Organic Dyes in Dye-Sensitized Solar Cells Featuring Back Reflector
Abstract
1. Introduction
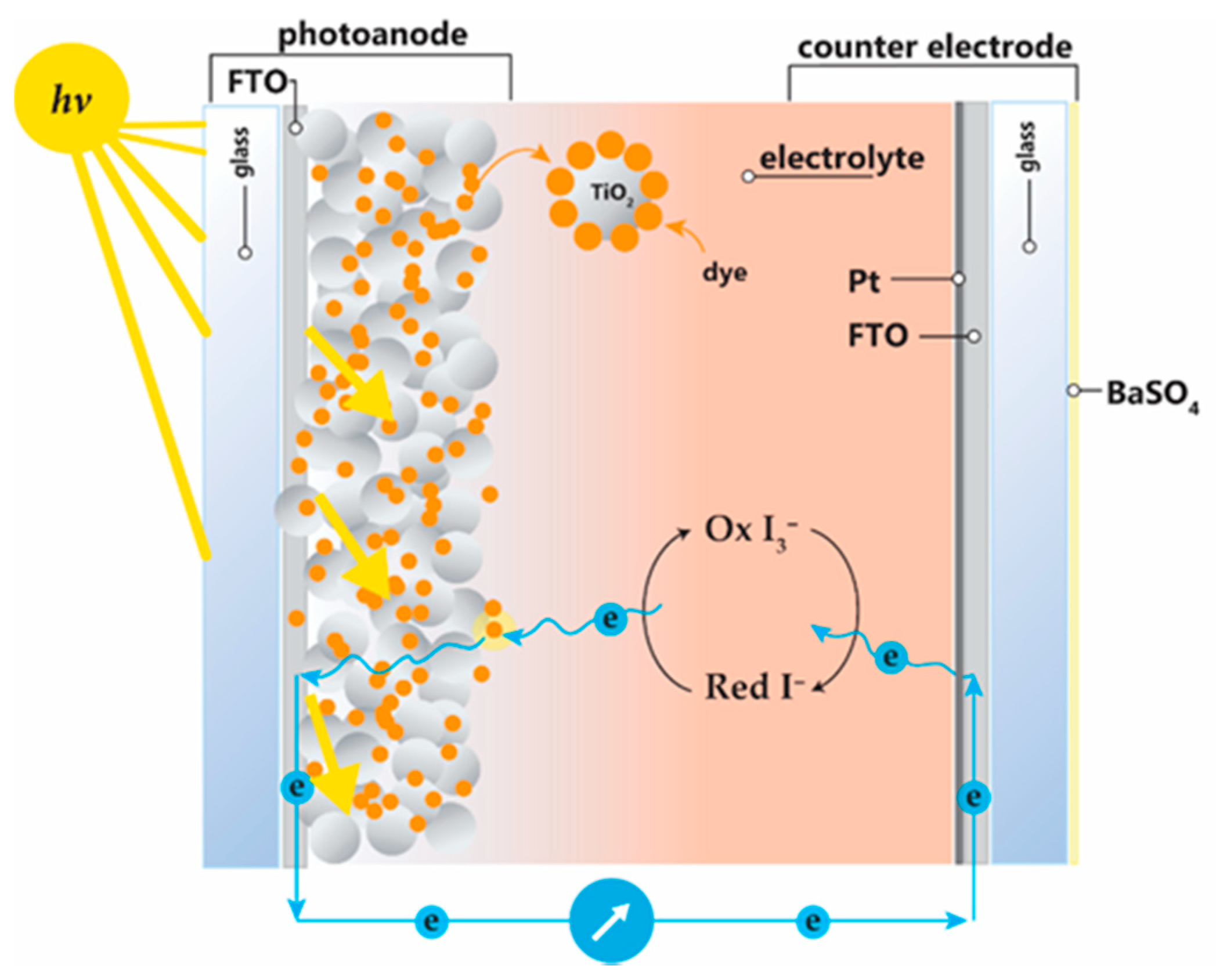
2. Materials and Methods
3. Results
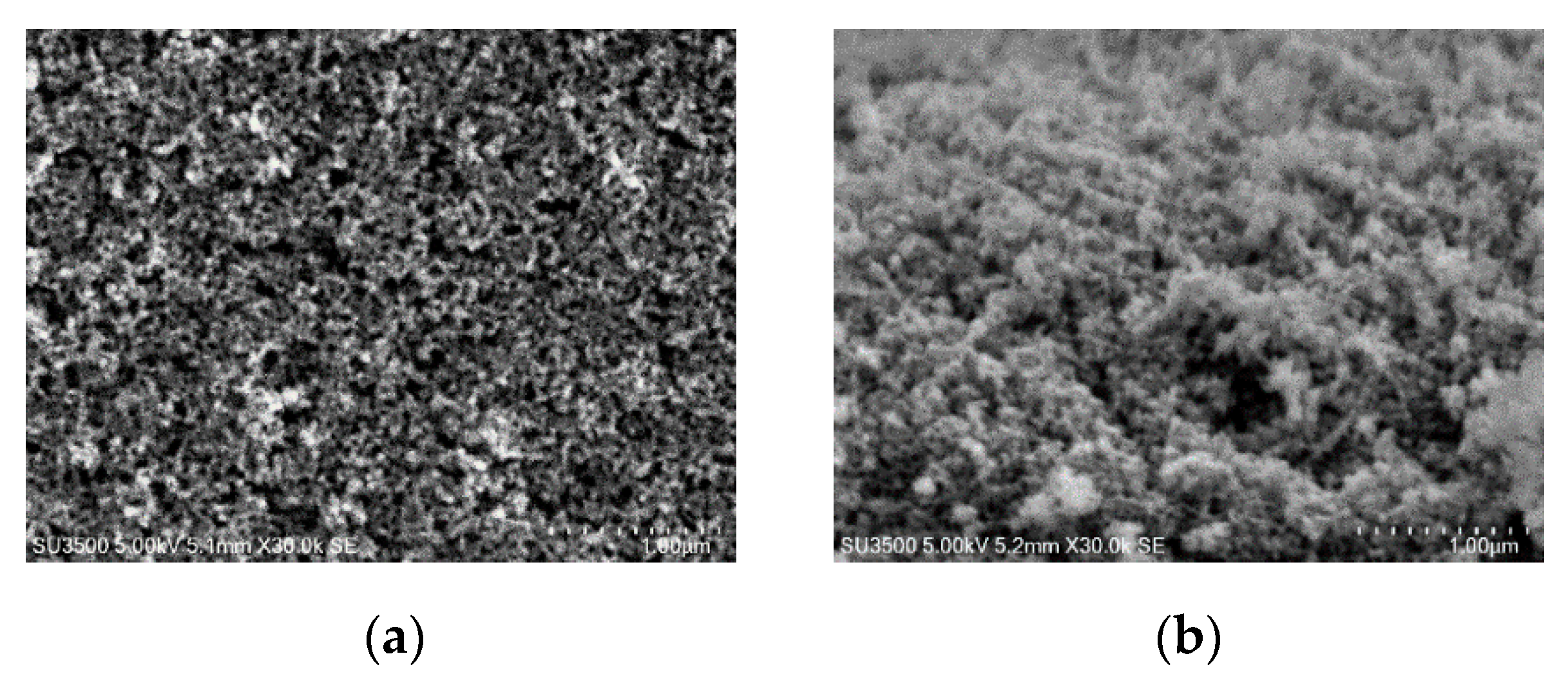
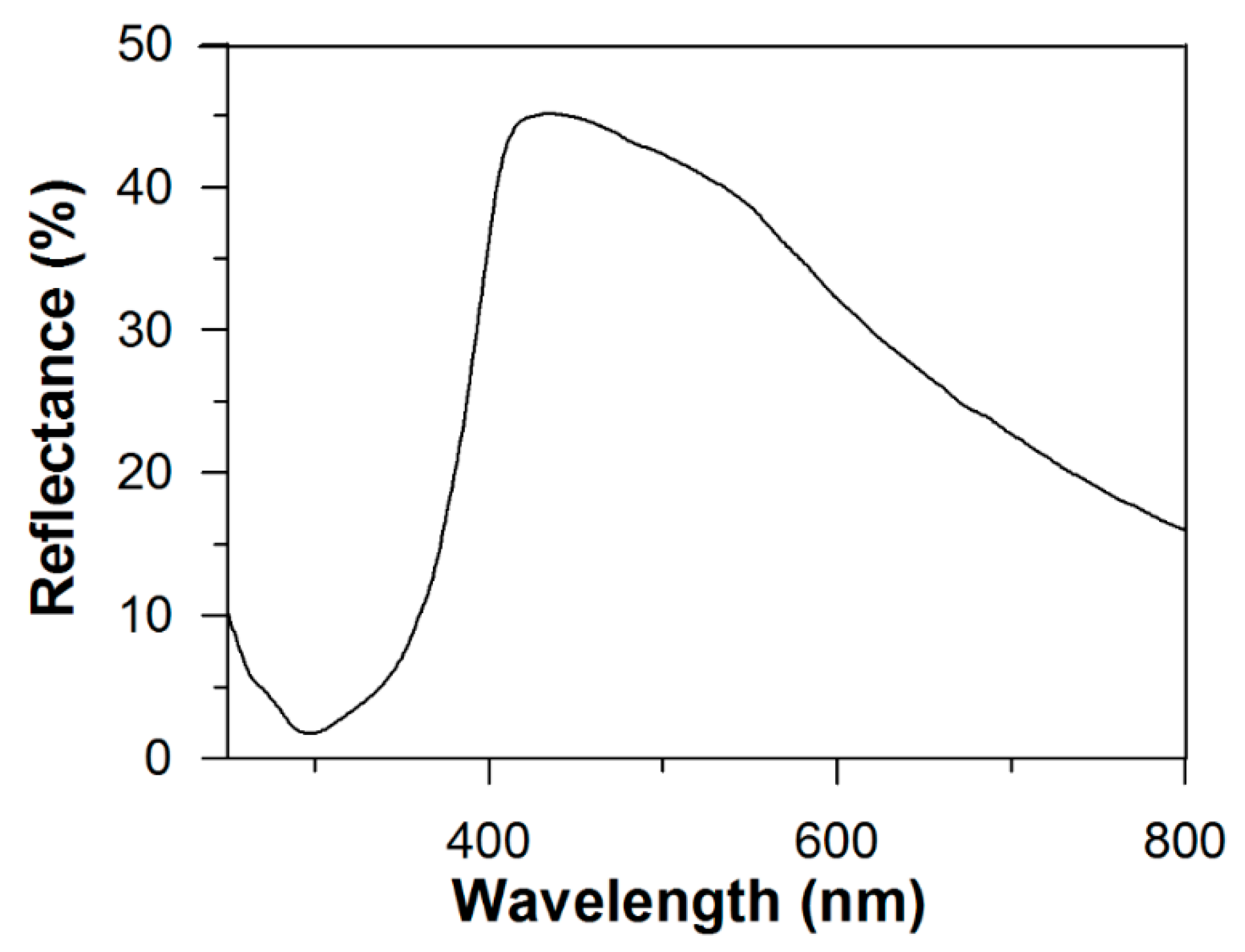
3.1. Characterization of Photoelectrode
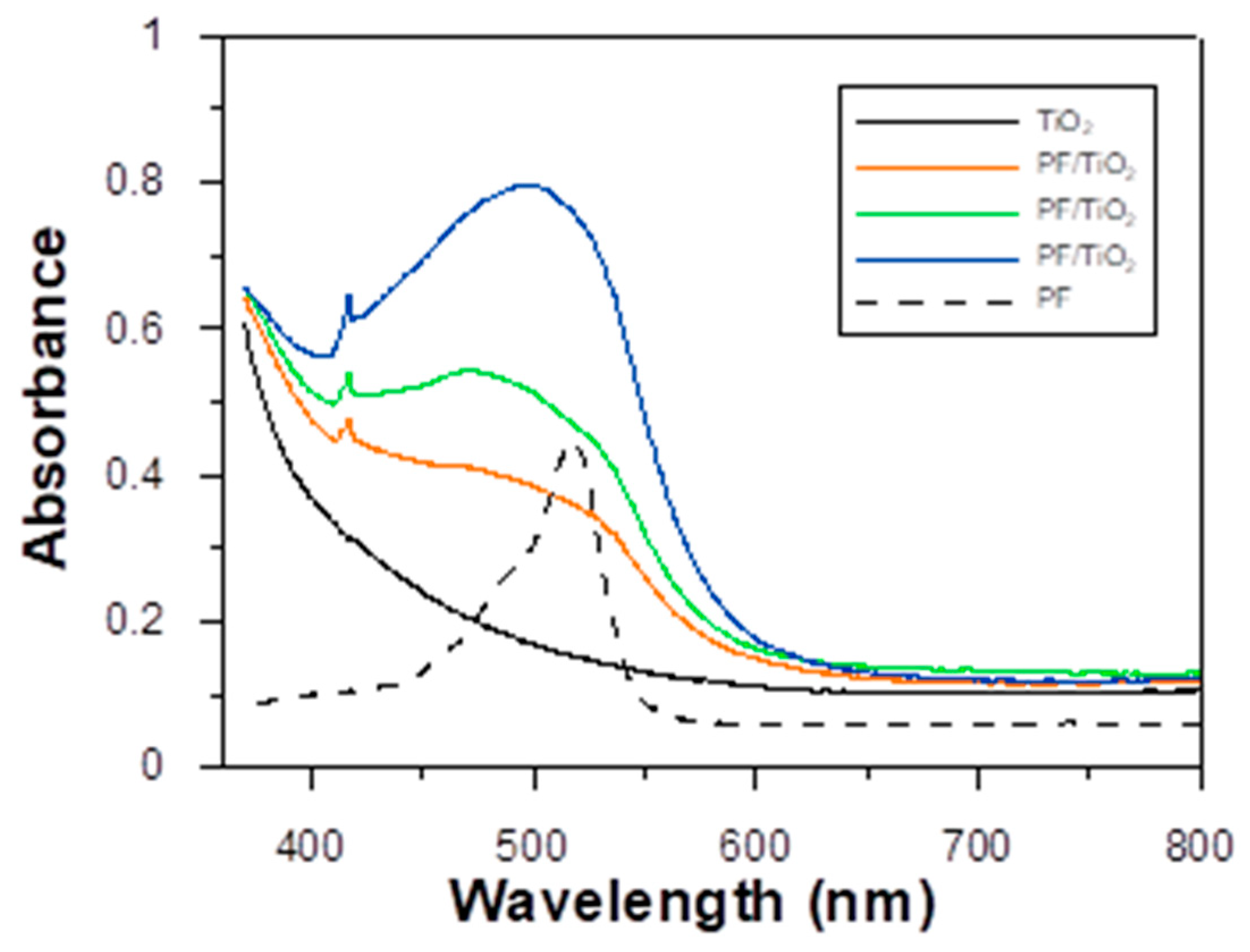
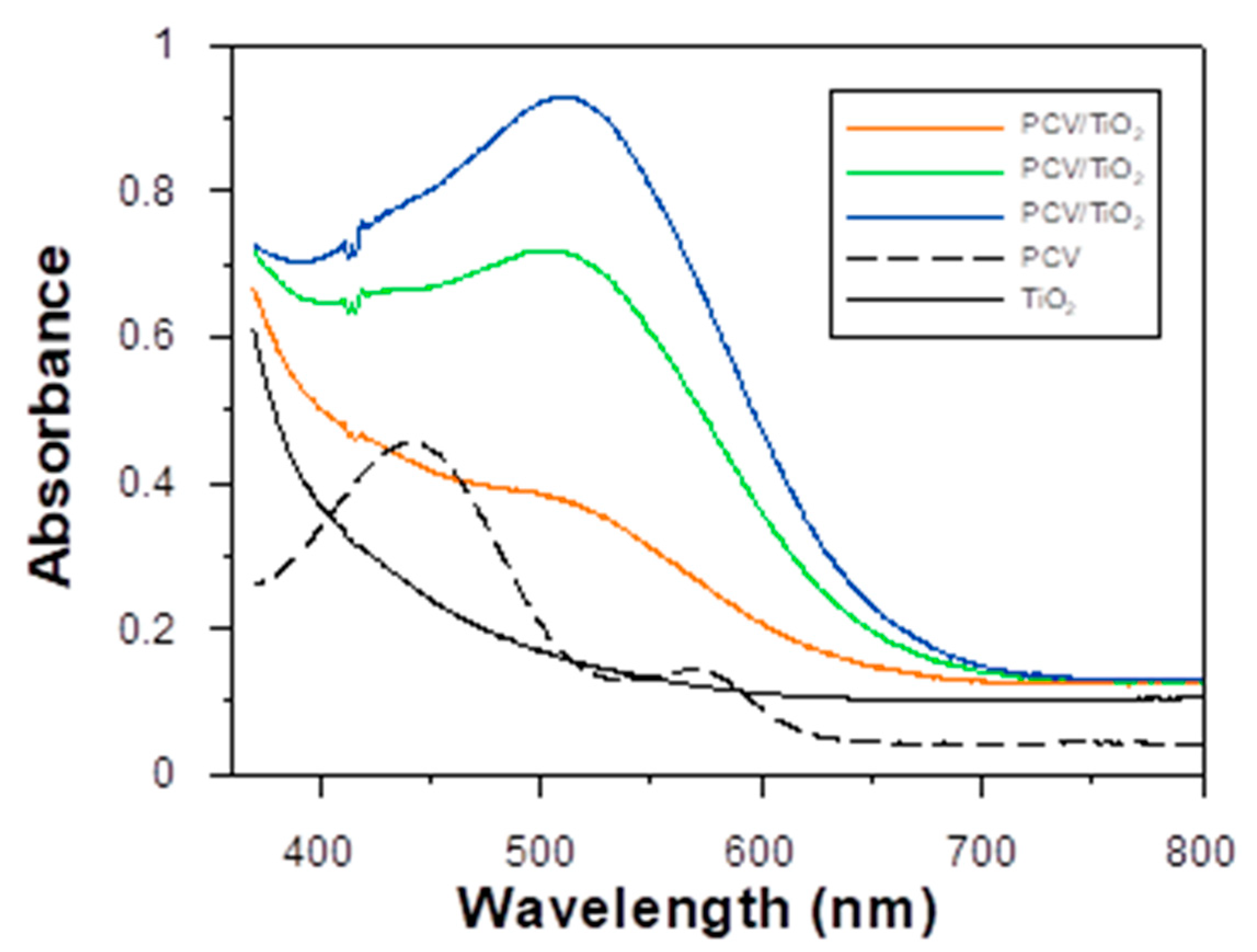
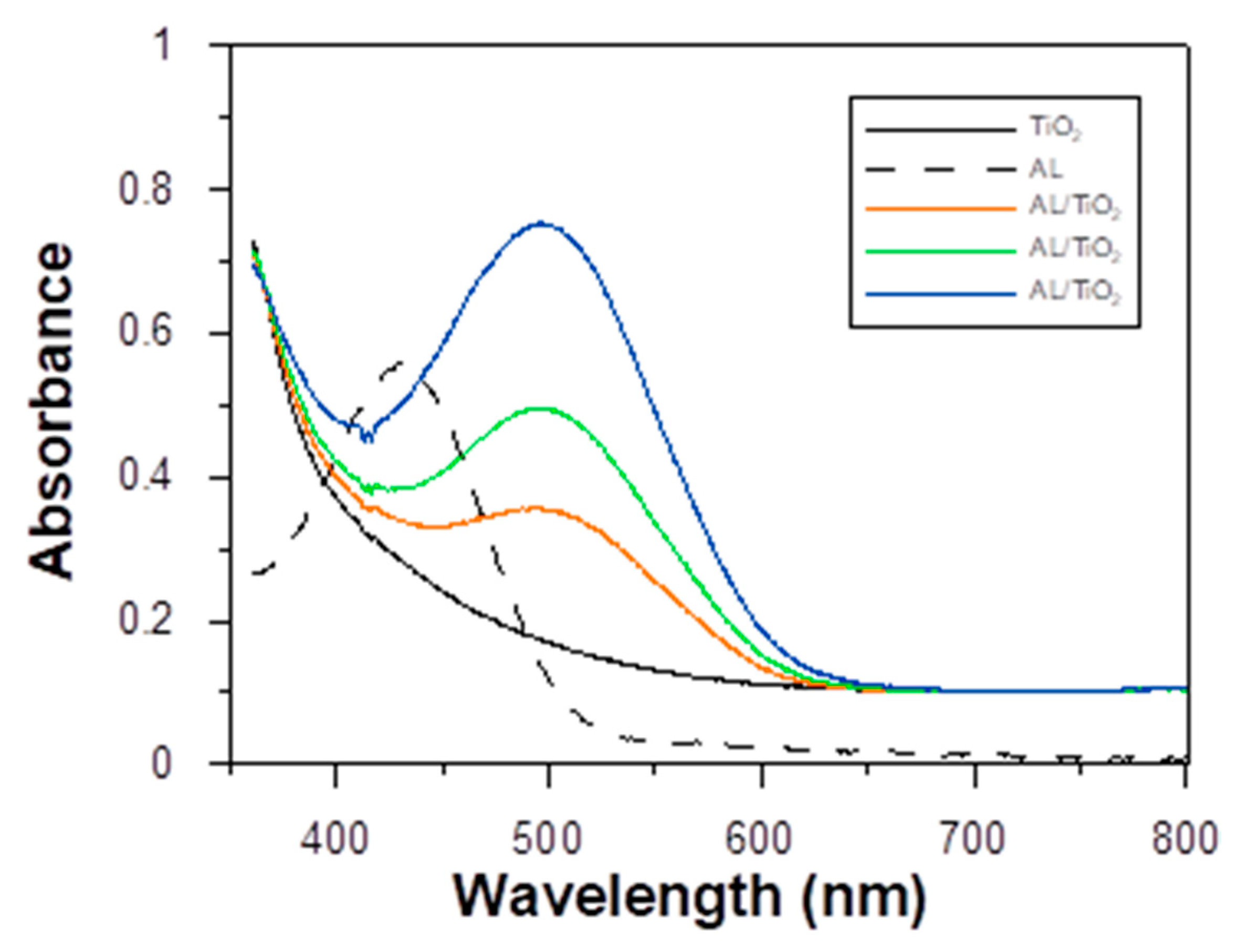
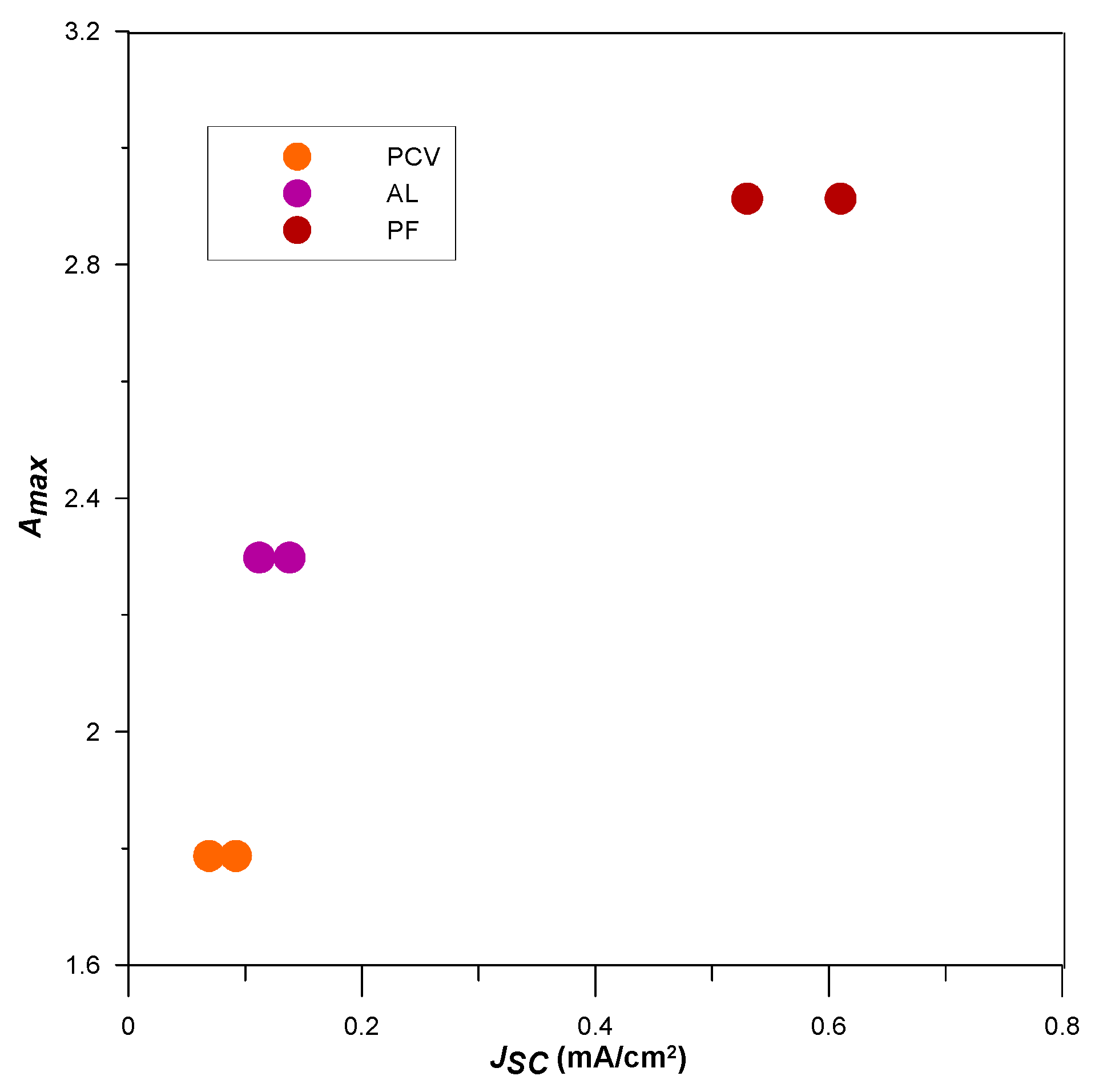
3.2. Photoelectrical Properties of the Dyes
3.3. TiO2/Dye/Electrolyte Interface
3.4. Photovoltaic Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hagfeldt, A.; Grätzel, M. Molecular Photovoltaics. Acc. Chem. Res. 2000, 33, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-de-Armas, R.; San-Miguel, M.; Oviedo, J.; Sanz, J.F. Direct vs. indirect mechanisms for electron injection in DSSC: Catechol and alizarin. Comput. Theor. Chem. 2011, 975, 99–105. [Google Scholar] [CrossRef]
- Krawczyk, S.; Nawrocka, A.; Zdyb, A. Charge-transfer excited state in pyrene-1-carboxylic acids adsorbed on titanium dioxide nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 198, 19–26. [Google Scholar] [CrossRef]
- Listorti, A.; O’Regan, B.; Durrant, J.R. Electron Transfer Dynamics in Dye-Sensitized Solar Cells. Chem. Mater. 2011, 23, 3381–3399. [Google Scholar] [CrossRef]
- Chitpakdee, C.; Namuangruk, S.; Suttisintong, K.; Jungsuttiwong, S.; Keawin, T.; Sudyoadsuk, T.; Sirithip, K.; Promarak, V.; Kungwan, N. Effects of π-linker, anchoring group and capped carbazole at meso-substituted zinc-porphyrins on conversion efficiency of DSSCs. Dye. Pigment. 2015, 118, 64–75. [Google Scholar] [CrossRef]
- Keawin, T.; Tarsang, R.; Sirithip, K.; Prachumrak, N.; Sudyoadsuk, T.; Namuangruk, S.; Roncali, J.; Kungwan, N.; Promarak, V.; Jungsuttiwong, S. Anchoring number-performance relationship of zinc-porphyrin sensitizers for dye-sensitized solar cells: A combined experimental and theoretical study. Dye. Pigment. 2017, 136, 697–706. [Google Scholar] [CrossRef]
