The Review of Carbon Capture-Storage Technologies and Developing Fuel Cells for Enhancing Utilization
Abstract
:1. Introduction
2. Role of CCS
- Improvement of energy transfer and usage efficiency;
- The ratio of CO2 pollution to primary consumption by transitioning to carbon-free electricity technology;
- Trap and store CO2 at large industrial point sources in geological formations;
- To accommodate alternative sources of fuel, which include a cleaner production process without carbon emissions.
- Energy demand may be minimized either by replacing it with capital and labor in a macro-economic output mechanism or by growing energy efficiency in an endogenous way. Investments in conventional and clean energy markets decide the carbon balance. The performance of production in renewables improves by learning. Alternatively, fossil dioxide may be collected and deposited in natural structures that are susceptible to leakage. Utility per capita depends on per capita consumption with declining returns.
2.1. Technological Aspects
2.1.1. Post Combustion Capture and Pre Combustion Capture
2.1.2. Artificial Intelligence in Carbon Capture
3. CO2 Storage
3.1. Studying the Subsurface Storage of CO2 and Related Challenges
3.2. The Capacity Available for Storage and the Role Played by CO2 in Different Energy Systems
3.3. Different CO2 Storage Methods
3.3.1. Geological Sequestration
3.3.2. Bioenergy-Based Aspect of Carbon Capture and Storage
3.3.3. Artificial Intelligence in Carbon Storage
3.3.4. Storage Using Metal-Organic Frameworks
4. Transporting the Stored CO2
4.1. Available Modes for Transporting the CO2
4.1.1. The Pipeline Mode for Transportation
4.1.2. Ship-Based Transportation
5. Development of CO2 Utilization Technology
5.1. Development of a Newer Fuel-Cell Technology That Produces Electric Current from CO2
5.1.1. Development of a Novel Fuel Cell: Generating Electricity and Mitigating CO2 Levels Simultaneously
5.1.2. The Progress in Oxygen Regeneration Owing to the Integration of Solid Oxide Co-Electrolyzer (SOCE) and Carbon Formation Reactor (CFR)
5.1.3. Operational Mode of Solid Oxide Co-Electrolyzer Cell
5.1.4. Carbon Formation Reactor (CFR) and its Mode of Operation
5.2. Electrochemical Conversion of CO2
6. Efficient CCUS Technologies and Their Economic Benefits
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anderson, T.R.; Hawkins, E.; Jones, P.D. CO2, the greenhouse effect and global warming: From the pioneering work of Arrhenius and Callendar to today’s Earth System Models. Endeavour 2016, 40, 178–187. [Google Scholar] [CrossRef] [Green Version]
- Ritchie, H.; Roser, M. CO₂ and Greenhouse Gas Emissions. 2020. Available online: https://ourworldindata.org/co2-and-other-greenhouse-gas-emissions#citation (accessed on 10 August 2021).
- Le Quéré, C.; Andrew, R.M.; Friedlingstein, P.; Sitch, S.; Hauck, J.; Pongratz, J.; Pickers, P.A.; Korsbakken, J.I.; Peters, G.P.; Canadell, J.G.; et al. Global Carbon Budget 2018. Earth Syst. Sci. Data 2018, 10, 2141–2194. [Google Scholar] [CrossRef] [Green Version]
- Dicks, A.L. The role of carbon in fuel cells. J. Power Sources 2006, 156, 128–141. [Google Scholar] [CrossRef]
- Bai, Y.; Liu, Y.; Tang, Y.; Xie, Y.; Liu, J. Direct carbon solid oxide Fuel Cell—A potential high performance battery. Int. J. Hydrogen Energy 2011, 36, 9189–9194. [Google Scholar] [CrossRef]
- Dudek, M.; Tomczyk, P. Composite fuel for direct carbon fuel cell. Catal. Today 2011, 176, 388–392. [Google Scholar] [CrossRef]
- Abedini, A.; Torabi, F. Parametric study of the cyclic CO2 injection process in light oil systems. Ind. Eng. Chem. Res. 2013, 52, 15211–15223. [Google Scholar] [CrossRef]
- Aycaguer, A.C.; Lev-On, M.; Winer, A.M. Reducing carbon dioxide emissions with enhanced oil recovery projects: A life cycle assessment approach. Energy Fuels 2001, 15, 303–308. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Ben-Mansour, R.; Habib, M.A. An efficient CO2 adsorptive storage using MOF-5 and MOF-177. Appl. Energy 2018, 210, 317–326. [Google Scholar] [CrossRef]
- Zheng, B.; Yun, R.; Bai, J.; Lu, Z.; Du, L.; Li, Y. Expanded porous MOF-505 analogue exhibiting large hydrogen storage capacity and selective carbon dioxide adsorption. Inorg. Chem. 2013, 52, 2823–2829. [Google Scholar] [CrossRef]
- Bielicki, J.M.; Peters, C.A.; Fitts, J.P.; Wilson, E.J. An examination of geologic carbon sequestration policies in the context of leakage potential. Int. J. Greenh. Gas Control 2015, 37, 61–75. [Google Scholar] [CrossRef] [Green Version]
- Bielicki, J.M.; Pollak, M.F.; Deng, H.; Wilson, E.J.; Fitts, J.P.; Peters, C.A. The Leakage Risk Monetization Model for Geologic CO2 Storage. Environ. Sci. Technol. 2016, 50, 4923–4931. [Google Scholar] [CrossRef] [PubMed]
- Vinca, A.; Emmerling, J.; Tavoni, M. Bearing the cost of stored carbon leakage. Front. Energy Res. 2018, 6, 40. [Google Scholar] [CrossRef] [Green Version]
- Marshall, C. Can Stored Carbon Dioxide Leak?—Scientific American. Available online: https://www.scientificamerican.com/article/can-stored-carbon-dioxide-leak/ (accessed on 10 June 2020).
- Day, M.J.; Reinke, R.F.; Thomson, J.A.M. Fate and transport of fuel components below slightly leaking underground storage tanks. Environ. Forensics 2001, 2, 21–28. [Google Scholar] [CrossRef]
- Gür, T.M.; Huggins, R.A. Direct Electrochemical Conversion of Carbon to Electrical Energy in a High Temperature Fuel Cell. J. Electrochem. Soc. 1992, 139, L95–L97. [Google Scholar] [CrossRef]
- Zecevic, S.; Patton, E.M.; Parhami, P. Carbon-air fuel cell without a reforming process. Carbon 2004, 42, 1983–1993. [Google Scholar] [CrossRef]
- CCS Institute, G. Transporting CO2. 2015. Available online: https://www.globalccsinstitute.com/resources/publications-reports-research/transporting-co2/ (accessed on 10 August 2021).
- Herzog, H.; Eliasson, B.; Kaarstad, O. Capturing greenhouse gases. Sci. Am. 2000, 282, 72–79. [Google Scholar] [CrossRef]
- Herzog, H.; Drake, E.; Adams, E. US Department of Energy Report: CO2 Capture, Reuse and Storage Technologies for Mitigating Global Climate Change. 1997. Available online: https://sequestration.mit.edu/pdf/WhitePaper.pdf (accessed on 10 August 2021).
- Saitaptim, S. Waste Water Treatment of Recycling Industry by Algae for Producing Biodiesel 2011. Available online: http://library1.nida.ac.th/termpaper6/sd/2554/19755.pdf (accessed on 10 August 2021).
- Jędrusik, J.; Kalinowski, E.; Jędrusik, M. A Method of Reducing the SO2 Emission from Power Boilers. Chem. Prot. Environ. 1998, 3, 79–85. [Google Scholar] [CrossRef]
- National Research Council; Commission on Natural Resources. Air Quality and Stationary Source Emission Control; US Government Printing Office: Washington, DC, USA, 1975. [CrossRef]
- Koebel, M.; Elsener, M.; Kleemann, M. Urea-SCR: A promising technique to reduce NOx emissions from automotive diesel engines. Catal. Today 2000, 59, 335–345. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, L.; Zeng, S.; Huang, J.; Roskilly, A.P.; He, Y.; Huang, X.; Li, S. Application of the Miller cycle to reduce NOx emissions from petrol engines. Appl. Energy 2008, 85, 463–474. [Google Scholar] [CrossRef]
- Sindhu, R.; Rao, G.A.P.; Murthy, K.M. Effective reduction of NOx emissions from diesel engine using split injections. Alex. Eng. J. 2018, 57, 1379–1392. [Google Scholar] [CrossRef]
- The 9 Most Common Air Pollutants—Business 2 Community. Available online: https://www.business2community.com/health-wellness/the-9-most-common-air-pollutants-0342288 (accessed on 10 June 2020).
- Buis, A. The Atmosphere: Getting a Handle on Carbon Dioxide—Climate Change: Vital Signs of the Planet. Available online: https://climate.nasa.gov/news/2915/the-atmosphere-getting-a-handle-on-carbon-dioxide/ (accessed on 10 June 2020).
- Mobjörk, M.; Smith, D. Translating Climate Security Policy into Practice. Available online: https://www.sipri.org/publications/2017/other-publications/translating-climate-security-policy-practice (accessed on 10 August 2021).
- Bauer, N.; Edenhofer, O.; Held, H.; Kriegler, E. Uncertainty of the role of carbon capture and sequestration within climate change mitigation strategies. Greenh. Gas Control Technol. 2005, 7, 931–939. [Google Scholar] [CrossRef] [Green Version]
- Carbon Management: Implications for R&D in the Chemical Sciences and Technology—National Research Council, Division on Earth and Life Studies, Board on Chemical Sciences and Technology, Chemical Sciences Roundtable—Google Books. Available online: https://www.nap.edu/catalog/10153/carbon-management-implications-for-rd-in-the-chemical-sciences-and (accessed on 10 June 2020).
