Electrocatalytic Activity of Reduced Graphene Oxide Supported Cobalt Cinnamate for Oxygen Evolution Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagent
2.2. Preparation of Electrocatalyst
2.3. Electrochemical Characterization
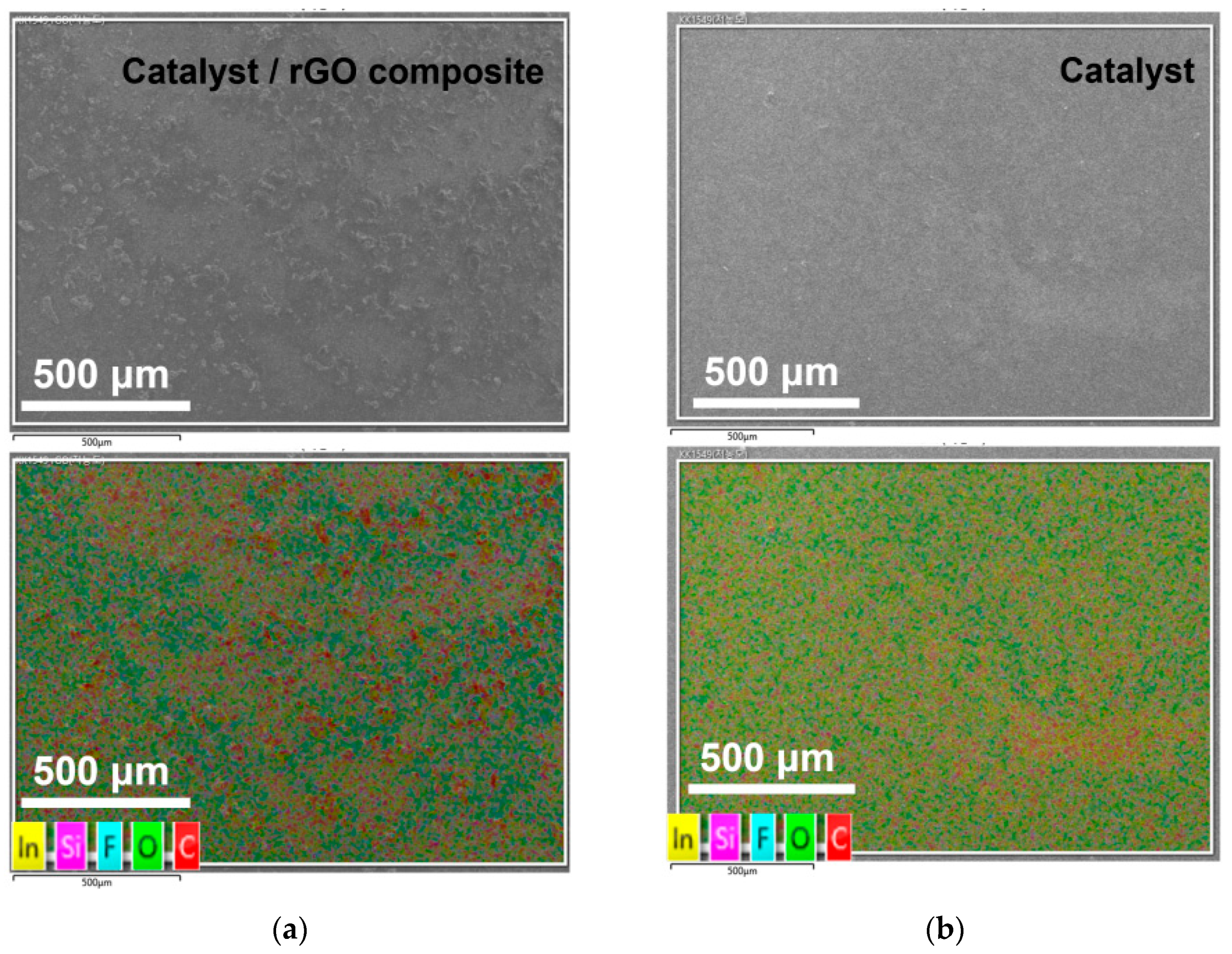
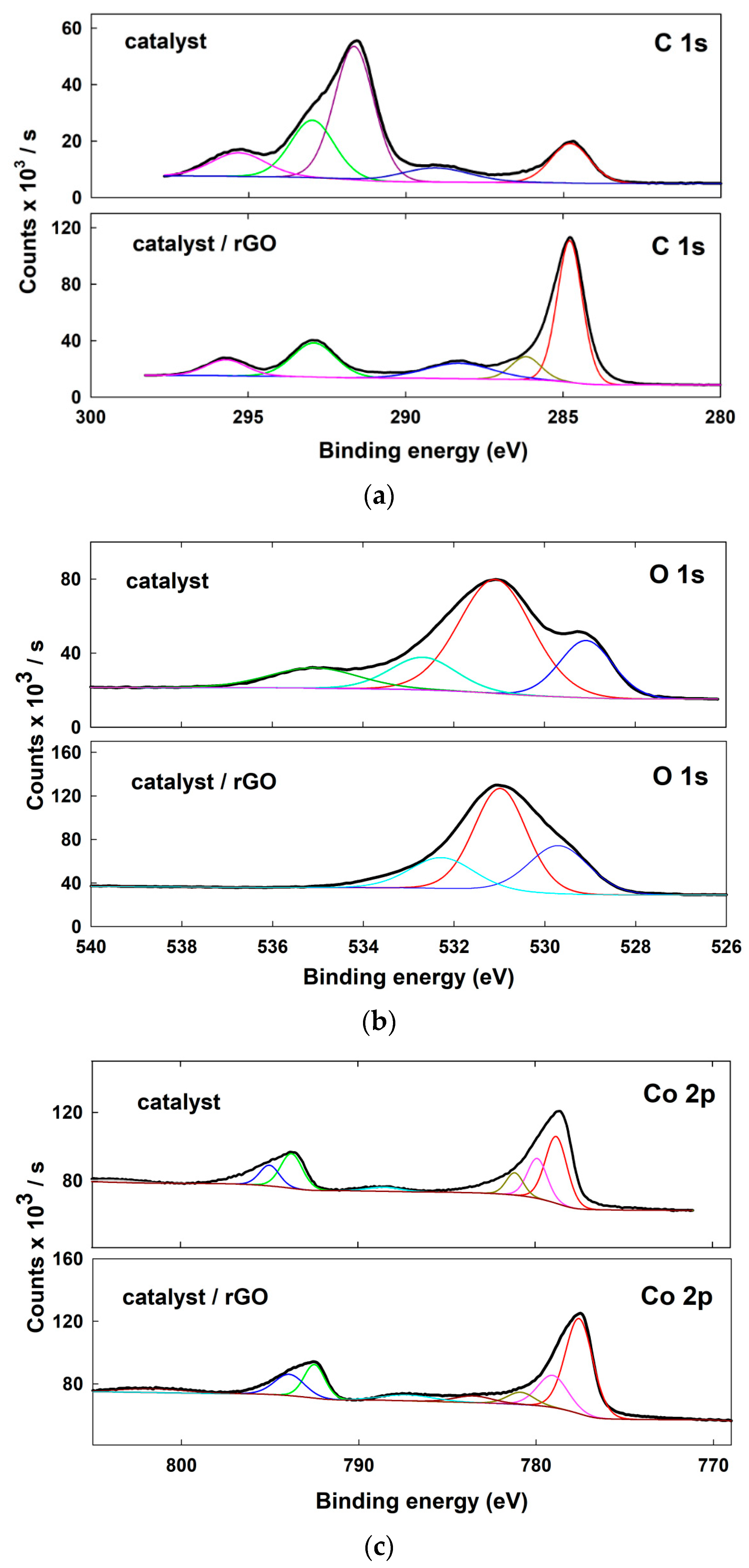
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chalgin, A.; Song, C.; Tao, P.; Shang, W.; Deng, T.; Wu, J. Effect of supporting materials on the electrocatalytic activity, stability and selectivity of noble metal-based catalysts for oxygen reduction and hydrogen evolution reactions. Prog. Nat. Sci. Mater. 2020, 30, 289–297. [Google Scholar] [CrossRef]
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, K.S.; Mishler, J.; Cho, S.C.; Adroher, X.C. A review of polymer electrolyte membrane fuel cells: Technology, applications, and needs on fundamental research. Appl. Energy 2011, 88, 981–1007. [Google Scholar] [CrossRef] [Green Version]
- Romeiro, F.C.; Rodrigues, M.A.; Silva, L.A.; Catto, A.C.; Silva, L.F.; Longo, E.; Nossol, E.; Lima, R.C. rGO-ZnO nanocomposites for high electrocatalytic effect on water oxidation obtained by microwave-hydrothermal method. Appl. Surf. Sci. 2017, 423, 743–751. [Google Scholar] [CrossRef] [Green Version]
- Reier, T.; Oezaslan, M.; Strasser, P. Electrocatalytic oxygen evolution reaction (OER) on Ru, Ir, and Pt catalysts: A comparative study of nanoparticles and bulk materials. ACS Catal. 2012, 2, 1765–1772. [Google Scholar] [CrossRef]
