Recent Development of Two Alternative Gases to SF6 for High Voltage Electrical Power Applications †
Abstract
1. Introduction
2. Performance in Dielectric Applications
2.1. Properties of Pure Novec Insulating Gases
2.2. Properties of Gas Mixtures
3. Safety Considerations
4. Environmental Considerations
4.1. Global Warming Potentials
4.2. Greenhouse Gas Emissions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hyrenbach, M.; Paul, T.; Owens, J. Environmental and safety aspects of AirPlus insulated GIS. CIRED Open Access Proc. J. 2017, 1, 132–135. [Google Scholar] [CrossRef][Green Version]
- Kieffel, Y.; Biquez, F.; Vigouroux, D.; Ponchon, P.; Schlernitzauer, A.; Magous, R.; Cros, G.; Owens, J. Characteristics of g3—An alternative to SF6. CIRED Open Access Proc. J. 2017, 1, 54–57. [Google Scholar] [CrossRef]
- Kieffel, Y.; Irwin, T.; Ponchon, P.; Owens, J. Green Gas to Replace SF6 in Electrical Grids. IEEE Power Energy Mag. 2016, 14, 32–39. [Google Scholar] [CrossRef]
- Cigré. Technical Brochure 802—Application of Non-SF6 Gases or Gas-Mixtures in Medium and High Voltage Gas-Insulated Switchgear; Cigré: Paris, France, 2020. [Google Scholar]
- Xiao, A.; Owens, J.; Bonk, J.; Zhang, A.; Wang, C.; Tu, Y. Environmentally Friendly Insulating Gases as SF6 Alternatives for Power Utilities. In Proceedings of the ICEMPE 2019—2nd International Conference on Electrical Materials and Power Equipment, Guangzhou, China, 7–10 April 2019; pp. 42–48. [Google Scholar]
- Wooton, R.E.; Kegelman, M.R. Gases Superior to SF6 for Insulation and Interruption; Report EL-2620; Electric Power Research Institute (EPRI): Washington, DC, USA, 1982. [Google Scholar]
- Pohlink, K.; Meyer, F.; Kieffel, Y.; Biquez, F.; Ponchon, P.; Owens, J.; Van San, R. Characteristics of Fluoronitrile/CO2 Mixture—An Alternative to SF6; Cigré: Paris, France, 2016; Paper D1-204. [Google Scholar]
- Stoller, P.; Hengstler, J.; Doiron, C.; Scheel, S.; Simka, P.; Müller, P. Environmental Aspects of High Voltage Gas Insulated Switchgear That Uses Alternatives to SFf6 and Monitoring and Long-Term Performance of a Pilot Installation; Cigré: Paris, France, 2018; Paper D1-202. [Google Scholar]
- Laruelle, E.; Maksoud, L.; Kieffel, Y.; Lüscher, R.; Ficheux, A. SF6 Alternative—What to Learn from the High Voltage Experience; Cigré: Madrid, Spain, 2019; Paper 0028. [Google Scholar]
- Material Toxicity Summary Sheet, 3M™ Novec™ 4710 Insulating Gas; 3M Company: St. Paul, MN, USA, 2019.
- Material Toxicity Summary Sheet, 3M™ Novec™ 5110 Insulating Gas; 3M Company: St. Paul, MN, USA, 2019.
- Ramboll US Consulting, Inc. Global Product Safety and Stewardship Practice, “CMR Self Classification”. Available online: https://bit.ly/2LQHkEO (accessed on 14 August 2021).