- Goebel, K. The Role of Permanent Monitoring Panels; World Scientific: Singapore, 1999; ISBN 978-981-02-3927-5. [Google Scholar] [CrossRef]
- Rosenberg, N.J.; Izaurralde, R.C. Storing Carbon in Agricultural Soils: A Multi-Purpose Environmental Strategy; Springer: Dordrecht, The Netherlands, 2001. [Google Scholar]
- Dooley, J.J.; Edmonds, J.A.; Wise, M.A. The Role of Carbon Capture & Sequestration in a Long-Term Technology Strategy of Atmospheric Stabilization. Environ. Sci. 1999. [Google Scholar] [CrossRef]
- Liu, J.; Wei, Y.; Zhao, Y. Trace Carbon Dioxide Capture by Metal–Organic Frameworks. ACS Sustain. Chem. Eng. 2019, 7, 82–93. [Google Scholar] [CrossRef]
- Ding, M.; Flaig, R.W.; Jiang, H.-L.; Yaghi, O.M. Carbon capture and conversion using metal–organic frameworks and MOF-based materials. Chem. Soc. Rev. 2019, 48, 2783–2828. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, J.; Mosleh, I.; Abolhassani, M.; Greenlee, L.F.; Beitle, R.R.; Beyzavi, M.H. Covalent Organic Frameworks for the Capture, Fixation, or Reduction of CO2. Front. Energy Res. 2019, 7, 77. [Google Scholar] [CrossRef] [Green Version]
- Trickett, C.A.; Helal, A.; Al-Maythalony, B.A.; Yamani, Z.H.; Cordova, K.E.; Yaghi, O.M. The chemistry of metal–organic frameworks for CO2 capture, regeneration and conversion. Nat. Rev. Mater. 2017, 2, 17045. [Google Scholar] [CrossRef]
- Belmabkhout, Y.; Guillerm, V.; Eddaoudi, M. Low concentration CO2 capture using physical adsorbents: Are metal–organic frameworks becoming the new benchmark materials? Chem. Eng. J. 2016, 296, 386–397. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-Y.; Park, S.-J. A review on solid adsorbents for carbon dioxide capture. J. Ind. Eng. Chem. 2015, 23, 1–11. [Google Scholar] [CrossRef]
- Jiang, Y.; Ling, J.; Xiao, P.; He, Y.; Zhao, Q.; Chu, Z.; Liu, Y.; Li, Z.; Webley, P.A. Simultaneous biogas purification and CO2 capture by vacuum swing adsorption using zeolite NaUSY. Chem. Eng. J. 2018, 334, 2593–2602. [Google Scholar] [CrossRef]
- Drage, T.C.; Snape, C.E.; Stevens, L.A.; Wood, J.; Wang, J.; Cooper, A.I.; Dawson, R.; Guo, X.; Satterley, C.; Irons, R. Materials challenges for the development of solid sorbents for post-combustion carbon capture. J. Mater. Chem. 2012, 22, 2815–2823. [Google Scholar] [CrossRef] [Green Version]
- Aresta, M.; Ballivet-Tkatchenko, D.; Dell’Amico, D.B.; Bonnet, M.C.; Boschi, D.; Calderazzo, F.; Faure, R.; Labella, L.; Marchetti, F. Isolation and structural determination of two derivatives of the elusive carbamic acid. Chem. Commun. 2000, 1099–1100. [Google Scholar] [CrossRef]
- Gurkan, B.E.; de la Fuente, J.C.; Mindrup, E.M.; Ficke, L.E.; Goodrich, B.F.; Price, E.A.; Schneider, W.F.; Brennecke, J.F. Equimolar CO2 Absorption by Anion-Functionalized Ionic Liquids. J. Am. Chem. Soc. 2010, 132, 2116–2117. [Google Scholar] [CrossRef]
- Wang, C.; Luo, H.; Jiang, D.; Li, H.; Dai, S. Carbon Dioxide Capture by Superbase-Derived Protic Ionic Liquids. Angew. Chem. Int. Ed. 2010, 49, 5978–5981. [Google Scholar] [CrossRef]
- Luo, X.Y.; Lv, X.Y.; Shi, G.L.; Meng, Q.; Li, H.R.; Wang, C.M. Designing amino-based ionic liquids for improved carbon capture: One amine binds two CO2. AIChE J. 2019, 65, 230–238. [Google Scholar] [CrossRef] [Green Version]
- Milner, P.J.; Siegelman, R.L.; Forse, A.C.; Gonzalez, M.I.; Runčevski, T.; Martell, J.D.; Reimer, J.A.; Long, J.R. A Diaminopropane-Appended Metal–Organic Framework Enabling Efficient CO2 Capture from Coal Flue Gas via a Mixed Adsorption Mechanism. J. Am. Chem. Soc. 2017, 139, 13541–13553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forse, A.C.; Milner, P.J.; Lee, J.-H.; Redfearn, H.N.; Oktawiec, J.; Siegelman, R.L.; Martell, J.D.; Dinakar, B.; Porter-Zasada, L.B.; Gonzalez, M.I.; et al. Elucidating CO2 Chemisorption in Diamine-Appended Metal–Organic Frameworks. J. Am. Chem. Soc. 2018, 140, 18016–18031. [Google Scholar] [CrossRef] [Green Version]
- The Case for Carbon Capture and Storage|Issues in Science and Technology. Available online: http://issues.org/22-1/stephens/ (accessed on 10 June 2020).
- Praetorius, B.; Schumacher, K. Greenhouse gas mitigation in a carbon constrained world: The role of carbon capture and storage. Energy Policy 2009, 37, 5081–5093. [Google Scholar] [CrossRef] [Green Version]
- Linga, P.; Kumar, R.; Englezos, P. The clathrate hydrate process for post and pre-combustion capture of carbon dioxide. J. Hazard. Mater. 2007, 149, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Lucquiaud, M.; Gibbins, J. Retrofitting CO2 capture ready fossil plants with post-combustion capture. Part 1: Requirements for supercritical pulverized coal plants using solvent-based flue gas scrubbing. Proc. Inst. Mech. Eng. Part A J. Power Energy 2009, 223, 213–226. [Google Scholar] [CrossRef]
- Sanpasertparnich, T.; Idem, R.; Bolea, I.; deMontigny, D.; Tontiwachwuthikul, P. Integration of post-combustion capture and storage into a pulverized coal-fired power plant. Int. J. Greenh. Gas Control 2010, 4, 499–510. [Google Scholar] [CrossRef]
- Wang, M.; Lawal, A.; Stephenson, P.; Sidders, J.; Ramshaw, C. Post-combustion CO2 capture with chemical absorption: A state-of-the-art review. Chem. Eng. Res. Des. 2011, 89, 1609–1624. [Google Scholar] [CrossRef] [Green Version]
- Oexmann, J.; Kather, A. Minimising the regeneration heat duty of post-combustion CO2 capture by wet chemical absorption: The misguided focus on low heat of absorption solvents. Int. J. Greenh. Gas Control 2010, 4, 36–43. [Google Scholar] [CrossRef]
- Lawal, A.; Wang, M.; Stephenson, P.; Koumpouras, G.; Yeung, H. Dynamic modelling and analysis of post-combustion CO2 chemical absorption process for coal-fired power plants. Fuel 2010, 89, 2791–2801. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.J.; Lee, J.D.; Linga, P.; Englezos, P.; Kim, Y.S.; Lee, M.S.; Kim, Y.D. Gas hydrate formation process for pre-combustion capture of carbon dioxide. Energy 2010, 35, 2729–2733. [Google Scholar] [CrossRef]
- Babu, P.; Linga, P.; Kumar, R.; Englezos, P. A review of the hydrate based gas separation (HBGS) process forcarbon dioxide pre-combustion capture. Energy 2015, 85, 261–279. [Google Scholar] [CrossRef]
- Kawabata, M.; Kurata, O.; Iki, N.; Tsutsumi, A.; Furutani, H. System modeling of exergy recuperated IGCC system with pre- and post-combustion CO2 capture. Appl. Therm. Eng. 2013, 54, 310–318. [Google Scholar] [CrossRef]
- Kim, S.M.; Lee, J.D.; Lee, H.J.; Lee, E.K.; Kim, Y. Gas hydrate formation method to capture the carbon dioxide for pre-combustion process in IGCC plant. Int. J. Hydrogen Energy 2011, 36, 1115–1121. [Google Scholar] [CrossRef]
- Kunze, C.; Spliethoff, H. Assessment of oxy-fuel, pre- and post-combustion-based carbon capture for future IGCC plants. Appl. Energy 2012, 94, 109–116. [Google Scholar] [CrossRef]
- Gazzani, M.; Macchi, E.; Manzolini, G. CO2 capture in natural gas combined cycle with SEWGS. Part A: Thermodynamic performances. Int. J. Greenh. Gas Control 2013, 12, 493–501. [Google Scholar] [CrossRef]
- Gazzani, M.; Macchi, E.; Manzolini, G. CO2 capture in integrated gasification combined cycle with SEWGS—Part A: Thermodynamic performances. Fuel 2013, 105, 206–219. [Google Scholar] [CrossRef]
- Garcia, S.; Gil, M.V.; Pis, J.J.; Rubiera, F.; Pevida, C. Cyclic operation of a fixed-bed pressure and temperature swing process for CO2 capture: Experimental and statistical analysis. Int. J. Greenh. Gas Control 2013, 12, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Casas, N.; Schell, J.; Joss, L.; Mazzotti, M. A parametric study of a PSA process for pre-combustion CO2 capture. Sep. Purif. Technol. 2013, 104, 183–192. [Google Scholar] [CrossRef]
- Agarwal, A.; Biegler, L.T.; Zitney, S.E. Superstructure-Based Optimal Synthesis of Pressure Swing Adsorption Cycles for Precombustion CO2 Capture. Ind. Eng. Chem. Res. 2010, 49, 5066–5079. [Google Scholar] [CrossRef]
- Xianhao, C.; Wu, X.; Lee, K. The mutual benefits of renewables and carbon capture: Achieved by an artificial intelligent scheduling strategy. Energy Convers. Manag. 2021, 233, 113856. [Google Scholar] [CrossRef]
- Galeczka, I.; Wolff-Boenisch, D.; Oelkers, E.H.; Gislason, S.R. An experimental study of basaltic glass-H2O-CO2 interaction at 22 and 50 °C: Implications for subsurface storage of CO2. Geochim. Cosmochim. Acta 2014, 126, 123–145. [Google Scholar] [CrossRef] [Green Version]
- Brandvoll, Ø.; Regnault, O.; Munz, I.A.; Iden, I.K.; Johansen, H. Fluid-solid interactions related to subsurface storage of CO2 Experimental tests of well cement. Energy Procedia 2009, 1, 3367–3374. [Google Scholar] [CrossRef] [Green Version]
- Bøe, R.; Magnus, C. CO2 Point Sources and Subsurface Storage Capacities for CO2 in Aquifers in Norway. Available online: https://openarchive.ngu.no/ngu-xmlui/bitstream/handle/11250/2665194/2002_010.pdf?sequence=1&isAllowed=y (accessed on 10 August 2021).
