Thermal Switch Based on an Adsorption Material in a Heat Pipe
Abstract
:1. Introduction
2. Experimental Setup
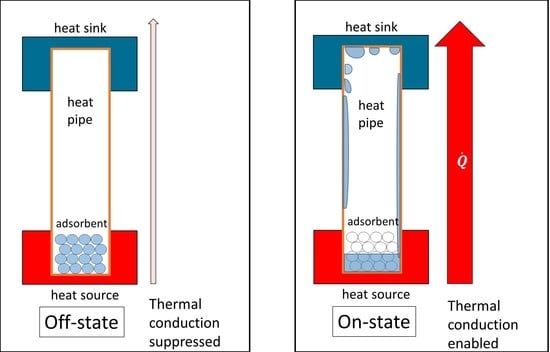
2.1. Basic Concept
2.2. Preparation and Characterization of Adsorbent
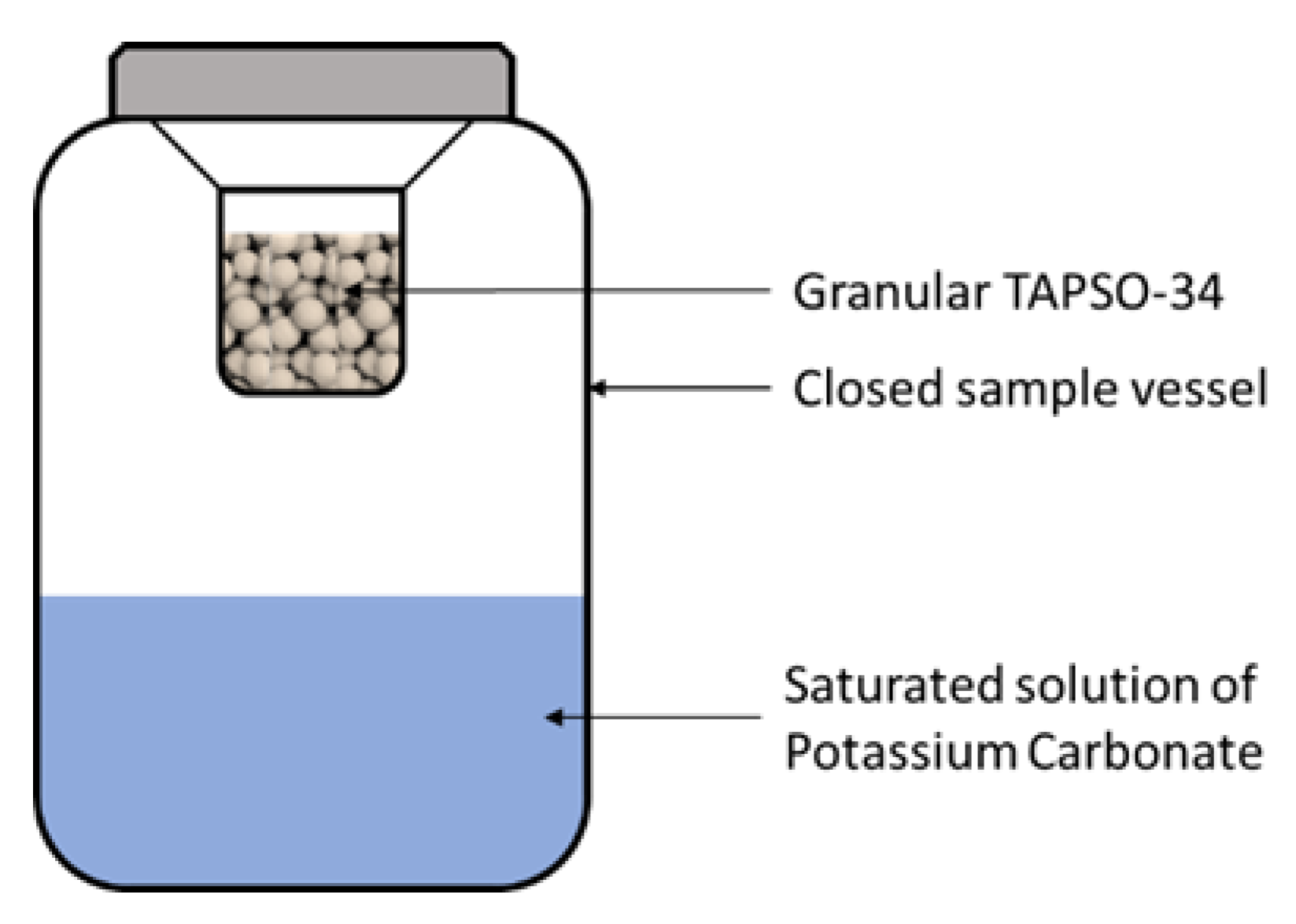
2.2.1. Pre-Treatment of the Adsorbent
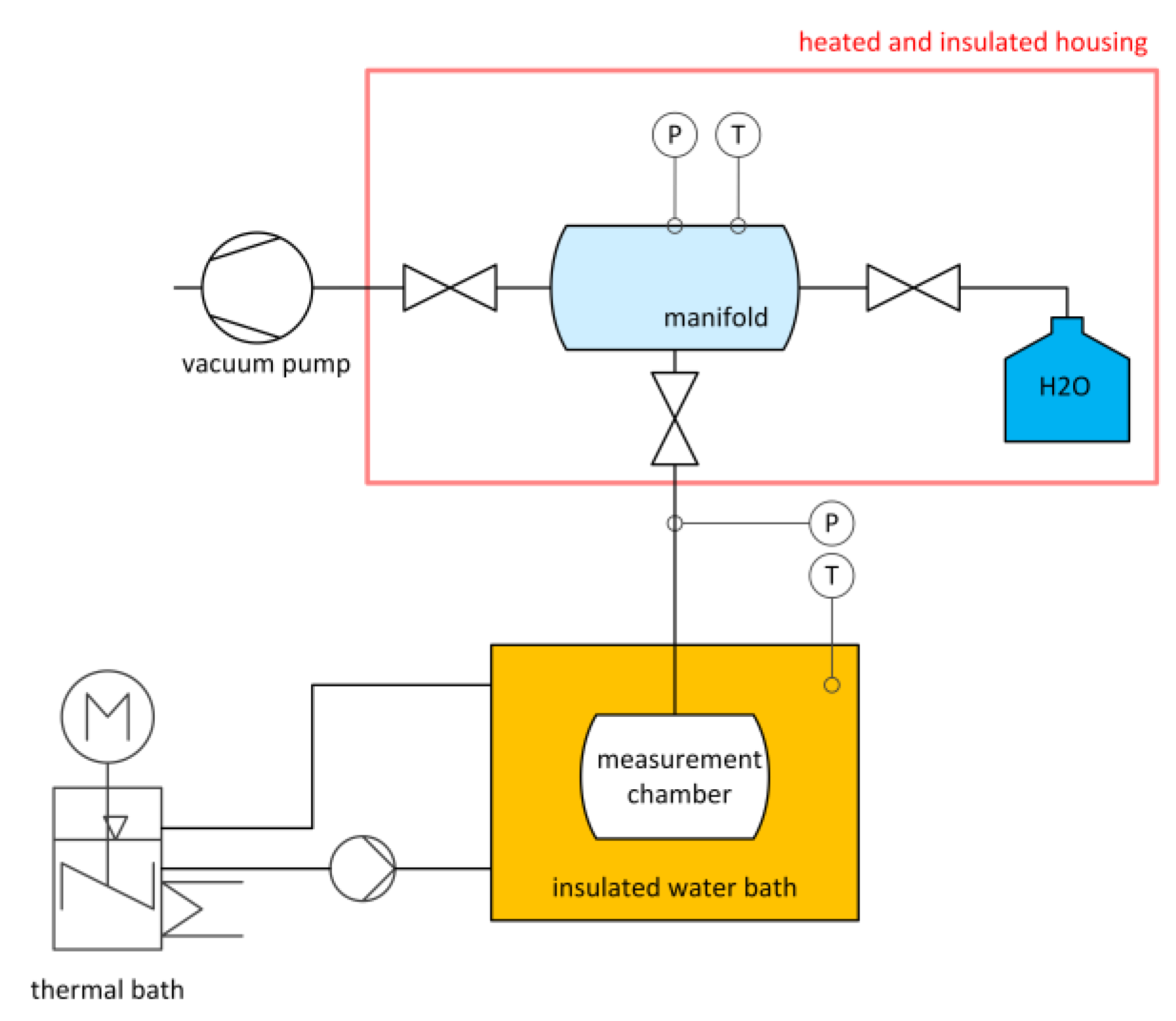
2.2.2. Measurement of Adsorption Isotherms
2.2.3. Measurement of Initial Water Loading and Adsorption Isobars
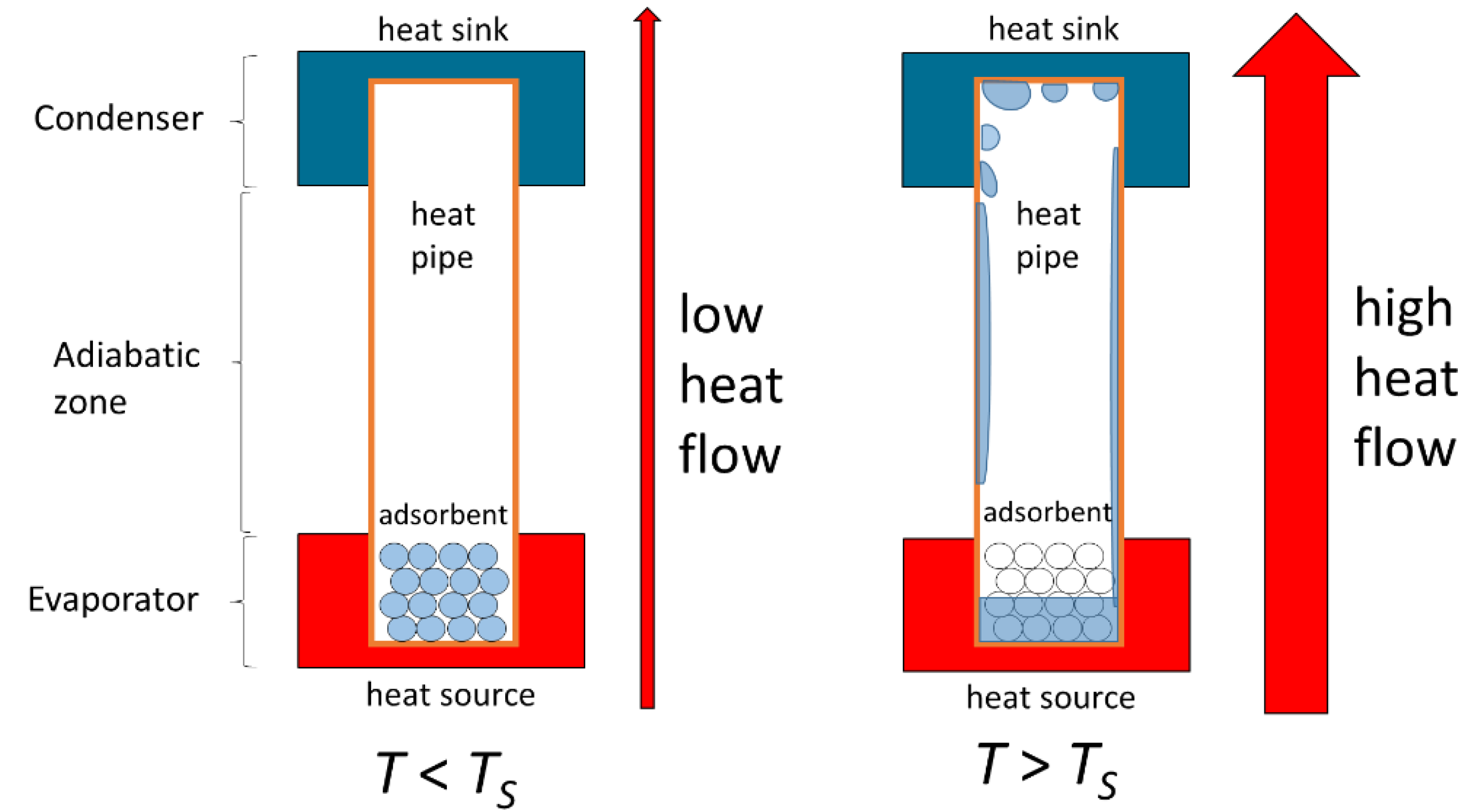
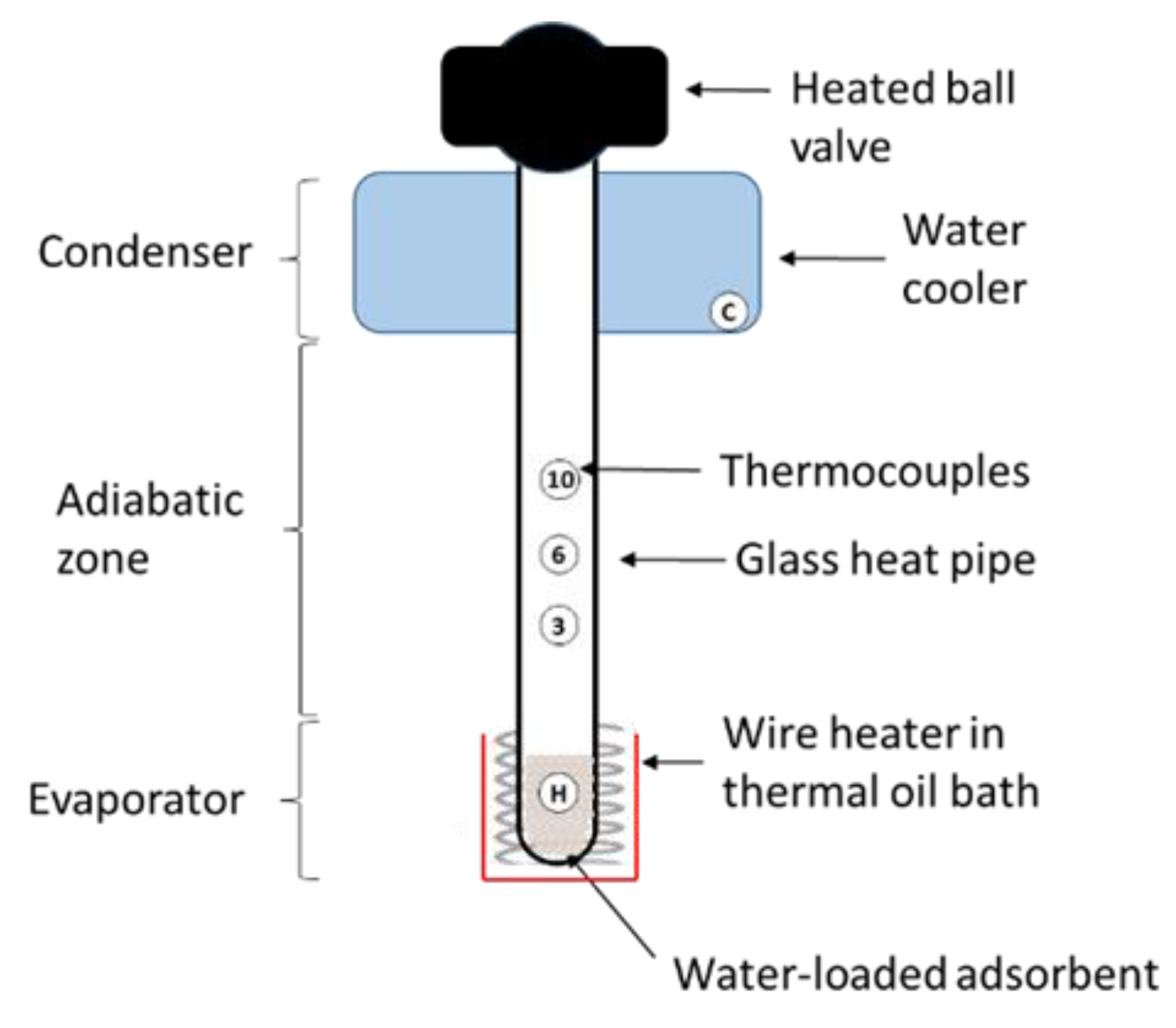
2.3. Setup for Heat Pipe Demonstrator
2.3.1. Setup
2.3.2. Experimental Procedure
