Optimization of Thermal Management System with Water and Phase Change Material Cooling for Li-Ion Battery Pack
Abstract
:1. Introduction
2. Materials and Methods
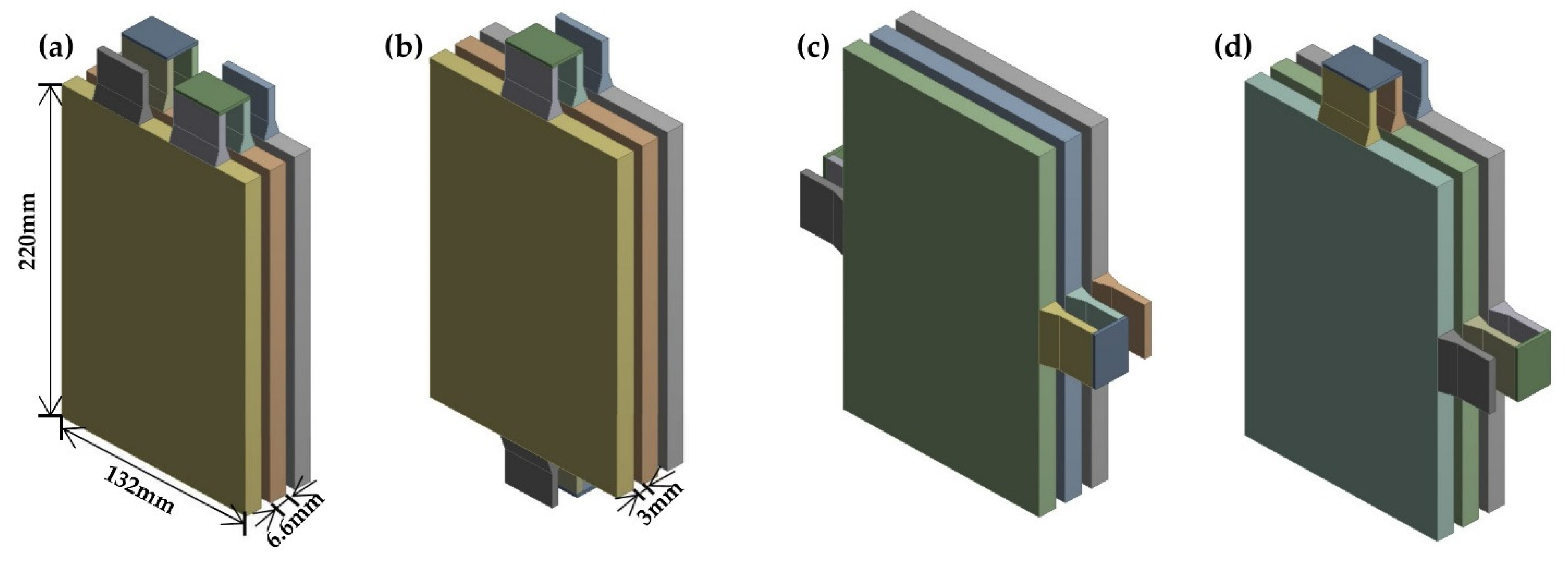
2.1. System Description
2.2. Lithium-ion battery (LIB) Reaction Mechanism
2.3. Heat Generation Model
2.4. Lithium-Ion Battery (LIB) Heat Transfer Model
- Although the LIB components theoretically have different thermal-physical properties, each LIB is thought to be a homogenous body.
- The boundary condition between the terminal and cell body is defined as a coupled state to maintain temperature continuity through this section [17].
- Heat transfer is isotropic in all directions.
- The flow condition for a melted PCM is not considered.
- The radiation heat transfer is negligible, owing to the small radiation heat.
- The heat transfer method at the terminal is defined as free convection.