- Jie, J.; Xu, Q.; Yang, G.; Feng, Y.; Zhang, B. Porphyrin sensitizers involving a fluorine-substituted benzothiadiazole as auxiliary acceptor and thiophene as π bridge for use in dye-sensitized solar cells (DSSCs). Dye. Pigment. 2020, 174, 107984. [Google Scholar] [CrossRef]
- Ambre, R.B.; Mane, S.B.; Chang, G.-F.; Hung, C.-H. Effects of Number and Position of Meta and Para Carboxyphenyl Groups of Zinc Porphyrins in Dye-Sensitized Solar Cells: Structure–Performance Relationship. ACS Appl. Mater. Interfaces 2015, 7, 1879–1891. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Yella, A.; Gao, P.; Humphry-Baker, R.; Curchod, B.F.E.; Ashari-Astani, N.; Tavernelli, I.; Rothlisberger, U.; Nazeeruddin, K.; Grätzel, M. Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat. Chem. 2014, 6, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Narayanaswamy, K.; Swetha, T.; Kapil, G.; Pandey, S.S.; Hayase, S.; Singh, S.P. Simple Metal-Free Dyes Derived from Triphenylamine for DSSC: A Comparative Study of Two Different Anchoring Group. Electrochim. Acta 2015, 169, 256–263. [Google Scholar] [CrossRef]
- Seo, K.D.; Song, H.M.; Lee, M.J.; Pastore, M.; Anselmi, C.; De Angelis, F.; Nazeeruddin, M.K.; Gräetzel, M.; Kim, H.K. Coumarin dyes containing low-band-gap chromophores for dye-sensitised solar cells. Dye. Pigment. 2011, 90, 304–310. [Google Scholar] [CrossRef]
- Han, L.; Kang, R.; Zu, X.; Cui, Y.; Gao, J. Novel coumarin sensitizers based on 2-(thiophen-2-yl)thiazole π-bridge for dye-sensitized solar cells. Photochem. Photobiol. Sci. 2015, 14, 2046–2053. [Google Scholar] [CrossRef] [PubMed]
- Naik, P.; Elmorsy, M.R.; Su, R.; Babu, D.D.; El-Shafei, A.; Adhikari, A.V. New carbazole based metal-free organic dyes with D-π-A-π-A architecture for DSSCs: Synthesis, theoretical and cell performance studies. Sol. Energy 2017, 153, 600–610. [Google Scholar] [CrossRef]
- Naik, P.; Su, R.; Elmorsy, M.; El-Shafei, A.; Adhikari, A.V. Investigation of new carbazole based metal-free dyes as active photo-sensitizers/co-sensitizers for DSSCs. Dye. Pigment. 2018, 149, 177–187. [Google Scholar] [CrossRef]