- Nordbotten, J.M.; Celia, M.A. Geological Storage of CO2: Modeling Approaches for Large-Scale Simulation—Jan Martin Nordbotten; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Duan, Z.; Sun, R.; Zhu, C.; Chou, I.M. An improved model for the calculation of CO2 solubility in aqueous solutions containing Na+, K+, Ca2+, Mg2+, Cl−, and SO42−. Mar. Chem. 2006, 98, 131–139. [Google Scholar] [CrossRef]
- Spycher, N.; Pruess, K. A Phase-partitioning model for CO2-brine mixtures at elevated temperatures and pressures: Application to CO2-enhanced geothermal systems. Transp. Porous Media 2010, 82, 173–196. [Google Scholar] [CrossRef] [Green Version]
- Edenhofer, O.; Pichs-Madruga, R.; Sokona, Y.; Minx, J.C.; Farahani, E.; Kadner, S.; Seyboth, K. Climate Change 2014 Mitigation of Climate Change Summary for Policymakers and Technical Summary, Working Group III Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (IPCC). Annu. Rev. Environ. Resour. 2015, 40, 363–394. [Google Scholar]
- Pacala, S.; Socolow, R. Stabilization wedges: Solving the climate problem for the next 50 years with current technologies. Science 2004, 305, 968–972. [Google Scholar] [CrossRef] [Green Version]
- Koelbl, B.S.; van den Broek, M.A.; Faaij, A.P.C.; van Vuuren, D.P. Uncertainty in Carbon Capture and Storage (CCS) deployment projections: A cross-model comparison exercise. Clim. Chang. 2014, 123, 461–476. [Google Scholar] [CrossRef]
- Mahgerefteh, H.; Brown, S.; Denton, G. Modelling the impact of stream impurities on ductile fractures in CO 2 pipelines. Chem. Eng. Sci. 2012, 74, 200–210. [Google Scholar] [CrossRef]
- Nordbotten, J.M.; Celia, M.A.; Bachu, S. Injection and storage of CO2 in deep saline aquifers: Analytical solution for CO2 plume evolution during injection. Transp. Porous Media 2005, 58, 339–360. [Google Scholar] [CrossRef]
- Zhou, Q.; Birkholzer, J.T.; Tsang, C.F.; Rutqvist, J. A method for quick assessment of CO2 storage capacity in closed and semi-closed saline formations. Int. J. Greenh. Gas Control 2008, 2, 626–639. [Google Scholar] [CrossRef] [Green Version]
- Mathias, S.A.; Hardisty, P.E.; Trudell, M.R.; Zimmerman, R.W. Approximate solutions for pressure buildup during CO2 injection in brine aquifers. Transp. Porous Media 2009, 79, 265–284. [Google Scholar] [CrossRef]
- Golding, M.J.; Neufeld, J.A.; Hesse, M.A.; Huppert, H.E. Two-phase gravity currents in porous media. J. Fluid Mech. 2011, 678, 248–270. [Google Scholar] [CrossRef]
- Birkholzer, J.T.; Oldenburg, C.M.; Zhou, Q. CO2 migration and pressure evolution in deep saline aquifers. Int. J. Greenh. Gas Control 2015, 40, 203–220. [Google Scholar] [CrossRef] [Green Version]
- Agada, S.; Jackson, S.; Kolster, C.; Dowell, N.M.; Williams, G.; Vosper, H.; Williams, J.; Krevor, S. The impact of energy systems demands on pressure limited CO2 storage in the Bunter Sandstone of the UK Southern North Sea. Int. J. Greenh. Gas Control 2017, 65, 128–136. [Google Scholar] [CrossRef] [Green Version]
- Kolster, C.; Agada, S.; Mac Dowell, N.; Krevor, S. The impact of time-varying CO2 injection rate on large scale storage in the UK Bunter Sandstone. Int. J. Greenh. Gas Control 2018, 68, 77–85. [Google Scholar] [CrossRef] [Green Version]
- Benson, S.M.; Surles, T. Carbon dioxide capture and storage: An overview with emphasis on capture and storage in deep geological formations. Proc. IEEE 2006, 94, 1795–1804. [Google Scholar] [CrossRef] [Green Version]
- Aydin, G.; Karakurt, I.; Aydiner, K. Evaluation of geologic storage options of CO2: Applicability, cost, storage capacity and safety. Energy Policy 2010, 38, 5072–5080. [Google Scholar] [CrossRef]
- Bajpai, S.; Tiwary, S.K.; Sonker, M.; Joshi, A.; Gupta, V.; Kumar, Y.; Shreyash, N.; Biswas, S. Recent Advances in Nanoparticle-Based Cancer Treatment: A Review. ACS Appl. Nano Mater. 2021, 4, 6441–6470. [Google Scholar] [CrossRef]
- D’Amato, G.; Cecchi, L.; D’Amato, M.; Annesi-Maesano, I. Climate change and respiratory diseases. Eur. Respir. Rev. 2014, 23, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Erickson, L.E. Reducing greenhouse gas emissions and improving air quality: Two global challenges. Environ. Prog. Sustain. Energy 2017, 36, 982–988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hickman, L. Timeline: How BECCS Became Climate Change’s ‘Saviour’ Technology. Available online: http://www.carbonbrief.org/beccs-the-story-of-climate-changes-saviour-technology?utm_content=buffer79430&utm_medium=social&utm_source=twitter.com&utm_campaign=buffer (accessed on 10 June 2020).
- Fridahl, M. Socio-political prioritization of bioenergy with carbon capture and storage. Energy Policy 2017, 104, 89–99. [Google Scholar] [CrossRef] [Green Version]
- Bellamy, R.; Lezaun, J.; Palmer, J. Perceptions of bioenergy with carbon capture and storage in different policy scenarios. Nat. Commun. 2019, 10, 743. [Google Scholar] [CrossRef] [Green Version]
- National Academies of Sciences. Bioenergy with Carbon Capture and Storage Approaches for Carbon Dioxide Removal and Reliable Sequestration; The National Academies Press: Washington, DC, USA, 2018. [Google Scholar] [CrossRef]
- Chung, Y.G.; Gómez-Gualdrón, D.A.; Li, P.; Leperi, K.T.; Deria, P.; Zhang, H.; Vermeulen, N.A.; Stoddart, J.F.; You, F.; Hupp, J.T.; et al. In silico discovery of metal-organic frameworks for precombustion CO2 capture using a genetic algorithm. Sci. Adv. 2016, 2, e1600909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furukawa, H.; Ko, N.; Go, Y.B.; Aratani, N.; Choi, S.B.; Choi, E.; Yazaydin, A.O.; Snurr, R.Q.; O’Keefe, M.; Kim, J.; et al. Ultrahigh Porosity in Metal-Organic Frameworks. Science 2010, 329, 424. [Google Scholar] [CrossRef] [Green Version]
- Farha, O.K.; Yazaydin, A.O.; Eryazici, I.; Malliakas, C.D.; Hauser, B.G.; Kanatzidis, M.G.; Nguyen, S.T.; Snurr, R.Q.; Hupp, J.T. De novo synthesis of a metal–organic framework material featuring ultrahigh surface area and gas storage capacities. Nat. Chem. 2010, 2, 944. [Google Scholar] [CrossRef]
- Xue, M.; Liu, Y.; Schaffino, R.M.; Xiang, S.; Zhao, X.; Zhu, G.-S.; Qiu, S.-L.; Chen, B. New Prototype Isoreticular Metal−Organic Framework Zn4O(FMA)3 for Gas Storage. Inorg. Chem. 2009, 48, 4649. [Google Scholar] [CrossRef]
- Botas, J.A.; Calleja, G.; Sánchez-Sánchez, M.; Orcajo, M.G. Cobalt Doping of the MOF-5 Framework and Its Effect on Gas-Adsorption Properties. Langmuir 2010, 26, 5300. [Google Scholar] [CrossRef] [PubMed]
- Tubert-Brohman, I.; Sherman, W.; Repasky, M.; Beuming, T. Improved Docking of Polypeptides with Glide. J. Chem. Inf. Model. 2013, 53, 1689–1699. [Google Scholar] [CrossRef] [PubMed]
- Herm, Z.R.; Swisher, J.A.; Smit, B.; Krishna, R.; Long, J.R. Metal−Organic Frameworks as Adsorbents for Hydrogen Purification and Precombustion Carbon Dioxide Capture. J. Am. Chem. Soc. 2011, 133, 5664. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Huang, S.; Xian, S.; Xi, H.; Li, Z. Adsorption Equilibrium and Kinetics of CO2 on Chromium Terephthalate MIL-101. Energy Fuels 2011, 25, 835. [Google Scholar] [CrossRef]
- Dietzel, P.D.C.; Besikiotis, V.; Blom, R. Application of metal–organic frameworks with coordinatively unsaturated metal sites in storage and separation of methane and carbon dioxide. J. Mater. Chem. 2009, 19, 7362. [Google Scholar] [CrossRef]
- Bao, Z.; Yu, L.; Ren, Q.; Lu, X.; Deng, S. Adsorption of CO2 and CH4 on a magnesium-based metal organic framework. J. Colloid Interface Sci. 2011, 353, 549. [Google Scholar] [CrossRef]
- Yazaydin, A.Ö.; Snurr, R.Q.; Park, T.-H.; Koh, K.; Liu, J.; LeVan, M.D.; Benin, A.I.; Jakubczak, P.; Lanuza, M.; Galloway, D.B.; et al. Screening of Metal−Organic Frameworks for Carbon Dioxide Capture from Flue Gas Using a Combined Experimental and Modeling Approach. J. Am. Chem. Soc. 2009, 131, 18198. [Google Scholar] [CrossRef]
- Moellmer, J.; Moeller, A.; Driesbach, F.; Glaeser, R.; Staudt, R. High pressure adsorption of hydrogen, nitrogen, carbon dioxide and methane on the metal–organic framework HKUST-1. Microporous Mesoporous Mater. 2011, 138, 140. [Google Scholar] [CrossRef]
- Aprea, P.; Caputo, D.; Gargiulo, N.; Iucolano, F.; Pepe, F. Modeling Carbon Dioxide Adsorption on Microporous Substrates: Comparison between Cu-BTC Metal−Organic Framework and 13X Zeolitic Molecular Sieve. J. Chem. Eng. Data 2010, 55, 3655. [Google Scholar] [CrossRef]
