for Liquid Fuel. Fuel Process. Technol. 1999, 60, 135–143. [Google Scholar] [CrossRef]
- Lu, J.; Liu, Z.; Zhang, Y.; Savage, P.E. 110th Anniversary: Influence of Solvents on Biocrude from Hydrothermal Liquefaction of Soybean Oil, Soy Protein, Cellulose, Xylose, and Lignin, and Their Quinary Mixture. Ind. Eng. Chem. Res. 2019, 58, 13971–13976. [Google Scholar] [CrossRef]
- Anouti, S.; Haarlemmer, G.; Déniel, M.; Roubaud, A. Analysis of Physicochemical Properties of Bio-Oil from Hydrothermal Liquefaction of Blackcurrant Pomace. Energy Fuels 2015, 30, 398–406. [Google Scholar] [CrossRef]
- Karagöz, S.; Bhaskar, T.; Muto, A.; Sakata, Y.; Oshiki, T.; Kishimoto, T. Low-Temperature Catalytic Hydrothermal Treatment of Wood Biomass: Analysis of Liquid Products. Chem. Eng. J. 2005, 108, 127–137. [Google Scholar] [CrossRef]
- Wang, B.; Huang, Y.; Zhang, J. Hydrothermal Liquefaction of Lignite, Wheat Straw and Plastic Waste in Sub-Critical Water for Oil: Product Distribution. J. Anal. Appl. Pyrolysis 2014, 110, 382–389. [Google Scholar] [CrossRef]
- Zhang, B.; Von Keitz, M.; Valentas, K. Thermal Effects on Hydrothermal Biomass Liquefaction. Appl. Biochem. Biotechnol. 2008, 147, 143–150. [Google Scholar] [CrossRef]
- Alba, L.G.; Torri, C.; Samori’, C.; Van Der Spek, J.; Fabbri, D.; Kersten, S.R.A.; Brilman, D.W.F. Hydrothermal Treatment (HTT) of Microalgae: Evaluation of the Process as Conversion Method in an Algae Biorefinery Concept. Energy Fuels 2011, 26, 642–657. [Google Scholar] [CrossRef]
- Jena, U.; Das, K.; Kastner, J. Effect of Operating Conditions of Thermochemical Liquefaction on Biocrude Production from Spirulina Platensis. Bioresour. Technol. 2011, 102, 6221–6229. [Google Scholar] [CrossRef]
- Zhang, L.; Champagne, P.; Xu, C. Bio-Crude Production from Secondary pulp/Paper-Mill Sludge and Waste Newspaper via Co-Liquefaction in Hot-Compressed Water. Energy 2011, 36, 2142–2150. [Google Scholar] [CrossRef]
- Akalın, M.K.; Tekin, K.; Karagöz, S. Hydrothermal Liquefaction of Cornelian Cherry Stones for Bio-Oil Production. Bioresour. Technol. 2012, 110, 682–687. [Google Scholar] [CrossRef]
- Singh, R.; Chaudhary, K.; Biswas, B.; Balagurumurthy, B.; Bhaskar, T. Hydrothermal Liquefaction of Rice Straw: Effect of Reaction Environment. J. Supercrit. Fluids 2015, 104, 70–75. [Google Scholar] [CrossRef]
- Isa, K.M.; Snape, C.; Uguna, C.N.; Meredith, W. High Conversions of Miscanthus Using Sub- and Supercritical Water above 400 °C. J. Anal. Appl. Pyrolysis 2015, 113, 646–654. [Google Scholar] [CrossRef]
- Yang, L.; Nazari, L.; Yuan, Z.; Corscadden, K.; Xu, C.; He, Q. Hydrothermal Liquefaction of Spent Coffee Grounds in Water Medium for Bio-Oil Production. Biomass Bioenergy 2016, 86, 191–198. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.-C.; Chen, C.-P.; Yang, C.-S.; Chen, Y.-H.; Huang, M.; Chang, C.-Y.; Shie, J.-L.; Yuan, M.-H.; Chen, Y.-H.; Ho, C.; et al. Conversion of Waste Bamboo Chopsticks to Bio-Oil via Catalytic Hydrothermal Liquefaction Using K2CO3. Sustain. Environ. Res. 2016, 26, 262–267. [Google Scholar] [CrossRef] [Green Version]
- Déniel, M.; Haarlemmer, G.; Roubaud, A.; Weiss-Hortala, E.; Fages, J. Optimisation of Bio-Oil Production by Hydrothermal Liquefaction of Agro-Industrial Residues: Blackcurrant Pomace (Ribes nigrum L.) As an Example. Biomass Bioenergy 2016, 95, 273–285. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; He, Q.; Havard, P.; Corscadden, K.; Xu, C.; Wang, X. Co-Liquefaction of Spent Coffee Grounds and Lignocellulosic Feedstocks. Bioresour. Technol. 2017, 237, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Seshasayee, M.S.; Savage, P.E. Oil from Plastic via Hydrothermal Liquefaction: Production and Characterization. Appl. Energy 2020, 278. [Google Scholar] [CrossRef]
- Durak, H.; Genel, S. Catalytic Hydrothermal Liquefaction of Lactuca Scariola with a Heterogeneous Catalyst: The Investigation of Temperature, Reaction Time and Synergistic Effect of Catalysts. Bioresour. Technol. 2020, 309, 123375. [Google Scholar] [CrossRef] [PubMed]
- Evcil, T.; Tekin, K.; Ucar, S.; Karagoz, S. Hydrothermal Liquefaction of Olive Oil Residues. Sustain. Chem. Pharm. 2021, 22, 100476. [Google Scholar] [CrossRef]
- Akhtar, J.; Amin, N.A.S. A Review on Process Conditions for Optimum Bio-Oil Yield in Hydrothermal Liquefaction of Biomass. Renew. Sustain. Energy Rev. 2011, 15, 1615–1624. [Google Scholar] [CrossRef]
- Minowa, T.; Zhen, F.; Ogi, T. Cellulose Decomposition in Hot-Compressed Water with Alkali or Nickel Catalyst. J. Supercrit. Fluids 1998, 13, 253–259. [Google Scholar] [CrossRef]
- Ogi, T.; Yokoyama, S.-Y.; Koguchi, K. Direct Liquefaction of Wood by Catalyst. Part 1. Effects of Pressure, Temperature, Holding Time and wood/catalyst/Water Ratio on Oil Yield. J. Jpn. Pet. Inst. 1985, 28, 239–245. [Google Scholar] [CrossRef] [Green Version]
- Qu, Y.; Wei, X.; Zhong, C. Experimental Study on the Direct Liquefaction of Cunninghamia Lanceolata in Water. Energy 2003, 28, 597–606. [Google Scholar] [CrossRef]
- Zhang, B.; von Keitz, M.; Valentas, K. Thermochemical Liquefaction of High-Diversity Grassland Perennials. J. Anal. Appl. Pyrolysis 2009, 84, 18–24. [Google Scholar] [CrossRef]
- Demirbaş, A.; Balat, M.; Bozbas, K. Direct and Catalytic Liquefaction of Wood Species in Aqueous Solution. Energy Sources 2005, 27, 271–277. [Google Scholar] [CrossRef]
- Zheng, J.-L.; Zhu, M.-Q.; Wu, H.-T. Alkaline Hydrothermal Liquefaction of Swine Carcasses to Bio-Oil. Waste Manag. 2015, 43, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Hwang, H.; Moon, J.; Choi, J.W. Characterization of Hydrothermal Liquefaction Products from Coconut Shell in the Presence of Selected Transition Metal Chlorides. J. Anal. Appl. Pyrolysis 2016, 122, 415–421. [Google Scholar] [CrossRef]
- Khampuang, K.; Boreriboon, N.; Prasassarakich, P. Alkali Catalyzed Liquefaction of Corncob in Supercritical ethanol–water. Biomass Bioenergy 2015, 83, 460–466. [Google Scholar] [CrossRef]
- Yin, S.; Dolan, R.; Harris, M.; Tan, Z. Subcritical Hydrothermal Liquefaction of Cattle Manure to Bio-Oil: Effects of Conversion Parameters on Bio-Oil Yield and Characterization of Bio-Oil. Bioresour. Technol. 2010, 101, 3657–3664. [Google Scholar] [CrossRef]
- Xu, C.; Lancaster, J. Conversion of Secondary pulp/Paper Sludge Powder to Liquid Oil Products for Energy Recovery by Direct Liquefaction in Hot-Compressed Water. Water Res. 2008, 42, 1571–1582. [Google Scholar] [CrossRef]
- Yang, Y.; Gilbert, A.; Xu, C. Production of Bio-Crude from Forestry Waste by Hydro-Liquefaction in Sub-/Super-Critical Methanol. AIChE J. 2009, 55, 807–819. [Google Scholar] [CrossRef]
- Brand, S.; Susanti, R.F.; Kim, S.K.; Lee, H.-S.; Kim, J.; Sang, B.-I. Supercritical Ethanol as an Enhanced Medium for Lignocellulosic Biomass Liquefaction: Influence of Physical Process Parameters. Energy 2013, 59, 173–182. [Google Scholar] [CrossRef]
- Kabyemela, B.M.; Takigawa, M.; Adschiri, T.; Malaluan, R.M.; Arai, K. Mechanism and Kinetics of Cellobiose Decomposition in Sub- and Supercritical Water. Ind. Eng. Chem. Res. 1998, 37, 357–361. [Google Scholar] [CrossRef]
- Cantero, D.A.; Tapia, Á.S.; Bermejo, M.D.; Cocero, M.J. Pressure and Temperature Effect on Cellulose Hydrolysis in Pressurized Water. Chem. Eng. J. 2015, 276, 145–154. [Google Scholar] [CrossRef]
- Kruse, A.; Gawlik, A. Biomass Conversion in Water at 330−410 °C and 30−50 MPa. Identification of Key Compounds for Indicating Different Chemical Reaction Pathways. Ind. Eng. Chem. Res. 2003, 42, 267–279. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, W.-T.; Zhang, P.; Luo, Z.; Zhang, Y. Hydrothermal Liquefaction of Chlorella Pyrenoidosa in Sub- and Supercritical Ethanol with Heterogeneous Catalysts. Bioresour. Technol. 2013, 133, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Yim, S.C.; Quitain, A.T.; Yusup, S.; Sasaki, M.; Uemura, Y.; Kida, T. Metal Oxide-Catalyzed Hydrothermal Liquefaction of Malaysian Oil Palm Biomass to Bio-Oil under Supercritical Condition. J. Supercrit. Fluids 2017, 120, 384–394. [Google Scholar] [CrossRef]
- Tran, K.-Q.; Klemsdal, A.J.; Zhang, W.; Sandquist, J.; Wang, L.; Skreiberg, Ø. Fast Hydrothermal Liquefaction of Native and Torrefied Wood. Energy Procedia 2017, 105, 218–223. [Google Scholar] [CrossRef]
- Bach, Q.-V.; Sillero, M.V.; Tran, Q.; Skjermo, J. Fast Hydrothermal Liquefaction of a Norwegian Macro-Alga: Screening Tests. Algal Res. 2014, 6, 271–276. [Google Scholar] [CrossRef]
- Biller, P.; Riley, R.; Ross, A. Catalytic Hydrothermal Processing of Microalgae: Decomposition and Upgrading of Lipids. Bioresour. Technol. 2011, 102, 4841–4848. [Google Scholar] [CrossRef]
- Biller, P.; Ross, A. Potential Yields and Properties of Oil from the Hydrothermal Liquefaction of Microalgae with Different Biochemical Content. Bioresour. Technol. 2011, 102, 215–225. [Google Scholar] [CrossRef]
- Akhtar, J.; Kuang, S.K.; Amin, N.S. Liquefaction of Empty Palm Fruit Bunch (EPFB) in Alkaline Hot Compressed Water. Renew. Energy 2010, 35, 1220–1227. [Google Scholar] [CrossRef]
- Karagöz, S.; Bhaskar, T.; Muto, A.; Sakata, Y. Catalytic Hydrothermal Treatment of Pine Wood Biomass: Effect of RbOH and CsOH on Product Distribution. J. Chem. Technol. Biotechnol. 2005, 80, 1097–1102. [Google Scholar] [CrossRef]
- Karagöz, S.; Bhaskar, T.; Muto, A.; Sakata, Y. Effect of Rb and Cs Carbonates for Production of Phenols from Liquefaction of Wood Biomass. Fuel 2004, 83, 2293–2299. [Google Scholar] [CrossRef]
- Tekin, K.; Karagöz, S. T-BuOK Catalyzed Bio-Oil Production from Woody Biomass under Sub-Critical Water Conditions. Environ. Chem. Lett. 2013, 11, 25–31. [Google Scholar] [CrossRef]
- Chen, Y.; Mu, R.; Yang, M.; Fang, L.; Wu, Y.; Wu, K.; Liu, Y.; Gong, J. Catalytic Hydrothermal Liquefaction for Bio-Oil Production over CNTs Supported Metal Catalysts. Chem. Eng. Sci. 2017, 161, 299–307. [Google Scholar] [CrossRef]
- Basar, I.A.; Liu, H.; Carrere, H.; Trably, E.; Eskicioglu, C. A Review on Key Design and Operational Parameters to Optimize and Develop Hydrothermal Liquefaction of Biomass for Biorefinery Applications. Green Chem. 2021, 23, 1404–1446. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, X.; Zhu, S.; Tian, F.; Xu, Y.; Zhu, C.; Dong, L. Synergistic Hydrothermal Liquefaction of Wheat Stalk With Homogeneous and Heterogeneous Catalyst at Low Temperature. Bioresour. Technol. 2019, 278, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Aysu, T.; Durak, H. Bio-Oil Production via Catalytic Supercritical Liquefaction of Syrian Mesquite (Prosopis farcta). J. Supercrit. Fluids 2016, 109, 26–34. [Google Scholar] [CrossRef]
- Durak, H.; Aysu, T. Effects of Catalysts and Solvents on Liquefaction of Onopordum Heteracanthum for Production of Bio-Oils. Bioresour. Technol. 2014, 166, 309–317. [Google Scholar] [CrossRef]
- Karagoz, S.; Bhaskar, T.; Muto, A.; Sakata, Y. Hydrothermal Upgrading of Biomass: Effect of KCO Concentration and biomass/Water Ratio on Products Distribution. Bioresour. Technol. 2006, 97, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Miyata, Y.; Yamazaki, Y.; Hirano, Y.; Kita, Y. Quantitative Analysis of the Aqueous Fraction from the Fe-Assisted Hydrothermal Liquefaction of Oil Palm Empty Fruit Bunches. J. Anal. Appl. Pyrolysis 2018, 132, 72–81. [Google Scholar] [CrossRef]
- Zhou, C.; Zhu, X.; Qian, F.; Shen, W.; Xu, H.; Zhang, S.; Chen, J. Catalytic Hydrothermal Liquefaction of Rice Straw in water/Ethanol Mixtures for High Yields of Monomeric Phenols Using Reductive CuZnAl Catalyst. Fuel Process. Technol. 2016, 154, 1–6. [Google Scholar] [CrossRef]