3. Results and Discussion
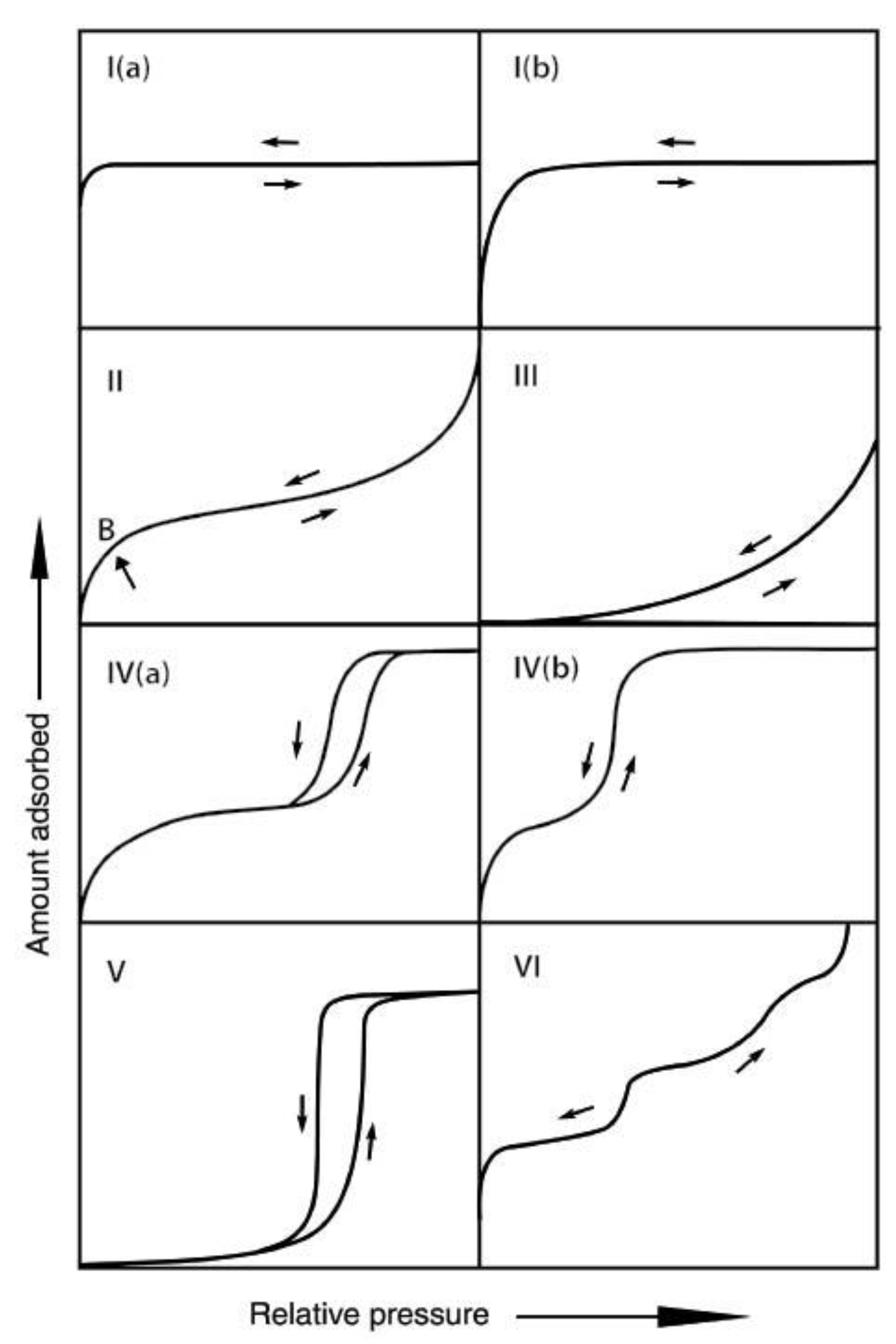
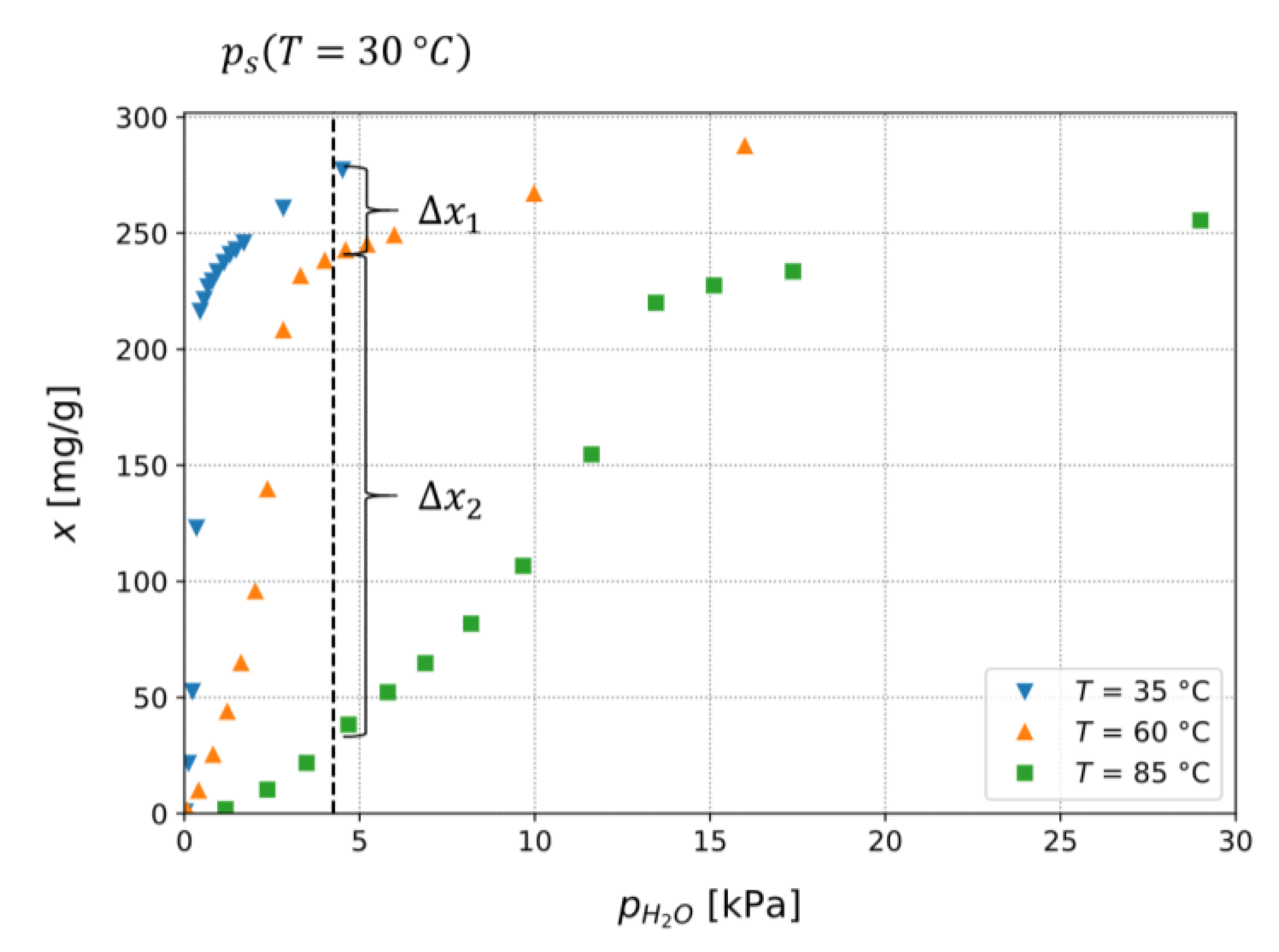
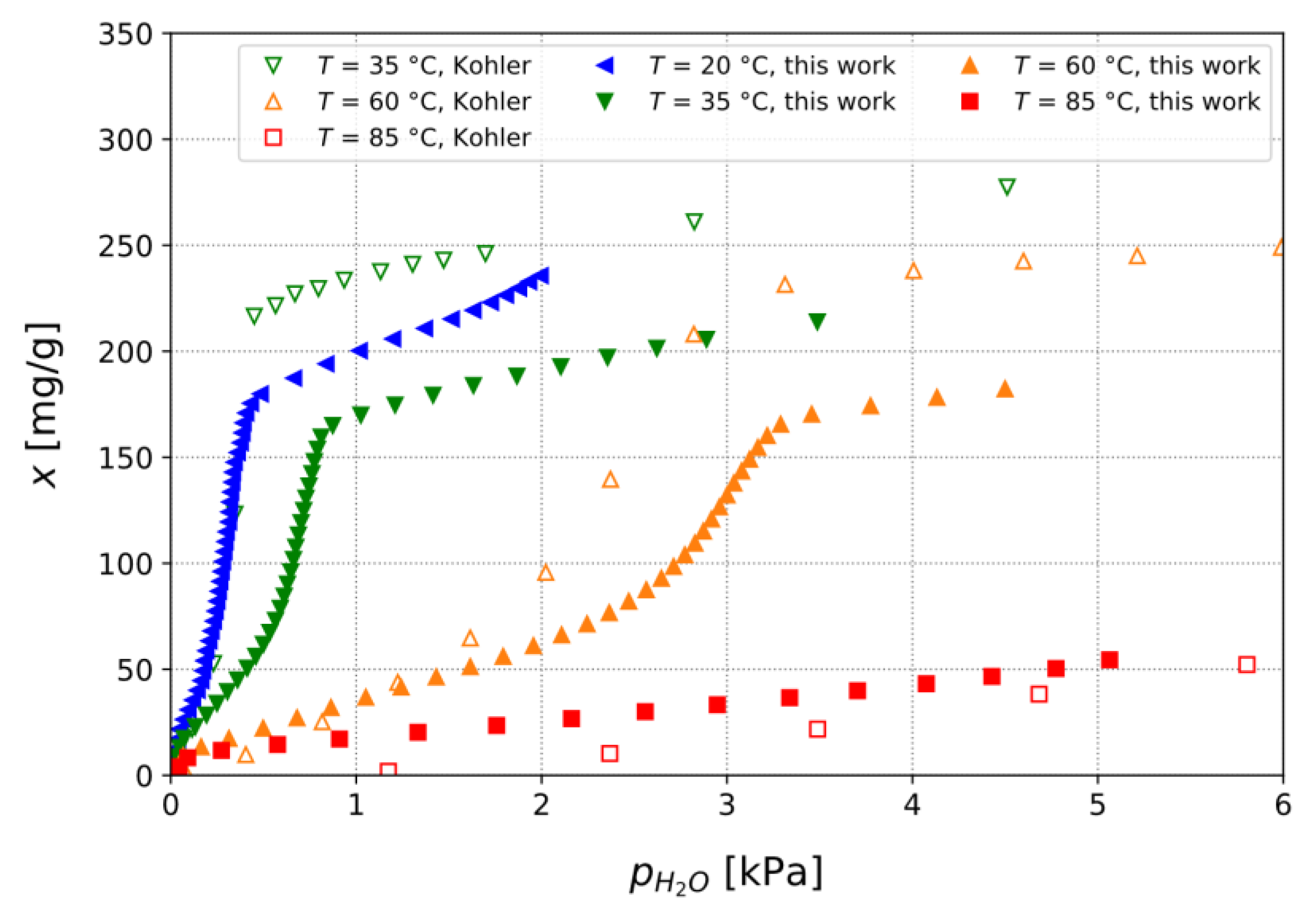
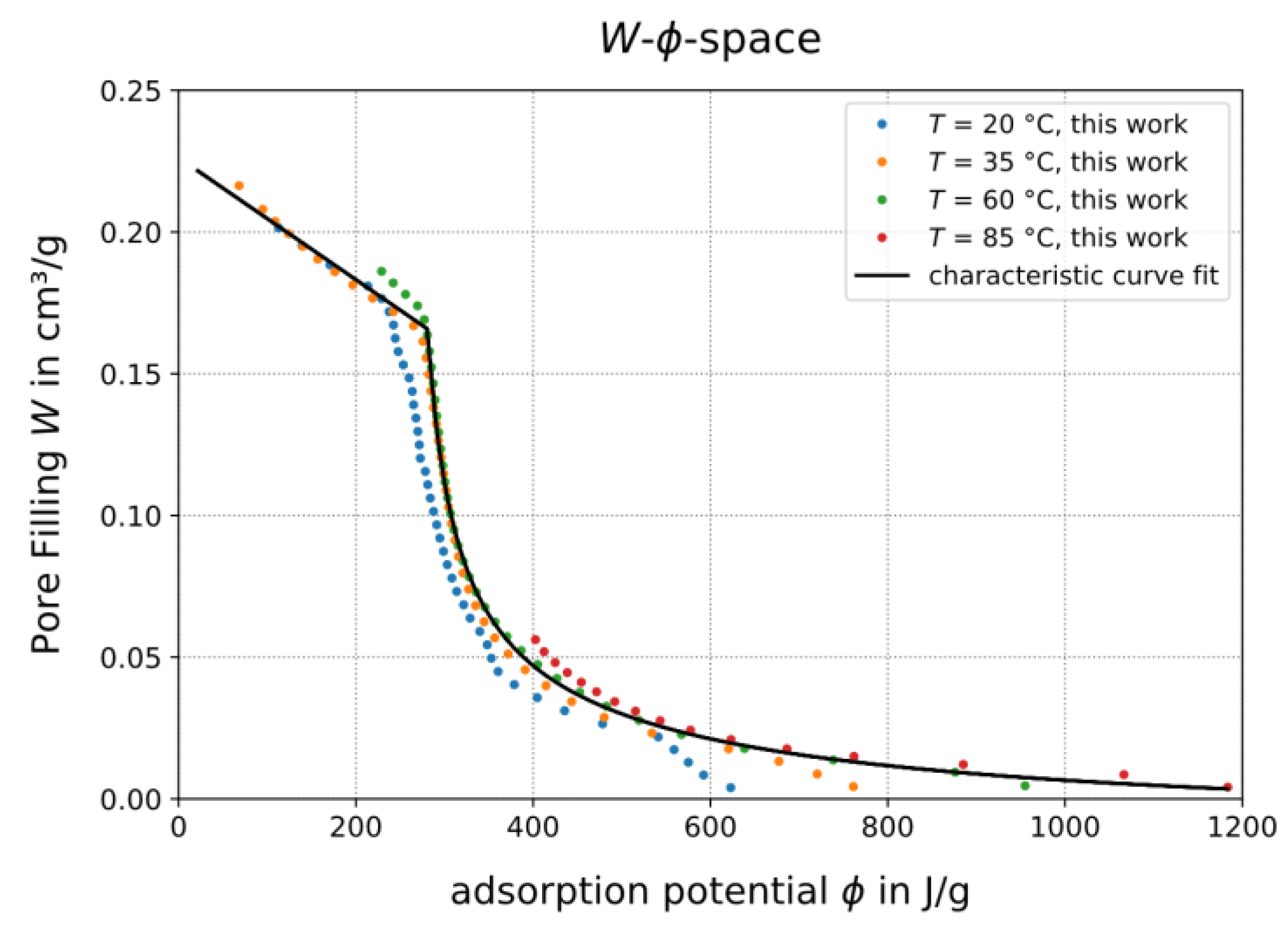
3.1. Adsorption Isotherms of TAPSO-34 and Estimation of Switching Temperature TS
3.1.1. Adsorption Isotherms and Their Role in a Heat Pipe-Based Thermal Switch
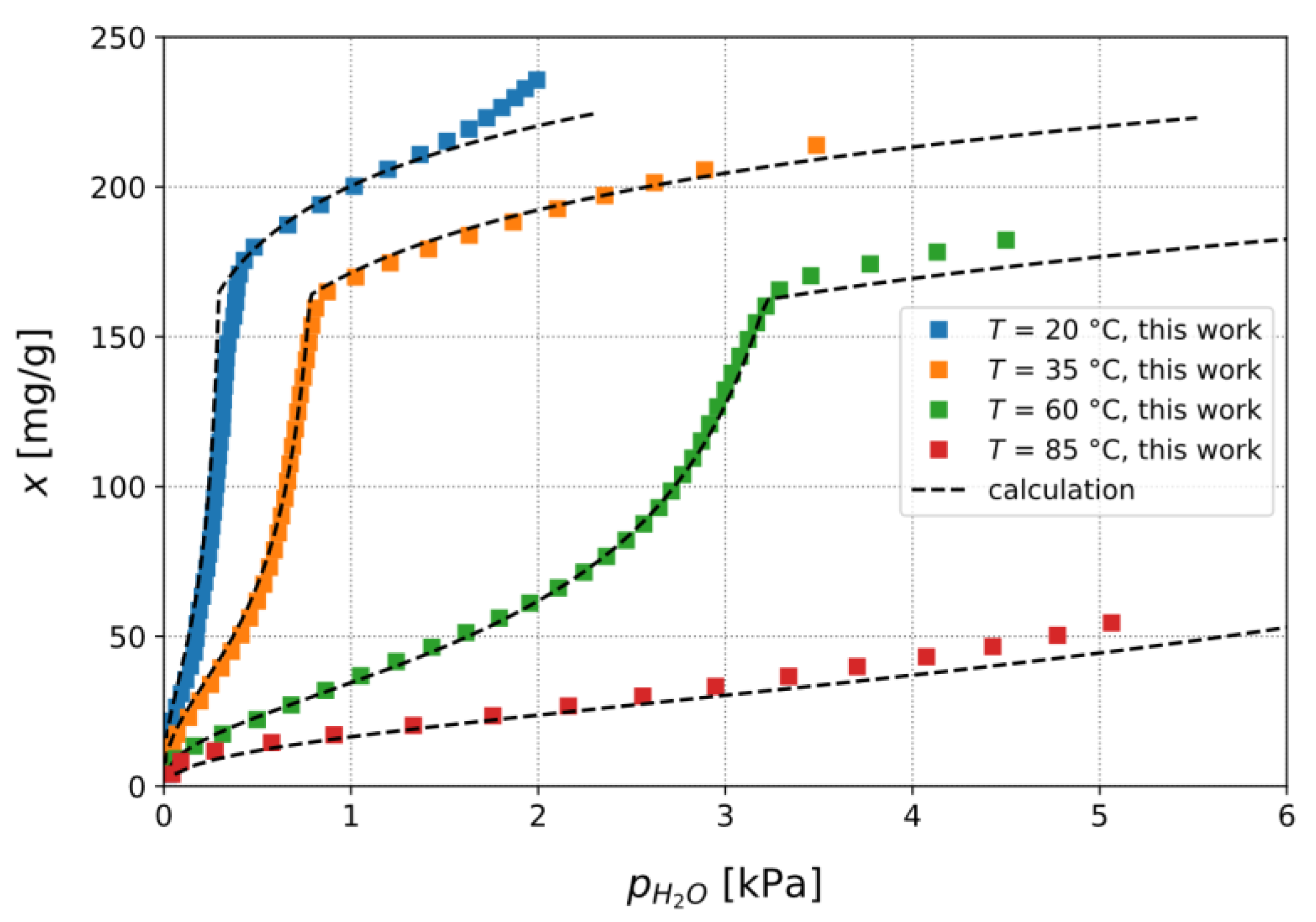
3.1.2. Calculation of Isobars from Measured Isotherms
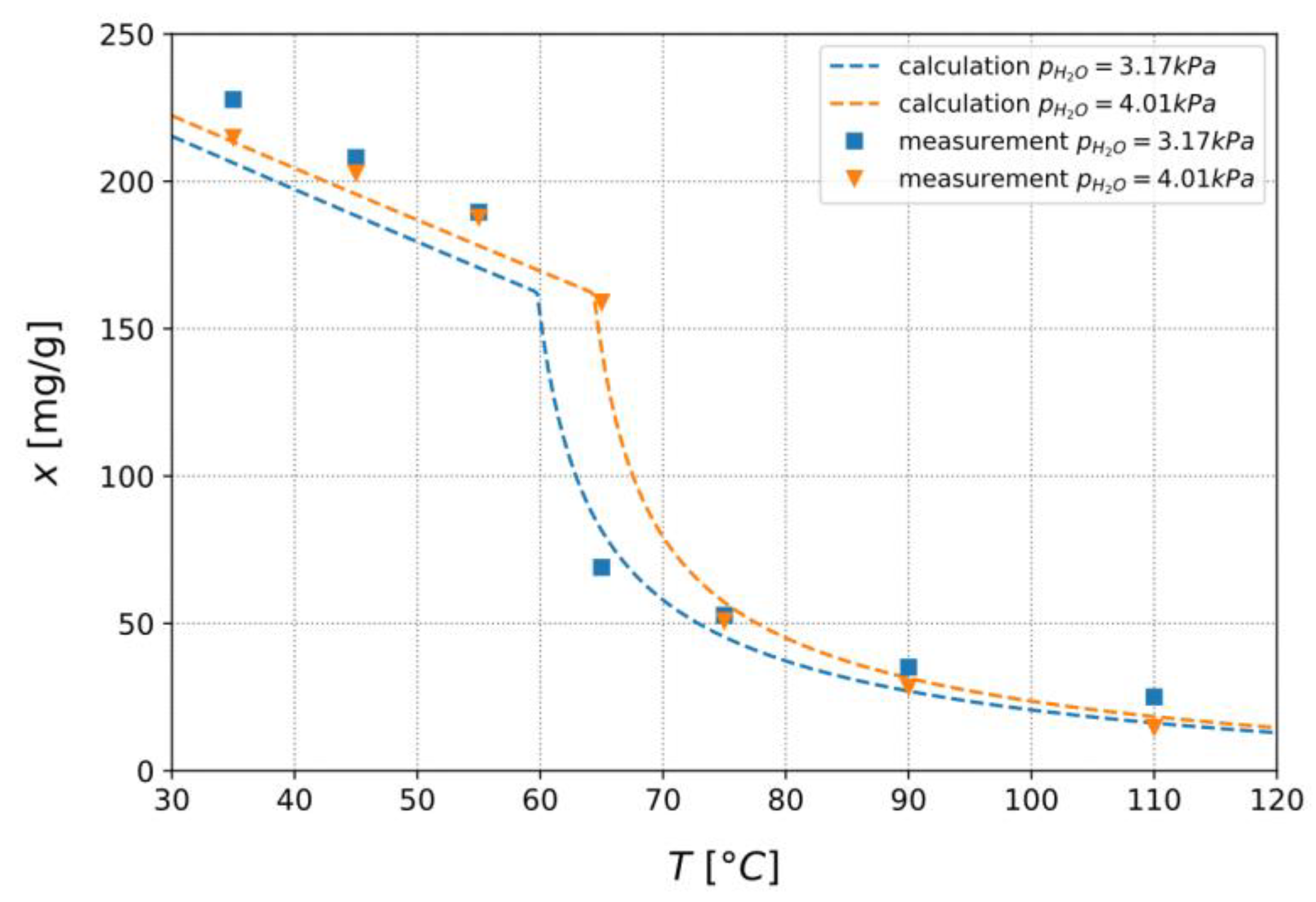
3.1.3. Confirmation of Loading Behavior
3.2. Experimental Confirmation of Heat Switching Using Adsorbent TAPSO-34 in a Heat Pipe