- Dai, P.-P.; Zhu, Y.-Z.; Liu, Q.-L.; Yan, Y.-Q.; Zheng, J.-Y. Novel indeno[1,2-b]indole-spirofluorene donor block for efficient sensitizers in dye-sensitized solar cells. Dye. Pigment. 2020, 175, 108099. [Google Scholar] [CrossRef]
- Ghann, W.; Kang, H.; Emerson, E.; Oh, J.; Chavez-Gil, T.; Nesbitt, F.; Williams, R.; Uddin, J. Photophysical properties of near-IR cyanine dyes and their application as photosensitizers in dye sensitized solar cells. Inorg. Chim. Acta 2017, 467, 123–131. [Google Scholar] [CrossRef]
- Ren, Y.; Sun, D.; Cao, Y.; Tsao, H.N.; Yuan, Y.; Zakeeruddin, S.M.; Wang, P.; Grätzel, M. A Stable Blue Photosensitizer for Color Palette of Dye-Sensitized Solar Cells Reaching 12.6% Efficiency. J. Am. Chem. Soc. 2018, 140, 2405–2408. [Google Scholar] [CrossRef]
- Kakiage, K.; Aoyama, Y.; Yano, T.; Oya, K.; Fujisawa, J.-I.; Hanaya, M. Highly-efficient dye-sensitized solar cells with collaborative sensitization by silyl-anchor and carboxy-anchor dyes. Chem. Commun. 2015, 51, 15894–15897. [Google Scholar] [CrossRef] [PubMed]
- Richhariya, G.; Kumar, A.; Tekasakul, P.; Gupta, B. Natural dyes for dye sensitized solar cell: A review. Renew. Sustain. Energy Rev. 2017, 69, 705–718. [Google Scholar] [CrossRef]
- Al-Alwani, M.A.; Ludin, N.A.; Mohamad, A.B.; Kadhum, A.A.H.; Mukhlus, A. Application of dyes extracted from Alternanthera dentata leaves and Musa acuminata bracts as natural sensitizers for dye-sensitized solar cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 192, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Al-Alwani, M.A.; Abu Hasan, H.; Al-Shorgani, N.K.N.; Al-Mashaan, A.B.S. Natural dye extracted from Areca catechu fruits as a new sensitiser for dye-sensitised solar cell fabrication: Optimisation using D-Optimal design. Mater. Chem. Phys. 2020, 240, 122204. [Google Scholar] [CrossRef]
- Ferreira, F.; Babu, R.S.; Barros, A.; Raja, S.; da Conceição, L.; Mattoso, L. Photoelectric performance evaluation of DSSCs using the dye extracted from different color petals of Leucanthemum vulgare flowers as novel sensitizers. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 233, 118198. [Google Scholar] [CrossRef]