2.5. Numerical Model
3. Results
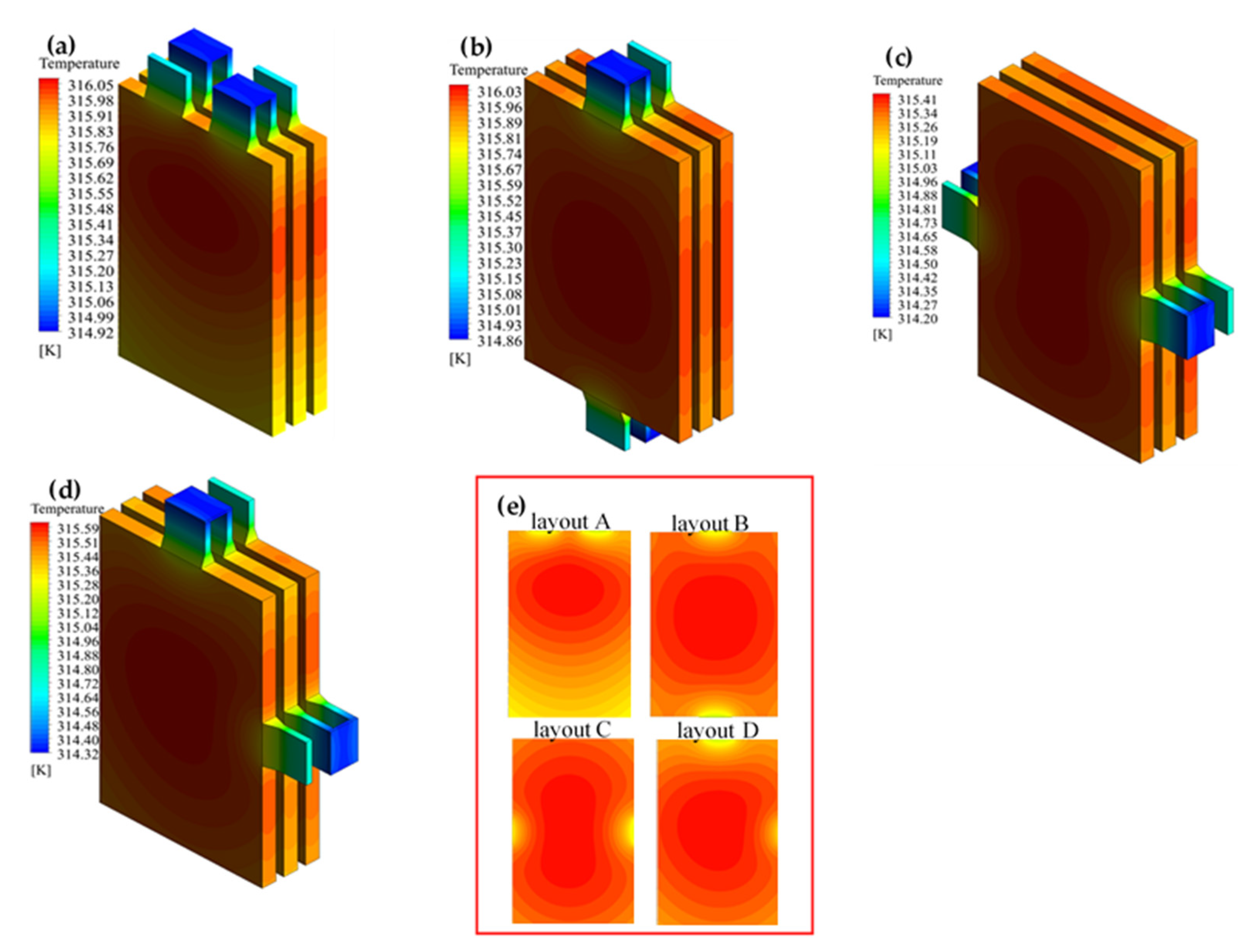
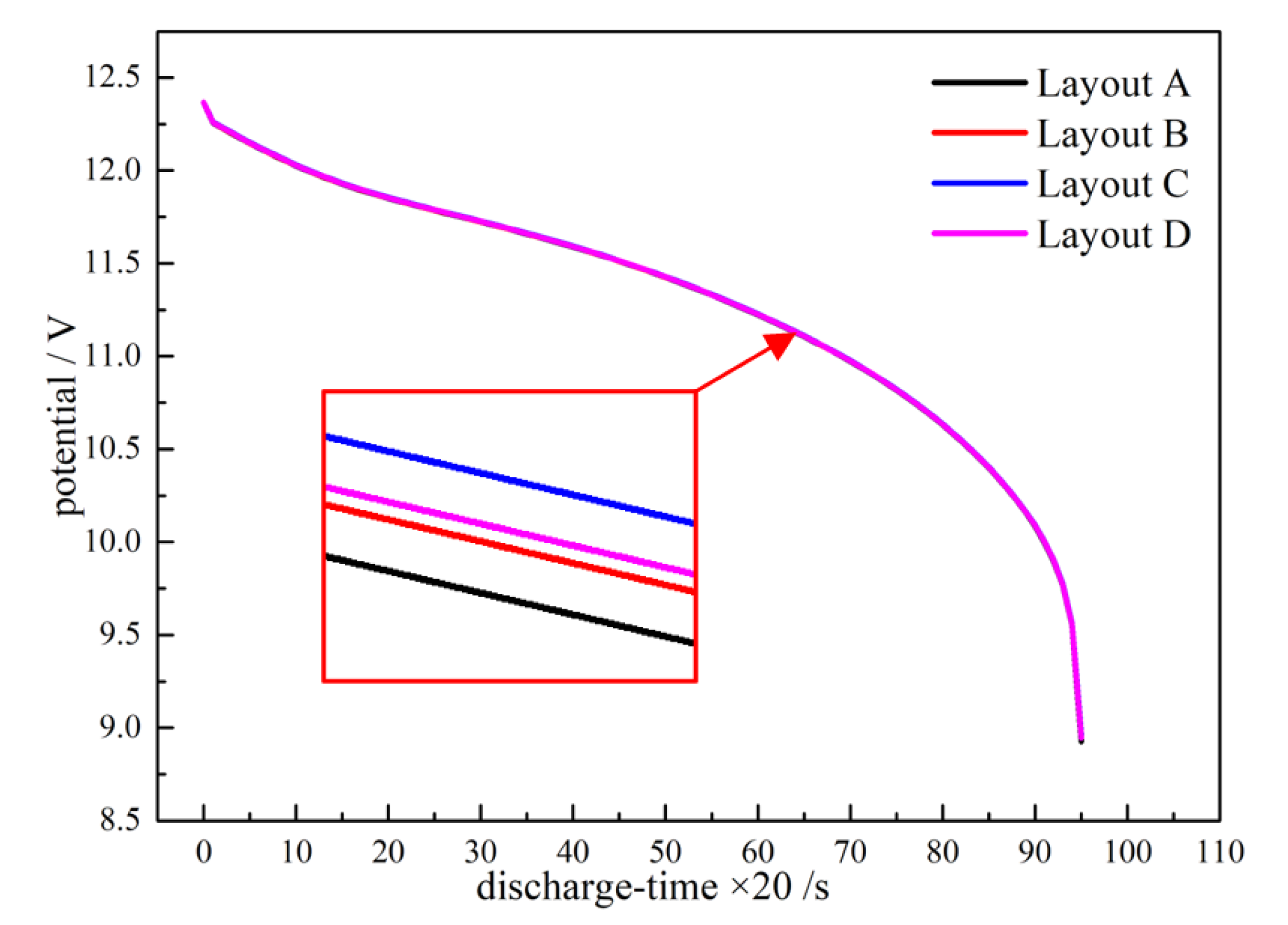
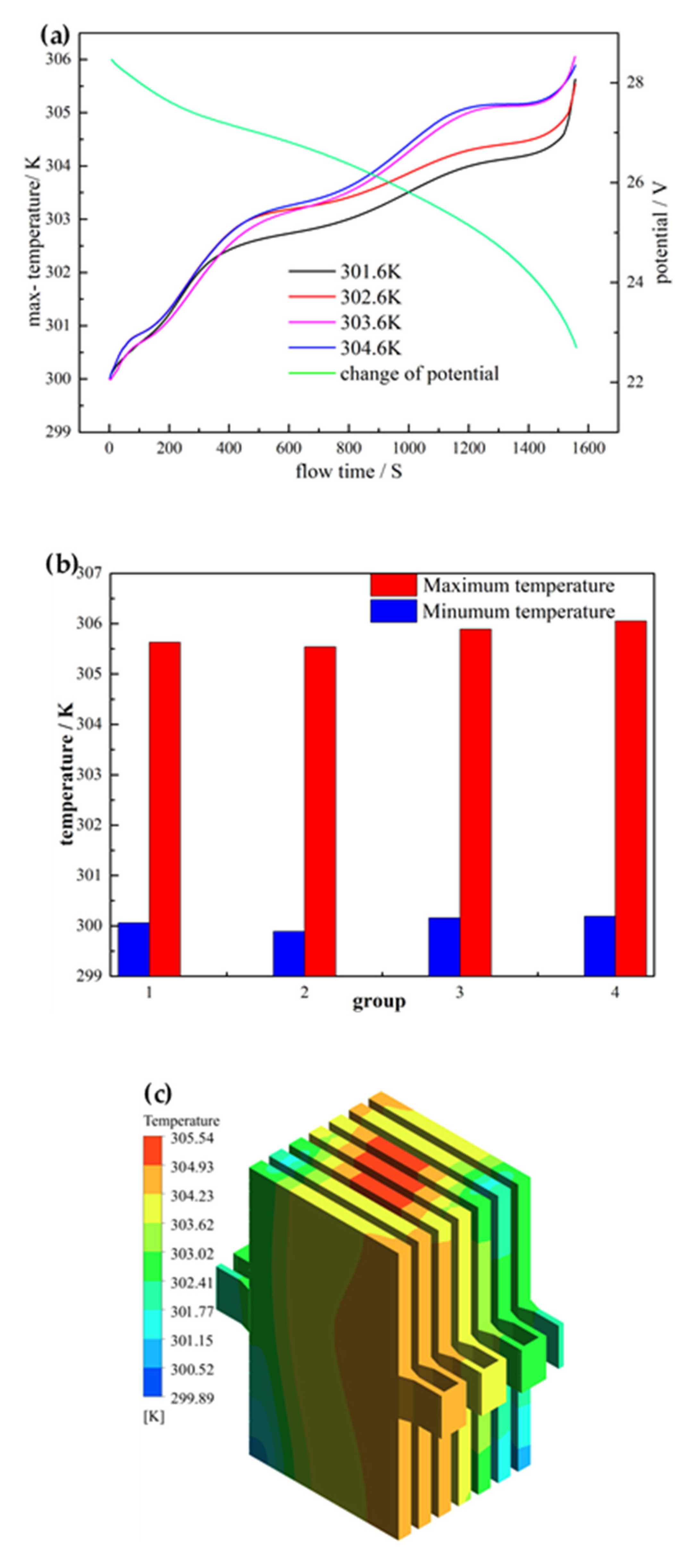
3.1. Effect of Terminal for Heat Generation
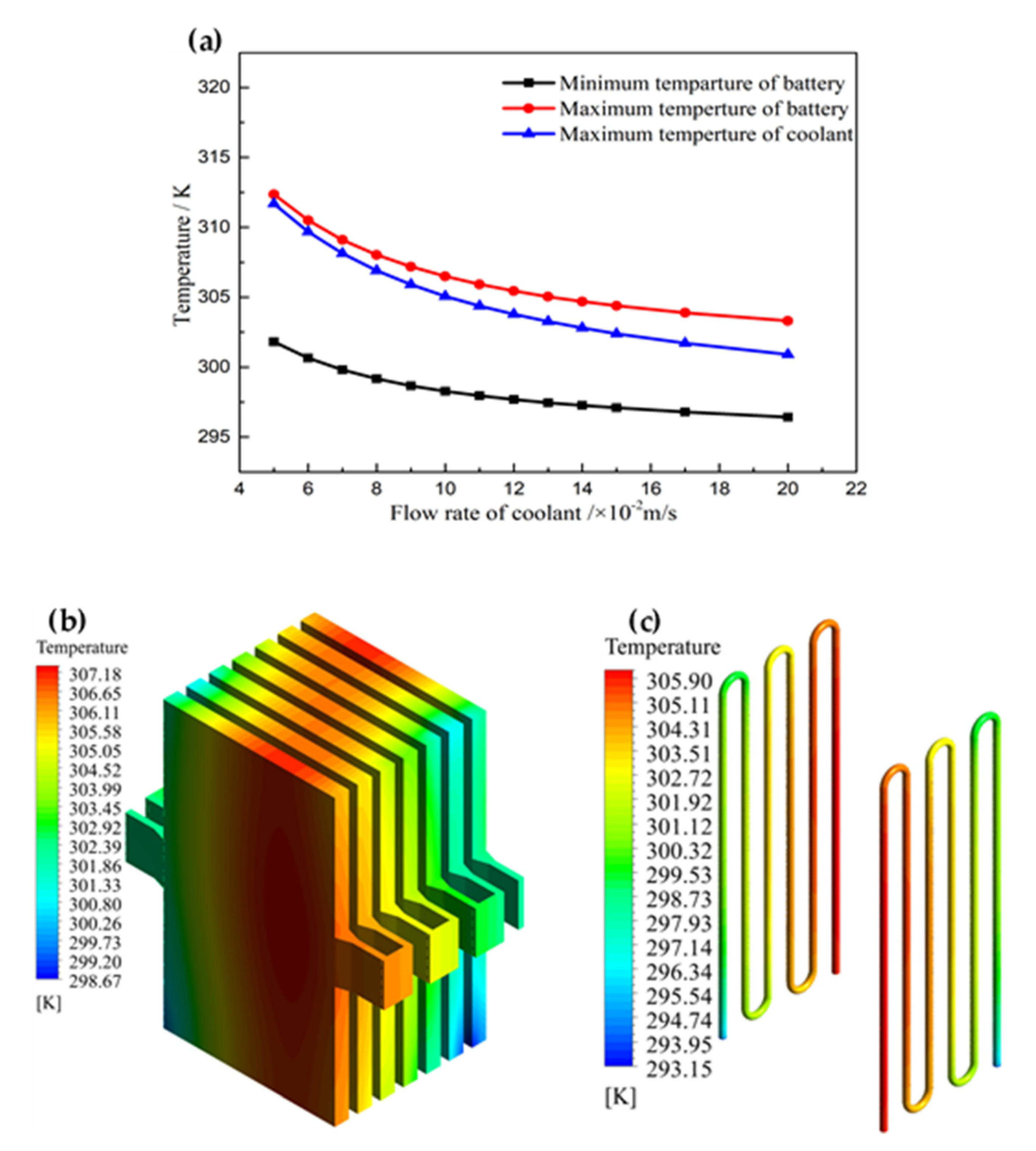
3.2. Effect of Flow Rate of Coolant
3.3. Effect of Section and Position of Cooling Pipe
3.4. Effect of Coupling the Liquid Cooling and Phase Change Material (PCM)
4. Conclusions
- A different terminal layout results in different internal current paths. Thus, the different positions involved in the reaction were affected. An optimized layout results in a lower potential decrease than the other layouts. The maximum temperature decreased slightly; however, the temperature distribution was more even in the body area for a single battery.
- The maximum temperature can be reduced by increasing the inlet flow rate. However, the cooling efficiency decreased when the flow rate exceeded a particular range. When the flow rate was beyond the range, the downward trend of the temperature became smoother, and the extra energy consumption increased. Therefore, a flow rate of 0.09 m/s was utilized in this system, and the deviation in the maximum temperature was smaller than 0.25%, which is comparable to 0.1 m/s. More heat can be removed by the unit mass coolant when using the appropriately selected flow rate.
- The cross-section of the pipe has an effect on the interflow rate of the coolant, which causes a difference in the cooling performance. Thus, the temperature difference was affected by the cross-section. Appropriate cross-section selection can decrease the temperature difference to below 10 K. Moreover, the positions of the cooling pipes were reasonably arranged, according to the main heat-generation area. This arrangement enables the dissipation of heat in a timely manner at the edge of the high-temperature zone. Moreover, it can maintain a more uniform temperature for the battery pack. It is necessary to arrange the cooling pipes in the main heating area for heat dissipation.