- Liu, D.; Li, Q.; Zhao, A.; Song, L.; Wu, P.; Yan, Z. Hydro-Liquefaction of Sawdust and Its Three Components in Supercritical Ethanol With [BMIM]Cl/NiCl2 Catalyst. Chem. Eng. J. 2015, 279, 921–928. [Google Scholar] [CrossRef]
- Ding, Y.-J.; Zhao, C.-X.; Liu, Z.-C. Catalytic Hydrothermal Liquefaction of Rice Straw for Production of Monomers Phenol over Metal Supported Mesoporous Catalyst. Bioresour. Technol. 2019, 294, 122097. [Google Scholar] [CrossRef]
- Lee, J.H.; Hwang, H.; Choi, J.W. Effects of Transition Metals on Hydrothermal Liquefaction of Empty Fruit Bunches (EFB) for Conversion to Biofuel and Valuable Chemicals. Energy 2018, 162, 1–9. [Google Scholar] [CrossRef]
- Cheng, S.; Wei, L.; Rabnawaz, M. Catalytic Liquefaction of Pine Sawdust and in-Situ Hydrogenation of Bio-Crude over Bifunctional Co-Zn/HZSM-5 Catalysts. Fuel 2018, 223, 252–260. [Google Scholar] [CrossRef]
- Alper, K.; Tekin, K.; Karagoz, S. Hydrothermal Liquefaction of Lignocellulosic Biomass Using Potassium Fluoride-Doped Alumina. Energy Fuels 2019, 33, 3248–3256. [Google Scholar] [CrossRef]
- Bian, J.; Zhang, Q.; Zhang, P.; Feng, L.; Li, C. Supported Fe2O3 Nanoparticles for Catalytic Upgrading of Microalgae Hydrothermal Liquefaction Derived Bio-Oil. Catal. Today 2017, 293-294, 159–166. [Google Scholar] [CrossRef]
- Bhalkikar, A.; Gernhart, Z.C.; Cheung, C.L. Recyclable Magnetite Nanoparticle Catalyst for One-Pot Conversion of Cellobiose to 5-Hydroxymethylfurfural in Water. J. Nanomater. 2015, 2015, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.; D’Cruz, I.; Wang, M.; Leitch, M.; Xu, C. Highly Efficient Liquefaction of Woody Biomass in Hot-Compressed Alcohol−Water Co-solvents. Energy Fuels 2010, 24, 4659–4667. [Google Scholar] [CrossRef]
- Chumpoo, J.; Prasassarakich, P. Bio-Oil from Hydro-Liquefaction of Bagasse in Supercritical Ethanol. Energy Fuels 2010, 24, 2071–2077. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Y.; Zhang, P.; Hua, D.; Yang, M.; Li, C.; Chen, Z.; Liu, J. Direct Liquefaction of Dunaliella Tertiolecta for Bio-Oil in sub/Supercritical ethanol–water. Bioresour. Technol. 2012, 124, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yuan, X.-Z.; Huang, H.-J.; Wang, X.-L.; Wang, H.; Zeng, G.-M. Thermochemical Liquefaction of Rice Husk for Bio-Oil Production in Mixed Solvent (ethanol–water). Fuel Process. Technol. 2013, 112, 93–99. [Google Scholar] [CrossRef]
- Zhu, W.-W.; Zong, Z.-M.; Yan, H.-L.; Zhao, Y.-P.; Lu, Y.; Wei, X.-Y.; Zhang, D. Cornstalk Liquefaction in methanol/Water Mixed Solvents. Fuel Process. Technol. 2014, 117, 1–7. [Google Scholar] [CrossRef]
- Patil, P.T.; Armbruster, U.; Martin, A. Hydrothermal Liquefaction of Wheat Straw in Hot Compressed Water and Subcritical water–alcohol Mixtures. J. Supercrit. Fluids 2014, 93, 121–129. [Google Scholar] [CrossRef]
- Kosinkova, J.; Ramirez, J.A.; Nguyen, J.; Ristovski, Z.; Brown, R.J.; Lin, C.S.K.; Rainey, T.J. Hydrothermal Liquefaction of Bagasse Using Ethanol and Black Liquor as Solvents. Biofuels Bioprod. Biorefining 2015, 9, 630–638. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.-L.; Zong, Z.-M.; Zhu, W.-W.; Li, Z.-K.; Wang, Y.-G.; Wei, Z.; Li, Y.; Wei, X.-Y. Poplar Liquefaction in Water/Methanol Cosolvents. Energy Fuels 2015, 29, 3104–3110. [Google Scholar] [CrossRef]
- He, Y.; Liang, X.; Jazrawi, C.; Montoya, A.; Yuen, A.; Cole, A.J.; Neveux, N.; Paul, N.; de Nys, R.; Maschmeyer, T.; et al. Continuous Hydrothermal Liquefaction of Macroalgae in the Presence of Organic Co-Solvents. Algal Res. 2016, 17, 185–195. [Google Scholar] [CrossRef]
- Feng, S.; Wei, R.; Leitch, M.; Xu, C.C. Comparative Study on Lignocellulose Liquefaction in Water, Ethanol, and water/Ethanol Mixture: Roles of Ethanol and Water. Energy 2018, 155, 234–241. [Google Scholar] [CrossRef]
- Pan, Z.-Q.; Huang, H.-J.; Zhou, C.-F.; Xiao, X.-F.; He, X.-W.; Lai, F.-Y.; Xiong, J.-B. Highly Efficient Conversion of Camphor Tree Sawdust into Bio-Oil and Biochar Products by Liquefaction in Ethanol-Water Cosolvent. J. Anal. Appl. Pyrolysis 2018, 136, 186–198. [Google Scholar] [CrossRef]
- Lai, F.-Y.; Chang, Y.-C.; Huang, H.-J.; Wu, G.-Q.; Xiong, J.-B.; Pan, Z.-Q.; Zhou, C.-F. Liquefaction of Sewage Sludge in Ethanol-Water Mixed Solvents for Bio-Oil and Biochar Products. Energy 2018, 148, 629–641. [Google Scholar] [CrossRef]
- Li, R.; Ma, Z.; Yang, T.; Li, B.; Wei, L.; Sun, Y. Sub-supercritical Liquefaction of Municipal Wet Sewage Sludge to Produce Bio-Oil: Effect of Different organic–water Mixed Solvents. J. Supercrit. Fluids 2018, 138, 115–123. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, S.; Qian, L.; Barati, B.; Gong, X.; Abomohra, A.E.-F.; Wang, X.; Esakkimuthu, S.; Hu, Y.; Liu, L. Effect of Cosolvent and Addition of Catalyst (HZSM-5) on Hydrothermal Liquefaction of Macroalgae. Int. J. Energy Res. 2019, 43, 8841–8851. [Google Scholar] [CrossRef]
- Hu, Y.; Gu, Z.; Li, W.; Xu, C. Alkali-Catalyzed Liquefaction of Pinewood Sawdust in ethanol/Water Co-Solvents. Biomass Bioenergy 2020, 134, 105485. [Google Scholar] [CrossRef]
- Shie, J.-L.; Yang, W.-S.; Liau, Y.-R.; Liau, T.-H.; Yang, H.-R. Subcritical Hydrothermal Co-Liquefaction of Process Rejects at a Wastepaper-Based Paper Mill with Waste Soybean Oil. Energies 2021, 14, 2442. [Google Scholar] [CrossRef]
- Marcus, Y. Extraction by Subcritical and Supercritical Water, Methanol, Ethanol and Their Mixtures. Separations 2018, 5, 4. [Google Scholar] [CrossRef] [Green Version]
- Kus, N.S. Organic Reactions in Subcritical and Supercritical Water. Tetrahedron 2012, 68, 949–958. [Google Scholar] [CrossRef]
- Ogi, T.; Yokoyama, S.-Y. Liquid Fuel Production from Woody Biomass by Direct Liquefaction. J. Jpn. Pet. Inst. 1993, 36, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.; Li, H.; Zeng, G.; Tong, J.; Xie, W. Sub- and Supercritical Liquefaction of Rice Straw in the Presence of ethanol–water and 2-propanol–water Mixture. Energy 2007, 32, 2081–2088. [Google Scholar] [CrossRef]
- Li, H.; Yuan, X.; Zeng, G.; Tong, J.; Yan, Y.; Cao, H.; Wang, L.; Cheng, M.; Zhang, J.; Yang, D. Liquefaction of Rice Straw in Sub- and Supercritical 1,4-dioxane–water Mixture. Fuel Process. Technol. 2009, 90, 657–663. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, C.; Hao, S.; Luo, G.; Zhang, S.; Chen, J. Effect of Glycerol As Co-Solvent on Yields of Bio-Oil from Rice Straw through Hydrothermal Liquefaction. Bioresour. Technol. 2016, 220, 471–478. [Google Scholar] [CrossRef]
- Tomoko, O.; Minowa, T.; Dote, Y.; Yokoyama, S.-Y. Characterization of Oil Produced by the Direct Liquefaction of Japanese Oak in an Aqueous 2-Propanol Solvent System. Biomass Bioenergy 1994, 7, 193–199. [Google Scholar]
- Minami, E.; Saka, S. Decomposition Behavior of Woody Biomass in Water-Added Supercritical Methanol. J. Wood Sci. 2005, 51, 395–400. [Google Scholar] [CrossRef] [Green Version]
- Savage, P. Organic Chemical Reactions in Supercritical Water. Chem. Rev. 1999, 99, 603–622. [Google Scholar] [CrossRef] [PubMed]
- Balogh, D.; Curvelo, A.; De Groote, R. Solvent Effects on Organosolv Lignin from Pinus Caribaea Hondurensis. Holzforschung 1992, 46, 343–348. [Google Scholar] [CrossRef]
- Pasquini, D.; Pimenta, M.T.B.; Ferreira, L.H.; Curvelo, A.A.D.S. Extraction of Lignin from Sugar Cane Bagasse and Pinus Taeda Wood Chips Using ethanol–water Mixtures and Carbon Dioxide at High Pressures. J. Supercrit. Fluids 2005, 36, 31–39. [Google Scholar] [CrossRef]
- Valdez, P.J.; Dickinson, J.G.; Savage, P.E. Characterization of Product Fractions from Hydrothermal Liquefaction of Naninochloropsis Sp. And the Influence of Solvents. Energy Fuels 2011, 25, 3235–3243. [Google Scholar] [CrossRef]
- Yan, W.-H.; Duan, P.-G.; Wang, F.; Xu, Y.-P. Composition of the Bio-Oil from the Hydrothermal Liquefaction of Duckweed and the Influence of the Extraction Solvents. Fuel 2016, 185, 229–235. [Google Scholar] [CrossRef]
- Yang, X.; Lyu, H.; Chen, K.; Zhu, X.; Zhang, S.; Chen, J. Selective Extraction of Bio-Oil from Hydrothermal Liquefaction of Salix psammophila by Organic Solvents With Different Polarities through Multistep Extraction Separation. BioResources 2014, 9, 5219–5233. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; He, Q.; Yang, L. A Review on Hydrothermal Co-Liquefaction of Biomass. Appl. Energy 2019, 250, 926–945. [Google Scholar] [CrossRef]
- Jiang, J.; Savage, P.E. Influence of Process Conditions and Interventions on Metals Content in Biocrude from Hydrothermal Liquefaction of Microalgae. Algal Res. 2017, 26, 131–134. [Google Scholar] [CrossRef]
- Xu, D.; Savage, P. Characterization of Biocrudes Recovered with and Without Solvent After Hydrothermal Liquefaction of Algae. Algal Res. 2014, 6, 1–7. [Google Scholar] [CrossRef]
- Watson, J.; Lu, J.; de Souza, R.; Si, B.; Zhang, Y.; Liu, Z. Effects of the Extraction Solvents in Hydrothermal Liquefaction Processes: Biocrude Oil Quality and Energy Conversion Efficiency. Energy 2019, 167, 189–197. [Google Scholar] [CrossRef]
- Karagöz, S.; Bhaskar, T.; Muto, A.; Sakata, Y.; Uddin, A. Low-Temperature Hydrothermal Treatment of Biomass: Effect of Reaction Parameters on Products and Boiling Point Distributions. Energy Fuels 2004, 18, 234–241. [Google Scholar] [CrossRef]
- Zhu, Y.; Biddy, M.J.; Jones, S.B.; Elliott, D.; Schmidt, A.J. Techno-Economic Analysis of Liquid Fuel Production from Woody Biomass via Hydrothermal Liquefaction (HTL) and Upgrading. Appl. Energy 2014, 129, 384–394. [Google Scholar] [CrossRef]
- Taghipour, A.; Ramirez, J.A.; Brown, R.J.; Rainey, T.J. A Review of Fractional Distillation to Improve Hydrothermal Liquefaction Biocrude Characteristics; Future Outlook and Prospects. Renew. Sustain. Energy Rev. 2019, 115, 109355. [Google Scholar] [CrossRef]
- Pedersen, T.; Jensen, C.; Sandström, L.; Rosendahl, L. Full Characterization of Compounds Obtained from Fractional Distillation and Upgrading of a HTL Biocrude. Appl. Energy 2017, 202, 408–419. [Google Scholar] [CrossRef] [Green Version]
- Tzanetis, K.F.; Posada, J.A.; Ramirez, A. Analysis of Biomass Hydrothermal Liquefaction and Biocrude-Oil Upgrading for Renewable Jet Fuel Production: The Impact of Reaction Conditions on Production Costs and GHG Emissions Performance. Renew. Energy 2017, 113, 1388–1398. [Google Scholar] [CrossRef]
- Yang, S.; Yuan, T.-Q.; Li, M.-F.; Sun, R.-C. Hydrothermal Degradation of Lignin: Products Analysis for Phenol Formaldehyde Adhesive Synthesis. Int. J. Biol. Macromol. 2015, 72, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Kumar, A.; Adamopoulos, S. Liquefaction of Lignocellulosic Materials and Its Applications in Wood adhesives—A Review. Ind. Crop. Prod. 2018, 124, 325–342. [Google Scholar] [CrossRef]
- Wang, M.; Xu, C.C.; Leitch, M. Liquefaction of Cornstalk in Hot-Compressed phenol–water Medium to Phenolic Feedstock for the Synthesis of phenol–formaldehyde Resin. Bioresour. Technol. 2009, 100, 2305–2307. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Yuan, Z.; Leitch, M.; Shui, H.; Xu, C.C. Effects of Bark Extraction before Liquefaction and Liquid Oil Fractionation After Liquefaction on Bark-Based Phenol Formaldehyde Resoles. Ind. Crops Prod. 2016, 84, 330–336. [Google Scholar] [CrossRef]
- Panisko, E.; Wietsma, T.; Lemmon, T.; Albrecht, K.; Howe, D. Characterization of the Aqueous Fractions from Hydrotreatment and Hydrothermal Liquefaction of Lignocellulosic Feedstocks. Biomass Bioenergy 2015, 74, 162–171. [Google Scholar] [CrossRef] [Green Version]