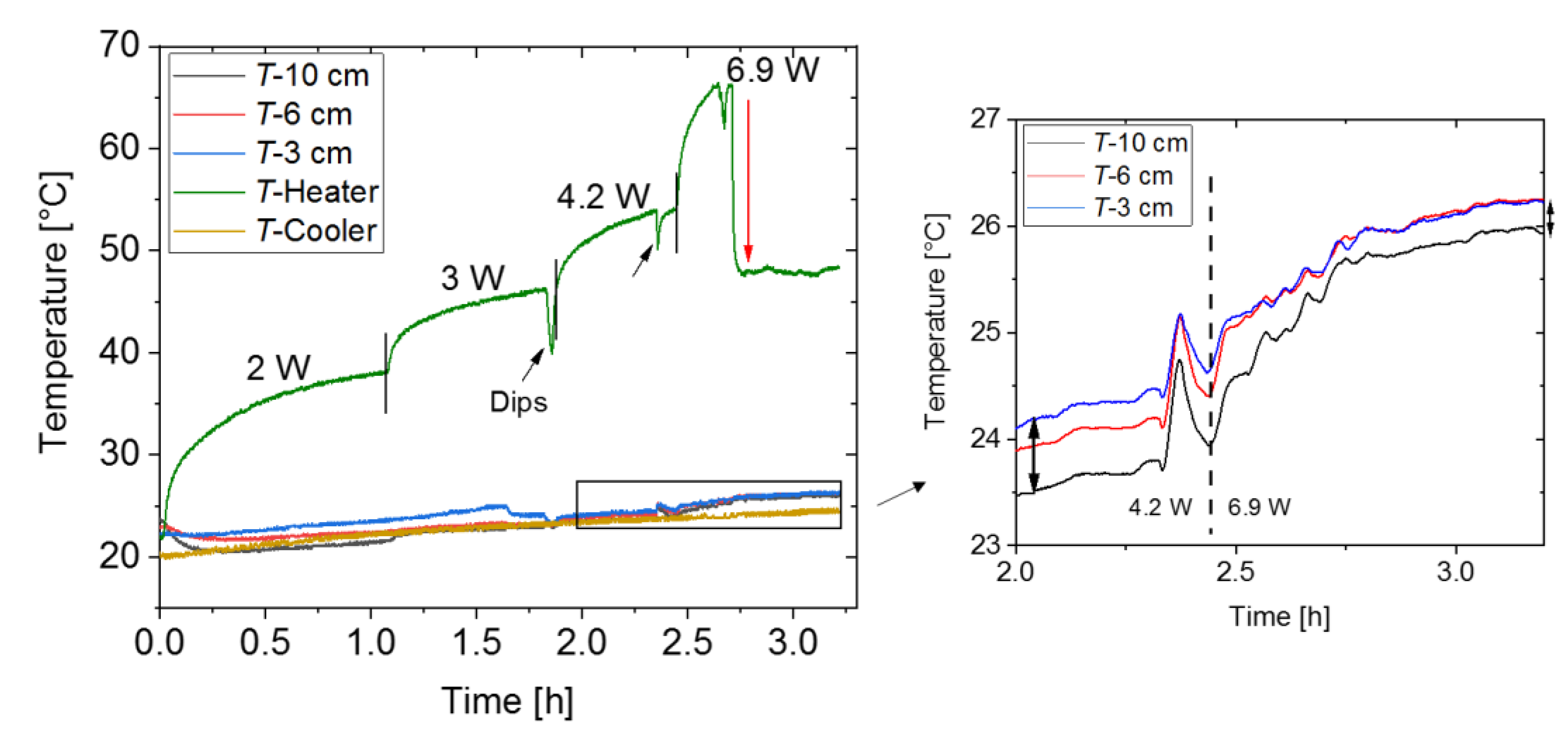
3.2.1. Temperatures vs. Time for Different Heating Powers
3.2.2. Comparison to Predictions on Activation Temperature
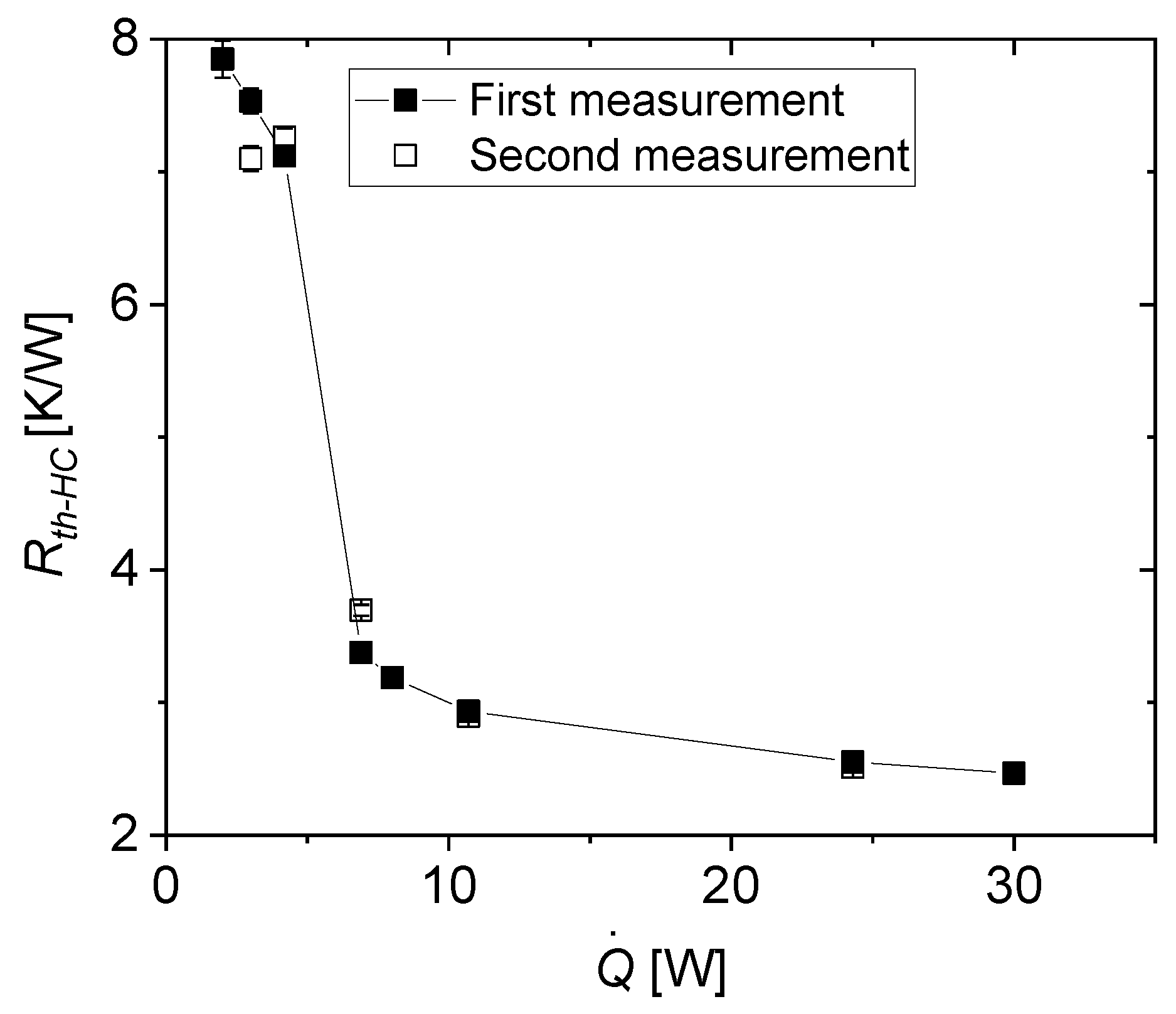
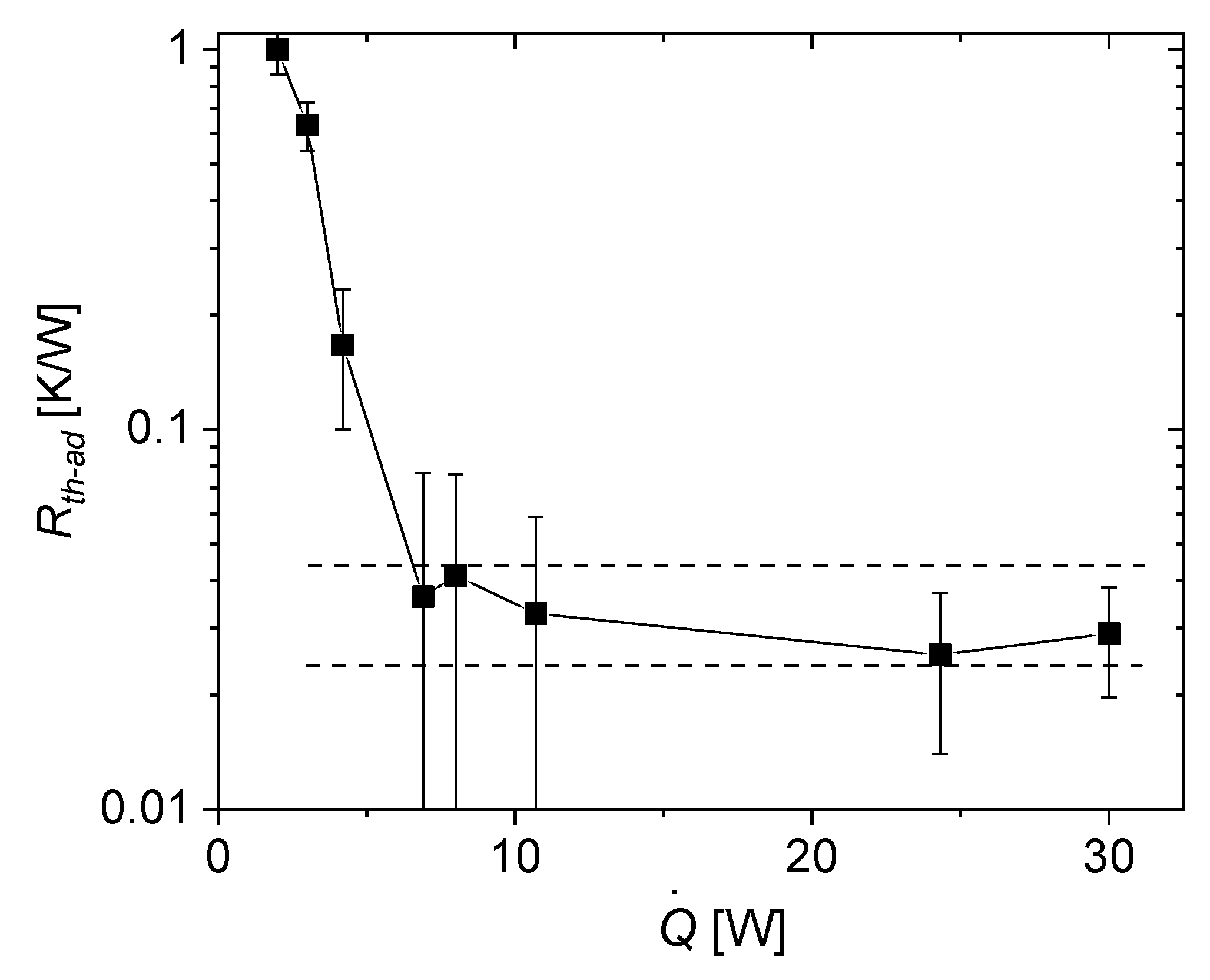
3.2.3. Thermal Resistance
- as thermal resistance between heater and cooler, where is the temperature difference between heater (evaporator) and cooler (condenser) at the end of the respective power plateau with heating power :
- as thermal resistance in the adiabatic zone where is the temperature difference between the thermocouples