- Castonguay, J. In-situ measurements of SF6 leak rates in indoor gas-insulated switchgears (GIS). In Gaseous Dielectrics IX; Christophorou, L., Olthoff, J., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2001; pp. 549–554. [Google Scholar]
- Li, Y.; Zhang, X.; Zhang, J.; Xiao, S.; Xie, B.; Chen, D.; Gao, Y.; Tang, J. Assessment on the toxicity and application risk of C4F7N: A new SF6 alternative gas. J. Hazard. Mater. 2019, 368, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Preve, C.; Maladen, R.; Piccoz, D. Innovative SF6 Free Load Break Switch with Shunt Vacuum Interruption (SVI) Technology; Cigré: Paris, France, 2020; Paper A3-116. [Google Scholar]
- Zhang, X.; Fanchao, Y.; Li, Y.; Tian, S.; Xie, B.; Gao, Y.; Xiao, S. Acute toxicity and health effect of perfluoroisobutyronitrile on mice: A promising substitute gas-insulating medium to SF6. J. Environ. Sci. Health Part A 2020, 14, 1646–1658. [Google Scholar] [CrossRef]
- Ten Berge, W.F.; Zwart, A.; Appelman, L.M. Concentration-time mortality response relationship of irritant and systemically acting vapors and gases. J. Hazard. Mater. 1986, 13, 301–309. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Ren, Y.; Bernard, F.; Daële, V.; Mellouki, A. Atmospheric fate and impact of perfluorinated butanone and pentanone. Environ. Sci. Technol. 2019, 53, 8862–8871. [Google Scholar] [CrossRef]
- 3M Company. Internal Report No. E11-0512; 3M Environmental Laboratory: St. Paul, MN, USA, 2015. [Google Scholar]
- Pinnock, S.; Hurley, M.D.; Shine, K.P.; Wallington, T.J.; Smyth, T.J. Radiative forcing of climate by hydrochlorofluorocarbons and hydrofluorocarbons. J. Geophys. Res. 1995, 100, 23227–23238. [Google Scholar] [CrossRef]
- Sulbaek Andsersen, M.; Kyte, M.; Andersen, S.T.; Neilsen, C.J.; Nielsen, O.J. Atmospheric chemistry of (CF3)2CFCN: A replacement compound for the most potent industrial greenhouse gas, SF6. Environ. Sci. Technol. 2017, 51, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Blázquez, S.; Antiñolo, M.; Nielsen, O.J.; Albaladejo, J.; Jiménez, E. Reaction kinetics of (CF3)2CFCN with OH radicals as a function of temperature (278–358 K): A good replacement for greenhouse SF6. Chem. Phys. Lett. 2017, 687, 297–302. [Google Scholar] [CrossRef]
- Billen, P.; Maes, B.; Larraín, M.; Braet, J. Replacing SF6 in Electrical Gas-Insulated Switchgear: Technological Alternatives and Potential Life Cycle Greenhouse Gas Savings in an EU-28 perspective. Energies 2020, 13, 1807. [Google Scholar] [CrossRef]
- European Commission Report C(2020) 6635. REPORT FROM THE COMMISSION: Assessing the Availability of Alternatives to Fluorinated Greenhouse Gases in Switchgear and Related Equipment, Including Medium-Voltage Secondary Switchgear. Available online: https://ec.europa.eu/clima/sites/clima/files/news/docs/c_2020_6635_en.pdf (accessed on 14 August 2021).





| Property at 1 Bar, 25 °C | Sulfur Hexafluoride | Novec 4710 | Novec 5110 |