- Keawwangchai, S.; Morakot, N.; Keawwangchai, T. Novel selective and sensitive optical chemosensor based on phenylfluorone derivative for detection of Ge(IV) ion in aqueous solution. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 202, 290–300. [Google Scholar] [CrossRef]
- Ricciu, A.; Secco, F.; Venturini, M.; Garcia, B.; Leal, J.M. Kinetics and Equilibria of the Interaction of Indium(III) with Pyrocathecol Violet by Relaxation Spectrometry. J. Phys. Chem. A 2000, 104, 7036–7043. [Google Scholar] [CrossRef]
- Sun, C.; Li, Y.; Han, J.; Cao, B.; Yin, H.; Shi, Y. Enhanced photoelectrical properties of alizarin-based natural dye via structure modulation. Sol. Energy 2019, 185, 315–323. [Google Scholar] [CrossRef]
- Nawrocka, A.; Zdyb, A.; Krawczyk, S. Stark spectroscopy of charge-transfer transitions in catechol-sensitized TiO2 nanoparticles. Chem. Phys. Lett. 2009, 475, 272–276. [Google Scholar] [CrossRef]
- Zdyb, A.; Krawczyk, S. Adsorption and electronic states of morin on TiO2 nanoparticles. Chem. Phys. 2014, 443, 61–66. [Google Scholar] [CrossRef]
- Zdyb, A.; Krawczyk, S. Characterization of adsorption and electronic excited states of quercetin on titanium dioxide nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 157, 197–203. [Google Scholar] [CrossRef]
- Pinto, A.L.; Cruz, L.; Gomes, V.; Cruz, H.; Calogero, G.; de Freitas, V.; Pina, F.; Parola, A.J.; Lima, J.C. Catechol versus carboxyl linkage impact on DSSC performance of synthetic pyranoflavylium salts. Dye. Pigment. 2019, 170, 107577. [Google Scholar] [CrossRef]
- Mikhailov, M.; Yuryev, S.; Lapin, A.; Lovitskiy, A. The effects of heating on BaSO4 powders’ diffuse reflectance spectra and radiation stability. Dye. Pigment. 2019, 163, 420–424. [Google Scholar] [CrossRef]
- Ito, S.; Chen, P.; Comte, P.; Nazeeruddin, M.K.; Liska, P.; Péchy, P.; Grätzel, M. Fabrication of screen-printing pastes from TiO2 powders for dye-sensitised solar cells. Prog. Photovolt. Res. Appl. 2007, 15, 603–612. [Google Scholar] [CrossRef]
- Mosurkal, R.; He, J.-A.; Yang, K.; Samuelson, L.A.; Kumar, J. Organic photosensitizers with catechol groups for dye-sensitized photovoltaics. J. Photochem. Photobiol. A Chem. 2004, 168, 191–196. [Google Scholar] [CrossRef]