- Biller, P.; Madsen, R.B.; Klemmer, M.; Becker, J.; Iversen, B.; Glasius, M. Effect of Hydrothermal Liquefaction Aqueous Phase Recycling on Bio-Crude Yields and Composition. Bioresour. Technol. 2016, 220, 190–199. [Google Scholar] [CrossRef]
- Miyata, Y.; Sagata, K.; Yamazaki, Y.; Teramura, H.; Hirano, Y.; Ogino, C.; Kita, Y. Mechanism of the Fe-Assisted Hydrothermal Liquefaction of Lignocellulosic Biomass. Ind. Eng. Chem. Res. 2018, 57, 14870–14877. [Google Scholar] [CrossRef]
- Elliott, D.; Hart, T.; Schmidt, A.J.; Neuenschwander, G.G.; Rotness, L.J.; Olarte, M.V.; Zacher, A.H.; Albrecht, K.O.; Hallen, R.T.; Holladay, J.E. Process Development for Hydrothermal Liquefaction of Algae Feedstocks in a Continuous-Flow Reactor. Algal Res. 2013, 2, 445–454. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Liu, L.; Wei, N.; Guo, Y.; Wang, S.; Wu, Z.; Duan, P. Catalytic Supercritical Water Gasification of Aqueous Phase Directly Derived from Microalgae Hydrothermal Liquefaction. Int. J. Hydrogen Energy 2019, 44, 26181–26192. [Google Scholar] [CrossRef]
- Demirbaş, A. Effect of Lignin Content on Aqueous Liquefaction Products of Biomass. Energy Convers. Manag. 2000, 41, 1601–1607. [Google Scholar] [CrossRef]
- Liu, Z.; Quek, A.; Hoekman, S.K.; Balasubramanian, R. Production of Solid Biochar Fuel from Waste Biomass by Hydrothermal Carbonization. Fuel 2013, 103, 943–949. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C. Hydrothermal carbonization (HTC) of lignocellulosic biomass. Energy Fuels 2011, 25, 1802–1810. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, F.-S. Removal of Lead from Water Using Biochars Prepared from Hydrothermal Liquefaction of Biomass. J. Hazard. Mater. 2009, 167, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Bargmann, I.; Rillig, M.; Kruse, A.; Greef, J.-M.; Kücke, M. Effects of Hydrochar Application on the Dynamics of Soluble Nitrogen in Soils and on Plant Availability. J. Plant Nutr. Soil Sci. 2014, 177, 48–58. [Google Scholar] [CrossRef]
- Baronti, S.; Alberti, G.; Camin, F.; Criscuoli, I.; Genesio, L.; Mass, R.; Vaccari, F.P.; Ziller, L.; Miglietta, F. Hydrochar Enhances Growth of Poplar for Bioenergy While Marginally Contributing to Direct Soil Carbon Sequestration. GCB Bioenergy 2017, 9, 1618–1626. [Google Scholar] [CrossRef] [Green Version]
- Kalderis, D.; Papameletiou, G.; Kayan, B. Assessment of Orange Peel Hydrochar as a Soil Amendment: Impact on Clay Soil Physical Properties and Potential Phytotoxicity. Waste Biomass Valorization 2019, 10, 3471–3484. [Google Scholar] [CrossRef]
- Ding, L.; Wang, Z.; Li, Y.; Du, Y.; Liu, H.; Guo, Y. A Novel Hydrochar and Nickel Composite for the Electrochemical Supercapacitor Electrode Material. Mater. Lett. 2012, 74, 111–114. [Google Scholar] [CrossRef]
- Ding, L.; Zou, B.; Li, Y.; Liu, H.; Wang, Z.; Zhao, C.; Su, Y.; Guo, Y. The Production of Hydrochar-Based Hierarchical Porous Carbons for Use as Electrochemical Supercapacitor Electrode Materials. Colloids Surfaces A Physicochem. Eng. Asp. 2013, 423, 104–111. [Google Scholar] [CrossRef]
- Reza, M.T.; Lynam, J.; Uddin, M.H.; Coronella, C.J. Hydrothermal Carbonization: Fate of Inorganics. Biomass Bioenergy 2013, 49, 86–94. [Google Scholar] [CrossRef]
- Smith, A.; Singh, S.; Ross, A.B. Fate of Inorganic Material During Hydrothermal Carbonisation of Biomass: Influence of Feedstock on Combustion Behaviour of Hydrochar. Fuel 2016, 169, 135–145. [Google Scholar] [CrossRef]
- Neveux, N.; Yuen, A.; Jazrawi, C.; Magnusson, M.; Haynes, B.; Masters, A.; Montoya, A.; Paul, N.; Maschmeyer, T.; de Nys, R. Biocrude Yield and Productivity from the Hydrothermal Liquefaction of Marine and Freshwater Green Macroalgae. Bioresour. Technol. 2014, 155, 334–341. [Google Scholar] [CrossRef]






| Feedstock | Cellulose | Hemicellulose | Lignin | Carbon | Hydrogen | Oxygen | H/C | O/C | Bio-Oil | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Agricultural Feedstock | ||||||||||
| Corn straw | 30.81 | 25.52 | 16.76 | 44.57 | 5.53 | 33.70 | 1.49 | 0.57 | 7.9 | [23] |
| Peanut straw | 36.56 | 20.27 | 18.36 | 41.42 | 5.51 | 35.21 | 1.60 | 0.64 | 14.6 | [23] |
| Rice straw | 46.33 | 31.09 | 10.17 | 41.34 | 5.33 | 34.29 | 1.55 | 0.62 | 15 | [23] |
| Soybean straw | 42.39 | 22.05 | 18.93 | 45.99 | 6.07 | 39.00 | 1.58 | 0.64 | 15.8 | [23] |
| Corn stover | 45.00 | 30.00 | 16.00 | 43.57 | 5.84 | 49.98 | 1.61 | 0.86 | 27.15 | [24,25] |
| Rice straw | 42.87 | 25.15 | 31.97 | 38.55 | 5.50 | 55.34 | 1.71 | 1.08 | 27.6 | [26] |
| Barley straw | 46.00 | 23.00 | 15.00 | 44.66 | 6.34 | 47.97 | 1.70 | 0.81 | 34.9 | [27] |
| Castor residue | 38.42 | 22.40 | 20.20 | 43.59 | 5.56 | 46.16 | 1.53 | 0.79 | 15.8 | [28] |
| Pre-treated sorghum bagasse | 49.84 | 8.01 | 24.65 | 43.20 | 5.80 | 41.40 | 1.61 | 0.72 | 23.42 | [29] |
| Forest Feedstock | ||||||||||
| Oakwood | 38.10 | 23.00 | 32.00 | 50.20 | 7.00 | 42.80 | 1.67 | 0.64 | 23.17 | [30,31] |
| Palm kernel shell | 24.50 | 22.90 | 33.50 | 47.77 | 4.06 | 47.55 | 1.02 | 0.75 | 24 | [32] |
| Empty fruit bunch | 26.60 | 26.90 | 18.60 | 43.62 | 4.03 | 50.22 | 1.11 | 0.86 | 16 | [32] |
| Palm mesocarp fiber | 23.10 | 22.20 | 30.60 | 46.29 | 4.67 | 47.37 | 1.21 | 0.77 | 16 | [32] |
| Poplar wood | 52.16 | 18.92 | 22.97 | 47.04 | 5.60 | 43.20 | 1.43 | 0.69 | 28.49 | [33] |
| Birch sawdust | 45.30 | 24.20 | 22.90 | 48.50 | 6.30 | 45.20 | 1.56 | 0.70 | 22.3 | [34] |
| Aspen wood | 47.14 | 19.64 | 22.11 | 50.39 | 6.19 | 43.23 | 1.47 | 0.64 | 20.65 | [35] |
| Datura stramonium L. stem | 42.20 | 23.13 | 24.33 | 43.55 | 5.98 | 49.70 | 1.65 | 0.86 | 32 | [36] |
| Poplar wood | 44.95 | 34.05 | 25.85 | 46.72 | 6.18 | 46.96 | 1.59 | 0.75 | 17.5 | [37] |
| Furniture sawdust | 32.63 | 37.23 | 22.16 | 47.42 | 5.67 | 46.71 | 1.43 | 0.74 | 12.1 | [38] |
| Cypress | 46.30 | 27.60 | 28.80 | 48.90 | 6.00 | 44.80 | 1.47 | 0.69 | 27.5 | [39] |
| Pine | 39.54 | 20.61 | 30.15 | 49.52 | 6.49 | 43.89 | 1.57 | 0.66 | 24.2 | [40] |
| Paulownia | 42.35 | 25.22 | 23.44 | 45.50 | 6.30 | 48.20 | 1.66 | 0.79 | 27.01 | [41] |
| Oil palm shell | 39.70 | 21.80 | 32.50 | 50.01 | 7.66 | 29.02 | 1.84 | 0.44 | 18.5 | [42] |
| Beech wood | 45.05 | 31.50 | 22.25 | 44.68 | 6.08 | 49.24 | 1.63 | 0.83 | 22 | [43] |
| Scotch pine | 47.30 | 20.54 | 27.70 | 48.33 | 6.49 | 45.18 | 1.61 | 0.70 | 24.6 | [44] |
| S. No | Year | Description/Objective | Results/Observations/Major Findings | Ref. |
|---|---|---|---|---|
| 1 | 2011 | HTT of microalgae: evaluation of the process as conversion method in an algae biorefinery concept. To understand the effect of wide range of condition (175–450 °C, up to 60 min) to obtain the optimum range. | The maximum bio-oil yield (49 wt%) was obtained at 375 °C, 5 min. Up to 75% of the calorific value of algal biomass can be retrieved in oil phase. With high oil yield, its pronounced increase in nitrogen content was observed (6 wt%). The dissolved inorganic N and P content in the aqueous phase points at recycling of the nutrients for next generation algal growth. | [73] |
| 2 | 2011 | Effect of operating conditions of thermochemical liquefaction on bio-crude production from Spirulina platensis. | Bio-crude obtained in the study has properties in range with the petroleum crude and could be further refined to obtain transportation fuel. Carbon conversion efficiency of 98.3% was obtained at 350 °C, 60 min, and 20% solid concentration. | [74] |
| 3 | 2011 | Bio-crude production from secondary pulp/paper mill sludge and waste newspaper via co-liquefaction in hot-compressed water. Investigate the effect of temperature and selected catalyst (HCOOH, KOH, and FeS) on product yield. | Addition of catalyst at 300 °C was found to increase the yield of heavy oil. Synergistic effect was observed on the yield of bio-oil when secondary pulp/paper mill sludge and newspaper waste was used. | [75] |
| 4 | 2012 | To understand the effects of different reaction conditions on HTT of cornelian cherry stones and bio-oil composition. | It was observed that at a temperature of 200 °C, longer retention time of 30 min gave higher yield, whereas at 250 °C and 300 °C, shorter retention time of 0 min was more effective. The major compound observed in heavy bio-oil was linoleic acid at both 250 and 300 °C. | [76] |
| 5 | 2012 | Characterization of products from HTL of cellulose. Effect of temperature and residence time on HTL process. | Longer retention time did not facilitate increased production of oil. The residual char had core-shell structure, the core contained ketone and ether groups and the shell contained carboxylic and carbonyl groups. | [50] |
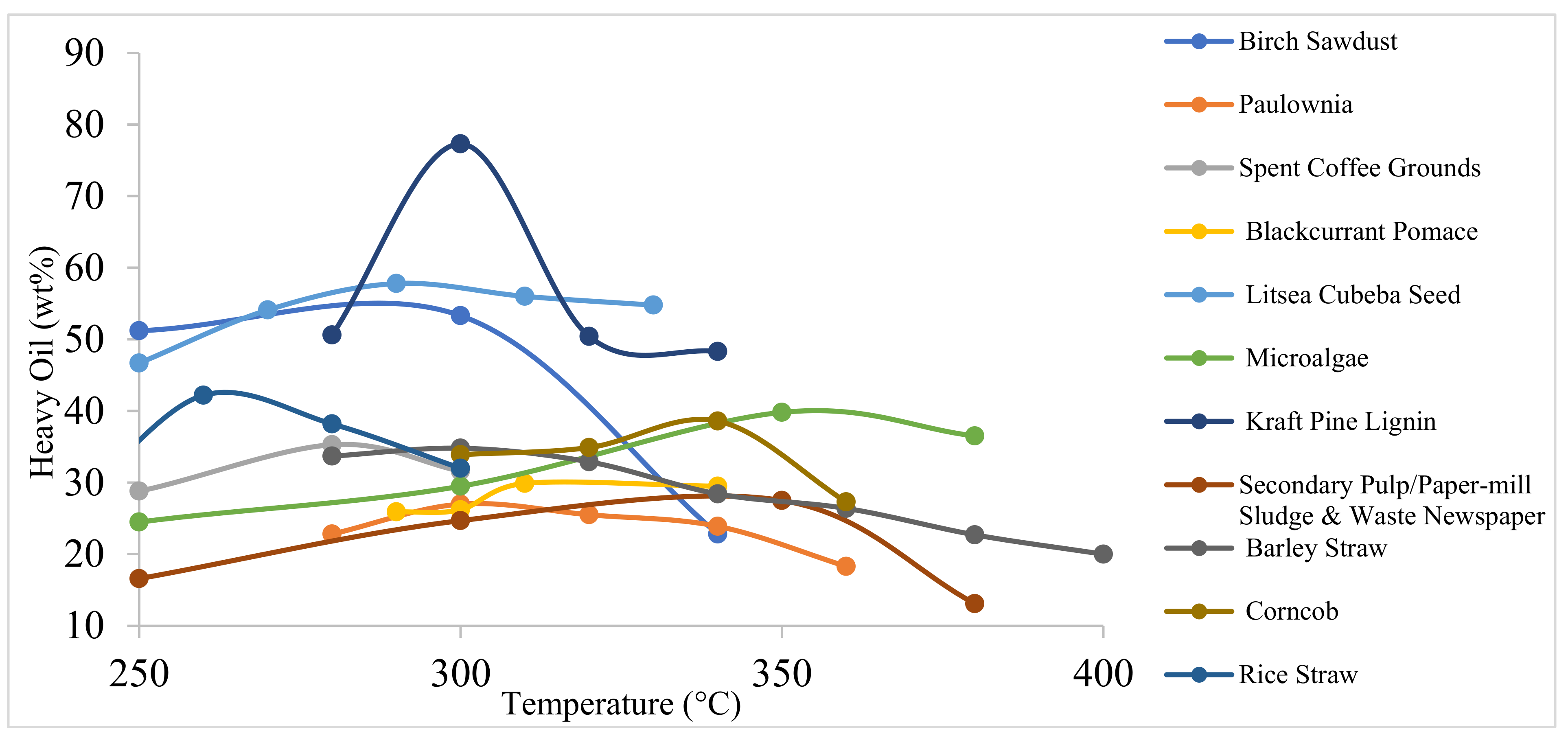
| 6 | 2013 | HTL of Litsea cubeba seed to produce bio-oils. Effect of temperature, residence time, reactor loading, and catalyst concentration was studied to maximize production. | In this study, Na2CO3 suppressed the polymerization pathways and, hence, negatively impacted the yield of bio-oil, which is contradictory to other studies. The bio-oil yield increased with increase in reactor loading up to a certain extent. | [57] |
| 7 | 2014 | Effect of heating rate on biomass liquefaction: Differences between subcritical water and supercritical ethanol. | In case of subcritical water, no significant effect of heating rate was observed for lower temperature of 250–280 °C, whereas significant change in conversion and bio-crude was observed for temperature above 315 °C. Only a marginal effect of supercritical ethanol as solvent was observed on HTL conversion and bio-crude yield. | [58] |
| 8 | 2014 | Alkaline pretreatment and HTL of cypress for high yield bio-oil production. Understand the reaction mechanism. | Alkaline pretreatment disrupts surface barriers and increases surface availability of biomass. Alkaline pretreatment helps to suppress the re-polymerization reactions during HTL. It markedly enhanced the bio-oil yield at optimum temperature. | [39] |
| 9 | 2014 | The effect of temperature on the catalytic conversion of Kraft lignin using near critical water. The catalytic conversion was studied using ZrO2/K2CO3 catalyst and phenol as co-solvent and char suppressing agent. The reaction temperature studied were 290–370 °C in a continuous flow reactor with feed flow of 1 kg h−1. | As the temperature increased the yield of water-soluble organics increased (5–11 wt%), bio-oil decreased (87–69 wt%) and that of char increased (16–22 wt%). The yield of catechols, alkylphenols, and anisoles increased with increase in temperature whereas the mass fraction of guaiacols and phenol dimers decreased. Total mass fraction of phenol-free compounds increased with increase in temperature in both water-soluble fraction and lignin bio-oil. | [56] |
| 10 | 2014 | Bio-oil production from oil palm biomass (empty fruit bunch, palm mesocarp fiber, and palm kernel shell) via subcritical and supercritical HTL. | The optimum condition for HTL of all oil palm biomass was 390 °C and 25 MPa with bio-oil yield of palm kernel shell (38.5 wt%) > empty fruit bunch (37.4 wt%) > palm mesocarp fiber (34.3 wt%). | [32] |
| 11 | 2015 | HTL of rice straw: effect of reaction environment. Effect of N2, O2, and CO2 on product distribution. | HTL in N2 environment produced highest bio-oil (17 wt%), whereas it was lowest in case of O2. In O2 atmosphere oxidation of some phenolic derivative occurred. | [77] |
| 12 | 2015 | High conversion of miscanthus using sub- and supercritical water above 400 °C. | At 410 °C, highest conversion of 90% with heavy oil yield of 22 wt% and light oil yield of 12 wt% was obtained. The gaseous fraction increased significantly at a temperature of 460 °C. | [78] |