- Millward, A.R.; Yaghi, O.M. Metal−Organic Frameworks with Exceptionally High Capacity for Storage of Carbon Dioxide at Room Temperature. J. Am. Chem. Soc. 2005, 127, 17998. [Google Scholar] [CrossRef]
- Chowdhury, P.; Bikkina, C.; Meister, D.; Dreisbach, F.; Gumma, S. Comparison of adsorption isotherms on Cu-BTC metal organic frameworks synthesized from different routes. Microporous Mesoporous Mater. 2009, 117, 406. [Google Scholar] [CrossRef]
- Yazaydin, A.Ö.; Benin, A.I.; Faheem, S.A.; Jakubczak, P.; Low, J.L.; Willis, R.R.; Snurr, R.Q. Enhanced CO2 Adsorption in Metal-Organic Frameworks via Occupation of Open-Metal Sites by Coordinated Water Molecules. Chem. Mater. 2009, 21, 1425. [Google Scholar] [CrossRef]
- Lee, Y.-G.; Moon, H.R.; Cheon, Y.E.; Suh, M.P. A comparison of the H2 sorption capacities of isostructural metal-organic frameworks with and without accessible metal sites: [{Zn2(abtc)(dmf)2}3] and [{Cu2(abtc)(dmf)2}3] versus [{Cu2(abtc)}3]. Angew. Chem. Int. Ed. 2008, 47, 7741. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Xiang, S.; Zhang, W.; Zhang, Z.; Wang, L.; Bai, J.; Chen, B. A new MOF-505 analog exhibiting high acetylene storage. Chem. Commun. 2009, 7551–7553. [Google Scholar] [CrossRef]
- Aspelund, A.; Mølnvik, M.J.; De Koeijer, G. Ship transport of CO2: Technical solutions and analysis of costs, energy utilization, exergy efficiency and CO2 emissions. Chem. Eng. Res. Des. 2006, 84, 847–855. [Google Scholar] [CrossRef]
- Aspelund, A.; Jordal, K. Gas conditioning-The interface between CO2 capture and transport. Int. J. Greenh. Gas Control 2007, 1, 343–354. [Google Scholar] [CrossRef]
- McCoy, S.T.; Rubin, E.S. An engineering-economic model of pipeline transport of CO2 with application to carbon capture and storage. Int. J. Greenh. Gas Control 2008, 2, 219–229. [Google Scholar] [CrossRef]
- Brown, S.; Mahgerefteh, H.; Martynov, S.; Sundara, V.; Dowell, N.M. A multi-source flow model for CCS pipeline transportation networks. Int. J. Greenh. Gas Control 2015, 43, 108–114. [Google Scholar] [CrossRef]
- Skaugen, G.; Roussanaly, S.; Jakobsen, J.; Brunsvold, A. Techno-economic evaluation of the effects of impurities on conditioning and transport of CO2 by pipeline. Int. J. Greenh. Gas Control 2016, 54, 627–639. [Google Scholar] [CrossRef] [Green Version]
- Middleton, R.S.; Bielicki, J.M. A scalable infrastructure model for carbon capture and storage: SimCCS. Energy Policy 2009, 37, 1052–1060. [Google Scholar] [CrossRef]
- Alhajaj, A.; Mac Dowell, N.; Shah, N. Multiscale design and analysis of CO2 capture, transport and storage networks. Energy Procedia 2013, 37, 2552–2561. [Google Scholar] [CrossRef] [Green Version]
- Lazic, T.; Oko, E.; Wang, M. Case study on CO2 transport pipeline network design for Humber region in the UK. Proc. Inst. Mech. Eng. Part E J. Process Mech. Eng. 2014, 228, 210–225. [Google Scholar] [CrossRef]
- Fimbres Weihs, G.A.; Wiley, D.E. Steady-state design of CO 2 pipeline networks for minimal cost per tonne of CO2 avoided. Int. J. Greenh. Gas Control 2012, 8, 150–168. [Google Scholar] [CrossRef]
- Middleton, R.S.; Kuby, M.J.; Bielicki, J.M. Generating candidate networks for optimization: The CO2 capture and storage optimization problem. Comput. Environ. Urban Syst. 2012, 36, 18–29. [Google Scholar] [CrossRef]
- Chandel, M.K.; Pratson, L.F.; Williams, E. Potential economies of scale in CO2 transport through use of a trunk pipeline. Energy Convers. Manag. 2010, 51, 2825–2834. [Google Scholar] [CrossRef]
- Wetenhall, B.; Race, J.M.; Downie, M.J. The Effect of CO2 Purity on the Development of Pipeline Networks for Carbon Capture and Storage Schemes. Int. J. Greenh. Gas Control 2014, 30, 197–211. [Google Scholar] [CrossRef] [Green Version]
- Knoope, M.M.J.; Guijt, W.; Ramírez, A.; Faaij, A.P.C. Improved cost models for optimizing CO2 pipeline configuration for point-to-point pipelines and simple networks. Int. J. Greenh. Gas Control 2014, 22, 25–46. [Google Scholar] [CrossRef]
- Wang, Z.; Fimbres Weihs, G.A.; Cardenas, G.I.; Wiley, D.E. Optimal pipeline design for CCS projects with anticipated increasing CO2 flow rates. Int. J. Greenh. Gas Control 2014, 31, 165–174. [Google Scholar] [CrossRef]
- Liljemark, S.; Arvidsson, K.; McCann, M.T.P.; Tummescheit, H.; Velut, S. Dynamic simulation of a carbon dioxide transfer pipeline for analysis of normal operation and failure modes. Energy Procedia 2011, 4, 3040–3047. [Google Scholar] [CrossRef] [Green Version]
- Chaczykowski, M.; Osiadacz, A.J. Dynamic simulation of pipelines containing dense phase/supercritical CO 2-rich mixtures for carbon capture and storage. Int. J. Greenh. Gas Control 2012, 9, 446–456. [Google Scholar] [CrossRef]
- Noothout, P.; Wiersma, F.; Hurtado, O.; Macdonald, D.; Kemper, J.; Van Alphen, K. CO2 pipeline infrastructure—lessons learnt. Energy Procedia 2014, 63, 2481–2492. [Google Scholar] [CrossRef] [Green Version]
- Cooper, R.; Barnett, J. Pipelines for transporting CO2 in the UK. Energy Procedia 2014, 63, 2412–2431. [Google Scholar] [CrossRef] [Green Version]
- Gale, J.; Davison, J. Transmission of CO2-safety and economic considerations. Energy 2004, 29, 1319–1328. [Google Scholar] [CrossRef]
- Shuter, D.; Bilio, M.; Wilday, J.; Murray, L.; Whitbread, R. Safety issues and research priorities for CCS systems and Infrastructure. Energy Procedia 2011, 4, 2261–2268. [Google Scholar] [CrossRef] [Green Version]
- Connolly, S.; Cusco, L. Hazards from High Pressure Carbon Dioxide Releases During Carbon Dioxide Sequestration Processes. IChemE Symp. Ser. 2007, 153, 1–5. [Google Scholar]
- Woolley, R.M.; Fairweather, M.; Wareing, C.J.; Proust, C.; Hebrard, J.; Jamois, D.; Narasimhamurthy, V.D.; Storvik, I.E.; Skjold, T.; Falle, S.A.E.G.; et al. An integrated, multi-scale modelling approach for the simulation of multiphase dispersion from accidental CO2 pipeline releases in realistic terrain. Int. J. Greenh. Gas Control 2014, 27, 221–238. [Google Scholar] [CrossRef]
- Witlox, H.W.M.; Stene, J.; Harper, M.; Nilsen, S.H. Modelling of discharge and atmospheric dispersion for carbon dioxide releases including sensitivity analysis for wide range of scenarios. Energy Procedia 2011, 4, 2253–2260. [Google Scholar] [CrossRef] [Green Version]
- Woodhill Engineering Consultants. Pipeline Transmission of CO2 and Energy. Transmission Study Report; IEA GHG. Report No.: PH4/6. 2002. Available online: https://ieaghg.org/docs/General_Docs/Reports/PH4_6%20TRANSMISSION%20REPORT.pdf (accessed on 13 November 2020).
- Hamelinck, C.N.; Faaij, A.P.C.; Turkenburg, W.C.; Van Bergen, F.; Pagnier, H.J.M.; Barzandji, O.H.M.; Wolf, K.H.A.A.; Ruijg, G.J. CO2 enhanced coalbed methane production in the Netherlands. Energy 2002, 27, 647–674. [Google Scholar] [CrossRef]
- Tian, Q.; Zhao, D.; Li, Z.; Zhu, Q. Robust and stepwise optimization design for CO2 pipeline transportation. Int. J. Greenh. Gas Control 2017, 58, 10–18. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Wang, G.X.; Massarotto, P.; Rudolph, V. Optimization of pipeline transport for CO2 sequestration. Energy Convers. Manag. 2006, 47, 702–715. [Google Scholar] [CrossRef]
- Vandeginste, V.; Piessens, K. Pipeline design for a least-cost router application for CO2 transport in the CO2 sequestration cycle. Int. J. Greenh. Gas Control 2008, 2, 571–581. [Google Scholar] [CrossRef]
- Ogden, J.M. Conceptual Design of Optimized Fossil Energy Systems with Capture and Sequestration of Carbon Dioxide. Available online: https://www.osti.gov/servlets/purl/1057457 (accessed on 10 August 2021).
- Energy Institute. Good Plant Design and Operation for Onshore Carbon Capture Installations and Onshore Pipelines; Energy Institute: London, UK, 2010; 137p, Available online: https://publishing.energyinst.org/__data/assets/file/0007/71395/WEB-VERSION-Good-plant-design-and-operation-for-onshore-carbon-capture-installations-and-onshore-pipelines-Sept-2010.pdf (accessed on 10 August 2021).
- Munkejord, S.T.; Hammer, M.; Løvseth, S.W. CO2 transport: Data and models—A review. Appl. Energy 2016, 169, 499–523. [Google Scholar] [CrossRef]
- House, M. Scottish Carbon Capture & Storage Ship transport of CO2 for Enhanced Oil Recovery-Literature Survey. Available online: http://www.sccs.org.uk/images/expertise/reports/co2-eor-jip/SCCS-CO2-EOR-JIP-WP15-Shipping.pdf (accessed on 10 August 2021).