- Xie, M.; Wang, J.; Xia, H.-Q.; Bai, F.-Q.; Jia, R.; Rim, J.-G.; Zhang, H.-X. Theoretical studies on the spectroscopic properties of porphyrin derivatives for dye-sensitized solar cell application. RSC Adv. 2015, 5, 33653–33665. [Google Scholar] [CrossRef]
- Ruiz-Anchondo, T.; Flores-Holguín, N.; Glossman-Mitnik, D. Natural Carotenoids as Nanomaterial Precursors for Molecular Photovoltaics: A Computational DFT Study. Molecules 2010, 15, 4490–4510. [Google Scholar] [CrossRef] [PubMed]
- Zevallos, J.; Toro-Labbé, A. A Theoretical Analysis of the Kohn-Sham and Hartree-Fock Orbitals and Their Use in the Determination of Electronic Properties. J. Chil. Chem. Soc. 2003, 48, 39–47. [Google Scholar] [CrossRef]
- Parr, R.G.; Szentpály, L.V.; Liu, S. Electrophilicity Index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Parthasarathi, R.; Padmanabhan, J.; Elango, M.; Subramanian, V.; Chattaraj, P. Intermolecular reactivity through the generalized philicity concept. Chem. Phys. Lett. 2004, 394, 225–230. [Google Scholar] [CrossRef]
- Grätzel, M. Recent Advances in Sensitized Mesoscopic Solar Cells. Acc. Chem. Res. 2009, 42, 1788–1798. [Google Scholar] [CrossRef]
- Krawczak, E.; Zdyb, A. The Effect of Electrode Immersion Time and Ageing on N719 Dye-Sensitized Solar Cells Performance. J. Ecol. Eng. 2020, 21, 53–60. [Google Scholar] [CrossRef]
- Wang, W.; Yuan, H.; Xie, J.; Xu, D.; Chen, X.; He, Y.; Zhang, T.; Chen, Z.; Zhang, Y.; Shen, H. Enhanced efficiency of large-area dye-sensitized solar cells by light-scattering effect using multilayer TiO2 photoanodes. Mater. Res. Bull. 2018, 100, 434–439. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Hsieh, T.-L. Dyes Amount and Light Scattering Influence on the Photocurrent Enhancement of Titanium Dioxide Hierarchically Structured Photoanodes for Dye-Sensitized Solar Cells. Coatings 2020, 10, 13. [Google Scholar] [CrossRef]