| 13 | 2015 | HTL of barley straw to bio-crude oil. Understanding the effect of reaction temperature and aqueous phase recirculation. | Maximum bio-crude yield of 34.9 wt% was obtained at 300 °C with pure water. On aqueous phase recirculation at 300 °C, the bio-crude yield increased to 38.4 wt% after three aqueous phase re-circulations. The aqueous phase was composed of alcohols, polyols, and -oic acids. Major compounds in bio-crude oil were phenolic, carboxylic acids, aldehydes, and alcohols. Recirculation facilitated higher carbon content in solid char. | [27] |
| 14 | 2016 | HTL of spent coffee grounds in water medium for bio-oil production. Effect of temperature, retention time, pressure, and biomass/water ratio. | The highest yield of crude bio-oil was experimented to be 47.3 wt% at 275 °C, 10 min, 1/20 (biomass/water ratio) and initial pressure of 2 MPa. Crude bio-oil molecular weight decreased from 200 °C (769 g mol−1) to 300 °C (479 g mol−1). Similarly, crude bio-oil m. wt. decreased with increase in retention time 10 min (479 g mol−1) to 30 min (433 g mol−1). | [79] |
| 15 | 2016 | Conversion of waste bamboo chopsticks to bio-oil via HTL using K2CO3. Understanding the effect of temperature, retention time, and catalyst on HTL. | The simulated distillation characteristics of bio-oil obtained in the study are close to diesel. K2CO3 was an effective catalyst for HTL of feedstock such as bamboo chopsticks, which have very high lignin content 25.5 wt%. | [80] |
| 16 | 2016 | Optimization of bio-oil production by HTL of agro-industrial residues: blackcurrant pomace as an example. The objective is to study temperature, retention time, slurry concentration, and pH effect on bio-oil yield. | The bio-oil obtained in the study has high heating value, low ash content and the boiling point lies in range for upgrading to useable fuels. However, the acidity and viscosity of oil suggest a need for further upgradation of oil. The low influence of retention time suggest continuous reactor of shorted residence can be adapted. | [81] |
| 17 | 2017 | HTL of lignin in a new batch reactor in near critical water: influence of phenol and temperature. Demonstration of a new batch reactor encompassing fast biomass heating, pressure control, and product quenching. | Most of the obtained compounds in products can be classified in five categories: methoxybenzene, guaiacols, catechols, alkylphenols (methyl, ethyl), and phenolic dimers. With increased temperature, the fraction of phenol-free components increased in both water-soluble organics and bio-oil. With an increase in the phenol fraction as a co-solvent in the feed, total solid residue formation decreased and the phenol-free yield of lignin derived 1-ring aromatic compound increased. | [53] |
| 18 | 2017 | Bio-crude production via supercritical Hydrothermal co-liquefaction of spent mushroom compost and aspen wood sawdust. The work focuses on supercritical catalyzed and non-catalyzed HTL at 400 °C, 15 min. Study on co-liquefaction with aspen wood and acid leaching aimed at solid reduction. | A bio-crude yield of 48 wt% was obtained using spent mushroom compost at 400 °C, 15 min in the presence of K2CO3 catalyst. It also produced 50 wt% solid fractions. Co-liquefaction of spent mushroom compost along with aspen wood sawdust in a ratio of 1:3 helped to reduce solid fraction to 24.5 wt%. | [35] |
| 19 | 2017 | Co-liquefaction of spent coffee grounds and lignocellulosic feedstocks. Exploring the co-liquefaction of spent coffee grounds with paper filter, corn stalk, and white pine bark. The main objective was to obtain possible synergistic effect of different feedstock to obtain bio-crude economically. | The optimum temperature was 250 °C, with mixing biomass ratio of 1:1. Spent coffee grounds and corn stalks were identified as the best feedstock combination for co-liquefaction with a significant positive synergistic effect. It was also found that the overall oil quality improved in terms of viscosity and relative molecular mass. | [82] |
| 20 | 2020 | HTL of lignocellulosic biomass feedstock to produce biofuels: parametric study and product characterization. | Highest yield of heavy oil (29. 5 wt%) was observed at 300 °C, final pressure 2200 psi and 0 min retention time. The highest HHV was 35.5 MJ kg−1 obtained for heavy oil yielded at 375 °C. | [24] |
| 21 | 2020 | Oil from plastic via HTL: production and characterization. Effect of resident time and temperature was studied for HTL of polypropylene (PP), polystyrene (PS), polycarbonate (PC), and polyethylene terephthalate (PET). | The highest oil yield for different plastic material was found to be 32 wt% for PP (425 °C, 30 min), 16 wt% for PET (450 °C, 30 min), 60 wt% for PC (425 °C, 30 min), and 86 wt% for PS (350 °C, 30 min). Depolymerization of plastic was fastest in supercritical water regime. The HHV value of oil from PS and PP was comparable to gasoline. | [83] |
| 22 | 2020 | Catalytic HTL of Lactuca scariola with a heterogeneous catalyst: the investigation of temperature, reaction time, and synergistic effect of catalysts. | The yield of light bio-oil increased on increasing temperatures, from 220 °C to 280 °C, and decreased afterwards. The yield of heavy bio-oil increased with temperature, whereas that of solid residue decreased. | [84] |
| 23 | 2021 | HTL of olive oil residue. The objective was to obtain optimum process parameters for HTL of olive oil residue. | Bio-oil and solid residue yield of 30.8 wt% and 31.8 wt% was observed at optimum condition 300 °C and 15 min, respectively. | [85] |
| 24 | 2021 | Fast hydrothermal co-liquefaction of corn stover (CS) and cow manure (CW) for bio-crude and hydrochar production. Several reaction parameters, such as residence time, reaction temperature, and feedstock mass ratio were studied. | The highest yield of bio-crude was found to be over 24 w% at 400 °C, 16 min, and CS:CW ratio of 1:1. For a fixed temperature of 400 °C, the yield of bio-crude was maximum at CS:CM ratio of 1:1. The percent of phenolic compound was found to increase with residence time and was approximately 42.92% for 30 min residence time at 400 °C and mass ratio 1:1. | [8] |
| Feedstock | Process Condition | Product Yield (wt%) | HHV * (MJ kg−1) | C * (wt%) | H * (wt%) | O * (wt%) | Oil Properties | Ref. |
|---|---|---|---|---|---|---|---|---|
| Wood (konara) | T *: 250–400 °C Pi *: 2 MPa Rt *: 30 min | HO *: 15–28.4 | 17.4–35.5 | 63.8–77.9 | 1.4–9.4 | 14–34.7 | - | [88] |
| Cunninghamia lanceolata | T: 280–360 °C Rt: 10 min | HO: 19.3–23.8 | 27.1–30.2 | 69.6–75.1 | 5.6–5.9 | 19.2–24.8 | - | [89] |
| High diversity biomass | 300–450 °C | LP *: 64–74 | - | Acetic acid (27%), ketones (20%), aldehydes (4%), benzenediols (17%), phenolics (26%), phenyl derivatives (7%) | [90] | |||
| Wood (Picea orientalis) | 277–377 °C Rt: 25 min | HO: 13.8–25.8 | 28.3–31.9 | 75.4 | 6.1 | 18.8 | - | [91] |
| Wood (Quercus robur) | HO: 15.9–27.1 | 27.9–31.6 | 75.2 | 6.1 | 18.9 | - | ||
| Wood (Fagus orientalis) | HO: 16.8–28.4 | 27.6–31.3 | 75.1 | 6.0 | 19.1 | - | ||
| Wood (Paulownia) | 280–360 °C Rt: 10 min 60 g/360 mL | HO: 18.6–27 | 26.2 | 66.8 | 5.9 | 27.3 | - | [41] |
| Spirulina platensis | 200–380 °C Rt: 60 min 20% solid concentration | BC *: 18–39.9 | 25.2–39.9 | 55.5–82.1 | 8.6–9.8 | 0.6–28.9 | - | [74] |
| Cornelian cherry stones | 200–300 °C Rt: 0 min | LO *: ~3–6 | 20.8–22.5 | 58–60 | 5.3–5.8 | 34–36 | Acetic acid (1.3–5%), phenolics (10.8–39%), furfurals (11–59%), vanillin (~3%) | [76] |
| HO: ~10–22 | 25.5–25.6 | 59.2–61.3 | 7.2–7.9 | 30.9–32.3 | Furfurals (0.6–3.9%), phenolics (~5%), -oic acids (45–61%) | |||
| Microcrystalline cellulose | 200–400 °C Rt: 30 min | HO: 5–14.75 | 25.01 | 69.9 | 4.2 | 25.9 | Acids (14%), aldehydes (10%), furans (4%), esters (28%), phenolics (7%), ketones (10%) | [50] |
| Palm kernel shell | 330–360 °C P: 25 MPa | BO *: 22.8–38.5 | - | - | - | - | Phenolics (80.7%), other aromatics (6.4%), ketones (6.7%), alcohols (3%), esters (3.3%) | [32] |
| Empty fruit bunch | 330–360 °C P: 25 MPa | BO: 15.7–37.4 | - | - | - | - | Phenolics (72.8%), other aromatics (10.7%), ketones (16.5%) | |
| Swine carcasses | T: 150–400 °C Rt: 60 min pH: 11 | BO: 40.1–58.2 | 32.3 | 75 | 13.7 | 8.4 | Phenyl der (21%), ketones (8.5%), furfurals (5%), aldehydes (3.7%), fatty acids (13) | [92] |
| Barley straw | T: 280–400 °C Catalyzed | BC: 19.9–34.9 | 26.8–35.5 | - | - | - | Phenolics (5%), FAAE * (20%), ketones (3-4%), cresol (2%), catechol (3%), alcohols | [27] |
| Spent coffee grounds | T: 200–300 °C Pi: 2 MPa, Rt: 10 min | BC: 16.6–35.3 | 31 | 71.2 | 7.1 | 18.7 | Hexadecanoic acid (48%), octadecanoic acid (15%), octadecadienoic acid (35%) | [79] |
| Waste bamboo chopstick | T: 290–380 °C Catalyzed | BO: 7.1–21.2 | 29.7–31 | 66.4–74.2 | 6.7–7.4 | 18.4–25.1 | - | [80] |
| Blackcurrant pomace | T: 290–335 °C Rt: 60 min | BO: 25.5–30 | 35.9 | 73.3 | 9.6 | 13.6 | Ketones (6%), FAAE (3%), FA * (30%), FAM (10%), phenolics (9%) | [81] |
| Coconut shell | T: 240–330 °C Rt: 30 min | BO: 7–13.9 | 29.9–31.1 | - | - | - | Ketones (0.1–2.7%), phenol (1.1–6%), acids (0.3–1.7%), furfurals (4–6%), polyols and alcohols (0.4–6.3%) | [93] |
| Spent coffee ground + corn stalk | T: 225–325 °C Rt: 10 min | BC: 10.2–21.6 | 33.3 | - | - | - | Long chain carboxylic acids | [82] |
| Rice straw | T: 200–300 °C Rt: 120 min | LO: 4.9–11.7 | 22.2–24.9 | 58.6–61.1 | 5.9–6.9 | 31.4–34.4 | Fatty acids, carboxylic acids, furan, aldehydes, esters, phenols, ketones, alcohols, alkenes, alkanes | [26] |
| HO: 8.8–15.9 | 22.4–31.9 | 58.6–72.7 | 6.5–7.5 | 18.8–34.8 | Carboxylic acids, ketones, alkanes, alcohols, amines, phenols | |||
| Corn stover | T: 250–375 °C Rt: 15 min, Pi: 4 MPa | HO: 14.3–27.2 | 27.5–35.1 | 64.9–76.3 | 7.2–8.2 | 13.6–26.5 | Ketones (2.1–9.6%), linear saturated and unsaturated HC (1–12%), phenyl compounds (3–7%), phenolic derivatives (56–82%), aldehydes, fatty acids, and fatty acid alkyl esters. | [14,24] |
| Olive oil residue | T: 250–330 °C, Rt: 15 min | BO: 14.79–30.75 | 25.6–31.8 | 63.8–70.9 | 6.6–7.9 | 19.9–28.5 | Ketones (17.81%), phenols (43.56%), acids (0.69%), and esters (10.47%) | [85] |
| Feedstock | Process Condition | Product Yield (wt%) | HHV * (MJ kg−1) | C *(wt%) | H * (wt%) | O * (wt%) | Oil Properties/Comments | Ref. |
|---|---|---|---|---|---|---|---|---|
| Wood (konara) | Pi *: 0.5–10 MPa T *: 350 °C Rt *: 30 min | HO *: 5.9–20.1 | 31.6–35.5 | 73.6–76.9 | 7–8.1 | 13.9–19.4 | The yield was remarkably dependent on pressure and maxed at initial pressure of 4.0 MPa. | [88] |
| Birch powder | Pi: 0–10 MPa T: 200 °C, Rt: 30 min | HO: 3–5.8 | - | - | - | - | There was a sharp increase in oil yield on increasing pressure from 0 to 2 MPa followed by a decrease in yield on further increasing pressure until 9 MPa. | [97] |
| Pi: 0–10 MPa T: 300 °C, Rt: 30 min | HO: 9.2–12.7 | - | - | - | - | |||
| Red pine sawdust | Pi: 0.4–7.5 MPaT: 370 °C, Rt: 30 min | BC *: 52–56 | - | - | - | - | H/C: 1.43–1.47, O/C: 0.28–0.34 The initial pressure head had negligible effect on conversion, bio-crude, and solid yield. | [98] |
| Palm mesocarp fiber | P: 25–35 MPa T: 390 °C | BO *: 29.4–38.5 | - | - | - | - | Phenolics (89.4%), other aromatics (5.3%), ketones (5.3%). The decreased bio-oil yield at higher pressure in supercritical condition was attributed to be the result of gasification due to increased free radical reaction. | [32] |