- Lee, Y.; Suntivich, J.; May, K.J.; Perry, E.E.; Shao-Horn, Y. Synthesis and activities of rutile IrO2 and RuO2 nanoparticles for oxygen evolution in acid and alkaline solutions. J. Phys. Chem. Lett. 2012, 3, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Campbell, M.G.; Dincâ, M. Electrically conductive porous metal-organic frameworks. Angew. Chem. Int. Ed. 2016, 55, 3566–3579. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.S.; Sun, L.; Wan, R.; Park, S.S.; DeGayner, J.A.; Hendon, C.H.; Dincâ, M. Tunable mixed-valence doping toward record electrical conductivity in a three-dimensional metal-organic framework. J. Am. Chem. Soc. 2018, 140, 7411–7414. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-L.; Xu, Q. Metal-organic frameworks as platforms for clean energy. Energy Environ. Sci. 2013, 6, 1656–1683. [Google Scholar] [CrossRef]
- Jiao, L.; Wang, Y.; Jiang, H.; Xu, Q. Metal-organic frameworks as platforms for catalytic applications. Adv. Mater. 2018, 30, 1703663–1703685. [Google Scholar] [CrossRef] [PubMed]
- Morozan, A.; Jaouen, F. Metal organic frameworks for electrochemical applications. Energy Environ. Sci. 2012, 5, 9269–9290. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, H.; Li, A.; Li, Z.; Liu, S.; Zhang, Q.; Gong, Y.; Zheng, L.; Zhu, Y.; Chen, C.; et al. Constructing NiCo/Fe3O4 heteroparticles within MOF-74 for efficient oxygen evolution reactions. J. Am. Chem. Soc. 2018, 140, 15336–15341. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Gong, Y.; Lin, J. Metal-organic framework-derived Co9S8-CoS-CoO-C nanoparticles as efficient electro and photo-catalysts for the oxygen evolution reaction. J. Mater. Chem. A 2017, 5, 10495–10509. [Google Scholar] [CrossRef]
