Impact of Low-Pressure UV Lamp on Swimming Pool Water Quality and Operating Costs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Facility
2.2. Sampling and Data Acquisition
2.3. Analytical Methods and Statistical Analysis
3. Results and Discussion
3.1. Swimming Pool Water Quality
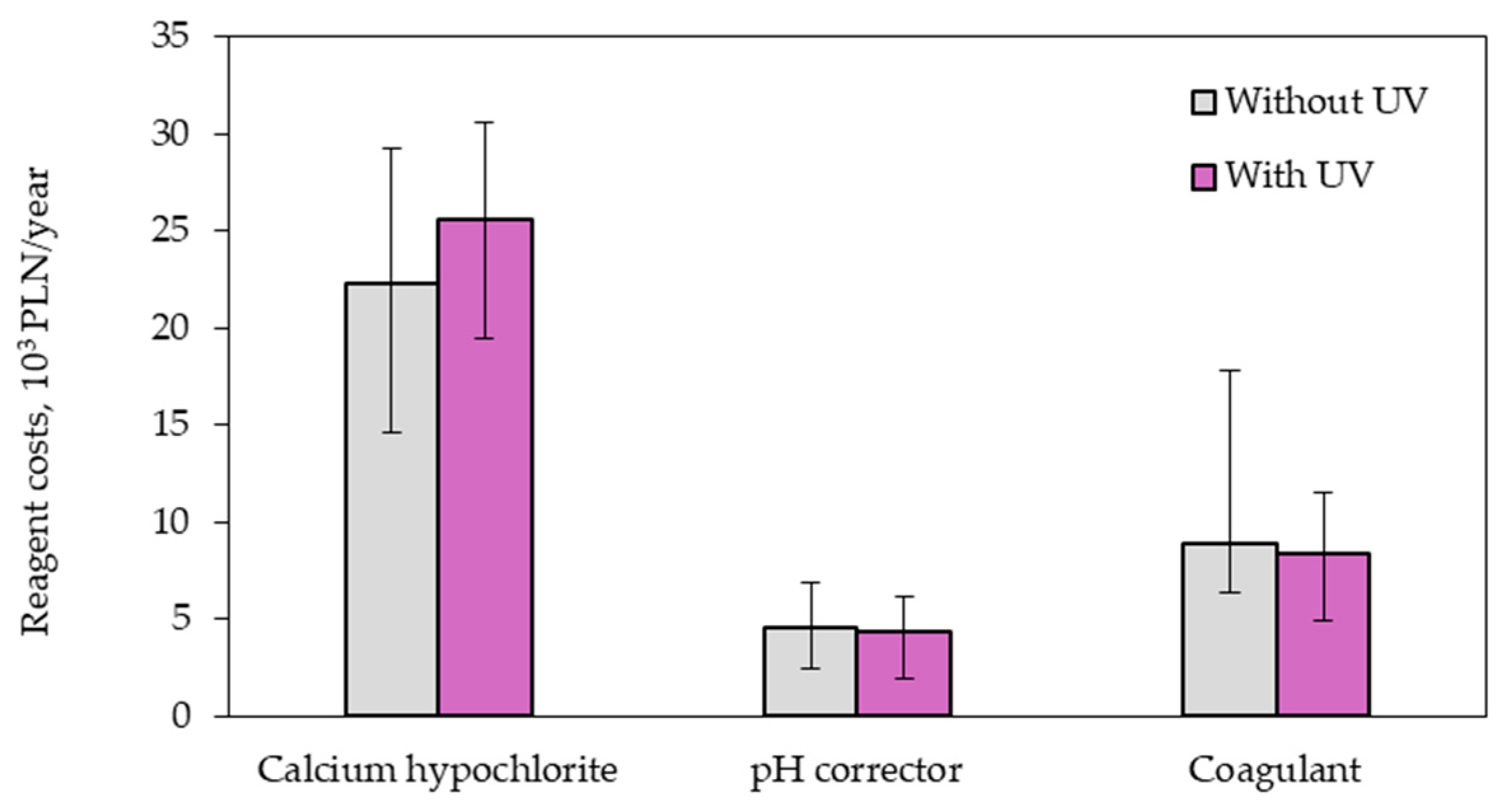
3.2. Operating Costs
4. Conclusions
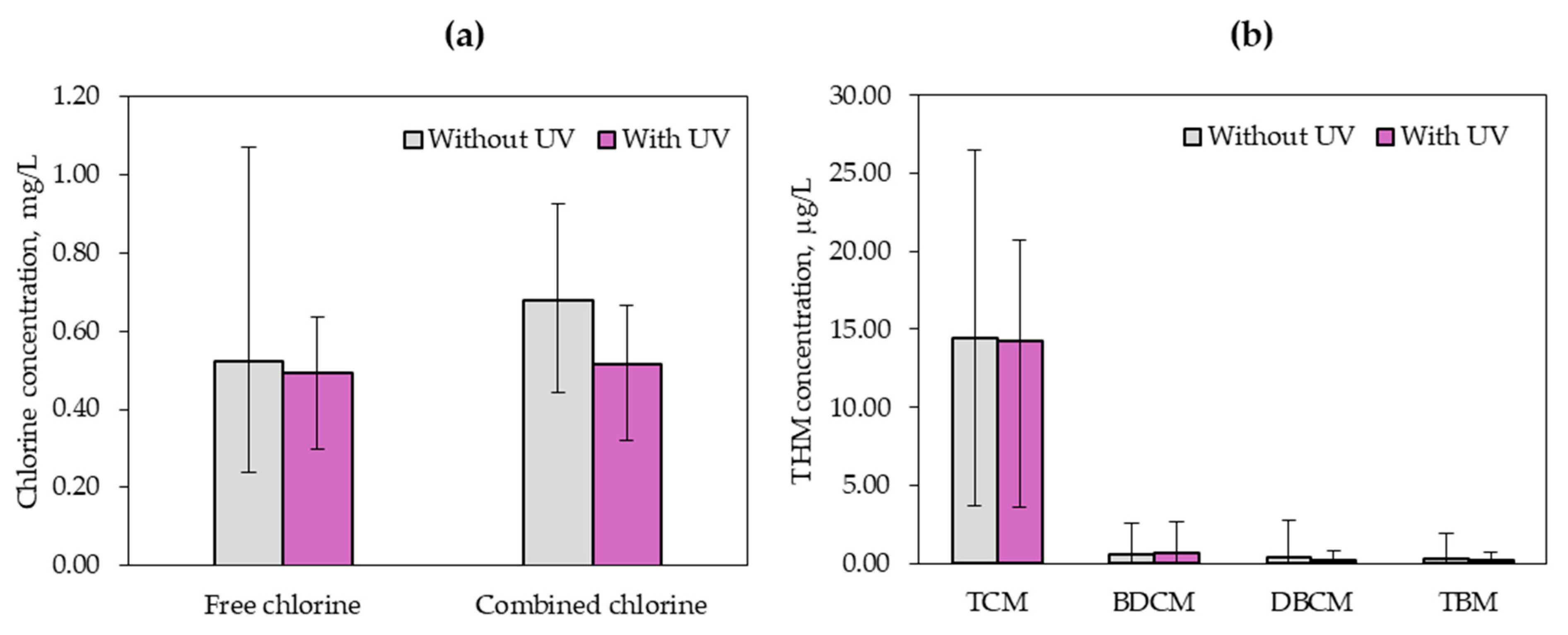
- Swimming pool water quality in terms of physicochemical parameters and microbiology in the case of both considered variants (with the UV lamp on and off) did not differ statistically.
- A statistically significant difference was noted for the concentration of combined chlorine. The application of the UV lamp decreased the share of combined chlorine; however, the obtained concentration did reach the permissible standards imposed by the Regulation of the Ministry of Health on the requirements for swimming pool water [23].
- The application of a low-pressure UV lamp did not statistically significantly affect the concentration of THM and individual compounds from this group. However, it was observed that UV radiation used to disinfect swimming pool water decreased the concentration of TCM, DBCM and TBM. In the case of BDCM, higher average concentrations were obtained in the disinfected swimming pool water for the variant with the UV lamp switched on.
- Adding UV radiation to the swimming pool water treatment system can increase the chlorine demand and consequently cause the greater consumption of the chemical chlorinating agent, which increases operating costs.