- Gladkova, O.L.; Panarin, A.; Khodasevich, I.A.; Terekhov, S.N. Surface-enhanced Raman spectra of a complex of antimony with phenylfluorone and their interpretation. Opt. Spectrosc. 2012, 112, 489–496. [Google Scholar] [CrossRef]
- de Sousa, J.M.; Couto, M.T.; Cassella, R.J. Polyurethane foam functionalized with phenylfluorone for online preconcentration and determination of copper and cadmium in water samples by flame atomic absorption spectrometry. Microchem. J. 2018, 138, 92–97. [Google Scholar] [CrossRef]
- Balaji, T.; Matsunaga, H. Naked-eye detection of fluoride using Zr(IV)-EDTA complex and pyrocatechol violet. Anal. Sci. 2005, 21, 973–977. [Google Scholar] [CrossRef][Green Version]
- Lee, C.; Ko, Y.-J.; Lee, S.-Y. A pyrocatechol violet-titanium alkoxide complex for HF sensing: Study on the complex structure and application. Dye. Pigment. 2016, 127, 133–141. [Google Scholar] [CrossRef]
- Gomez, T.; Hermann, G.; Zarate, X.; Pérez-Torres, J.F.; Tremblay, J.C. Imaging the Ultrafast Photoelectron Transfer Process in Alizarin-TiO2. Molecules 2015, 20, 13830–13853. [Google Scholar] [CrossRef] [PubMed]
- Galappaththi, K.; Ekanayake, P.; Petra, M.I. A rational design of high efficient and low-cost dye sensitizer with exceptional absorptions: Computational study of cyanidin based organic sensitizer. Sol. Energy 2018, 161, 83–89. [Google Scholar] [CrossRef]
- Islam, A.; Sugihara, H.; Arakawa, H. Molecular design of ruthenium(II) polypyridyl photosensitizers for efficient nanocrystalline TiO2 solar cells. J. Photochem. Photobiol. A Chem. 2003, 158, 131–138. [Google Scholar] [CrossRef]
- Feng, S.; Li, Q.-S.; Niehaus, T.A.; Li, Z.-S. Effects of different electron donating groups on dye regeneration and aggregation in phenothiazine-based dye-sensitized solar cells. Org. Electron. 2017, 42, 234–243. [Google Scholar] [CrossRef]
- Macyk, W.; Szaciłowski, K.; Stochel, G.; Buchalska, M.; Kuncewicz, J.; Łabuz, P. Titanium(IV) complexes as direct TiO2 photosensitizers. Coord. Chem. Rev. 2010, 254, 2687–2701. [Google Scholar] [CrossRef]
- Dayan, S.; Kayaci, N.; Özpozan, N.K. Improved performance with molecular design of Ruthenium(II) complexes bearing diamine-based bidentate ligands as sensitizer for dye-sensitized solar cells (DSSC). J. Mol. Struct. 2020, 1209, 127920. [Google Scholar] [CrossRef]