- Cheetham, A.K.; Rao, C.N.R.; Feller, R.K. Structural diversity and chemical trends in hybrid inorganic-organic framework materials. Chem. Commun. 2006, 46, 4780–4795. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Lee, M.J.; Oh, N.G.; Shin, J.; Kwon, S.J.; Chun, H.; Kim, S.-J.; Yun, H.; Jo, H.; Ok, K.M.; et al. I3O0-type 3D framework of cobalt cinnamate and its efficient electrocatalytic activity toward oxygen evolution reaction. Chem. Mater. 2021, 33, 2804–2813. [Google Scholar] [CrossRef]
- Li, L.; Hu, L.; Li, J.; Wei, Z. Enhanced stability of Pt nanoparticle electrocatalysts for fuel cells. Nano Res. 2015, 8, 418–440. [Google Scholar] [CrossRef]
- Ma, W.; Wu, F.; Yu, P.; Mao, L. Carbon support tuned electrocatalytic activity of a single-site metal-organic framework toward the oxygen reduction reaction. Chem. Sci. 2021, 12, 7908–7917. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.S.; Choi, B.; Kim, Y.-B. Development of highly active bifunctional electrocatalyst using Co3O4 on carbon nanotubes for oxygen reduction and oxygen evolution. Sci. Rep. 2018, 8, 2543–2552. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Xiong, Z.; Yang, L.; Shi, H.; Fang, D.; Wang, M.; Shao, P.; Luo, X. Electrochemical approach toward reduced graphene oxide-based electrodes for environmental applications: A review. Sci. Total Environ. 2021, 778, 146301–146315. [Google Scholar] [CrossRef]