located 3 cm and 10 cm above the surface of the thermal oil bath (see Figure 7) at the end of the respective power plateau at heating power :
3.2.4. Switching Ratio
- At low power (mostly at 4.2 W and below), the heat pipe is in its non-conducting or off-state.
- Upon increasing the power, the thermal resistance decreases drastically. The drop in thermal conductivity is not perfectly sharp and can extend over more than one power level.
- After the transition to the conducting state, the thermal resistance reaches a relatively constant plateau and does not significantly decrease for higher power levels. Moreover, no significant increase of thermal resistance was observed during the experiment, which means that the heat pipe does not dry out for applied heating powers of at least 30 W.
- as ratio of thermal resistances and at the power level before the observed switching effect takes place (4.2 W), and the power level after switching (6.9 W). This yields a low value for the switching ratio since the transition is not perfectly sharp.
- as the ratio of all thermal resistances and averaged before switching (from 2 to 4.2 W) and those averaged after switching (from 6.9 W to 30 W.) This averaged switching ratio probably best represents the overall behavior of the heat pipe as a thermal switch.
4. Conclusions
- It is possible to predict the “activation temperature” of a heat pipe with integrated adsorbent based on the adsorbent’s isotherm equilibrium data.
- Polanyi’s potential theory can be used in this context to perform the necessary calculations.
- With the presented approach, it is possible to realize a heat switch with averaged thermal switching ratios of 3 and 18 (depending on whether evaporator/condenser or the adiabatic zone are considered).
- The used glass heat pipe demonstrator has its limitations and therefore we are planning to adapt the concept to conventional copper heat pipes and common diameters used in applications (typically around 10 mm).
- The main challenges will be the proper integration of the adsorbent into the heat pipe, determining the appropriate amount of adsorbent and heater position, and ensuring a gravity-independent operation.
- Furthermore, experiments will be carried out to yield defined switching temperatures that are adapted to the individual requirements of the technical application to be addressed (i.e., battery systems, fuel cells etc.).