- Santos, S. CO2 Transport via Pipeline and Ship. Available online: https://ieaghg.org/docs/General_Docs/IEAGHG_Presentations/3._CO2_Transport_Overview_-_S._Santos_IEAGHG.pdf (accessed on 10 August 2021).
- European Technology Platform for Zero Emission Fossil Fuel Power Plants (ZEP). The Costs of CO2 Transport: Post-demonstration CCS in the EU—Global CCS Institute. Available online: https://www.globalccsinstitute.com/archive/hub/publications/119811/costs-co2-transport-post-demonstration-ccs-eu.pdf (accessed on 10 August 2021).
- Doctor, R.; States, U.; Palmer, A.; Coleman, D.; Davison, J.; Kingdom, U. 4 Transport of CO2 Coordinating Lead Authors. Available online: https://www.ipcc.ch/site/assets/uploads/2018/03/srccs_chapter4-1.pdf (accessed on 10 August 2021).
- Kjärstad, J.; Skagestad, R.; Eldrup, N.H.; Johnsson, F. Ship transport—A low cost and low risk CO2 transport option in the Nordic countries. Int. J. Greenh. Gas Control 2016, 54, 168–184. [Google Scholar] [CrossRef]
- Gao, L.; Fang, M.; Li, H.; Hetland, J. Cost analysis of CO2 transportation: Case study in China. Energy Procedia 2011, 4, 5974–5981. [Google Scholar] [CrossRef] [Green Version]
- Metz, B.; Davidson, O.; de Coninck, H.; Loos, M.; Meyer, L. IPCC Special Report on Carbon Dioxide Capture and Storage. Available online: https://www.ipcc.ch/site/assets/uploads/2018/03/srccs_wholereport-1.pdf (accessed on 10 August 2021).
- Vasanthakumari, R. Design and Development of Thermoplastic Polyurethane Based Composite Membranes. In Proceedings of the ASME 2010 8th International Fuel Cell Science, Engineering and Technology Conference, Brooklyn, NY, USA, 14–16 June 2010; pp. 21–26. [Google Scholar]
- Debnath, S.; Tiwary, S.K.; Ojha, U. Dynamic Carboxylate Linkage Based Reprocessable and Self-Healable Segmented Polyurethane Vitrimers Displaying Creep Resistance Behavior and Triple Shape Memory Ability. ACS Appl. Polym. Mater. 2021, 3, 2166–2177. [Google Scholar] [CrossRef]
- Andersen, S.O. We can and must govern climate engineering. Nature 2017, 551, 415. [Google Scholar] [CrossRef]
- Keith, D.W.; Holmes, G.; Angelo, D.S.; Heidel, K. A Process for Capturing CO2 from the Atmosphere. Joule 2018, 2, 1573–1594. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Naghavi, M.; Allen, C.; Barber, R.M.; Carter, A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z.; Coates, M.M.; Coggeshall, M.; et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Zhang, Q.; Chen, Y.; Bao, J.; Zhou, X.; Xie, Z.; Wei, J.; Zhou, Z. The first introduction of graphene to rechargeable Li-CO2 batteries. Angew. Chem. Int. Ed. 2015, 54, 6550–6553. [Google Scholar] [CrossRef]
- Qie, L.; Lin, Y.; Connell, J.W.; Xu, J.; Dai, L. Highly Rechargeable Lithium-CO2 Batteries with a Boron- and Nitrogen-Codoped Holey-Graphene Cathode. Angew. Chem. Int. Ed. 2017, 56, 6970–6974. [Google Scholar] [CrossRef]
- Hu, X.; Sun, J.; Li, Z.; Zhao, Q.; Chen, C.; Chen, J. Rechargeable room-temperature Na-CO2 batteries. Angew. Chem. Int. Ed. 2016, 55, 6482–6486. [Google Scholar] [CrossRef]
- Al Sadat, W.I.; Archer, L.A. The O2-assisted Al/CO2 electrochemical cell: A system for CO2 capture/conversion and electric power generation. Sci. Adv. 2016, 2, e1600968. [Google Scholar] [CrossRef] [Green Version]
- Das, S.K.; Xu, S.; Archer, L.A. Carbon dioxide assist for non-aqueous sodium-oxygen batteries. Electrochem. Commun. 2013, 27, 59–62. [Google Scholar] [CrossRef]
- Kaur, G.; Kulkarni, A.P.; Fini, D.; Giddey, S.; Seeber, A. High-performance composite cathode for electrolysis of CO2 in tubular solid oxide electrolysis cells: A pathway for efficient CO2 utilization. J. CO2 Util. 2020, 41, 101271. [Google Scholar] [CrossRef]
- Chen, K.K.; Han, Y.; Tong, Z.; Gasda, M.; Ho, W.S.W. Membrane processes for CO2 removal and fuel utilization enhancement for solid oxide fuel cells. J. Memb. Sci. 2021, 620, 118846. [Google Scholar] [CrossRef]
- Xie, F.; Chen, R.; Zhu, X.; Liao, Q.; Ye, D.; Zhang, B.; Yu, Y.; Li, J. CO2 utilization: Direct power generation by a coupled system that integrates photocatalytic reduction of CO2 with photocatalytic fuel cell. J. CO2 Util. 2019, 32, 31–36. [Google Scholar] [CrossRef]
- Consonni, S.; Mastropasqua, L.; Spinelli, M.; Barckholtz, T.A.; Campanari, S. Low-carbon hydrogen via integration of steam methane reforming with molten carbonate fuel cells at low fuel utilization. Adv. Appl. Energy 2021, 2, 100010. [Google Scholar] [CrossRef]
- Shreyash, N.; Sonker, M.; Bajpai, S.; Tiwary, S.K. Review of the Mechanism of Nanocarriers and Technological Developments in the Field of Nanoparticles for Applications in Cancer Theragnostics. ACS Appl. Bio Mater. 2021, 4, 2307–2334. [Google Scholar] [CrossRef]
- Roberts, F.S.; Kuhl, K.P.; Nilsson, A. High selectivity for ethylene from carbon dioxide reduction over copper nanocube electrocatalysts. Angew. Chem. Int. Ed. 2015, 54, 5179–5182. [Google Scholar] [CrossRef]
- Kuhl, K.P.; Hatsukade, T.; Cave, E.R.; Abram, D.N.; Kibsgaard, J.; Jaramillo, T.F. Electrocatalytic conversion of carbon dioxide to methane and methanol on transition metal surfaces. J. Am. Chem. Soc. 2014, 136, 14107–14113. [Google Scholar] [CrossRef]
- Gao, S.; Lin, Y.; Jiao, X.; Sun, Y.; Luo, Q.; Zhang, W.; Li, D.; Yang, J.; Xie, Y. Partially oxidized atomic cobalt layers for carbon dioxide electroreduction to liquid fuel. Nature 2016, 529, 68–71. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Li, S.; Shu, C.; Fang, Y.; Song, B. Improved Charge Transfer in a Mn2O3@Co1.2Ni1.8O4 Hybrid for Highly Stable Alkaline Direct Methanol Fuel Cells with Good Methanol Tolerance. ACS Appl. Mater. Interfaces 2018, 10, 9485–9494. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Sun, Y.; Chen, L.; Hernandez, Y.; Feng, X.; Müllen, K. Porous iron oxide ribbons grown on graphene for high-performance lithium storage. Sci. Rep. 2012, 2, 427. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Y.; Ming, J.; Chen, L.; Shu, C.; Qu, T.; Tan, Q.; Liu, Y.; Asefa, T. Harvesting waste heat energy by promoting H+-ion concentration difference with a fuel cell structure Harvesting waste heat energy by promoting H+-ion concentration difference with a fuel cell structure. Nano Energy 2018, 57, 101–107. [Google Scholar] [CrossRef]
- Jaouen, F.; Proietti, E.; Lefèvre, M.; Chenitz, R.; Dodelet, J.-P.; Wu, G.; Chung, H.T.; Johnston, C.M.; Zelenay, P. Recent advances in non-precious metal catalysis for oxygen-reduction reaction in polymer electrolyte fuelcells. Energy Environ. Sci. 2011, 4, 114–130. [Google Scholar] [CrossRef]
- Das, V.; Padmanaban, S.; Venkitusamy, K.; Selvamuthukumaran, R.; Blaabjerg, F.; Siano, P. Recent advances and challenges of fuel cell based power system architectures and control—A review. Renew. Sustain. Energy Rev. 2017, 73, 10–18. [Google Scholar] [CrossRef]
- Bashyam, R.; Zelenay, P. A class of non-precious metal composite catalysts for fuel cells. Nature 2006, 443, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-W. A review of recent development: Transport and performance modeling of PEM fuel cells. Appl. Energy 2016, 165, 81–106. [Google Scholar] [CrossRef]
- Long, R.; Li, Y.; Liu, Y.; Chen, S.; Zheng, X.; Gao, C.; He, C.; Chen, N.; Qi, Z.; Song, L.; et al. Isolation of Cu Atoms in Pd Lattice: Forming Highly Selective Sites for Photocatalytic Conversion of CO2 to CH4. J. Am. Chem. Soc. 2017, 139, 4486–4492. [Google Scholar] [CrossRef]
- Bouchet, R.; Miller, S.; Duclot, M.; Souquet, J.L. A thermodynamic approach to proton conductivity in acid-doped polybenzimidazole. Solid State Ionics 2001, 145, 69–78. [Google Scholar] [CrossRef]
- Qiao, J.; Liu, Y.; Hong, F.; Zhang, J. A review of catalysts for the electroreduction of carbon dioxide to produce low-carbon fuels. Chem. Soc. Rev. 2014, 43, 631–675. [Google Scholar] [CrossRef]
- Kumar, B.; Brian, J.P.; Atla, V.; Kumari, S.; Bertram, K.A.; White, R.T.; Spurgeon, J.M. New trends in the development of heterogeneous catalysts for electrochemical CO2 reduction. Catal. Today 2016, 270, 19–30. [Google Scholar] [CrossRef]
- Gattrell, M.; Gupta, N.; Co, A. A review of the aqueous electrochemical reduction of CO2 to hydrocarbons at copper. J. Electroanal. Chem. 2006, 594, 1–19. [Google Scholar] [CrossRef]
- Wheeler, R.R.; Dewberry, R.H.; Mccurry, B.D.; Abney, M.B.; Greenwood, Z.W. Development of a Microwave Regenerative Sorbent-based Hydrogen Purifier. In Proceedings of the 46th International Conference on Environmental Systems, Vienna, Austria, 10–14 July 2016. [Google Scholar]
- NASA Technical Reports Server (NTRS)—Advanced Plasma Pyrolysis Assembly (PPA) Reactor and Process Development. Available online: https://ntrs.nasa.gov/search.jsp?R=20120015328 (accessed on 10 June 2020).