- Choi, S.; Kim, C.; Suh, J.M.; Jang, H.W. Reduced graphene oxide-based materials for electrochemical energy conversion reactions. Carbon Energy 2019, 1, 85–108. [Google Scholar] [CrossRef] [Green Version]
- Woldetinsay, M.; Soreta, T.R.; Maiyalagan, T.; Femi, O.E. Effect of support material on the electrocatalytic activity of palladium nanoparticle toward hydrogen evolution reaction. Mater. Res. Express 2021, 8, 025501–025508. [Google Scholar] [CrossRef]
- Ohn, S.; Kim, S.Y.; Mun, S.K.; Oh, J.; Sa, Y.J.; Park, S.; Joo, S.H.; Kwon, S.J.; Park, S. Molecularly dispersed nickel-containing species on the carbon nitride network as electrocatalysts for the oxygen evolution reaction. Carbon 2017, 124, 180–187. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2001. [Google Scholar]
- Alobaid, A.; Wang, C.; Adomaitis, R.A. Mechanism and kinetics of HER and OER on NiFe LDH films in an alkaline electrolyte. J. Electrochem. Soc. 2018, 165, J3395–J3404. [Google Scholar] [CrossRef]
- Jiao, L.; Zhou, Y.-X.; Jiang, H.-L. Metal-organic framework-based CoP/reduced graphene oxide: High-performance bifunctional electrocatalyst for overall water splitting. Chem. Sci. 2016, 7, 1690–1695. [Google Scholar] [CrossRef] [Green Version]
- Yaqoob, L.; Noor, T.; Iqbal, N.; Nasir, H.; Sohail, M.; Zaman, N.; Usman, M. Nanocomposites of cobalt benzene tricarboxylic acid MOF with rGO: An efficient and robust electrocatalyst for oxygen evolution reaction (OER). Renew. Energy. 2020, 156, 1040–1054. [Google Scholar] [CrossRef]