- The need to power the lamp with electricity and periodically replace filaments in UV lamps additionally increases the costs associated with the operation of the swimming pool water treatment system.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schwarzenbach, R.; Gschwend, P.; Imboden, D. Environmental Organic Chemistry, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Watts, M.; Linden, K. Chlorine photolysis and subsequent OH radical production during UV treatment of chlorinated water. Water Res. 2007, 41, 2871–2878. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; El-Din, M.; Bolton, J. Assessment of the UV/Chlorine process as an advanced oxidation process. Water Res. 2011, 45, 1890–1896. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, W.; Blatchley, E., III; Wang, X.; Ren, P. UV/chlorine process for ammonia removal and disinfection by-product reduction: Comparison with chlorination. Water Res. 2015, 68, 804–811. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Z.; Yang, X.; Xu, Y.; Liang, Y. Effects of UV irradiation and UV/chlorine co-exposure on natural organic matter in water. Sci. Total Environ. 2012, 414, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Lyon, B.; Dotson, A.; Linden, K.; Weinberg, H. The effect of inorganic precursors on disinfection byproduct formation during UV-chlorine/chloramine drinking water treatment. Water Res. 2012, 46, 4653–4664. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Wang, Y.; Yan, H.; Gao, B.; Ma, D.; Sun, S.; Ling, J.; Chu, Y. Photocatalysis of THM precursors in reclaimed water: The application of TiO2 in UV irradiation. Desalin. Water Treat. 2016, 57, 9136–9147. [Google Scholar] [CrossRef]
- WHO. Guidelines for Safe Recreational Water Environments. Vol. 2: Swimming Pools and Similar Environments; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Zwiener, C.; Richardson, S.; de Marini, D.; Grummt, T.; Glauner, T.; Frimmel, F. Drowning in disinfection byproducts? Assessing swimming pool water. Environ. Sci. Technol. 2007, 41, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Choi, Y.-J. The effects of UV disinfection on drinking water quality in distribution systems. Water Res. 2010, 44, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Murphy, H.; Payne, S.; Gagnon, G. Sequential UV- and chlorine-based disinfection to mitigate Escherichia coli in drinking water biofilms. Water Res. 2008, 42, 2083–2092. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.; Li, J.; Wood, K.; Kenttämaa, H.; Williams, P.; Amundson, L.; Blatchley, E., III. UV-induced effects on chlorination of creatinine. Water Res. 2013, 47, 4948–4956. [Google Scholar] [CrossRef] [PubMed]
- Cimetiere, N.; De Laat, J. Effects of UV-dechloramination of swimming pool water on the formation of disinfection by-products: A lab-scale study. Microchem. J. 2014, 112, 34–41. [Google Scholar] [CrossRef] [Green Version]
- Kristensen, G.H.; Klausen, M.M.; Andersen, H.R.; Erdinger, L.; Lauritsen, F.R.; Arvin, E.; Albrechtsen, H.-J. Full scale test of UV-based water treatment technologies at Gladsaxe Sportcentre—With and without advanced oxidation mechanisms. In Proceedings of the Third International Swimming Pool and Spa Conference, London, UK, 17–20 March 2009. [Google Scholar]
- Weng, S.C.; Li, J.; Blatchley, E.R., III. Effects of UV254 irradiation on residual chlorine and DBPs in chlorination of model organic-N precursors in swimming pools. Water Res. 2012, 46, 2674–2682. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.G.; Smith, D.W.; Bolton, J.R. Photolysis of aqueous free chlorine species (HOCl and OCl-) with 254 nm ultraviolet light. J. Environ. Eng. Sci. 2007, 6, 277–284. [Google Scholar] [CrossRef] [Green Version]
- Cassan, D.; Mercier, B.; Castex, F.; Rambaud, A. Effects of medium-pressure UV lamps radiation on water quality in a chlorinated indoor swimming pool. Chemosphere 2006, 62, 1507–1513. [Google Scholar] [CrossRef] [PubMed]
- Beyer, A.; Worner, H.; van Lierop, R. The Use of UV for Destruction of Combined Chlorine. Fact File of UV in Swimming Pools; USF Wallace & Tiernan: Pittsburgh, PA, USA, 2004. [Google Scholar]
- Afifi, M.; Blatchley, E., III. Effects of UV-based treatment on volatile disinfection byproducts in a chlorinated, indoor swimming pool. Water Res. 2016, 105, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Nitter, T.B.; Svendsen, K.H. UV treatment and air quality in a pool facility. Water Sci. Technol. 2019, 80, 499–506. [Google Scholar] [CrossRef] [PubMed]
- U.S. EPA. Method 551—Determination of Chlorination Disinfection Byproducts and Chlorinated Solvents in Drinking Water by Liquid-Liquid Extraction and Gas Chromatography with Electron-Capture Detection; Environmental Monitoring Systems Laboratory Office of Research and Development: Cincinnati, OH, USA, 1990.