- Finally, the heat pipe’s transient behavior will be examined in more detail. This means testing the thermal behavior for different holding times at each power level, going back from high temperatures to low temperatures etc.
5. Patents
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Blumenthal, P.; Raatz, A. Classification of electrocaloric cooling device types. EPL 2016, 115, 17004. [Google Scholar] [CrossRef]
- Wehmeyer, G.; Yabuki, T.; Monachon, C.; Wu, J.; Dames, C. Thermal diodes, regulators, and switches: Physical mechanisms and potential applications. Appl. Phys. Rev. 2017, 4, 41304. [Google Scholar] [CrossRef] [Green Version]
- Reay, D.A.; Kew, P.A.; McGlen, R.J. Heat Pipes: Theory, Design and Applications, 6th ed.; Butterworth-Heineman: Oxford, UK, 2013. [Google Scholar]
- Groll, M.; Munzel, W.D.; Supper, W.; Savage, C.J. Development of a Liquid-Trap Heat Pipe Thermal Diode. J. Spacecr. Rockets 1979, 16, 195–202. [Google Scholar] [CrossRef]
- Boreyko, J.B.; Zhao, Y.; Chen, C.-H. Planar jumping-drop thermal diodes. Appl. Phys. Lett. 2011, 99, 234105. [Google Scholar] [CrossRef] [Green Version]
- Tsukamoto, T.; Hirayanagi, T.; Tanaka, S. Micro thermal diode with glass thermal insulation structure embedded in a vapor chamber. J. Micromech. Microeng. 2017, 27, 45001. [Google Scholar] [CrossRef]
- Benafan, O.; Notardonato, W.U.; Meneghelli, B.J.; Vaidyanathan, R. Design and development of a shape memory alloy activated heat pipe-based thermal switch. Smart Mater. Struct. 2013, 22, 105017. [Google Scholar] [CrossRef]
- Leriche, M.; Harmand, S.; Lippert, M.; Desmet, B. An experimental and analytical study of a variable conductance heat pipe: Application to vehicle thermal management. Appl. Therm. Eng. 2012, 38, 48–57. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Sauerbeck, S.; Manoylova, O.; Tissler, A.; Dienersberger, M. Titano-Silico-Alumino-Phosphate. US 2013/0334460 AA, 12 April 2017. [Google Scholar]
- Freni, A.; Bonaccorsi, L.; Calabrese, L.; Caprì, A.; Frazzica, A.; Sapienza, A. SAPO-34 coated adsorbent heat exchanger for adsorption chillers. Appl. Therm. Eng. 2015, 82, 1–7. [Google Scholar] [CrossRef]
- Kayal, S.; Baichuan, S.; Saha, B.B. Adsorption characteristics of AQSOA zeolites and water for adsorption chillers. Int. J. Heat Mass Transf. 2016, 92, 1120–1127. [Google Scholar] [CrossRef]
- Goldsworthy, M.J. Measurements of water vapour sorption isotherms for RD silica gel, AQSOA-Z01, AQSOA-Z02, AQSOA-Z05 and CECA zeolite 3A. Microporous Mesoporous Mater. 2014, 196, 59–67. [Google Scholar] [CrossRef]
- Kohler, T.; Hinze, M.; Müller, K.; Schwieger, W. Temperature independent description of water adsorption on zeotypes showing a type V adsorption isotherm. Energy 2017, 135, 227–236. [Google Scholar] [CrossRef]
- Greenspan, L. Humidity fixed points of binary saturated aqueous solutions. J. Res. Natl. Bur. Stand. Sect. A 1977, 81A, 89. [Google Scholar] [CrossRef]
- Polanyi, M. Section III—Theories of the adsorption of gases. A general survey and some additional remarks. Trans. Faraday Soc. 1932, 28, 316–333. [Google Scholar] [CrossRef]
- Polanyi, M. Über die Adsorption vom Standpunkt des dritten Waermesatzes. Verh. Dtsch. Phys. Ges 1914, 16, 1012–1016. [Google Scholar]
- Dubinin, M.M. Theory of the physical adsorption of gases and vapors and adsorption properties of adsorbents of various natures and porous structures. translated version. Russ. Chem. B 1960, 9, 1072–1078. [Google Scholar] [CrossRef]
- Ruthven, D.M. Principles of Adsorption and Adsorption Processes; Wiley & Sons: New York, NY, USA, 1984; ISBN 0-471-86606-7. [Google Scholar]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Hauer, A. Beurteilung Fester Adsorbentien in Offenen Sorptionssystemen für Energetische Anwendungen. Ph.D. Thesis, Technische Universität Berlin, Berlin, Germany, 2002. [Google Scholar]
- Semprini, S.; Lehmann, C.; Beckert, S.; Kolditz, O.; Gläser, R.; Kerskes, H.; Nagel, T. Numerical modelling of water sorption isotherms of zeolite 13XBF based on sparse experimental data sets for heat storage applications. Energ. Convers. Manag. 2017, 150, 392–402. [Google Scholar] [CrossRef]
- Dubinin, M.M.; Astakhov, V.A. Description of Adsorption Equilibria of Vapors on Zeolites over Wide Ranges of Temperature and Pressure. Adv. Chem. 1971, 102, 69–85. [Google Scholar] [CrossRef]
- Tomás Núnez. Charakterisierung und Bewertung von Adsorbentien für Wärmetranformationsanwendungen. Ph.D. Thesis, Albert-Ludwigs-Universität, Freiburg im Breisgau, Germany, 2001.
- McKinney, W. Data Structures for Statistical Computing in Python. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, 28 June–3 July 2010; pp. 56–61. [Google Scholar]
- Harris, C.R.; Millman, K.J.; van der Walt, S.J.; Gommers, R.; Virtanen, P.; Cournapeau, D.; Wieser, E.; Taylor, J.; Berg, S.; Smith, N.J.; et al. Array programming with NumPy. Nature 2020, 585, 357–362. [Google Scholar] [CrossRef]
- Hunter, J.D. Matplotlib: A 2D Graphics Environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Incropera, F.P.; DeWitt, D.P.; Bergman, T.L.; Lavine, A.S. Incropera’s Principles of Heat and Mass Transfer, 1st ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2017; ISBN 9781119382911. [Google Scholar]




| UsedRth | |||
|---|---|---|---|
| 3.2 | 2.1 | 2.6 | |
| 35 | 4.6 | 18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winkler, M.; Teicht, C.; Corhan, P.; Polyzoidis, A.; Bartholomé, K.; Schäfer-Welsen, O.; Pappert, S. Thermal Switch Based on an Adsorption Material in a Heat Pipe. Energies 2021, 14, 5130. https://doi.org/10.3390/en14165130
Winkler M, Teicht C, Corhan P, Polyzoidis A, Bartholomé K, Schäfer-Welsen O, Pappert S. Thermal Switch Based on an Adsorption Material in a Heat Pipe. Energies. 2021; 14(16):5130. https://doi.org/10.3390/en14165130
Chicago/Turabian StyleWinkler, Markus, Christian Teicht, Patrick Corhan, Angelos Polyzoidis, Kilian Bartholomé, Olaf Schäfer-Welsen, and Sandra Pappert. 2021. "Thermal Switch Based on an Adsorption Material in a Heat Pipe" Energies 14, no. 16: 5130. https://doi.org/10.3390/en14165130
APA StyleWinkler, M., Teicht, C., Corhan, P., Polyzoidis, A., Bartholomé, K., Schäfer-Welsen, O., & Pappert, S. (2021). Thermal Switch Based on an Adsorption Material in a Heat Pipe. Energies, 14(16), 5130. https://doi.org/10.3390/en14165130