- Yun, S.; Lee, S.; Shin, C.; Park, S.; Kwon, S.J.; Park, H.S. One-pot self-assembled, reduced graphene oxide/palladium nanoparticle hybrid aerogels for electrocatalytic applications. Electrochim. Acta 2015, 180, 902–908. [Google Scholar] [CrossRef]
- Huang, X.; Qi, X.; Boey, F.; Zhang, H. Graphene-based composites. Chem. Soc. Rev. 2012, 41, 666–686. [Google Scholar] [CrossRef] [PubMed]
- Abidat, I.; Cazayus, E.; Loupias, L.; Morais, C.; Comminges, C.; Napporn, T.W.; Portehault, D.; Durupthy, O.; Mamede, A.-S.; Chanéac, C.; et al. Co3O4/rGO catalysts for oxygen electrocatalysis: On the role of the oxide/carbon interaction. J. Electrochem. Soc. 2019, 166, H94–H102. [Google Scholar] [CrossRef]
- Chlistunoff, J.; Sansiñena, J.-M. On the use of Nafion® in electrochemical studies of carbon supported oxygen reduction catalysts in aqueous media. J. Electroanal. Chem. 2016, 780, 134–146. [Google Scholar] [CrossRef] [Green Version]
- He, D.; Song, X.; Li, W.; Tang, C.; Liu, J.; Ke, Z.; Jiang, C.; Xiao, X. Active electron density modulation of Co3O4-based catalysts enhances their oxygen evolution performance. Angew. Chem. Int. Ed. 2020, 59, 6929–6935. [Google Scholar] [CrossRef]
- Weidler, N.; Paulus, S.; Schuch, J.; Klett, J.; Hoch, S.; Stenner, P.; Maljusch, A.; Brotz, J.; Wittich, C.; Kaiser, B.; et al. CoOx thin film deposited by CVD as efficient water oxidation catalyst: Change of oxidation state in XPS and its correlation to electrochemical activity. Phys. Chem. 2016, 18, 10708–10718. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lei, Y.-J.; Xin, Z.-J.; Xiang, R.-J.; Styring, S.; Thapper, A.; Wang, H.-Y. Ligand modification to stabilize the cobalt complexes for water oxidation. Int. J. Hydrogen Energy 2017, 42, 29716–29724. [Google Scholar] [CrossRef]
- Zheng, X.; Cao, Y.; Liu, D.; Cai, M.; Ding, J.; Liu, X.; Wang, J.; Hu, W.; Zhong, C. Bimetallic metal-organic-framework/reduced graphene oxide composites as bifunctional electrocatalysts for rechargeable Zn-air batteries. ACS Appl. Mater. Interfaces 2019, 11, 15662–15669. [Google Scholar] [CrossRef] [PubMed]
- Al-Gaashani, R.; Najjar, A.; Zakaria, Y.; Mansour, S.; Atieh, M.A. XPS and structural studies of high quality graphene oxide and reduced graphene oxide prepared by different chemical oxidation methods. Ceram. Int. 2019, 45, 14439–14448. [Google Scholar] [CrossRef]
- Meng, C.; Lin, M.; Sun, X.; Chen, X.D.; Chen, X.M.; Du, X.; Zhou, Y. Laser synthesis of oxygen vacancy-modified CoOOH for highly efficient oxygen evolution. Chem. Commun. 2019, 55, 2904–2907. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Lyu, F.; Bai, Y.; Gao, A.; Feng, J.; Cai, Z.; Yin, Y. Porous cobalt oxide nanoplates enriched with oxygen vacancies for oxygen evolution reaction. Nano Energy 2018, 43, 110–116. [Google Scholar] [CrossRef]
- Pan, S.; Mao, X.; Yu, J.; Hao, L.; Du, A.; Li, B. Remarkably improved oxygen evolution reaction activity of cobalt oxides by an Fe ion solution immersion process. Inorg. Chem. Front. 2020, 7, 3327–3339. [Google Scholar] [CrossRef]