| P: 25–35 MPa T: 360 °C | BO: 23.4–27.5 | |||||||
| P: 25–35 MPa T: 330 °C | BO: 22.8–25.6 | |||||||
| Poplar wood | P: 0–4 MPa | BO: 16–18 | 26.8 | 66.3 | 6.4 | 27.1 | Bio-oil yield increased while solid residue decreased with the increase in pressure. | [37] |
| Corn stover | Pi: 2–4 MPa T: 300 °C | HO: 21.8–27.2 | 29.6–30.3 | 68.3–69.4 | 7.4–7.5 | 21.5–22.7 | Effect of pressure was obvious in sub-critical temperature and negligible near supercritical condition. | [24] |
| Pi: 2–4 MPa T: 375 °C | HO: 14.3 (No change) | 35.1 | 76.3 | 8.2 | 13.6 |
| Feedstock | Process Condition | Product Yield wt%, | HHV * (MJ kg−1) | C * (wt%) | H * (wt%) | O * (wt%) | Comments/Findings | Ref. |
|---|---|---|---|---|---|---|---|---|
| Wood (konara) | Rt *: 0–180 min T *: 350 °C, Pi: 2 MPa | HO *: 14.7–24.6 | 30.3–35.8 | 71.6–77.9 | 6.9–7.9 | 14.1–21.5 | Highest oil yield was obtained at 0 min and yield decreased afterwards. | [88] |
| Cunninghamia lanceolata | Rt: 10–30 min T: 320 °C | HO: 17.5–23.8 | 28.9 | 73.3 | 5.6 | 21.1 | Optimum time was 10 min and the CHNS and HHV values are mentioned for the same. The yield of oil decreased with retention time. | [89] |
| Birch powder | Rt: 10–480 min T: 300 °C, methanol | HO: 10–26 | - | - | - | - | Total conversion and yield of all products increased monotonically with retention time. | [97] |
| Microalgae (Desmodesmus sp.) | Rt: 5–60 min T: 200 °C | BO *: 10–23 | 31.0–33.7 | 66.6–69.2 | 8.6–9.6 | 17.5–22.8 | The effect of retention time was more pronounced at lower temperature (200 °C). The effect of time was little at 300 °C and above. Water-soluble organics decreased with reaction time above 300 °C. | [73] |
| Rt: 5–60 min T: 300 °C | BO: 40.5–46.6 | 34.9–35.8 | 72.4–75 | 8.8–9 | 10.2–12.3 | |||
| Spirulina platensis | Rt: 0–120 min 350 °C, 20% solid concentration | BC *: 30–39.9 | 34.1–36.8 | 73.1–77.2 | 8.6–9.6 | 9.1–11.1 | The yield of bio-crude increased from 0 min to 60 min and decreased afterwards. WSO fraction decreased with increase in time. There was no significant effect on solid residue. | [74] |
| Cornelian cherry stones | Rt: 0–30 min T: 300 °C | LO *: ~4–6 | 22.5–23.9 | 59.9–61.3 | 5.8–6.3 | 32.2–34 | The total bio-oil yield was maximum at 0 min retention time for 250 °C and 300 °C, whereas at lower temperature of 200 °C it was maximum at 30 min. | [76] |
| HO: ~14–22 | 25.6–28.4 | 59.2–67.2 | 7.1–7.9 | 25.1–32.3 | ||||
| Litsea cubeba seed | Rt: 30–120 min T: 290 °C | BO: 53.5–56.9 | - | - | - | - | It was observed that retention time had limited effect on the product yield making it a thermally controlled process. | [57] |
| Spent coffee grounds | Rt: 5–30 min T: 275 °C, Pi: 2 MPa | BC: 22.7–31.7 | 31 | 71.2 | 7.1 | 18.7 | The highest yield of crude bio-oil was obtained at 10 min (31.7 wt%) followed by a decrease until 30 min (22.7 wt%). | [79] |
| Blackcurrant pomace | Rt: 0–240 min T: 300 °C | BO: 22.5–27 | 35.9 | 73.3 | 9.6 | 13.6 | The CHNS and HHV value was provided at 300 °C and 60 min.The holding time had no significant effect on the yield of products. | [81] |
| Waste furniture sawdust | Rt: 0–60 min T: 280 °C | BO: 7.2–12.7 | - | - | - | - | The maximum bio-oil yield was obtained at residence time of 15 min. The yield of solid residue decreased initially until 30 min and increased thereafter, whereas the gas yield increased with increase in retention time. | [38] |
| Empty fruit bunch | Rt: 15–120 min T: 360–450 °C P: 25 MPa | - | - | - | - | - | The relative yield of bio-oil increased throughout retention time for T: 360 °C. The relative yield of bio-oil increased until 60 min for temperatures 390 and 450 °C, and decreased steeply on further increase in time. | [103] |
| Olive oil residue | Rt: 5–60 min T: 300 °C | BO: 20.7–30.8 | 29.1–32.2 | 68.0–70.7 | 7.3–8.3 | 20–23.7 | The yield of bio-oil and solid residue increased from 5 to 15 min and decreased afterwards. The carbon percentage and HHV value of bio-oil was maximum at residence time of 5 min. | [85] |
| Type | Feedstock | Process Condition | Product Yield wt% | Comments/Findings | Ref. |
|---|---|---|---|---|---|
| Wood | Corn stover | 5–140 °C min−1 T *: 350 °C | LP *: 53.4–70.6 | [72] | |
| Aspen wood | LP: 50.3–72.4 | ||||
| High diversity grassland perennials | 5–140 °C min−1 T: 375 °C | LP: 61.1–73.1 | [90] | ||
| Red pine sawdust | 3–20 °C min−1 T: 350 °C | BC *: 15–27 | Maximum bio-crude was obtained by a combination of fast heating rate, high final temperature, and immediate quenching.There was no effect of heating rate in the presence of supercritical EtOH. | [58] | |
| Spruce wood | 66–179 °C min−1 T: 350 °C | BO *: 18.9–35.8 | The bio-oil yield increases with increasing heating rate, whereas the solid residue decreases. Maximum yield was obtained at heating rate of 179 °C min−1. | [104] | |
| Algae | Laminaria saccharina | 146–585 °C min−1 T: 350 °C | BO: 53–79 | Fast heating rate promoted bio-oil yield and decreased formation of gas and char. | [105] |
| Chlorella | 10–25 °C min−1 T: 350 °C | BO: 35.8 | There was no significant effect of heating rate on the yield of bio-oil. | [106,107] | |
| Nannochloropsis | BO: 34.3 |
| Feedstock | Process Condition | Product Yield (wt%) | HHV * (MJ kg−1) | C * (wt%) | H * (wt%) | O * (wt%) | Comments | Ref. |
|---|---|---|---|---|---|---|---|---|
| Wood (konara) | 1/16–1/6 g/mL T *: 300 °C, Pi *: 2 MPa Rt *: 30 min | HO *: 16.4–25.2 | 31.9–33.8 | 73.8–76.6 | 7.16–7.48 | 15.9–19 | Yield of oil was maximum at 5/50 g/mL of biomass/water ratio. | [88] |
| Cunninghamia lanceolata | 8/100–12.5/100 g/mLT: 320 °C, Rt: 10 min | HO: 16.3–23.8 | - | - | - | - | The fraction of heavy oil decreased with increasing biomass/water ratio. | [89] |
| Secondary pulp/paper sludge + newspaper | 4.8–16.7 wt% solid concentration T: 280 °C, Rt: 60 min | HO: 15–22 | - | - | - | - | There was a significant decrease in water-soluble organics with increased feed concentration. | [96] |
| Empty palm fruit bunch | 1/10–8/10 g/mL T: 270 °C, Pi: 2 MPa, Rt: 20 min | LP *: 50–68 | - | - | - | - | Phenols (0.6–60.0), methyl ester (0–94.3%), benzoic acid (0–24.3%) With increase in feed concentration fraction of methyl esters decreased initially and on further increase vanished completely. | [108] |
| Spirulina platensis | 10–50% solid concentration 350 °C, Rt: 60 min | BC *: 33–39.9 | 35.6–35.9 | 72.3–73.8 | 8.9–9.1 | 10.5–11.7 | Bio-crude yield increased as solid concentration increased from 10 to 20%. No change in product composition over solid concentration of 20%. | [74] |
| Litsea cubeba seed powder | 0.5–4.5 g T: 290 °C, Rt: 60 min | BO *: 42.4–56.9 | 40.8 | 76.2 | 11.9 | 10.4 | The bio-oil yield increased initially with solid fraction and started to decrease afterwards. | [57] |
| Miscanthus | 2/5–1/15 T: 410 °C, Rt: 60 min | HO: 9–22 | - | 77 | 6.9 | 16 | - | [78] |
| Spent coffee grounds | 1/20–1/5 g/mL T: 275 °C, Pi: 2 MPa, Rt: 10 min | BC: 35.3–47.3 | 31 | 71.2 | 7.1 | 18.7 | Larger water to spent coffee ground ratio has positive impact on conversion rate and bio-oil yield. | [79] |
| Blackcurrant pomace | 0.05–0.29 (dry mass fraction) T: 300 °C, Rt: 60 min | BO: 24–29 | - | - | - | - | Carbon recovery in bio-oil increased initially with increase in concentration and decreased after a point, whereas the yield of bio-oil decreased thoroughly. | [81] |
| S. No | Year | Description/Objective | Results/Observations/Major Findings | Ref. |
|---|---|---|---|---|
| 1 | 2005 | Low temperature catalytic hydrothermal treatment of wood biomass: analysis of liquid products. Effect of Na and K hydroxides and carbonates on liquefaction products. | Catalytic activity order K2CO3 > KOH > RbCO3 >RbOH > Na2CO3 > NaOH > CsCO3 > CsOH for bio-oil production. Oil yield increased from 8.6 wt% in water to 33.7 wt% in the presence of K2CO3. Boiling point of hydrocarbons ranged near n-C11 and mainly phenolic in nature. | [70,109,110] |
| 2 | 2010 | Direct liquefaction of paulownia in hot compressed water. Effect of Fe and Na2CO3 on heavy oil yield. | Both Fe and Na2CO3 effectively enhanced the formation of heavy oil, and the highest heavy oil yield of 36.34 wt% was observed with Fe catalyst at 340 °C. Both catalysts significantly promoted the gases formation. | [41] |
| 3 | 2012 | HTL of beech wood using a natural calcium borate mineral. | The total bio-oil yield at 300 °C, 0 min without and with colemanite was 21 wt% and 41 wt%. The use of colemanite had no significant effect on heating value and composition of oil. | [43] |
| 4 | 2013 | Effect of sodium perborate monohydrate concentrations on product distributions from HTL of scotch pine wood. | The highest heating value of resulting heavy oil was 32 MJ kg−1, at 350 °C with the catalyst. The product distribution significantly varied with temperature and additive concentration. | [111] |
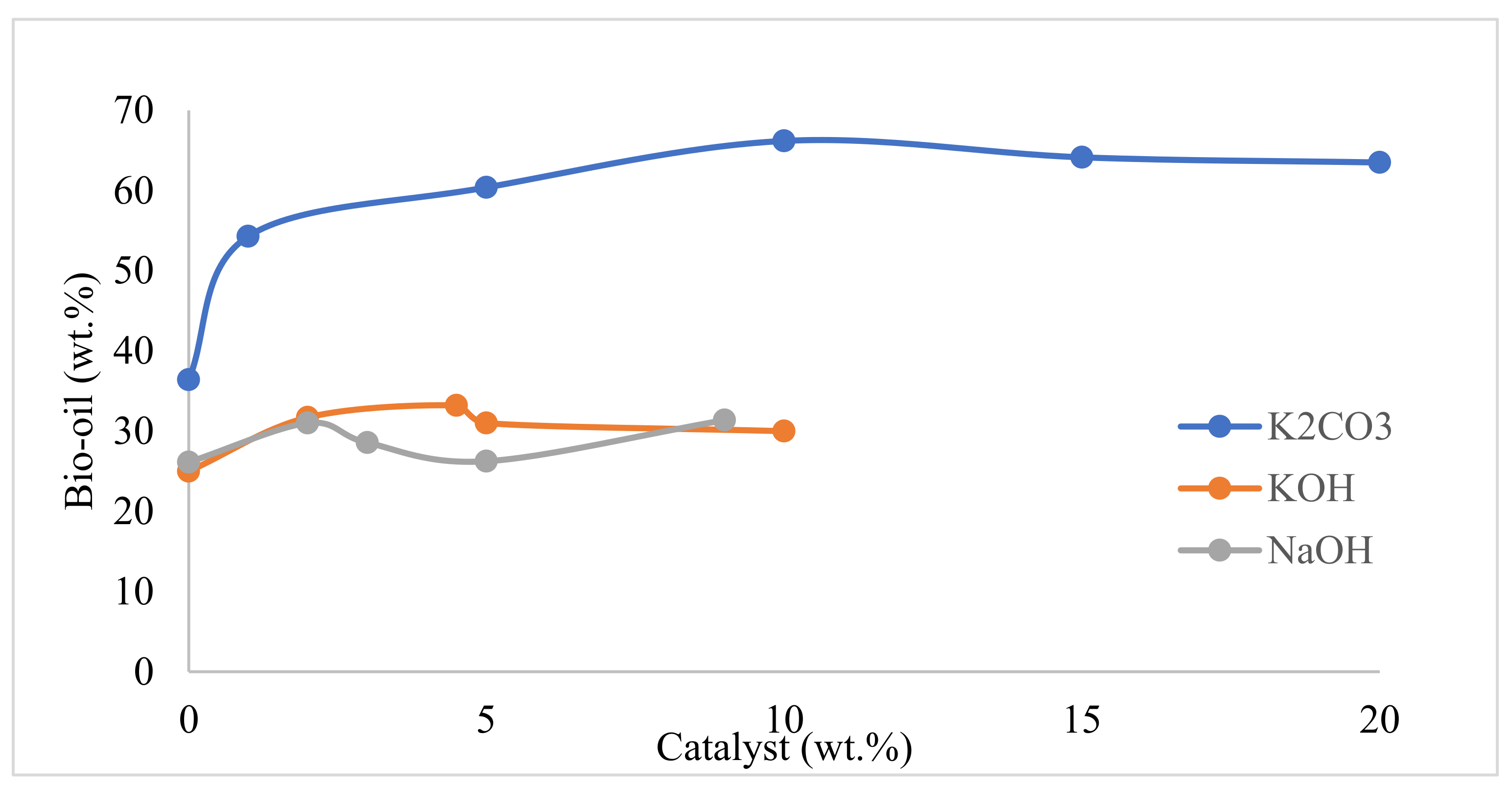
| 5 | 2015 | Influence of alkali catalyst on product yield and properties via HTL of barley straw. | Addition of K2CO3 increased bio-crude yield and inhibited solid char formation. Catalyst run bio-crude has better properties, such as higher HHV value and lower O/C ratio. | [15] |
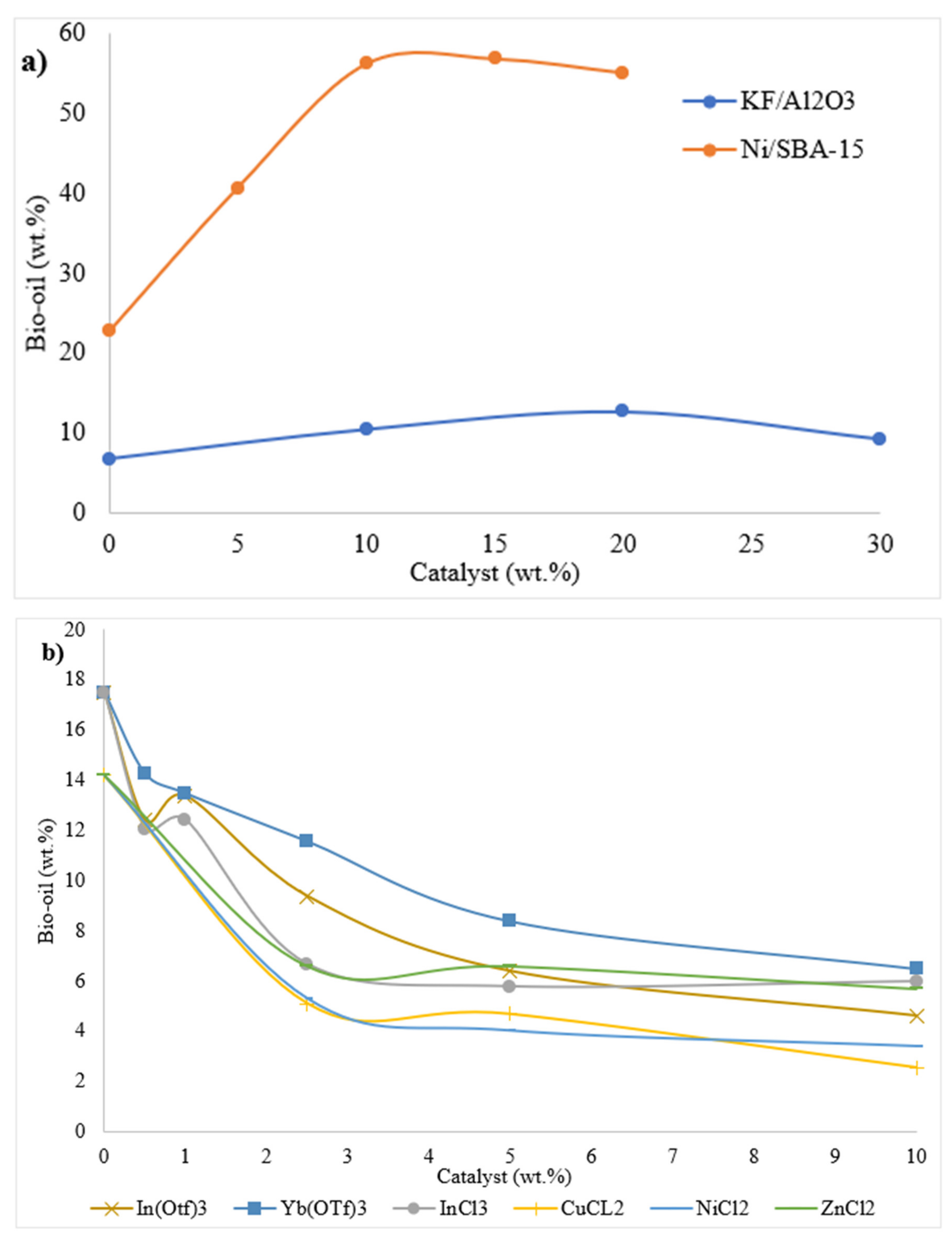
| 6 | 2016 | Characterization of HTL products from coconut shell in the presence of selected transition metal chlorides. To examine the effect of Zn, Cu and Ni on HTL at fixed temperature and to understand the effect of temperature. | The highest bio-oil yield was 13.9 wt% at 300 °C without the catalyst, where the water content was minimum (3 wt%) and HHV was highest (31.1 MJ kg−1). Main decomposition products of cellulose levulinic acid and γ-valerolactone were present in oil.The use of transition metals helped in decreasing the TAN value of oil. | [93] |
| 7 | 2016 | Structural analysis of bio-oil from subcritical and supercritical HTL of Datura stramonium L. plant stems. Understanding the effect of temperature and catalyst (colemanite and borax). | Conversion increased with increase in temperature. Colemanite catalyst was more effective than Borax. The GC–MS composition at all of the temperatures were provided. | [36] |
| 8 | 2016 | The effects of water tolerant Lewis acids on the HTL of lignocellulosic biomass. | The use of water tolerant Lewis acids had negative effects on bio-oil yields. The study suggest that low catalyst concentration promotes production of oil. Lewis acids have no significant effects on composition of oil. | [37] |
| 9 | 2017 | Production of bio-oil via HTL of birch sawdust. To understand the effect of biomass/water ratio, temperature, H2 pressure, residence time, catalysts (FeSO4, ZnSO4, NiSO4, Raney nickel, Ni65% -Al2O3, Na2CO3, NaOH). | The highest yield of bio-oil (54.1 wt%) was obtained at 300 °C, 5 min in the presence of NaOH catalyst. The order of catalyst to promote bio-oil yield was NaOH > Na2CO3 > Ni65% |SiO2–Al2O3 > Raney nickel > NiSO4 > ZnSO4 > FeSO4 > none. | [34] |
| 10 | 2017 | HTL of rice straw with NiO nanocatalyst for bio-oil production. | The tested catalyst (NiO) helped in increasing the overall oil yield but had no significant impact on the elemental composition and HHV of oil. | [26] |