- The PCM assumed an auxiliary role in optimizing the temperature distribution. Selecting the type of PCM according to the highest temperature of the battery pack can improve the cooling effect to reduce the battery temperature difference. It is beneficial to extend the working life of a battery pack.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Li, Q.; Yang, Q.; Zhao, Y.; Wan, B. Carbon-based coating containing ultrafine MoO2 nanoparticles as an integrated anode for high-performance lithium-ion batteries. J. Nanoparticle Res. 2017, 19, 332. [Google Scholar] [CrossRef]
- Saw, L.H.; Poon, H.M.; San Thiam, H.; Cai, Z.; Chong, W.T.; Pambudi, N.A.; King, Y.J. Novel thermal management system using mist cooling for lithium-ion battery packs. Appl. Energy 2018, 223, 146–158. [Google Scholar] [CrossRef] [Green Version]
- Peng, X.; Ma, C.; Garg, A.; Bao, N.; Liao, X. Thermal performance investigation of an air-cooled lithium-ion battery pack considering the inconsistency of battery cells. Appl. Therm. Eng. 2019, 153, 596–603. [Google Scholar] [CrossRef]
- Bahiraei, F.; Fartaj, A.; Nazri, G.A. Electrochemical-thermal modeling to evaluate active thermal management of a lithium-ion battery module. Electrochem. Acta 2017, 254, 59–71. [Google Scholar] [CrossRef]
- Li, Y.; Huang, G.; Xu, T.; Liu, X.; Wu, H. Optimal design of PCM thermal storage tank and its application for winter available open-air swimming pool. Appl. Energy 2018, 209, 224–235. [Google Scholar] [CrossRef]
- Li, Y.; Du, Y.; Xu, T.; Wu, H.; Zhou, X.; Ling, Z.; Zhang, Z. Optimization of thermal management system for Li-ion batteries using phase change material. Appl. Therm. Eng. 2018, 131, 766–778. [Google Scholar] [CrossRef]
- Mehrabi-Kermani, M.; Houshfar, E.; Ashjaee, M. A novel hybrid thermal management for Li-ion batteries using phase change materials embedded in copper foams combined with forced-air convection. Int. J. Therm. Sci. 2019, 141, 47–61. [Google Scholar] [CrossRef]
- Wang, Q.; Rao, Z.; Huo, Y.; Wang, S. Thermal performance of phase change material/oscillating heat pipe-based battery thermal management system. Int. J. Therm. Sci. 2016, 102, 9–16. [Google Scholar] [CrossRef]
- Bahiraei, F.; Ghalkhani, M.; Fartaj, A.; Nazri, G.A. A pseudo 3D electrochemical-thermal modeling and analysis of a lithium-ion battery for electric vehicle thermal management applications. Appl. Therm. Eng. 2017, 125, 904–918. [Google Scholar] [CrossRef]
- Ling, Z.; Cao, J.; Zhang, W.; Zhang, Z.; Fang, X.; Gao, X. Compact liquid cooling strategy with phase change materials for Li-ion batteries optimized using response surface methodology. Appl. Energy 2018, 228, 777–788. [Google Scholar] [CrossRef]
- Bai, F.; Chen, M.; Song, W.; Feng, Z.; Li, Y.; Ding, Y. Thermal management performances of PCM/water cooling-plate using for lithium-ion battery module based on non-uniform internal heat source. Appl. Therm. Eng. 2017, 126, 17–27. [Google Scholar] [CrossRef]
- Rao, Z.; Wang, Q.; Huang, C. Investigation of the thermal performance of phase change material/mini-channel coupled battery thermal management system. Appl. Energy 2016, 164, 659–669. [Google Scholar] [CrossRef]
- Qian, X.; Xuan, D.; Zhao, X.; Shi, Z. Heat dissipation optimization of lithium-ion battery pack based on neural networks. Appl. Therm. Eng. 2019, 162, 114289. [Google Scholar] [CrossRef]
- Thomas, K.E.; Newman, J. Thermal modeling of porous insertion electrodes. J. Electrochem. Soc. 2003, 150, A176–A192. [Google Scholar] [CrossRef]