- Wang, L.; Pérez-Fortes, M.; Madi, H.; Diethelm, S.; Van Herle, J.; Maréchal, F. Optimal design of solid-oxide electrolyzer based power-to-methane systems: A comprehensive comparison between steam electrolysis and co-electrolysis. Appl. Energy 2018, 211, 1060–1079. [Google Scholar] [CrossRef]
- Wang, L.; Chen, M.; Küngas, R.; Lin, T.E.; Diethelm, S.; Maréchal, F.; Van Herle, J. Power-to-fuels via solid-oxide electrolyzer: Operating window and techno-economics. Renew. Sustain. Energy Rev. 2019, 110, 174–187. [Google Scholar] [CrossRef]
- Ebbesen, S.D. Solid Oxide Electrolysis Cells. Available online: https://www.energy.dtu.dk/english/Research/Electrolysis-Cells/Solid-Oxide-Electrolysis-Cells (accessed on 10 June 2020).
- Matter, P.H.; Beachy, M.G.; Gaydos, J. Carbon Formation Reactor and Method of Using Same. Available online: https://www.mysciencework.com/patent/show/carbon-formation-reactor-method-using-same-US20180023200A1 (accessed on 10 August 2021).
- Stanley, C.; Barnett, B.; Matter, P.; Beachy, M.; Thompson, J.; Kelly, S.F. Alternative Carbon Formation Reactors for the Series-Bosch System. In Proceedings of the 48th International Conference on Environmental Systems, Albuquerque, NM, USA, 8–12 July 2018; pp. 1–7. [Google Scholar]
- NASA; CASI. General disclaimer. Stat. J. IAOS 2011, 27, 3. [Google Scholar] [CrossRef]
- Fussler, C. Solutions for a Circular Carbon Economy—Briefing Paper. Available online: https://www.wrforum.org/wp-content/uploads/2015/10/2_Circular-Carbon-Solutions-Claude-Fussler.pdf (accessed on 10 August 2021).
- EUROPA—CO2 as a Raw Material for Waxes and Fuels|SETIS—European Commission. Available online: https://setis.ec.europa.eu/publications/setis-magazine/carbon-capture-utilisation-and-storage/co2-raw-material-waxes-and-fuels (accessed on 9 June 2020).
- Benjaminsson, G.; Benjaminsson, J.; Rudberg, R.B. Power-to-Gas—A Technical Review; (El-till-Gas-System, Ekonomi och Teknik) SGC Rapport; Svenskt Gastekniskt Center: Malmö, Sweden, 2013; p. 284. [Google Scholar]
- Hydrogen from “Reverse Fuel” Cells—DTU. Available online: https://www.dtu.dk/english/news/2017/03/dynamo-theme4-hydrogen-from-reverse-fuel-cells?id=e804ab15-4822-4f1c-92be-09a3e5bece1e (accessed on 9 June 2020).
- Agarwal, A.S.; Zhai, Y.; Hill, D.; Guan, S.; Rode, E.; Sridhar, N. Technology development for large scale electrochemical conversion of CO2 to useful products. In Proceedings of the Clean Technology 2011 Conference & Expo, Boston, MA, USA, 13–16 June 2011; Volume 3, pp. 805–808. [Google Scholar]
- Weigel, M.C.; Tritscher, E.; Lisdat, F. Direct electrochemical conversion of bilirubin oxidase at carbon nanotube-modified glassy carbon electrodes. Electrochem. commun. 2007, 9, 689–693. [Google Scholar] [CrossRef]
- Frackowiak, E.; Béguin, F. Electrochemical storage of energy in carbon nanotubes and nanostructured carbons. Carbon 2002, 40, 1775–1787. [Google Scholar] [CrossRef]
- Albo, J.; Alvarez-Guerra, M.; Castaño, P.; Irabien, A. Towards the electrochemical conversion of carbon dioxide into methanol. Green Chem. 2015, 17, 2304–2324. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Q. Recent Advances in Breaking Scaling Relations for Effective Electrochemical Conversion of CO2. Adv. Energy Mater. 2016, 6, 1600463. [Google Scholar] [CrossRef]
- Ren, J.; Johnson, M.; Singhal, R.; Licht, J. Transformation of the greenhouse gas CO2 by molten electrolysis into a wide controlled selection of carbon nanotubes. J. CO2 Util. 2017, 18, 335–344. [Google Scholar] [CrossRef] [Green Version]
- Lau, J.; Dey, G.; Licht, S. Thermodynamic assessment of CO2 to carbon nanofiber transformation for carbon sequestration in a combined cycle gas or a coal power plant. Energy Convers. Manag. 2016, 122, 400–410. [Google Scholar] [CrossRef] [Green Version]
- Sunfire Green Syngas—Sunfire. Available online: https://www.sunfire.de/en/applications/syngas (accessed on 10 June 2020).
- Zheng, Y.; Wang, J.; Yu, B.; Zhang, W.; Chen, J.; Qiao, J.; Zhang, J. A review of high temperature co-electrolysis of H2O and CO2 to produce sustainable fuels using solid oxide electrolysis cells (SOECs): Advanced materials and technology. Chem. Soc. Rev. 2017, 46, 1427–1463. [Google Scholar] [CrossRef]
- The Reaction between Hydrogen and Oxygen on Platinum|Johnson Matthey Technology Review. Available online: https://www.technology.matthey.com/article/10/2/60-64/ (accessed on 10 June 2020).
- Campanari, S.; Chiesa, P.; Manzolini, G. CO2 capture from combined cycles integrated with Molten Carbonate Fuel Cells. Int. J. Greenh. Gas Control 2010, 4, 441–451. [Google Scholar] [CrossRef]
- DOE-DOD Workshop. Fuel Cell Power Plants Fuel Cell Power Plants Renewable and Waste Fuels Reliable, Efficient, Ultra-clean. Available online: https://www1.eere.energy.gov/hydrogenandfuelcells/pdfs/waste_wolak.pdf (accessed on 10 August 2021).
- Carbon Capture|Fuelcell Energy. Available online: https://www.fuelcellenergy.com/recovery-2/recovery-2/ (accessed on 8 June 2020).
- ExxonMobil, Fuel Cell Energy Expand Agreement to Optimize Carbonate Fuel Cell Technology for Large-Scale Carbon Capture—Green Car Congress. Available online: https://www.greencarcongress.com/2019/11/20191110-fcel.html (accessed on 10 June 2020).
- Eisler, M.N. Fuel Cells Finally Find a Killer App: Carbon Capture—IEEE Spectrum. Available online: https://spectrum.ieee.org/green-tech/fuel-cells/fuel-cells-finally-find-a-killer-app-carbon-capture (accessed on 8 June 2020).
- Le Quéré, C.; Moriarty, R.; Andrew, R.M.; Canadell, J.G.; Sitch, S.; Korsbakken, J.I.; Friedlingstein, P.; Peters, G.P.; Andres, R.J.; Boden, T.A.; et al. Global carbon budget 2015. Earth Syst. Sci. Data 2015, 7, 349–396. [Google Scholar] [CrossRef] [Green Version]
- Figueres, C.; Le Quéré, C.; Mahindra, A.; Bäte, O.; Whiteman, G.; Peters, G.; Guan, D. Emissions are still rising: Ramp up the cuts. Nature 2018, 564, 27–30. [Google Scholar] [CrossRef] [Green Version]
- Saadi, F.H.; Lewis, N.S.; McFarland, E.W. Relative costs of transporting electrical and chemical energy. Energy Environ. Sci. 2018, 11, 469–475. [Google Scholar] [CrossRef] [Green Version]
- De Luna, P.; Hahn, C.; Higgins, D.; Jaffer, S.A.; Jaramillo, T.F.; Sargent, E.H. What would it take for renewably powered electrosynthesis to displace petrochemical processes? Science 2019, 364, 6438. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.M.; Abdala, P.M.; Broda, M.; Hosseini, D.; Copéret, C.; Müller, C. Integrated CO2 Capture and Conversion as an Efficient Process for Fuels from Greenhouse Gases. ACS Catal. 2018, 8, 2815–2823. [Google Scholar] [CrossRef]
- Kar, S.; Goeppert, A.; Galvan, V.; Chowdhury, R.; Olah, J.; Prakash, G.K.S. A Carbon-Neutral CO2 Capture, Conversion, and Utilization Cycle with Low-Temperature Regeneration of Sodium Hydroxide. J. Am. Chem. Soc. 2018, 140, 16873–16876. [Google Scholar] [CrossRef]
- Zhang, X.; Song, Z.; Gani, R.; Zhou, T. Comparative Economic Analysis of Physical, Chemical, and Hybrid Absorption Processes for Carbon Capture. Ind. Eng. Chem. Res. 2020, 59, 2005–2012. [Google Scholar] [CrossRef]
- Styring, P.; Jansen, D.; De Coninick, H.; Reith, H.; Armstrong, K. Carbon Capture and Utilisation in the Green Economy; Centre for Low Carbon Futures: New York, NY, USA, 2011. [Google Scholar]
- von der Assen, N.; Jung, J.; Bardow, A. Life-cycle assessment of carbon dioxide capture and utilization: Avoiding the pitfalls. Energy Environ. Sci. 2013, 6, 2721–2734. [Google Scholar] [CrossRef]
- Fan, J.-L.; Xu, M.; Yang, L.; Zhang, X.; Li, F. How can carbon capture utilization and storage be incentivized in China? A perspective based on the 45Q tax credit provisions. Energy Policy 2019, 132, 1229–1240. [Google Scholar] [CrossRef]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef] [Green Version]
- Le Quéré, C.; Moriarty, R.; Andrew, R.M.; Peters, G.P.; Ciais, P.; Friedlingstein, P.; Jones, S.D.; Sitch, S.; Tans, P.; Arneth, A.; et al. Global carbon budget 2014. Earth Syst. Sci. Data 2015, 7, 47–85. [Google Scholar] [CrossRef] [Green Version]
- Wallace, J.; Palmer, F.D.; Ericson, A.; Bozzuto, C.; Krutka, H.; Tomski, P.; Angielski, S.; Phillips, J. Fossil Forward-Revitalizing CCS: Bringing Scale and Speed to CCS Deployment; National Coal Council: Washington, DC, USA, 2015; Available online: http://www.nationalcoalcouncil.org/studies/2015/News-Release-NCC-Study-January-29-2015.pdf (accessed on 10 August 2021).