| 11 | 2017 | Catalytic HTL for bio-oil production over carbon nanotubes (CNTs) supported metal catalysts. Effect of carbon nanotubes supported transition metals (Co, Ni, Pt) for HTL of Dunaliella tertiolecta to produce bio-oil. | The addition of catalysts was conducive for bio-oil yield and quality. All (Co, Ni, and Pt) gave almost equal bio-oil yield of 40.25 wt%, whereas the conversion of biomass was highest for Co|CNTs (95.8%) > Ni (93.3%) > Pt (92.4%) > None (~89%). Bio-oil obtained using Co catalyst had higher hydrocarbons, lower fatty acids, and nitrogen compounds. The selected catalysts helped in reducing the nitrogen content in bio-oil. | [112] |
| 12 | 2017 | Bio-crude from pretreated sorghum bagasse through catalytic HTL. To study liquefaction at different temperature and six different catalysts. | The highest bio-crude yield was obtained with K2CO3 (61.8 wt%) catalyst. The bio-crude had high carbon content and low nitrogen and sulfur contents. The relative high oxygen content ~15% in bio-crude requires it to be upgraded before refinery ready. | [29] |
| 13 | 2017 | Metal oxide catalyzed hydrothermal liquefaction of Malaysian oil palm biomass to bio-oil under supercritical condition. Effect of CaO, MgO, MnO, ZnO, NiO, SnO, CeO2, Al2O3, La2O3 was studied. Fitting kinetic formation to the modified Reverchon-Sesti Osseo model. | It was found that La2O3, CeO2, MnO and CaO, were four most active catalyst. The bio-oil yield in case of CeO2 was 1.44 times that of without catalyst. It was found that phenolics, ketones, other aromatics, and carboxylic acids were the major compounds. The bio-oil produced was easily volatilized and combusted in TGA study. | [103] |
| 14 | 2019 | Enhanced bio-crude yield and quality by reductive HTL of oak wood biomass: Effect of iron addition. Iron powder, hematite, and magnetite were investigated as catalyst in 1:5 biomass to water ratio for 260–330 °C. | Oxidation of Fe to produce H2 in water was a key step in the process as the produced H2 prevented the repolymerization and condensation of bio-oil precursors. Fe as catalyst increased the bio-oil yield by 30 wt% and improved the quality by reducing oxygen content, increasing the HHV value to 32.4 MJ kg−1. | [31] |
| 15 | 2020 | Catalytic HTL of lactuca scariola with a heterogeneous catalyst. Effect of Zn, Fe, and Zn + Fe was studied. | Fe was found to be most effective for the production of bio-oil. The yield of aqueous and gas fraction was maximum in the presence of Zn + Fe. | [84] |
| 16 | 2021 | HTL of olive oil residue Understanding the effect of metal chlorides on HTL products. | It was found that the yield of bio-oil decreased and that of solid residue increased with the addition of AlCl3 and SnCl3. | [85] |
| 17 | 2021 | Use of Lewis acid, a Brønsted acid, and their binary mixtures for the HTL of lignocellulose.HTL of teak wood was studied without and with the use of Mg(ClO4)2, HClO4, and HClO4/Mg(ClO4)2. | It was found that the catalyst had a negative effect on the yield of bio-oil; moreover, the yield of oil was found to decrease with the increase of catalyst loading. The use of catalyst increased the percentage of naphtha in bio-oils. The relative yield of ketones, phenols, and acids were affected in the presence of the catalyst. | [113] |
| Feedstock | Process Condition | Catalysts | Product Yield (wt%) | HHV *(MJ kg−1) | C * (wt%) | H * (wt%) | O * (wt%) | Oil Properties | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Wood (konara) | T *: 350 °C Pi *: 2 MPa Rt *: 30 min | - | HO *: 5.0 | 28.9 | 70.9 | 6.9 | 22.1 | - | [88] |
| K2CO3 | HO: 21.4–26.2 | 30.4–33.4 | 71.8–76.6 | 6.8–7.7 | 15.9–21.2 | - | |||
| Sawdust (pine wood) | T: 280 °C Rt: 15 min | - | BO *: 8.6 | - | - | - | - | EEO *: aldehydes (18%), ketones (8%), phenolics (45%), fatty acids (8%) EAO *: unsaturated chain (26%), ketones (11%), -oic acid (32%) | [70,109] |
| NaOH | BO: 22.4 | - | - | - | - | EEO: acetic acid (3–12%), phenolics (50–70%), benzenediols (10–25%) EAO: ketones (45–60%), unsaturated chains (15–25%) | |||
| Na2CO3 | BO: 23.0 | ||||||||
| KOH | BO: 28.7 | ||||||||
| K2CO3 | Total oil: 33.7 | ||||||||
| RbOH | BO: 23.8 | ||||||||
| CsOH | BO: 20.8 | ||||||||
| Empty palm fruit bunch | T: 270 °C Pi: 2 MPa Rt: 20 min | - | LP *: 30 | - | - | - | - | Methyl esters (26.44%) | [108] |
| NaOH | LP: ~58 | - | - | - | - | Phenols (6%), methyl ester (86.45%) | |||
| KOH | LP: ~58 | - | - | - | - | Phenols (18.4%), methyl ester (81.6%) | |||
| K2CO3 | LP: ~63 | - | - | - | - | Phenols (60.08%), methyl ester (39.92%) | |||
| Wood (Paulownia) | T: 300 °C Rt: 10 min | - | HO: 26 | 26.3 | - | - | - | Phenolics (37%), ketones (13%), carboxylic acid/ester (17%), phenyl derivatives (3%) | [41] |
| T: 300 °C Rt: 10 min | Na2CO3 | HO: 31.5 | 28.1 | - | - | - | Phenolics (39%), ketones (17%), carboxylic acid/ester (7%), phenyl derivatives (10%) | ||
| T: 340 °C Rt: 10 min | Fe | HO: 36 | 31.5 | - | - | - | Phenolics (38%), ketones (20%), carboxylic acid/ester (6%), phenyl derivatives (3%), long chain alkanes (2%) | ||
| Pulp/paper mill sludge + newspaper waste | T: 300 °C Rt: 20 min | - | BO: 24.9 | 29.5 | 70.5 | 6.8 | 22.4 | - | [75] |
| KOH | BO: 31.2 | 29.5 | 70.2 | 6.9 | 22.4 | - | |||
| HCOOH | BO: 34.4 | 29.5 | 69.9 | 6.9 | 22.1 | - | |||
| FeS | BO: 27.7 | 29.9 | 71.4 | 6.7 | 21.4 | - | |||
| Beech wood | T: 300 °C Rt: 0 min | - | LO *: 6.2 | 22.9 | 60 | 6 | 34 | Furfurals (13%), ketones (12.7%), phenolics (22%), benzenediols (5.2%) | [43] |
| HO: 14.8 | 24.4 | 60 | 7 | 33 | Acetic acid (2%), ketones (6.4%), phenolics (11.3%), FAAE * (3%) | ||||
| Colemanite | LO: 11.1 | 21.2 | 59.6 | 6.6 | 36.4 | Ketones (12%), phenolics (29.4%), benzenediols (7.3%) | |||
| HO: 29.8 | 25.2 | 62.5 | 6.7 | 31 | Acetic acid (2%), ketones (21.8%), phenolics (14%) | ||||
| Scotch pine wood | T: 300 °C Rt: 0 min | - | LO: 4.5 | 22.9 | 60 | 6.1 | 33.9 | Furfurals, ketones, phenolics, FAAE | [43] |
| HO: 19 | 27.1 | 66.6 | 6.6 | 26.8 | Ketones (16%), phenolics (9.6%), FAAE (18) | ||||
| NaBO3·H2O | LO: 7 | 24.2 | 61 | 6.6 | 32.5 | Acetic acid, ketones, phenolics, phenyl derivatives, FAAE | |||
| HO: 33 | 28.6 | 68 | 7.1 | 24.9 | Ketones (60), phenolics (4.4), FAAE (9%) | ||||
| Oil palm shell | T: 330 °C Rt: 60 min | - | BO: 14.2 | 33.1 | 79.4 | 6.3 | 13.3 | Hexadecanoic acid (54%), 6-octadecanoic acid (21.3%), oleic acid (26%) | [42] |
| K2CO3 | BO: 27.9 | 34.4 | 80.7 | 6.7 | 11.8 | Benzenediols (51%), catechols (24%), dihydroxytoluene (13%) | |||
| Na2CO3 | BO: 26.3 | 32.9 | 76.5 | 7.1 | 15.9 | Benzenediols (52%), catechols (24.5%), ethylene oxide (18%), methyl tetradecanoate (13%) | |||
| NaOH | BO: 23.9 | 31.1 | 69.9 | 8 | 21.5 | Benzenediols (73%), catechols (16.4%), dihydroxytoluene (8.3%), ethylene oxide (2.3%) | |||
| Cypress | T: 260–300 °C | - | BO: 27.5–31.1 | - | - | - | - | Ethanol insoluble organics, ethanol soluble organics, DEE soluble and insoluble oil | [39] |
| Alkaline pretreated | BO: 29.7–48.4 | - | - | - | - | ||||
| Barley straw | T: 300 °C | - | BC *: 17.88 | 24.9 | 62.6 | 6.4 | 29.8 | Phenolics + alcohols (12.6%), ketones (13%), -oic acids (29%), aldehydes (7.7%) | [15] |
| K2CO3 | BC: 34.85 | 27.3 | 67.9 | 7.6 | 23.2 | Phenolics + alcohols (12.8%), ketones (13%), -oic acids (24%), aldehydes (7%) | |||
| Waste bamboo chopstick | T: 290–380 °C Rt: 30 min | - | BO: 1.2–3.7 | 29.7–30.9 | 65.6–68.4 | 6.7–7.4 | 5.5–5.6 | - | [80] |
| K2CO3 | BO: 7.1–21.2 | 31.6–33.9 | 66.4–74.2 | 6.7–7.4 | 18.4–25.1 | - | |||
| Datura stramonium L. stems | T: 300 °C | - | BO: 32 | - | - | - | - | Cyclic HC * (15%), saturated chain HC (56%), acids (13.4%) | [36] |
| Borax | BO: 36 | - | - | - | - | Ketones (7.2%), cyclic HC (2%), saturated chain HC (52%), acids (4.2%) | |||
| Colemanite | BO: 40.2 | - | - | - | - | Ketones (6.7%), cyclic HC (0.5%), saturated chain HC (28%), acids (5.5%) | |||
| Coconut shell | T: 300 °C Rt: 30 min | - | BO: 13.9 | 31.1 | - | - | - | Ketones (2.5%), phenol (5%), acids (1.7%), furfurals (6%), polyols, and alcohols (5.3%) | [93] |
| ZnCl2 | BO: 5–6 | 28.7 | - | - | - | Ketones (2.1%), phenol (6.3%), acids (0.6%), furfurals (7.3%), polyols, and alcohols (3.7%) | |||
| CuCl2 | BO: 2–5 | 24 | - | - | - | Ketones (1.1%), phenol (5.1%), acids (0.8%), furfurals (6.8%), polyols, and alcohols (1.7%) | |||
| NiCl2 | BO: 3–5 | 28.3 | - | - | - | Ketones (1.4%), phenol (3.7%), acids (0.3%), furfurals (2.4%), polyols, and alcohols (3.4%) | |||
| Poplar wood | T: 300 °C Pi: 1 MPa Rt: 10 min | - | BO: 17.5 | 26.8 | 66.3 | 6.4 | 27.1 | Furfurals (4.6%), phenolics (39.1%), ketones (8.8%) | [37] |
| In(OTf)3 | BO: 13.37 | 25.5 | 65 | 6.1 | 28.8 | Furfurals (5.3%), phenolics (39.5%), ketones (10.1%) | |||
| Yb(OTf)3 | BO: 13.48 | 24.3 | 63.6 | 5.8 | 30.6 | Furfurals (6.7%), phenolics (38.1%), ketones (9.8%) | |||
| InCl3 | BO: 12.42 | 24.8 | 64.1 | 5.9 | 29.9 | Furfurals (3.4%), phenolics (36.6%), ketones (11.8%) | |||
| Spent mushroom compost | T: 300 °C Rt: 15 min | - | BC: 35 ± 12 | 34.5 | 73.7 | 8.5 | 13.1 | - | [35] |
| K2CO3 | BC: 48 ± 9 | 34.1 | 72 | 8.8 | 14.8 | - | |||
| Spent coffee ground | T: 250 °C Rt: 10 min | - | BC: 19.6 | 40.4 | 73.9 | 12.3 | 13 | Long chain carboxylic acids | [82] |
| NaOH | BC: 26.5 | 31.9 | 66.6 | 9.2 | 21.9 | Long chain carboxylic acids, aldehyde, ketones, alcohols, esters | |||
| Corn stalk | T: 250 °C Rt: 10 min | - | BC: 20.8 | 27.9 | 67.3 | 6.7 | 25.1 | Long chain carboxylic acids, aldehyde, ketones, alcohols, esters, cyclic oxygenates | |
| NaOH | BC: 21.4 | 27 | 64.7 | 6.9 | 27.3 | Long chain carboxylic acids, aldehyde, ketones, alcohols, esters, cyclic oxygenates | |||
| Spent coffee ground + corn stalk | T: 250 °C Rt: 10 min | - | BC: 21.4 | 33.3 | 71.9 | 8.5 | 17.9 | Long chain carboxylic acids | |
| NaOH | BC: 29.5 | 29.1 | 65 | 8.1 | 25.2 | Long chain carboxylic acids, aldehyde, ketones, alcohols, esters, cyclic oxygenates | |||
| Rice straw | T: 300 °C | - | LO: 11.7 | 24.9 | 61.1 | 6.9 | 31.4 | Phenols (39%), ketones (21%), alcohols (27%), alkanes (8%), esters (3%) | [26] |
| HO: 15.9 | 31.9 | 72.7 | 7.5 | 18.8 | Carboxylic acids (15.6%), ketones (9%), alcohols (12.5%), alkanes (28%), amines (9%) | ||||
| NiO | LO: 13.2 | 23.9 | 60.3 | 6.9 | 32.2 | Phenols (41%), ketones (24%), alcohols (27%), alkenes (2.4%), carboxylic acids (3%) | |||
| HO: 17.2 | 31.6 | 72.1 | 7.5 | 19.5 | Carboxylic acids (26.6%), ketones (7.3%), alcohols (10%), alkanes (36%), amines (11%) | ||||
| Dunaliella tertiolecta | T: 320 °C Rt: 30 min P: 12 MPa | - | BO: 34 | - | - | - | - | HCs (14%), ketones (20%), fatty acids (50%), N-containing compounds (15%) | [112] |
| Co|CNTs | BO: 40.25 | - | - | - | - | HCs (50%), ketones (13%), fatty acids (25%), N-containing compounds (8%) | |||
| Ni|CNTs | BO: 40.25 | - | - | - | - | HCs (25%), ketones (8%), fatty acids (65%), N-containing compounds (5%) | |||
| Pt|CNTs | BO: 40.25 | - | - | - | - | HCss (28%), ketones (10%), fatty acids (55%), N-containing compounds (12%) | |||
| Pretreated sorghum Bagasse | T: 300 °C | K2CO3 | BC: 61.8 | 33.1 | 73.2 | 7.7 | 15 | Ketones (3%), alcohols (1.5%), HCs (0.6%), phenolics (22%), esters (3.2%), phenyl deriv. (4%) | [29] |
| KOH | BC: 42.2 | 30 | 64.3 | 7.6 | 14.5 | Ketones (4%), alcohols (0.5%), HCs (0.8%), phenolics (23%), esters (10%), phenyl deri (1.7%) | |||
| Ni|Si-Al | BC: 45.5 | 21.5 | 50.2 | 5.9 | 21.5 | Ketones (5%), alcohols (2%), HCs (2.2), phenolics (15%), esters (2.7%), phenyl deri (4.2) | |||
| Spruce wood | T: 300 °C Pi: 2 MPa Rt: 30 min | - | BO: 6.6 | - | - | - | - | Ketones (29.4%), phenolics (40.6%), FAAE (9.6%) | [124] |
| KF/Al2O3 | BO: 13.9 | 28.42 | 68.3 | 6.8 | 24.8 | Ketones (23.7%), phenolics (44.7%), FAAE (8.7%) |
| S. No | Year | Description/Objective | Results/Observations/Major Findings | Ref. |