- Zhang, C.; Xie, Y.; Bai, T.; Hu, J.; Wang, J. Cooperation of multifunction composite structures and fluorescein for photovoltaic performance-enhanced ZnO-based dye-sensitized solar cells. J. Power Sources 2015, 297, 16–22. [Google Scholar] [CrossRef]
- Funabiki, K.; Sugiyama, N.; Iida, H.; Jin, J.-Y.; Yoshida, T.; Kato, Y.; Minoura, H.; Matsui, M. Ring-fluorinated fluoresceins as an organic photosensitizer for dye-sensitized solar cells using nanocrystalline zinc oxide. J. Fluor. Chem. 2006, 127, 257–262. [Google Scholar] [CrossRef]
- Sarker, A.K.; Kang, M.G.; Hong, J.-D. A near-infrared dye for dye-sensitized solar cell: Catecholate-functionalized zinc phthalocyanine. Dye. Pigment. 2012, 92, 1160–1165. [Google Scholar] [CrossRef]
- Manmeeta, S.D.; Saxena, D.; Sharma, G.D.; Roy, M.S. Improved performance of oxidized Alizarin based Quasi solid state Dye Sensitized solar cell by Surface Treatment. Res. J. Chem. Sci. 2012, 2, 61–71. [Google Scholar]
- Ramachandran, A.; Sreekala, C.O.; Sreelatha, K.S.; Jinchu, I. Photovoltaic studies of Dye Sensitized Solar cells Fabricated from Microwave Exposed Photo anodes. IOP Conf. Ser. Mater. Sci. Eng. 2018, 310, 012151. [Google Scholar] [CrossRef]
- Hamadanian, M.; Safaei-Ghomi, J.; Hosseinpour, M.; Masoomi, R.; Jabbari, V. Uses of new natural dye photosensitizers in fabrication of high potential dye-sensitized solar cells (DSSCs). Mater. Sci. Semicond. Process. 2014, 27, 733–739. [Google Scholar] [CrossRef]
- DeSilva, L.A.; Pitigala, P.K.D.D.P.; Gaquere-Parker, A.; Landry, R.; Hasbun, J.E.; Martin, V.; Bandara, T.M.W.J.; Perera, A.G.U. Broad absorption natural dye (Mondo-Grass berry) for dye sensitized solar cell. J. Mater. Sci. Mater. Electron. 2017, 28, 7724–7729. [Google Scholar] [CrossRef]
- Omar, A.; Ali, M.S.; Rahim, N.A. Electron transport properties analysis of titanium dioxide dye-sensitized solar cells (TiO2-DSSCs) based natural dyes using electrochemical impedance spectroscopy concept: A review. Sol. Energy 2020, 207, 1088–1121. [Google Scholar] [CrossRef]