- Von Der Assen, N.; Bardow, A. Life cycle assessment of polyols for polyurethane production using CO2 as feedstock: Insights from an industrial case study. Green Chem. 2014, 16, 3272–3280. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Zhuang, Y.; Tao, R.; Liu, L.; Zhang, L.; Du, J. Multi-objective optimization for the deployment of carbon capture utilization and storage supply chain considering economic and environmental performance. J. Clean. Prod. 2020, 270, 122481. [Google Scholar] [CrossRef]
- Hasan, M.M.F.; Boukouvala, F.; First, E.L.; Floudas, C.A. Nationwide, Regional, and Statewide CO2 Capture, Utilization, and Sequestration Supply Chain Network Optimization. Ind. Eng. Chem. Res. 2014, 53, 7489–7506. [Google Scholar] [CrossRef]
- Leonzio, G.; Foscolo, P.U.; Zondervan, E. An outlook towards 2030: Optimization and design of a CCUS supply chain in Germany. Comput. Chem. Eng. 2019, 125, 499–513. [Google Scholar] [CrossRef]
- d’Amore, F.; Bezzo, F. Optimal European cooperative supply chains for carbon capture, transport, and sequestration with costs share policies. AIChE J. 2020, 66, e16872. [Google Scholar] [CrossRef]
- Sherafati, M.; Bashiri, M.; Tavakkoli-Moghaddam, R.; Pishvaee, M.S. Supply chain network design considering sustainable development paradigm: A case study in cable industry. J. Clean. Prod. 2019, 234, 366–380. [Google Scholar] [CrossRef]


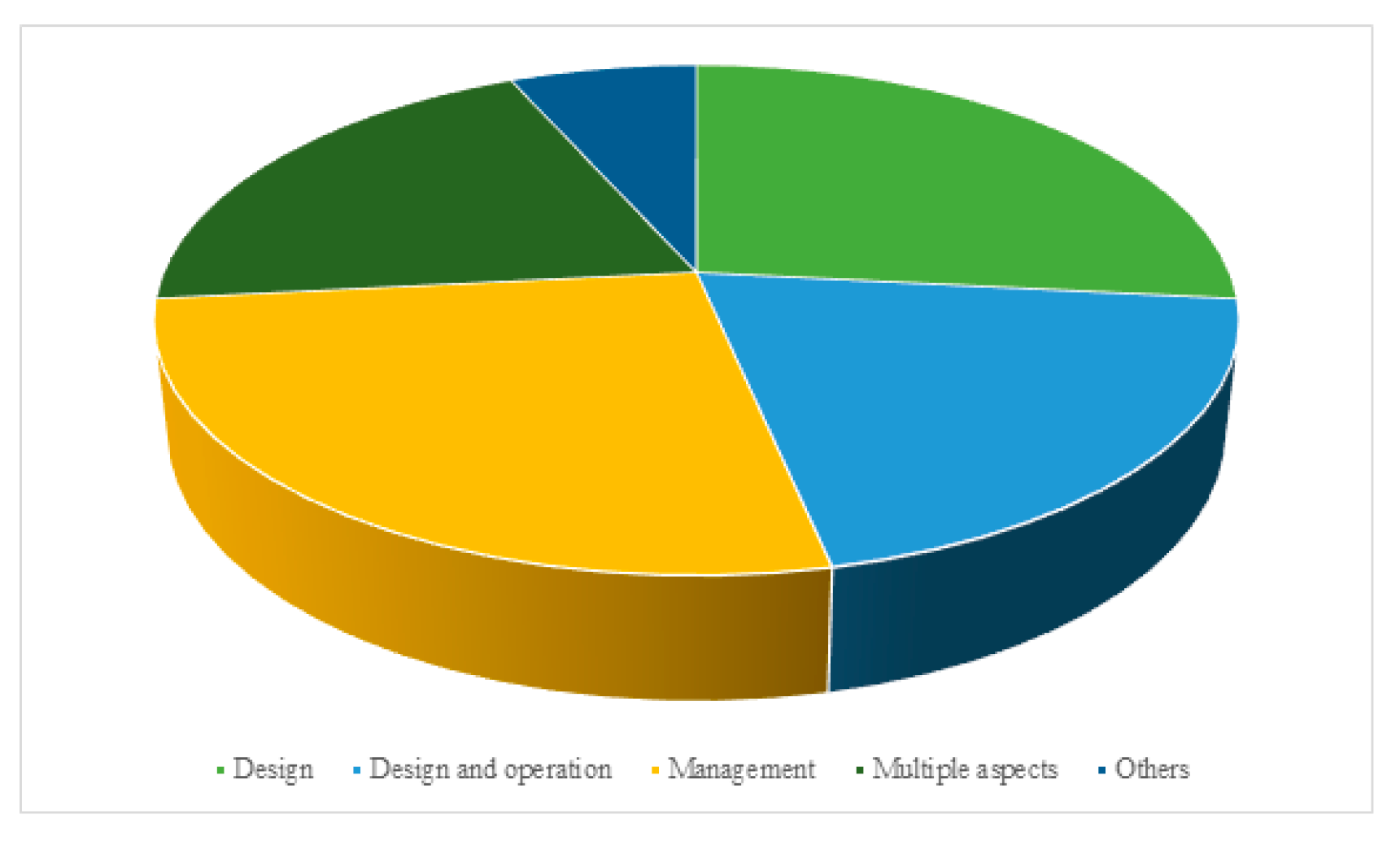

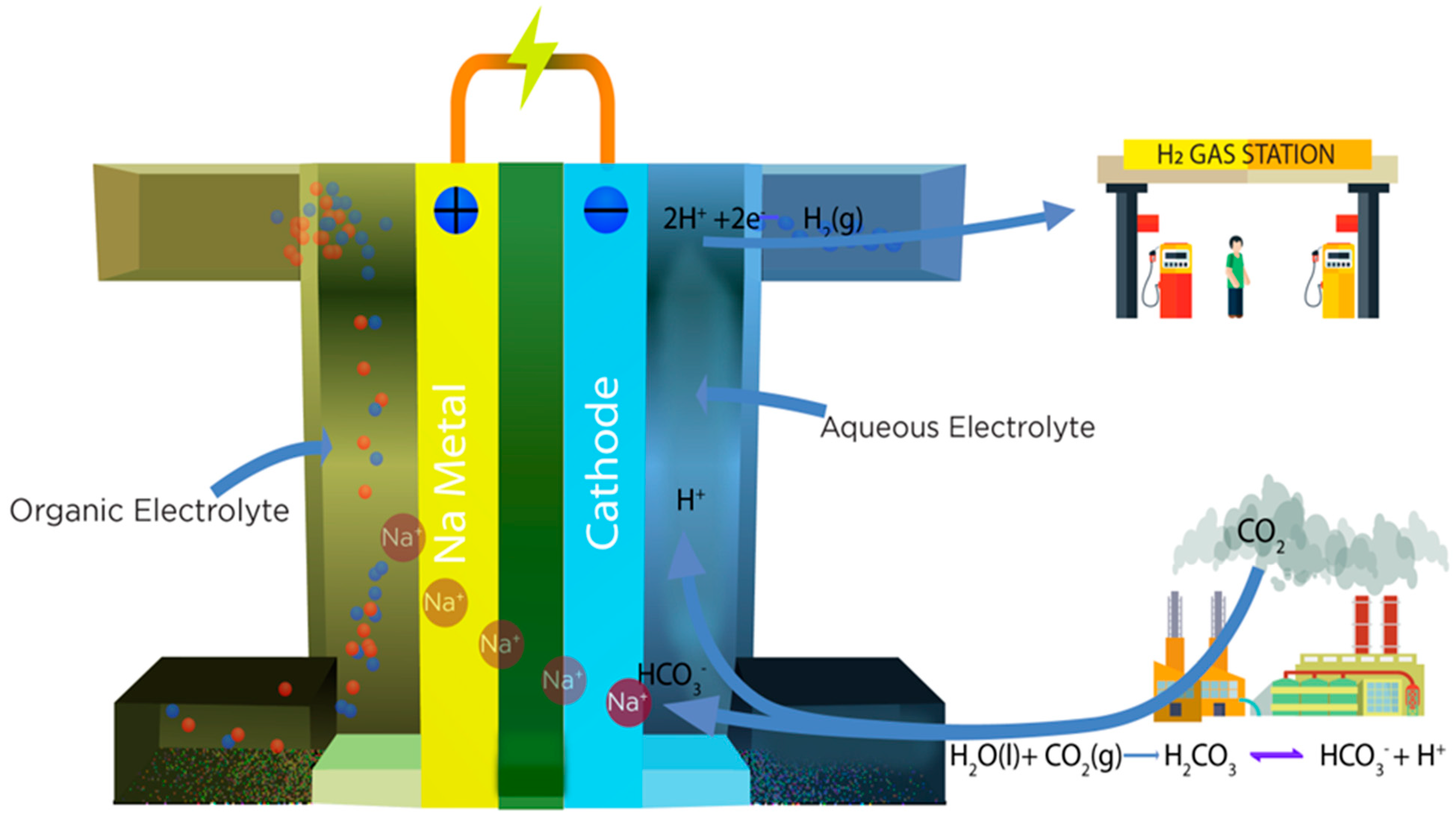


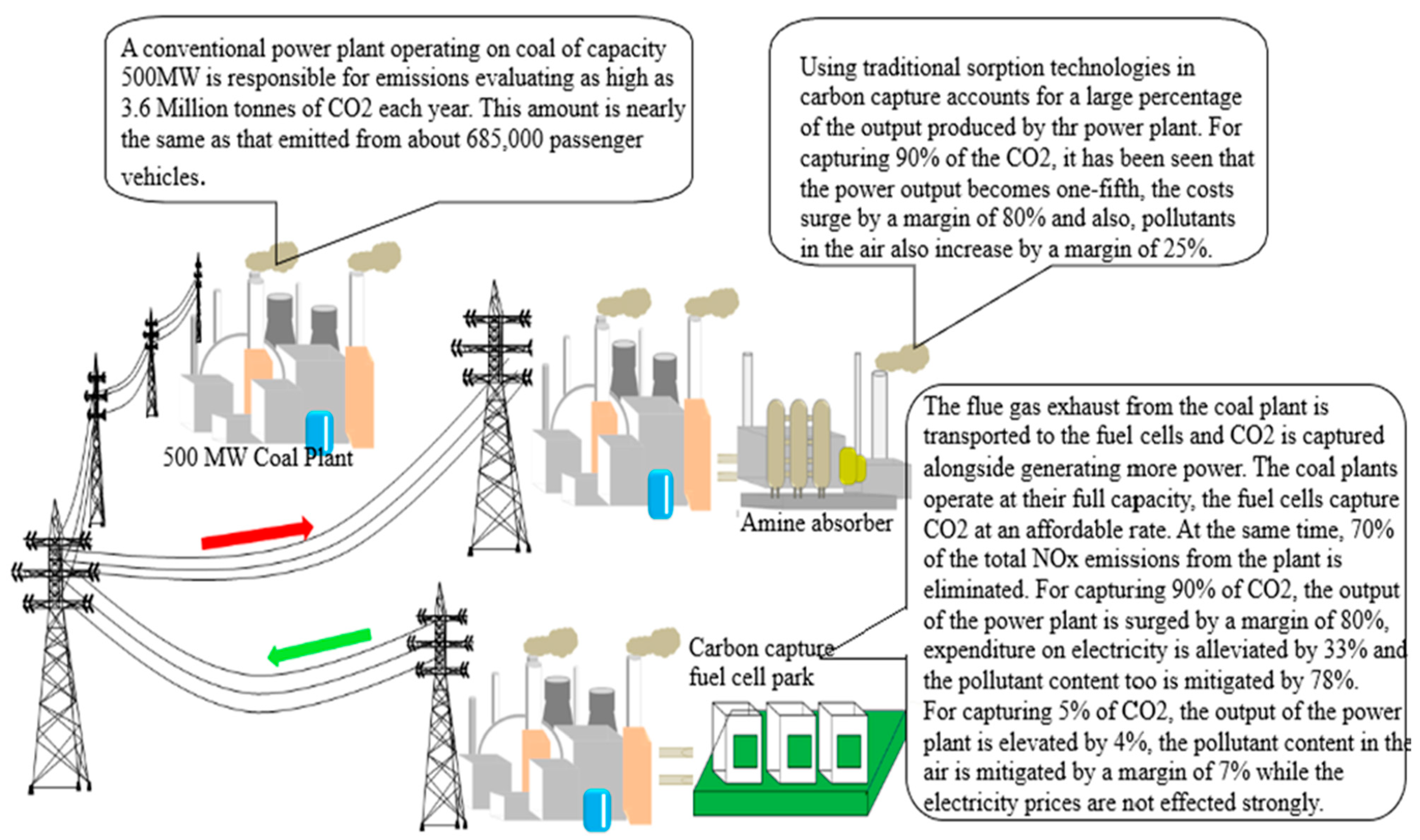
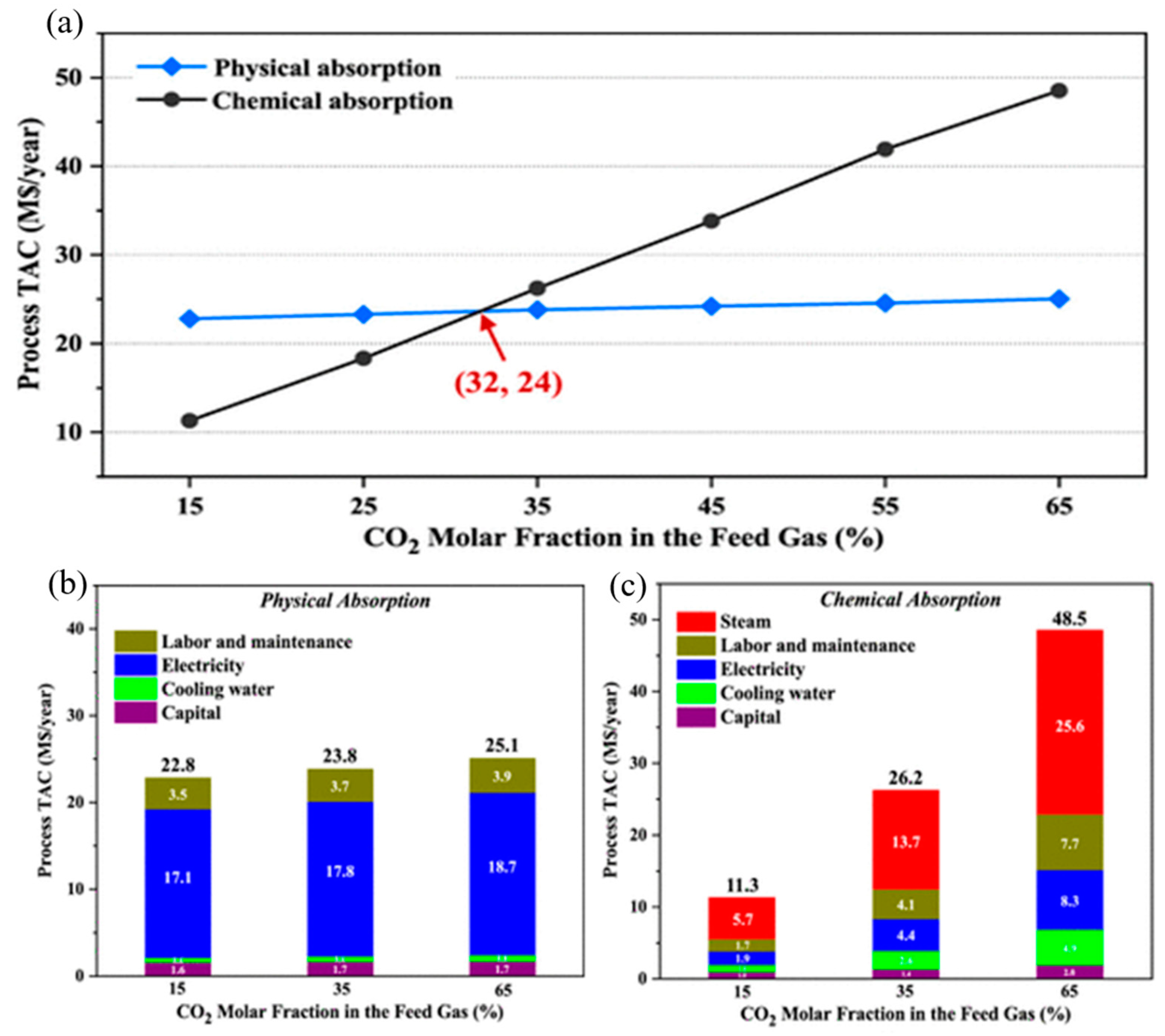
| MOFs | BET | Capacity | Pressure | Temperature | Reference |
|---|---|---|---|---|---|
| (m2/g) | (wt%) | (bar) | (K) | ||
| MOF-210 | 6240 | 74.2 | 50 | 298 | [95] |
| MOF-200 | 4530 | 73.9 | 50 | 298 | [95] |
| NU-100 | 6143 | 69.8 | 40 | 298 | [96] |
| Zn4O(FMA)3 | 1120 | 69.0 | 28 | 300 | [97] |
| Co-21-MOF-5 | - | 65 | 10 | 273 | [98] |