|---|---|---|---|---|
| 1 | 2010 | Highly efficient liquefaction of woody biomass in hot-compressed alcohol–water co-solvents. | Co-solvent mixture of ethanol–water is more attractive for biomass liquefaction since ethanol is a renewable source of energy, which is easily produced from fermentation of sugars. | [127] |
| 2 | 2010 | Bio-oil from hydro-liquefaction of bagasse in supercritical ethanol. | Increased concentrations of ethanol in ethanol–water mixture resulted in higher heavy oil yield and higher HHV. Compound groups of phenolic, esters, and aldehydes were mostly found in heavy oil. | [128] |
| 3 | 2012 | Direct liquefaction of Dunaliella tertiolecta for bio-oil in sub/supercritical ethanol–water. | Low ash content was found in heavy oil obtained using ethanol–water mixture, making the heavy oil useful as a cleaner fuel, when compared to heavy oil obtained using only pure water during liquefaction. Ethanol react with amides and/or acids to form ethyl esters, and act as hydrogen-donor during liquefaction process. | [129] |
| 4 | 2013 | Synergic effect of methanol and water on pine liquefaction. | 50 vol% water content enhanced solvolytic liquefaction of pine. Mixture of methanol and water promoted more decomposition of some plant tissue in pine when compared to using purely methanol or water. | [40] |
| 5 | 2013 | Thermochemical liquefaction of rice husk for bio-oil production in mixed solvent (ethanol–water). | Heavy oil obtained after liquefaction in ethanol–water mixture, showed mostly phenolic and ester compounds to be present. Presence of ethanol allowed high yields of heavy oil with less severity in operation during liquefaction. | [130] |
| 6 | 2014 | Cornstalk liquefaction in methanol/water mixed solvents. | 50:50 (v/v) of methanol–water mixture provided highest heavy oil at 300 °C and 15 min of liquefaction process. Increasing water content led to cornstalk hydrolysis and increasing methanol content led to esterification reactions. | [131] |
| 7 | 2014 | Hydrothermal liquefaction of wheat straw in hot compressed water and subcritical water-alcohol mixtures. | For liquefaction of wheat straw, the optimum water–ethanol mixture ratio of 50:50 (v/v) was found to be effective in producing high yield of heavy oil and HHV at 300 °C and 10 MPa. | [132] |
| 8 | 2015 | Hydrothermal liquefaction of bagasse using ethanol and black liquor as solvents. | Increasing trend of HHV was observed with increase in ethanol concentrations, with 95 vol% ethanol to give the highest HHV of heavy oil produced after liquefaction. Phenolic compounds and esters in heavy oil were observed to be decreasing with increase in water content. Alcohols, aromatics, and heterocyclic compounds in heavy oil, were maximum when 50 vol% ethanol was used for liquefaction. | [133] |
| 9 | 2015 | Poplar liquefaction in water/methanol co-solvents. | 70:30 (v/v) water–methanol mixtures resulted in complete degradation of cellulose and hemicellulose than for lignin. | [134] |
| 10 | 2016 | Continuous hydrothermal liquefaction of macroalgae in the presence of organic co-solvents. | Anisole–water mixture produced high yields of heavy oil when compared to those when liquefied using toluene–water or n-heptane–water mixtures. Viscosity of heavy oil when liquefaction was conducted with anisole–water mixture was much higher when compared to those obtained by using toluene–water and n-heptane–water mixtures. | [135] |
| 11 | 2018 | Comparative study on lignocellulose liquefaction in water, ethanol, and water/ethanol mixture: role of ethanol and water. | Mixed ethanol/water solvent promoted the permeation of the solvent into the lignocellulosic structure and increased solubility of intermediates. Hot compressed water was found to promote hydrolysis of cellulose and hemicellulose, and ethanol was found to accelerate degradation of lignin. | [136] |
| 12 | 2018 | Highly efficient conversion of camphor tree sawdust into bio-oil and biochar products by liquefaction in ethanol–water co-solvent. | 50:50 (v/v) ethanol–water mixture efficiently liquefied camphor tree sawdust at 280 °C for 30 min, with heavy oil yield of 61.5 wt%, higher conversion rate of 98.4%, and energy recovery rate of 94.5%. Heavy oil produced using ethanol–water mixture resulted in higher contents of esters and ketones and lower amounts of nitrogenous compounds. | [137] |
| 13 | 2018 | Liquefaction of sewage sludge in ethanol–water mixed solvents for bio-oil and biochar products. | Optimum ethanol–water mixture of 75:25 (v/v) provided the highest heavy oil yield at 220 °C and 30 min liquefaction conditions. The synergistic effect of ethanol–water mixture allowed liquefaction to be conducted at mild operating conditions with elevated yield and quality of heavy oil. | [138] |
| 14 | 2018 | Sub- and supercritical liquefaction of municipal wet sewage sludge to produce bio-oil: effect of different organic–water mixed solvents. | Methanol–water mixture promoted high content of ester due to esterification. n-hexane–water promoted more aliphatic compounds to be formed, due to its high solubility in n-hexane. Low-boiling point materials were found more in heavy oil obtained by methanol–water liquefaction. Oxygen content in heavy oil reduced significantly in n-hexane–water liquefaction. | [139] |
| 15 | 2019 | Effect of co-solvent and addition of catalyst (HZSM-5) on hydrothermal liquefaction of macroalgae. | Liquefaction of macroalgae at 300 °C and 45 min, using 75 vol% ethanol, provided the highest heavy oil yield of 46.75 wt%. Catalyst HZSM-5 failed to provide high yield of heavy oil; however, quality of heavy oil was improved with high amounts of ester compounds. | [140] |
| 16 | 2020 | Alkali-catalyzed liquefaction of pinewood sawdust in ethanol/water co-solvents. | Yield of heavy oil obtained by using ethanol–water mixture for liquefaction can be compromised by using Na2CO3 or NaOH as catalyst. | [141] |
| 17 | 2021 | HTL of olive oil residue. Understanding the effect of using supercritical methanol as solvent on HTL of olive oil residue. | At 300 °C and 15 min, the use of methanol as solvent increased the oil yield from 30.8 wt% to 33.5 wt%, which further increased to 40.3 wt% in the presence of AlCl3. | [85] |
| 18 | 2021 | Subcritical hydrothermal co-liquefaction of process rejects at a waste paper-based paper mill with waste soybean oil. Effect of solvent ratio of waste soybean oil (WSO)–water was studied. | The highest HHV value of product was 42.02 MJ kg−1 at WSO–water ratio of 25:75. The conversion was found to be highest at WSO–water ratio of 100:00. The percentage of alcohols, benzene, alkenyl, phenols, and ester decreased with the increase of WSO–water ratio, whereas the percentage of acids increased up to a WSO–water ratio of 50:50 on further increase of the WSO–water ratio. | [142] |
| Biomass | Operation Condition | Co-Solvents | Oil Yield (wt%) | HHV (MJ kg−1) | C (wt.) | H (wt.) | O (wt.) | Comments | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Rice straw | T: 300 °C R: 3 min | 50 vol% Ethanol | 38.35 | 29.66 | - | - | - | Bio-oil yield increased with solvent–water ratio, ranging from 0 to 50 vol%, and decreased afterwards. Yields of residue were higher when ethanol–water was used, when compared to 2-propanol–water mixture during the liquefaction process. | [146] |
| 50 vol% 2-Propanol | 39.70 | 30.75 | 63.81 | 7.10 | 27.29 | ||||
| Rice straw | T: 300 °C R: 5 min | Water | 18.51 | 30.77 | 72.57 | 6.88 | 20.55 | Phenolic compounds are majorly present in liquefied products along with ester derivatives, hydrocarbons, organic acids, and alcohols. | [147] |
| 20 vol% 1,4-dioxane | 22.80 | 37.17 | 79.25 | 8.69 | 12.06 | ||||
| 50 vol% 1,4-dioxane | 14.34 | 32.99 | 75.48 | 7.32 | 17.20 | ||||
| 80 vol% 1,4-dioxane | 17.33 | 32.28 | 74.74 | 7.12 | 18.14 | ||||
| 1,4-dioxane | 12.43 | 37.07 | 77.66 | 9.14 | 13.20 | ||||
| Pine wood | T: 300 °C R: 15 min | Water | 39.13 | - | - | - | - | Liquefaction conducted with 50 wt% methanol or ethanol in solvent–water mixture resulted in > 95 wt% conversion of biomass and yield of bio-oil was > 65wt% at 300 °C and a residence time of 15 min, compared to the runs using mono-solvents. | [127] |
| 50 wt% Ethanol | 65.74 | ||||||||
| Ethanol | 25.92 | ||||||||
| 50 wt% Methanol | 64.45 | ||||||||
| Methanol | 23.54 | ||||||||
| Sugarcane bagasse | T: 330 °C R: 40 min | 50 vol% Ethanol | 42.44 | 21.4 | - | - | - | For 95 vol% ethanol and 5 vol% water mixture, there was a decrease in the amount of gas and water products, resulting in a higher yield of heavy oil. HHV increased with increasing ethanol concentrations, until 95 vol% ethanol. | [128] |
| 70 vol% Ethanol | 41.48 | 24.6 | - | - | - | ||||
| 90 vol% Ethanol | 45.03 | 24.7 | - | - | - | ||||
| 95 vol% Ethanol | 48.24 | 27.1 | 56.5 | 6.8 | 34.7 | ||||
| Ethanol | 39.55 | 24.2 | - | - | - | ||||
| Rice husk | T: 260 °C R: 20 min | Water | 13.16 | 25.03 | 60.55 | 6.93 | 30.24 | Optimum ratio of 5:5 (v/v) ethanol–water mixture provided relatively high yield of oil with low oxygen content. Ethanol provided decomposition of macromolecules such as cellulose, hemicellulose, and lignin, at milder operating conditions. | [130] |
| 50 vol% Ethanol | 21.07 | 27.04 | 64.88 | 6.78 | 25.99 | ||||
| Ethanol | 18.44 | 27.56 | 63.63 | 7.46 | 26.23 | ||||
| Pine wood | T: 300 °C R: 15 min | Methanol | 24.25 | - | - | - | - | More heavy oil was converted to char at reaction temperatures above 300 °C. Heavy oil yield increased until 15 min residence time after which both gas and residue yields started increasing. | [40] |
| 20 vol% Methanol | 40.02 | ||||||||
| 50 vol% Methanol | 54.65 | ||||||||
| 80 vol% Methanol | 27.79 | ||||||||
| Water | 29.96 | ||||||||
| Wheat straw | T: 300 °C R: 120 min | Water | 25.8 | 20.5 | - | - | - | Water–ethanol mixture of 50:50 (v/v) used during liquefaction of wheat straw at 300 °C and 100 bar, effectively produced high heavy oil yield of 30.4 wt% with HHV of 27.8 MJ kg−1. | [132] |
| 25 vol% Ethanol | 22.2 | 25.5 | |||||||
| 50 vol% Ethanol | 30.4 | 27.8 | |||||||
| 75 vol% Ethanol | 32.7 | 28.5 | |||||||
| Ethanol | 43.2 | 28.2 | |||||||
| Cornstalk | T: 300 °C R: 30 min | Water | 45.15 | - | - | - | - | Isometric methanol/water 50:50 (v/v) showed the best synergistic effect on degradation of lignin. | [131] |
| 20 vol% Methanol | 45.71 | ||||||||
| 40 vol% Methanol | 48.07 | ||||||||
| 50 vol% Methanol | 52.40 | ||||||||
| 80 vol% Methanol | 43.92 | ||||||||
| Methanol | 28.10 | ||||||||
| Poplar | T: 300 °C R: 15 min | Water | 28.8 | - | - | - | - | More amounts of ketone, aldehyde, and carboxylic acid were found in heavy oil liquefied with 70:30 (v/v) water–methanol mixture. Yield of esters decreased with an increase in water content in the solvent mixture. | [134] |
| 20 vol% Methanol | 36.2 | ||||||||
| 30 vol% Methanol | 38.9 | ||||||||
| 40 vol% Methanol | 37.3 | ||||||||
| 60 vol% Methanol | 31.8 | ||||||||
| 80 vol% Methanol | 32.8 | ||||||||
| Methanol | 23.2 | ||||||||
| Rice straw | T: 260 °C R: 60 min | Glycerol–water (1:1) (mL) | 21.69 | 30.06 | 68.17 | 7.71 | 22.30 | 50 vol% glycerol decomposes the lignocellulosic components to produce free phenoxyl radicals that enable new compounds to be formed by condensation/repolymerization reactions, receding the residue formation. | [148] |
| Macroalgae | T: 350 °C R: 3 min | 10 wt% n-heptane | 7.23 | - | - | - | - | Anisole led to an increase in heavy oil yield when compared to n-heptane and toluene. | [135] |