- Putra, N.; Ariantara, B.; Pamungkas, R.A. Experimental investigation on performance of lithium-ion battery thermal management system using flat plate loop heat pipe for electric vehicle application. Appl. Therm. Eng. 2016, 99, 784–789. [Google Scholar] [CrossRef]
- Zhao, J.; Rao, Z.; Li, Y. Thermal performance of mini-channel liquid cooled cylinder based battery thermal management for cylindrical lithium-ion power battery. Energy Convers. Manag. 2015, 103, 157–165. [Google Scholar] [CrossRef]
- Javani, N.; Dincer, I.; Naterer, G.F.; Yilbas, B.S. Heat transfer and thermal management with PCMs in a Li-ion battery cell for electric vehicles. Int. J. Heat Mass Transf. 2014, 72, 690–703. [Google Scholar] [CrossRef]
- Agyenim, F.; Hewitt, N.; Eames, P.; Smyth, M. A review of materials, heat transfer and phase change problem formulation for latent heat thermal energy storage systems (LHTESS). Renew. Sustain. Energy Rev. 2010, 14, 615–628. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, H.; Sui, Y.; Yang, C. Numerical analysis and experimental visualization of phase change material melting process for thermal management of cylindrical power battery. Appl. Therm. Eng. 2018, 128, 489–499. [Google Scholar] [CrossRef]
- Liu, R.; Chen, J.; Xun, J.; Jiao, K.; Du, Q. Numerical investigation of thermal behaviors in lithium-ion battery stack discharge. Appl. Energy 2014, 132, 288–297. [Google Scholar] [CrossRef]
- Song, L.; Zhang, H.; Yang, C. Thermal analysis of conjugated cooling configurations using phase change material and liquid cooling techniques for a battery module. Int. J. Heat Mass Transf. 2019, 133, 827–841. [Google Scholar] [CrossRef]
- Chen, D.; Jiang, J.; Kim, G.H.; Yang, C.; Pesaran, A. Comparison of different cooling methods for lithium ion battery cells. Appl. Therm. Eng. 2016, 94, 846–854. [Google Scholar] [CrossRef] [Green Version]
- Thompson, D.L.; Hartley, J.M.; Lambert, S.M.; Shiref, M.; Harper, G.D.; Kendrick, E.; Anderson, P.; Ryder, K.S.; Gaines, L.; Abbott, A.P. The importance of design in lithium ion battery recycling—A critical review. Green Chem. 2020, 22, 7585–7603. [Google Scholar] [CrossRef]



| Parameters | Symbol (unit) | Value |
|---|---|---|
| Latent heat of PCM | (J kg−1) | 243,500 |
| Specific heat of PCM | (J kg−1 k−1) | 1800 (T ≤ T1) |
| 1800 + (600 × (T − T1)/2) (T1 < T < T2) | ||
| 2400 (T2 ≤ T) | ||
| Density of PCM | (kg m−3) | 785 (T ≤ T1) |
| 785 − (36 × (T − 301.6)/2) (T1 < T < T2) | ||
| 749 × (1 − 0.001 × (T − T2)) (T2 ≤ T) | ||
| Viscosity of PCM | (kg m−1 s−1) | 1 (T ≤ T1) |
| (303.6 − T)/2 (T1 < T < T2) | ||
| 0.000169 (T2 ≤ T) | ||
| Thermal conductivity of PCM | (W m−1 k−1) | 0.6 (T ≤ T2) |
| 0.6 − (0.01 × (T − T1)/2) (T1 < T < T2) | ||
| 0.59 (T2 ≤ T) | ||
| Specific heat of water | (J kg−1 k−1) | 4182 |
| Density of water | (kg m−3) | 996.5 |
| Viscosity of water | (kg m−1 s−1) | 0.001003 |
| Thermal conductivity of water | (W m−1 k−1) | 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Cho, J.-R.; Zhai, J. Optimization of Thermal Management System with Water and Phase Change Material Cooling for Li-Ion Battery Pack. Energies 2021, 14, 5312. https://doi.org/10.3390/en14175312
Li Q, Cho J-R, Zhai J. Optimization of Thermal Management System with Water and Phase Change Material Cooling for Li-Ion Battery Pack. Energies. 2021; 14(17):5312. https://doi.org/10.3390/en14175312
Chicago/Turabian StyleLi, Quanyi, Jong-Rae Cho, and Jianguang Zhai. 2021. "Optimization of Thermal Management System with Water and Phase Change Material Cooling for Li-Ion Battery Pack" Energies 14, no. 17: 5312. https://doi.org/10.3390/en14175312