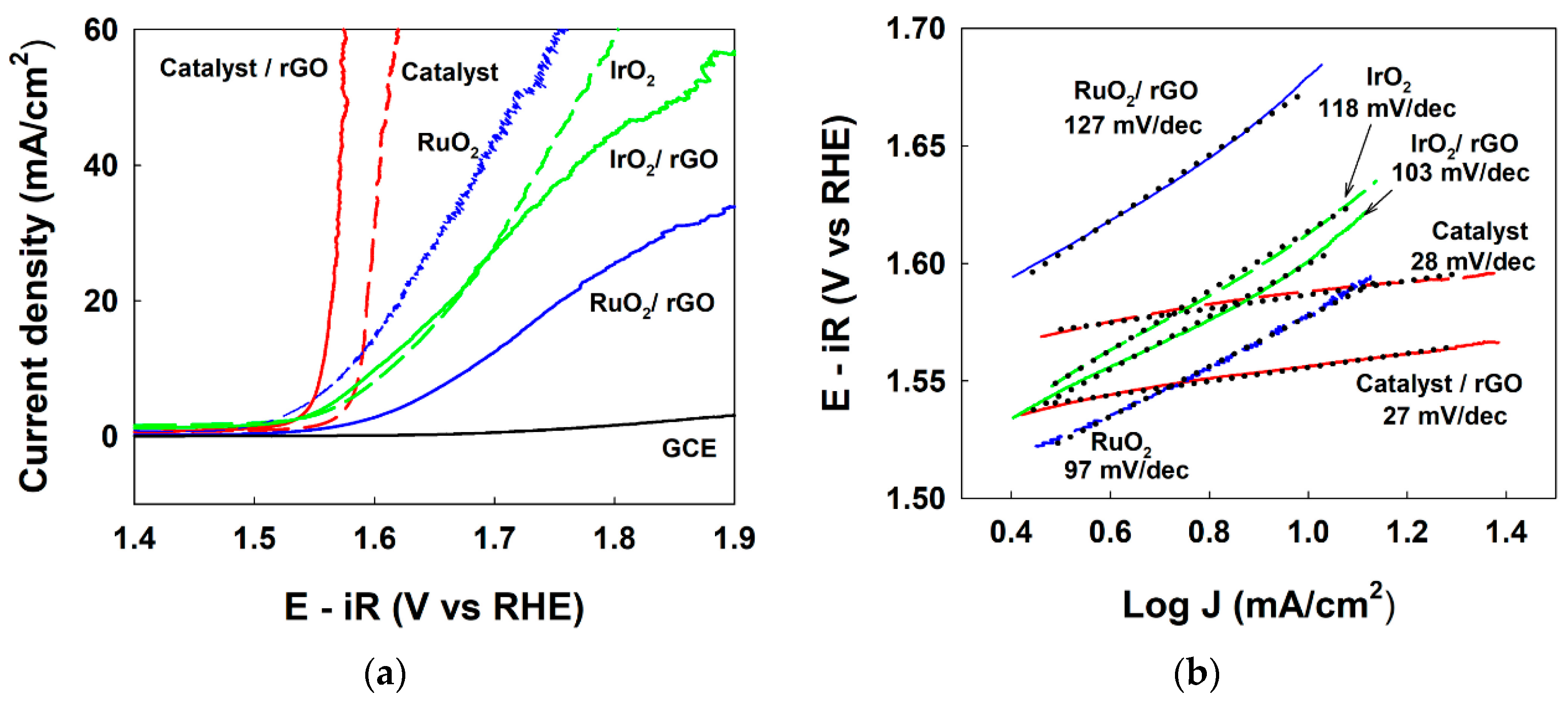

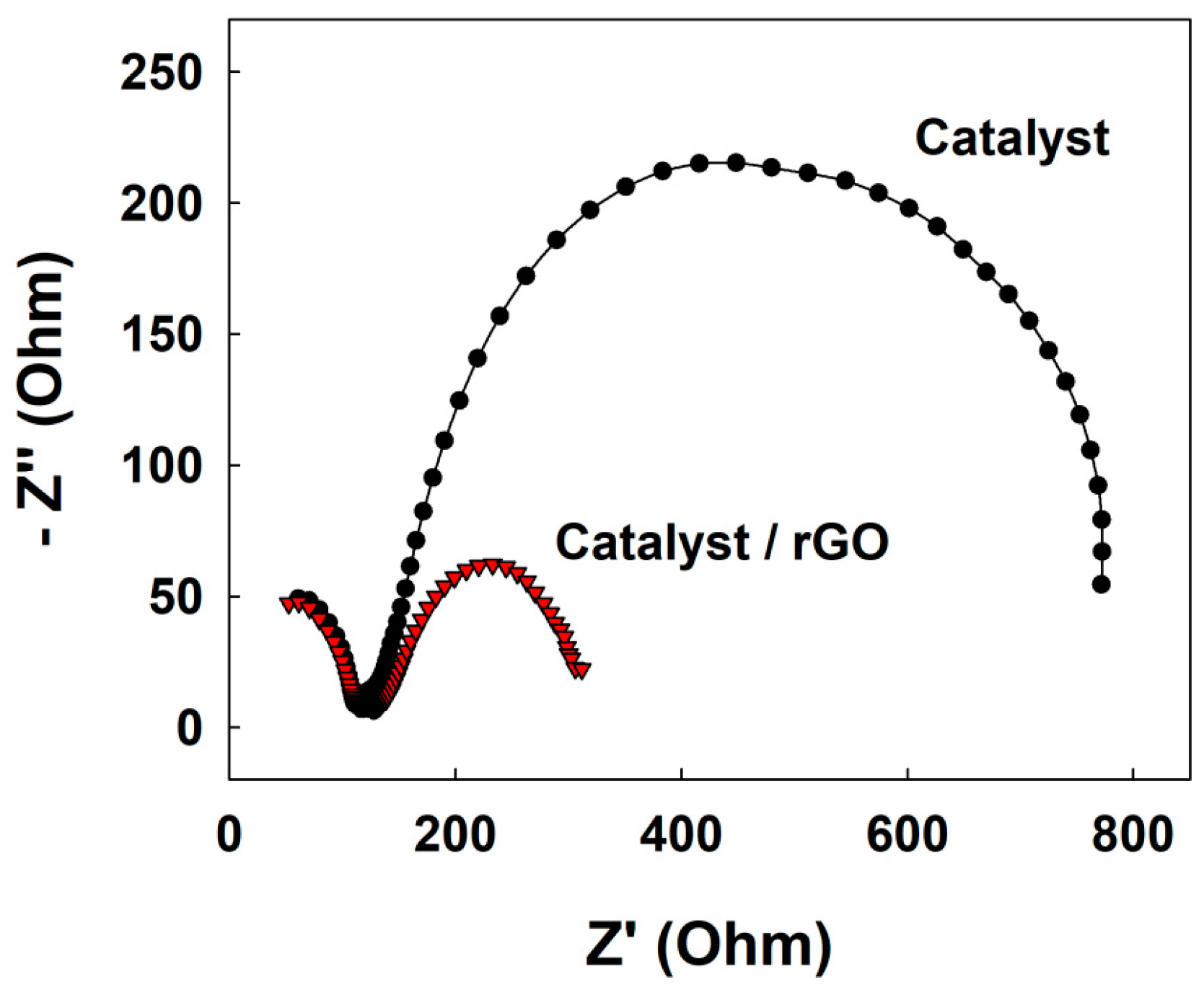
| Catalyst | VJ (V at J = 10 mA cmgeo−2) 1 | Tafel Slope (mV dec−1) | Rct (Ω) | CPE (mF/cm2) | Cdl (mF/cm2) 2 |
|---|---|---|---|---|---|
| catalyst/rGO | 327 mV | 27 | 220 | 30 | 5.9 |
| catalyst | 358 mV | 28 | 630 | 7 | 5.7 |
| RuO2/rGO | 560 mV | 127 | - | - | |
| RuO2 | 348 mV | 97 | - | - | |
| IrO2/rGO | 370 mV | 103 | - | - | |
| IrO2 | 385 mV | 118 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.J.; Kim, J.; Kang, J.; Shin, H.; Do, J.; Kwon, S.J. Electrocatalytic Activity of Reduced Graphene Oxide Supported Cobalt Cinnamate for Oxygen Evolution Reaction. Energies 2021, 14, 5020. https://doi.org/10.3390/en14165020
Lee MJ, Kim J, Kang J, Shin H, Do J, Kwon SJ. Electrocatalytic Activity of Reduced Graphene Oxide Supported Cobalt Cinnamate for Oxygen Evolution Reaction. Energies. 2021; 14(16):5020. https://doi.org/10.3390/en14165020
Chicago/Turabian StyleLee, Myung Jun, Junyeop Kim, Jaeun Kang, Hyewon Shin, Junghwan Do, and Seong Jung Kwon. 2021. "Electrocatalytic Activity of Reduced Graphene Oxide Supported Cobalt Cinnamate for Oxygen Evolution Reaction" Energies 14, no. 16: 5020. https://doi.org/10.3390/en14165020
APA StyleLee, M. J., Kim, J., Kang, J., Shin, H., Do, J., & Kwon, S. J. (2021). Electrocatalytic Activity of Reduced Graphene Oxide Supported Cobalt Cinnamate for Oxygen Evolution Reaction. Energies, 14(16), 5020. https://doi.org/10.3390/en14165020