- Pozos, N.; Scow, K.; Wuertz, S.; Darby, J. UV disinfection in a model distribution system: Biofilm growth and microbial community. Water Res. 2004, 38, 3083–3091. [Google Scholar] [CrossRef] [PubMed]
- Regulation of Polish Minister of Health of 9 November 2015 on the Requirements for Swimming Pool Water. Available online: http://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20150002016 (accessed on 15 August 2021).
- Zhao, Q.; Shang, C.; Zhang, X.; Ding, G.; Yang, X. Formation of halogenated organic byproducts during medium-pressure UV and chlorine coexposure of model compounds, NOM and bromide. Water Res. 2011, 45, 6545–6554. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.; Zortea, R.; Piketty, A.; Vaga, S.; Andersen, H. Photolytic removal of DBPs by medium pressure UV in swimming pool water. Sci. Total Environ. 2013, 443, 850–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peldszus, S.; Andrews, S.A.; Souza, R.; Smith, F.; Douglas, I.; Bolton, J.; Huck, P.M. Effect of medium-pressure UV irradiation on bromate concentrations in drinking water, a pilot-scale study. Water Res. 2004, 38, 211–217. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Concentration (Standard Deviation) | |
|---|---|---|
| Without UV Lamp | With UV Lamp | |
| Number of swimmers | 158 (69) | 155 (65) |
| Free chlorine, mg/L | 0.52 (0.22) | 0.49 (0.10) |
| Combined chlorine, mg/L | 0.68 (0.16) | 0.52 (0.10) |
| DOC, mg/L | 3.12 (1.26) | 3.02 (0.84) |
| SUVA, m−1·L/mg | 4.691 (0.958) | 1.292 (0.370) |
| Br−, mg/L | 0.43 (0.02) | 0.37 (0.11) |
| pH | 7.28 (0.10) | 7.28 (0.09) |
| Temperature, °C | 28 (1) | 28 (1) |
| Conductivity, mS/cm | 1.012 (0.226) | 0.966 (0.279) |
| Mesophilic bacteria, cfu 1/mL | 7 (12) | 6 (15) |
| Psychrophilic bacteria, cfu 1/mL | 6 (11) | 20 (62) |
| ∑THM, μg/L | 15.70 (7.00) | 15.26 (5.74) |
| TCM, μg/L | 14.40 (6.10) | 14.23 (5.17) |
| BDCM, μg/L | 0.58 (0.67) | 0.65 (0.64) |
| DBCM, μg/L | 0.42 (0.71) | 0.20 (0.23) |
| TBM, μg/L | 0.31 (0.45) | 0.18 (0.23) |
| Type of Cost | Operating Costs, PLN/Year | |
|---|---|---|
| Without UV Lamp | With UV Lamp | |
| Reagents | 35,853 | 38,455 |
| Electricity | 13,359 | 17,192 |
| Filaments of a UV lamp | - | 4031 |
| Total operating costs | 49,212 | 59,678 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Włodyka-Bergier, A.; Bergier, T. Impact of Low-Pressure UV Lamp on Swimming Pool Water Quality and Operating Costs. Energies 2021, 14, 5013. https://doi.org/10.3390/en14165013
Włodyka-Bergier A, Bergier T. Impact of Low-Pressure UV Lamp on Swimming Pool Water Quality and Operating Costs. Energies. 2021; 14(16):5013. https://doi.org/10.3390/en14165013
Chicago/Turabian StyleWłodyka-Bergier, Agnieszka, and Tomasz Bergier. 2021. "Impact of Low-Pressure UV Lamp on Swimming Pool Water Quality and Operating Costs" Energies 14, no. 16: 5013. https://doi.org/10.3390/en14165013