|---|---|---|---|
| Chemical Formula | SF6 | (CF3)2CFCN | (CF3)2CFC(O)CF3 |
| Molecular Weight | 146 | 195 | 266 |
| Boiling Point (°C) | −63.9 a | −5 | 27 |
| Vapor Pressure (kPa) | 2372 | 297 | 94 |
| Freezing Point (°C) | −50.8 | −118 | −110 |
| Flash Point (°C) | none | none | none |
| Gas Density (kg/m3) | 5.9 | 7.9 | 10.7 |
| Thermal Conductivity (W/m∙K) | 0.013 | 0.025 | 0.004 |
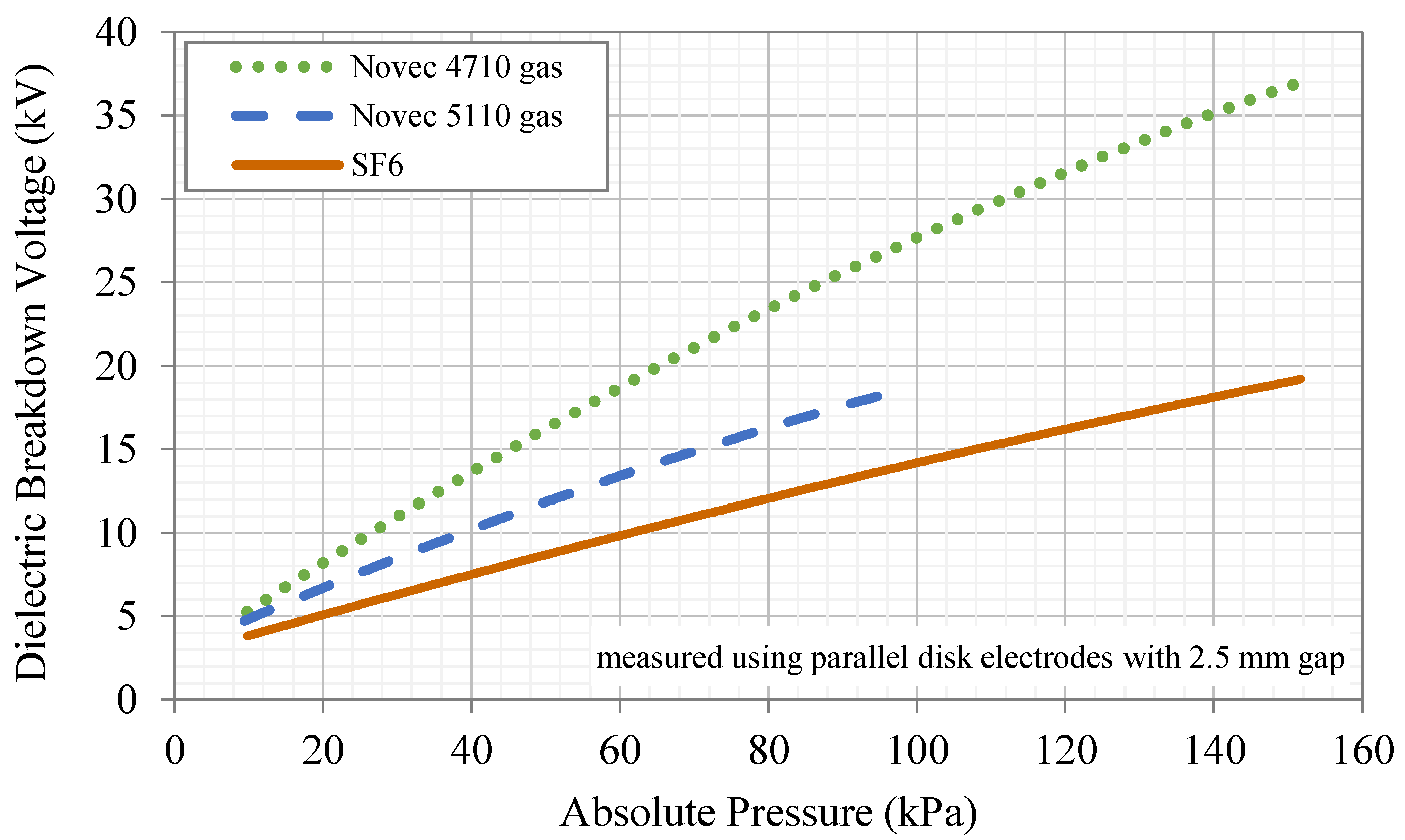
| Breakdown Voltage (kV) 2.5 mm gap with parallel electrodes | 14.0 | 27.5 | 18.4 b |
| Atmospheric Lifetime (year) | 3200 | 30 | 0.04 (15 days) |
| Ozone Depletion Potential | zero | zero | zero |
| GWP (100-year ITH) | 23,500 | 2100 | <1 |
| Gas Formulation (mole%) | 100% SF6 | 5% Novec 4710/95% CO2 | 5% Novec 5110/95% Air |
|---|---|---|---|
| Typical GIS Pressure (bar) | 4 | 6 | 6.5 |
| Gas Density @ 25 °C (kg/m3) | 24.75 | 12.48 | 10.67 |
| Condensation temperature (°C) | −38 | −27 | 0 |
| Dielectric breakdown voltage relative to SF6 | ― | ~1 | ~1 |
| Novec 4710 Gas | Novec 5110 Gas |
|---|---|
| Low acute inhalation toxicity (4-h LC50 > 10,000, <15,000 ppmv) | Low acute inhalation toxicity (4-h LC50 > 148, <213 mg/L) 1 |
| Low repeated-dose inhalation toxicity (based upon 28-day study) | Low repeated-dose inhalation toxicity (based upon 28-day study) |
| Negative for in vivo genotoxicity using both micronucleus and Comet assays | Not mutagenic in bacterial reverse mutation assays |
| Negative for reproductive and developmental toxicity | Expected to be negative for reproductive and developmental toxicity based upon read across from next nearest homologue |
| Gas Formulation (mole%) | 100% SF6 | 5% Novec 4710/95% CO2 | 5% Novec 5110/95% Air |
|---|---|---|---|
| Pressure (bar) | 4 | 6 | 6.5 |
| GWP of gas mixture | 23,500 | 398 | <1 |
| GWP reduction vs SF6 | ― | 98.3% | >99.9% |
| GHG content (kg CO2e/m3) | 553,929 | 4969 | 3.5 |
| GHG emission reduction from discrete emission relative to SF6 | ― | 99.1% | >99.9% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Owens, J.; Xiao, A.; Bonk, J.; DeLorme, M.; Zhang, A. Recent Development of Two Alternative Gases to SF6 for High Voltage Electrical Power Applications. Energies 2021, 14, 5051. https://doi.org/10.3390/en14165051
Owens J, Xiao A, Bonk J, DeLorme M, Zhang A. Recent Development of Two Alternative Gases to SF6 for High Voltage Electrical Power Applications. Energies. 2021; 14(16):5051. https://doi.org/10.3390/en14165051
Chicago/Turabian StyleOwens, John, Ang Xiao, Jason Bonk, Michael DeLorme, and Agnes Zhang. 2021. "Recent Development of Two Alternative Gases to SF6 for High Voltage Electrical Power Applications" Energies 14, no. 16: 5051. https://doi.org/10.3390/en14165051
APA StyleOwens, J., Xiao, A., Bonk, J., DeLorme, M., & Zhang, A. (2021). Recent Development of Two Alternative Gases to SF6 for High Voltage Electrical Power Applications. Energies, 14(16), 5051. https://doi.org/10.3390/en14165051