| 10 wt% Toluene | 14.88 | ||||||||
| 10 wt% Anisole | 24.44 | ||||||||
| Camphor tree sawdust (CTS) | T: 280 °CR: 30 min | 50:50 (v/v) Water–ethanol | 61.5 | 24.6 | 61.6 | 6.5 | 31.6 | Conversion rate can be arranged from high to low efficiency of liquefaction as ethanol–water > only water > only ethanol. | [135] |
| Sewage sludge | T: 220 °C R: 30 min | Water | 55.38 | 20.30 | 45.90 | 7.50 | 33.30 | Heavy oil obtained by using ethanol–water mixture showed less content of long chain carbon compounds (>C70), when compared to using pure water as a solvent. | [138] |
| 25 vol% Ethanol | 55.52 | - | - | - | - | ||||
| 50 vol% Ethanol | 34.87 | 25.10 | 54.50 | 7.90 | 26.40 | ||||
| 75 vol% Ethanol | 58.15 | - | - | - | - | ||||
| Ethanol | 66.41 | 27.00 | 58.40 | 8.00 | 24.10 | ||||
| Sewage sludge | T: 340 °C R: 20 min | 50 vol% Methanol | 39.4 | 34.14 ± 0.48 | 73.94 ± 0.34 | 10.55 ± 0.22 | 9.34 ± 0.32 | Effect of the reaction temperature on the yield of heavy oil using different solvents showed fewer changes, whereas the effect of the residence time created significant changes to heavy oil yield. | [139] |
| 50 vol% n-hexane | 38.6 | 36.45 ± 0.38 | 73.08 ± 0.23 | 10.75 ± 0.12 | 7.65 ± 0.26 | ||||
| Macroalgae | T: 300 °C R: 45 min | Water | 13.93 | - | - | - | - | Using 75 vol% ethanol required high pressures of 20 MPa; hence, 50 vol% ethanol can be considered as optimum alcohol–water mixture that produced a similar yield of heavy oil with reduced operating pressure required for liquefaction. | [140] |
| 25 vol% Ethanol | 23.22 | - | - | - | |||||
| 50 vol% Ethanol | 44.94 | 63.48 | 5.88 | 22.18 | |||||
| 75 vol% Ethanol | 46.75 | - | - | - | |||||
| Ethanol | 42.29 | - | - | - | |||||
| Pinewood sawdust | T: 300 °C R: 30 min | 50 wt% Ethanol | 47.63 ± 0.09 | 25.89 | 65.60 | 6.13 | 28.24 | Heavy oil obtained by using ethanol–water mixture contains high content of mild boiling point materials (range of 343-538 °C) than those obtained by using pure water for liquefaction. | [141] |
| S. No | Year | Description/Objective | Results/Observations/Major Findings | Ref. |
|---|---|---|---|---|
| 1 | 2011 | Characterization of product fractions from hydrothermal liquefaction of Nannochloropsis sp. and the influence of solvents. | Non-polar solvents have been observed to provide high gravimetric yields of heavy oil, but these oils contain low carbon content and show less energy density. Polar solvents have shown low yields of heavy oil, but these oils contain high fatty acid content. | [154] |
| 2 | 2014 | Selective extraction of bio-oil from hydrothermal liquefaction of Salix psammophila by organic solvents with different polarities through multistep extraction separation. | Heavy oil was extracted using 9 organic solvents with different polarities in the order of high to low efficiency—THF > toluene > EAC > acetone > ether > DCM > methanol > petroleum ether > n-hexane. | [32] |
| 3 | 2016 | Analysis of physicochemical properties of bio-oil from HTL of blackcurrant pomace. | Highest yield of heavy oil was obtained by using acetone as an extraction solvent. Heavy oil extracted by hexane was suggested to be the best performing fuel in terms of quality since the heavy oil showed low carbon residue, volatility close to that of diesel and HHV close to those fuels used in marine applications. | [69] |
| 4 | 2016 | Composition of the bio-oil from the HTL of duckweed and the influence of the extraction solvents. | Polarity and molecular structure of extraction solvent greatly affects the yield and composition of heavy oil. Heavy oil extracted by using polar solvents showed high yields, high C and H content, and high energy density when compared to those extracted by using non-polar solvents. | [155] |
| 5 | 2019 | 110th anniversary: influence of solvents on bio-crude from hydrothermal liquefaction of soybean oil, soy protein, cellulose, xylose, and lignin, and their quinary mixture. | Solvent type for extraction was confirmed to affect the yield of heavy oil recovered from two different HTL processes. Polarity of solvent cannot be the sole determinant of evaluating its performance during the extraction process. | [68] |
| Biomass | Operation Conditions | Extraction Solvent | Oil (wt%) | HHV (MJ kg−1) | C (wt%) | H (wt%) | O (wt%) | Comments | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Marine algae | T: 350 °C R: 60 min | Hexadecane | 38.66 | - | - | - | - | DCM and chloroform were found more effective in extraction of light ends and fatty acids derivatives. | [154] |
| Decane | 39.39 | - | 68.80 | 9.37 | |||||
| Hexane | 31.79 | - | 70.45 | 9.80 | |||||
| Cyclohexane | 33.68 | - | 64.87 | 9.76 | |||||
| Methoxycyclopentane | 31.91 | - | 72.27 | 9.70 | |||||
| Chloroform | 34.75 | - | 73.68 | 9.85 | |||||
| DCM | 30.14 | - | 75.76 | 10.57 | |||||
| Willow branches | T: 300 °C R: 30 min | Petroleum ether | 12.95 | - | - | - | - | Ketones and phenolic compounds were easily extracted with high polarity solvents such as THF, EAC, acetone, and methanol. Multistep-extraction procedure provided efficient separation of bio-oil components and sequential analysis of components. | [156] |
| n-hexane | 8.27 | - | |||||||
| Toluene | 38.31 | - | |||||||
| Ether | 28.10 | - | |||||||
| DCM | 26.78 | - | |||||||
| THF | 45.34 | - | |||||||
| EAC | 30.55 | - | |||||||
| Acetone | 30.00 | - | |||||||
| Methanol | 23.32 | - | |||||||
| Blackcurrant pomace | T: 300 °C R: 60 min | EAC | 28 ± 3 | 33.4 | 75 ± 1 | - | - | Heavy oil extracted using hexane resulted in having high quality with high C-content and HHV. | [69] |
| Hexane | 17 ± 3 | 38.4 | 80 ± 1 | ||||||
| Acetone | 32 ± 3 | 35.0 | 74 ± 1 | ||||||
| Isopropyl alcohol | 29 ± 3 | 34.6 | 73 ± 1 | ||||||
| Duckweed | T: 350 °C R: 30 min | Isopropanol | 26 ± 1 | 34 ± 2 | 70 ± 0.7 | 9 ± 0.5 | 14 ± 0.7 | Highest yield of heavy oil was produced by using isopropanol as the extraction solvent. Isopropanol was found to be miscible with water, and hence only one phase was separated since water-soluble products did not create a separate phase. Carbon disulfide being a non-polar solvent was able to extract high yields of heavy oil when compared to those extracted by n-hexane, cyclohexane, and petroleum ether. | [155] |
| Dichloroethane | 19 ± 1 | 36 ± 2 | 74 ± 0.7 | 9 ± 0.4 | 10 ± 0.5 | ||||
| DCM | 24 ± 1 | 37 ± 2 | 76 ± 0.8 | 9 ± 0.4 | 8 ± 0.4 | ||||
| EAC | 20 ± 1 | 37 ± 2 | 76 ± 0.8 | 9 ± 0.4 | 8 ± 0.4 | ||||
| DEE | 17 ± 0.8 | 37 ± 2 | 76 ± 0.8 | 9 ± 0.5 | 8 ± 0.4 | ||||
| Benzene | 18 ± 0.9 | 38 ± 2 | 77 ± 0.8 | 9 ± 0.5 | 7 ± 0.4 | ||||
| Carbon disulfide | 17 ± 0.9 | 38 ± 2 | 76 ± 0.8 | 9 ± 0.5 | 7 ± 0.3 | ||||
| n-Hexane | 3 ± 0.2 | 40 ± 2 | 78 ± 0.8 | 10 ± 0.5 | 6 ± 0.3 | ||||
| Cyclohexane | 9 ± 0.4 | 39 ± 2 | 78 ± 0.8 | 10 ± 0.5 | 7 ± 0.3 | ||||
| Petroleum ether | 4 ± 0.2 | 40 ± 2 | 78 ± 0.8 | 10 ± 0.5 | 6 ± 0.3 | ||||
| Microalgae | T: 400 °C R: 60 min | DCM | 29.5 ± 2.1 | - | - | - | - | Heavy oil yield increased when DCM was used as an extraction solvent. HHV of heavy oil extracted by either DCM or MTBE did not have a major difference. | [160] |
| T: 400 °C R: 60 min | MTBE | 24.5 ± 2.3 | - | ||||||
| Chlorella sp. | T: 260 °C R: 60 min | Acetone | 28.22 | 35.5 | 70.0 ± 1.8 | 10.5 ± 0.3 | 17.8 | Acetone-extracted heavy oil shows high N and S content from liquefaction of high-carbohydrate and high-ash content biomass. DCM and toluene-extracted heavy oil showed high N and S content from liquefaction of high-protein content biomass. | [159] |
| DCM | 48.70 | 39.5 | 74.6 ± 0.2 | 11.6 ± 0.1 | 13.1 | ||||
| Toluene | 28.60 | 37.4 | 72.0 ± 0.8 | 11.2 ± 0.1 | 16.0 | ||||
| Spent coffee grounds (SCG) | T: 290 °C R: 10 min | Hexane | 15.2 ± 1.0 | DCM, THF, and acetone were effective solvents for extraction, giving higher yields of heavy oil when compared to hexane. Hexane was an effective solvent during Soxhlet separation method and was able to recover more heavy oil when compared to the rest three solvents. Yield of heavy oil extracted by filtration is mentioned here since filtration was the most efficient separation method. | [157] | ||||
| Acetone | 32.3 ± 1.0 | ||||||||
| DCM | 32.8 ± 1.2 | ||||||||
| THF | 38.1 ± 2.2 | ||||||||
| Cellulose | T: 350 °C R: 30 min | DCM | 4.40 | 32.3 | 72.8 | 6.8 | 20.1 | Individual feedstock containing cellulose, xylose, and lignin in high amounts, showed that the heavy oil extracted did not depend on solvent type. It is difficult to achieve the high yields and high HHV from heavy oil extracted by a certain kind of solvent. | [68] |
| Acetone | 10.92 | 30.2 | 70.8 | 6.3 | 25.3 | ||||
| MTBE | 5.71 | 32.8 | 71.6 | 7.6 | 21.4 | ||||
| Xylose | DCM | 6.36 | 31.9 | 73.2 | 6.5 | 20.2 | |||
| Acetone | 10.52 | 30.2 | 70.1 | 6.3 | 23.4 | ||||
| MTBE | 8.15 | 32.4 | 71.6 | 7.3 | 21.8 | ||||
| Lignin | DCM | 1.22 | 32.1 | 70.1 | 7.5 | 22.1 | |||
| Acetone | 14.35 | 17.9 | 46.8 | 5.4 | 46.3 | ||||
| MTBE | 2.77 | 19.9 | 51.7 | 5.2 | 41.2 |
| Biomass | Gas Yield (wt%) | Composition | Comments | Ref. |
|---|---|---|---|---|
| Birch wood | 1–12 | CO2, CO, C2H4, C2H6, C3H8 | CO2 dominates near 300 °C, and CO starts to dominate over 325 °C. Fraction of methane increases significantly above 350 °C. | [97] |
| Wet and Dry distiller’s grains and solubles | ~15% | CO2, H2, N2, CO, CH4, light alkanes and alkenes | 96% of the gas was CO2 and 1.6% H2. | [59] |
| Red pine sawdust | 0.2–8.7 (mol%) | CO2, H2, CO, CH4, C2H6, C2H4, C3–C6 (negligible) | General trend of gas fraction CO > CO2 > C2H4 > CH4 > C2H6. The amount of H2 and C3–C6 was negligible. At 400 °C, compounds like C2–C6 were also present. | [98] |
| Miscanthus | ~20–37 | CO2, CO, CH4, H2, C2H4, C2H6, C3H6, C3H8, C4H10 | CO diminished with increase in water ratio in the reactor. | [78] |
| Corn straw | 16.5 | H2 (1.9%), CO (6.2%), CH4 (0.9%), CO2 (90.8%), C2H6 (0.2%), C2H8 (0.1%) | [23] | |
| Peanut straw | 19.9 | H2 (2.7%), CO (4.2%), CH4 (1.1%), CO2 (91.6%), C2H6 (0.1%), C2H8 (0.2%) | ||
| Soybean straw | 19.7 | H2 (3.1%), CO (5.5%), CH4 (0.6%), CO2 (90.6%), C2H6 (0.1%), C2H8 (0.1%) | ||
| Rice straw | 22.9 | H2 (2.2%), CO (6.2%), CH4 (1.1%), CO2 (90.2%), C2H6 (0.1%), C2H8 (2.2%) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathanker, A.; Das, S.; Pudasainee, D.; Khan, M.; Kumar, A.; Gupta, R. A Review of Hydrothermal Liquefaction of Biomass for Biofuels Production with a Special Focus on the Effect of Process Parameters, Co-Solvents, and Extraction Solvents. Energies 2021, 14, 4916. https://doi.org/10.3390/en14164916
Mathanker A, Das S, Pudasainee D, Khan M, Kumar A, Gupta R. A Review of Hydrothermal Liquefaction of Biomass for Biofuels Production with a Special Focus on the Effect of Process Parameters, Co-Solvents, and Extraction Solvents. Energies. 2021; 14(16):4916. https://doi.org/10.3390/en14164916
Chicago/Turabian StyleMathanker, Ankit, Snehlata Das, Deepak Pudasainee, Monir Khan, Amit Kumar, and Rajender Gupta. 2021. "A Review of Hydrothermal Liquefaction of Biomass for Biofuels Production with a Special Focus on the Effect of Process Parameters, Co-Solvents, and Extraction Solvents" Energies 14, no. 16: 4916. https://doi.org/10.3390/en14164916
APA StyleMathanker, A., Das, S., Pudasainee, D., Khan, M., Kumar, A., & Gupta, R. (2021). A Review of Hydrothermal Liquefaction of Biomass for Biofuels Production with a Special Focus on the Effect of Process Parameters, Co-Solvents, and Extraction Solvents. Energies, 14(16), 4916. https://doi.org/10.3390/en14164916