| MOF-205 | 4460 | 62.6 | 50 | 298 | [95] |
| DUT-9 | - | 62.1 | 47 | 298 | [99] |
| MOF-177 | 4500 | 60.8 | 50 | 298 | [95] |
| Be-BTB | 4030 | 58.5 | 40 | 313 | [100] |
| PCN-68 | 5109 | 57.2 | 35 | 298 | [101] |
| Mg-MOF-74 | 1542 | 68.9 | 36 | 278 | [102] |
| 1174 | 27.5 | 1 | 298 | [103] | |
| - | 27.2 | 1 | 298 | [104] | |
| HKSUT-1 | 1270 | 42.8 | 300 | 313 | [105] |
| HKSUT-1 | 1400 | 19.8 | 1 | 293 | [106] |
| HKSUT-1 | 1781 | 15.2 | 1 | 298 | [107] |
| HKSUT-1 | 1482 | 15 | 1 | 295 | [108] |
| HKSUT-1 (4 wt% H2O) | - | 27 | 1 | 298 | [109] |
| SNU-5 | - | 38.5 | 1 | 273 | [110] |
| Cu-EBTC | 1852 | 25.9 | 1 | 273 | [111] |
| Co-MOF-74 | 957 | 24.9 | 1 | 298 | [104] |
| Ni-MOF-74 | 936 | 23.9 | 1 | 298 | [104] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shreyash, N.; Sonker, M.; Bajpai, S.; Tiwary, S.K.; Khan, M.A.; Raj, S.; Sharma, T.; Biswas, S. The Review of Carbon Capture-Storage Technologies and Developing Fuel Cells for Enhancing Utilization. Energies 2021, 14, 4978. https://doi.org/10.3390/en14164978
Shreyash N, Sonker M, Bajpai S, Tiwary SK, Khan MA, Raj S, Sharma T, Biswas S. The Review of Carbon Capture-Storage Technologies and Developing Fuel Cells for Enhancing Utilization. Energies. 2021; 14(16):4978. https://doi.org/10.3390/en14164978
Chicago/Turabian StyleShreyash, Nehil, Muskan Sonker, Sushant Bajpai, Saurabh Kr Tiwary, Mohd Ashhar Khan, Subham Raj, Tushar Sharma, and Susham Biswas. 2021. "The Review of Carbon Capture-Storage Technologies and Developing Fuel Cells for Enhancing Utilization" Energies 14, no. 16: 4978. https://doi.org/10.3390/en14164978
APA StyleShreyash, N., Sonker, M., Bajpai, S., Tiwary, S. K., Khan, M. A., Raj, S., Sharma, T., & Biswas, S. (2021). The Review of Carbon Capture-Storage Technologies and Developing Fuel Cells for Enhancing Utilization. Energies, 14(16), 4978. https://doi.org/10.3390/en14164978