- Błaszczyk, A.; Joachimiak-Lechman, K.; Sady, S.; Tański, T.; Szindler, M.; Drygała, A. Environmental performance of dye-sensitized solar cells based on natural dyes. Sol. Energy 2021, 215, 346–355. [Google Scholar] [CrossRef]
- Guermit, N.; Remache, L.; Lorrain, N.; Guendouz, M.; Charrier, J. Theoretical and experimental study of Fermi Bragg reflector as application for thin silicon solar cells. Opt. Mater. 2020, 100, 109615. [Google Scholar] [CrossRef]
- Ren, R.; Zhong, Z. Enhanced light absorption of silicon solar cells with dielectric nanostructured back reflector. Opt. Commun. 2018, 417, 110–114. [Google Scholar] [CrossRef]
- Wang, M.; Ye, X.; Wan, X.; Liu, Y.; Xie, X. Brilliant white polystyrene microsphere film as a diffuse back reflector for solar cells. Mater. Lett. 2015, 148, 122–125. [Google Scholar] [CrossRef]
- Berger, O.; Inns, D.; Aberle, A.G. Commercial white paint as back surface reflector for thin-film solar cells. Sol. Energy Mater. Sol. Cells 2007, 91, 1215–1221. [Google Scholar] [CrossRef]
- Gouillart, L.; Cattoni, A.; Goffard, J.; Donsanti, F.; Patriarche, G.; Jubault, M.; Naghavi, N.; Collin, S. Development of reflective back contacts for high-efficiency ultrathin Cu(In,Ga)Se2 solar cells. Thin Solid Film. 2019, 672, 1–6. [Google Scholar] [CrossRef]
- S., I.R.; Xu, X.; Yang, W.; Yang, F.; Hou, L.; Li, Y. Highly active and reflective MoS2 counter electrode for enhancement of photovoltaic efficiency of dye sensitized solar cells. Electrochim. Acta 2016, 212, 614–620. [Google Scholar] [CrossRef]
- Mohammadian-Sarcheshmeh, H.; Arazi, R.; Mazloum-Ardakani, M. Application of bifunctional photoanode materials in DSSCs: A review. Renew. Sustain. Energy Rev. 2020, 134, 110249. [Google Scholar] [CrossRef]
- Ramasamy, P.; Kim, J. Combined plasmonic and upconversion rear reflectors for efficient dye-sensitized solar cells. Chem. Commun. 2014, 50, 879–881. [Google Scholar] [CrossRef] [PubMed]
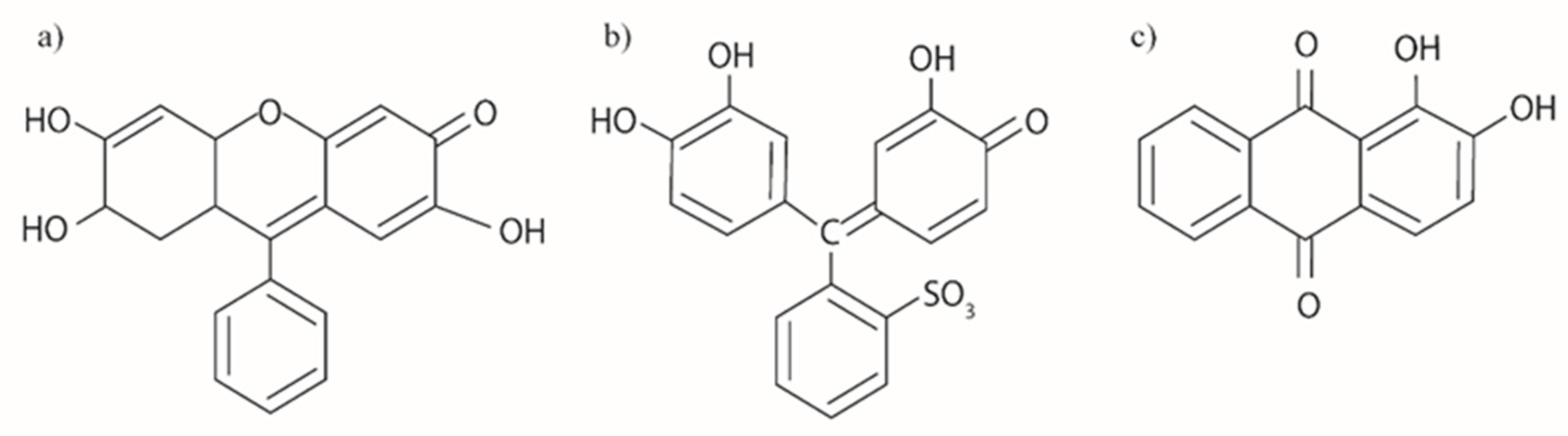
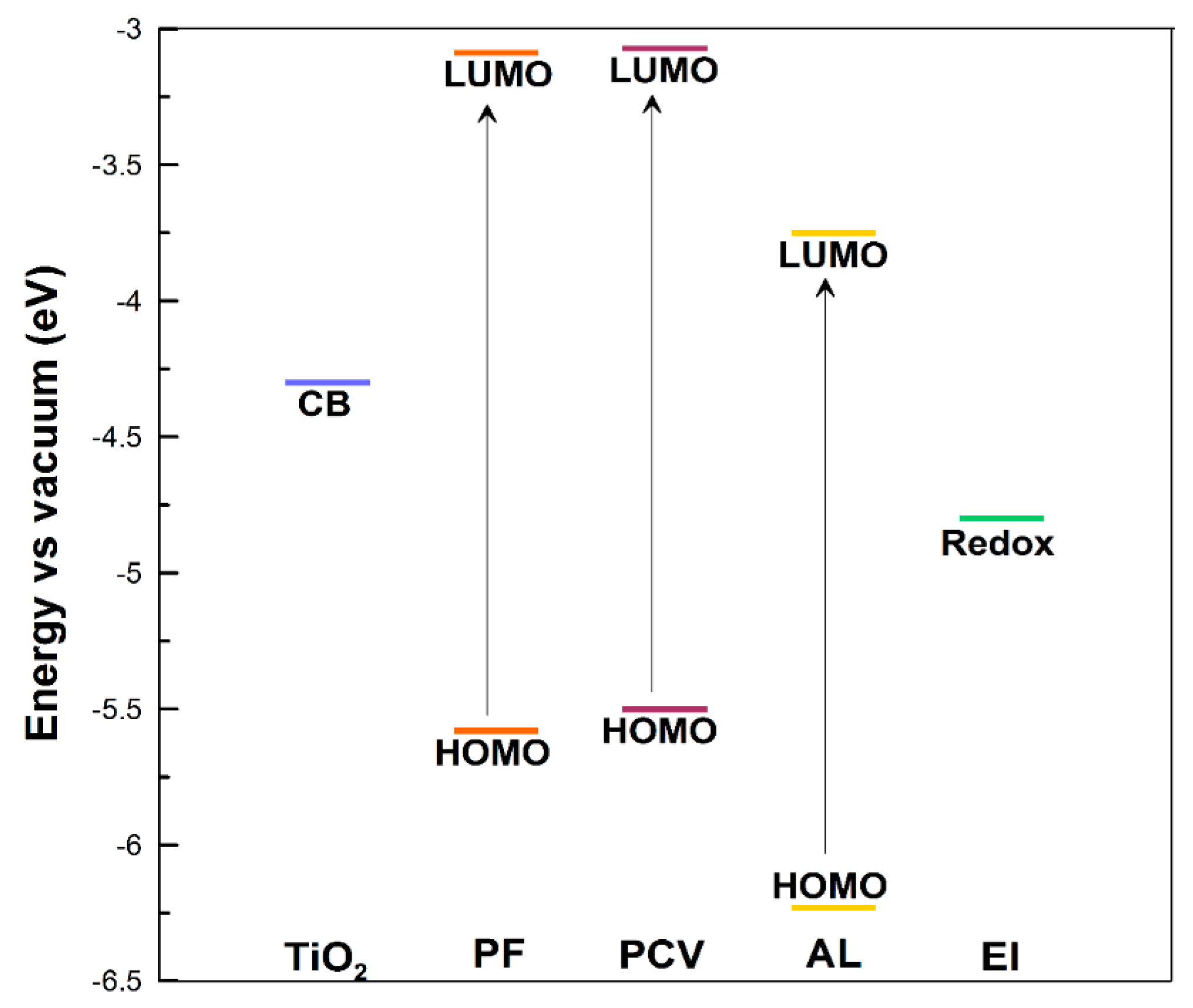
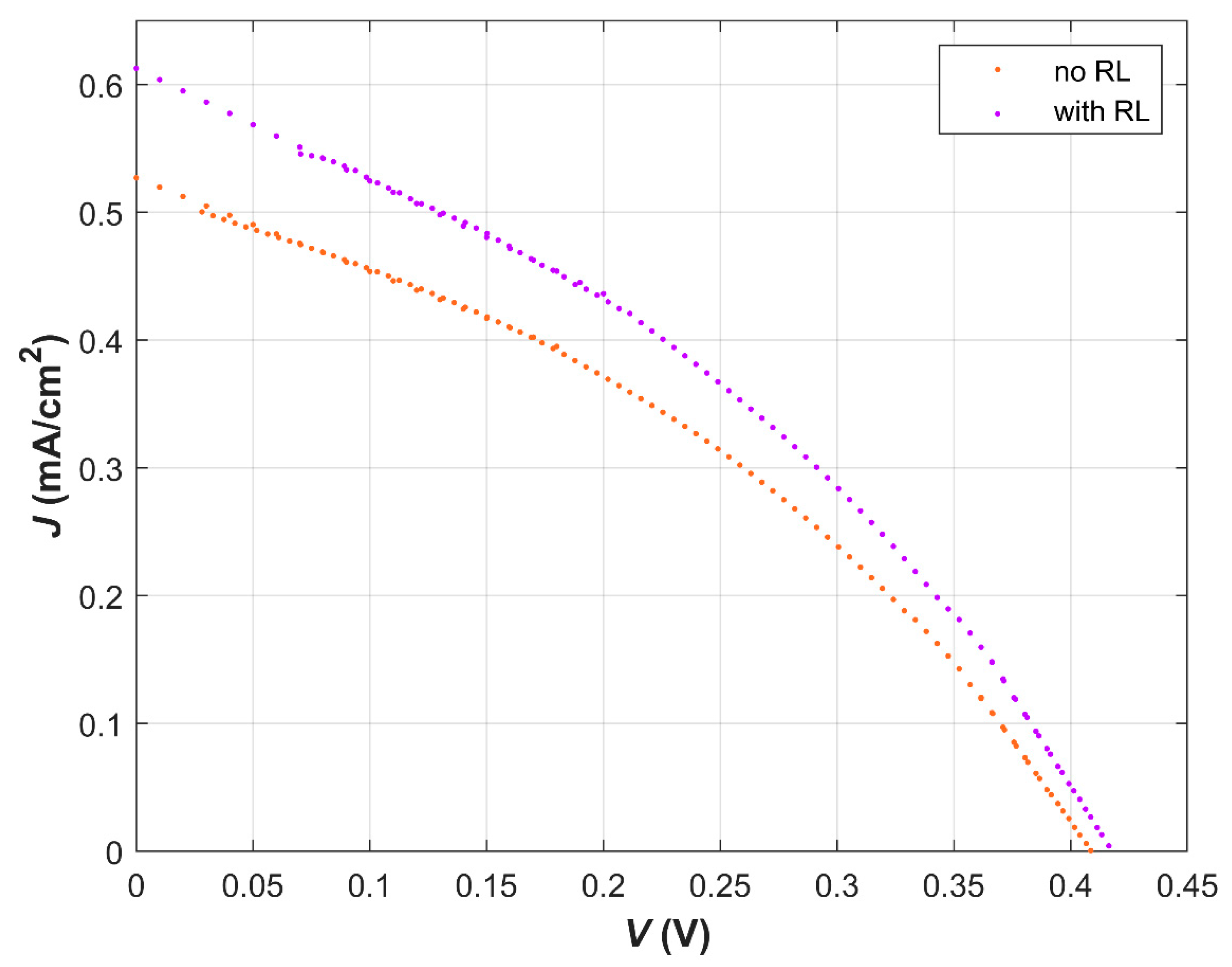
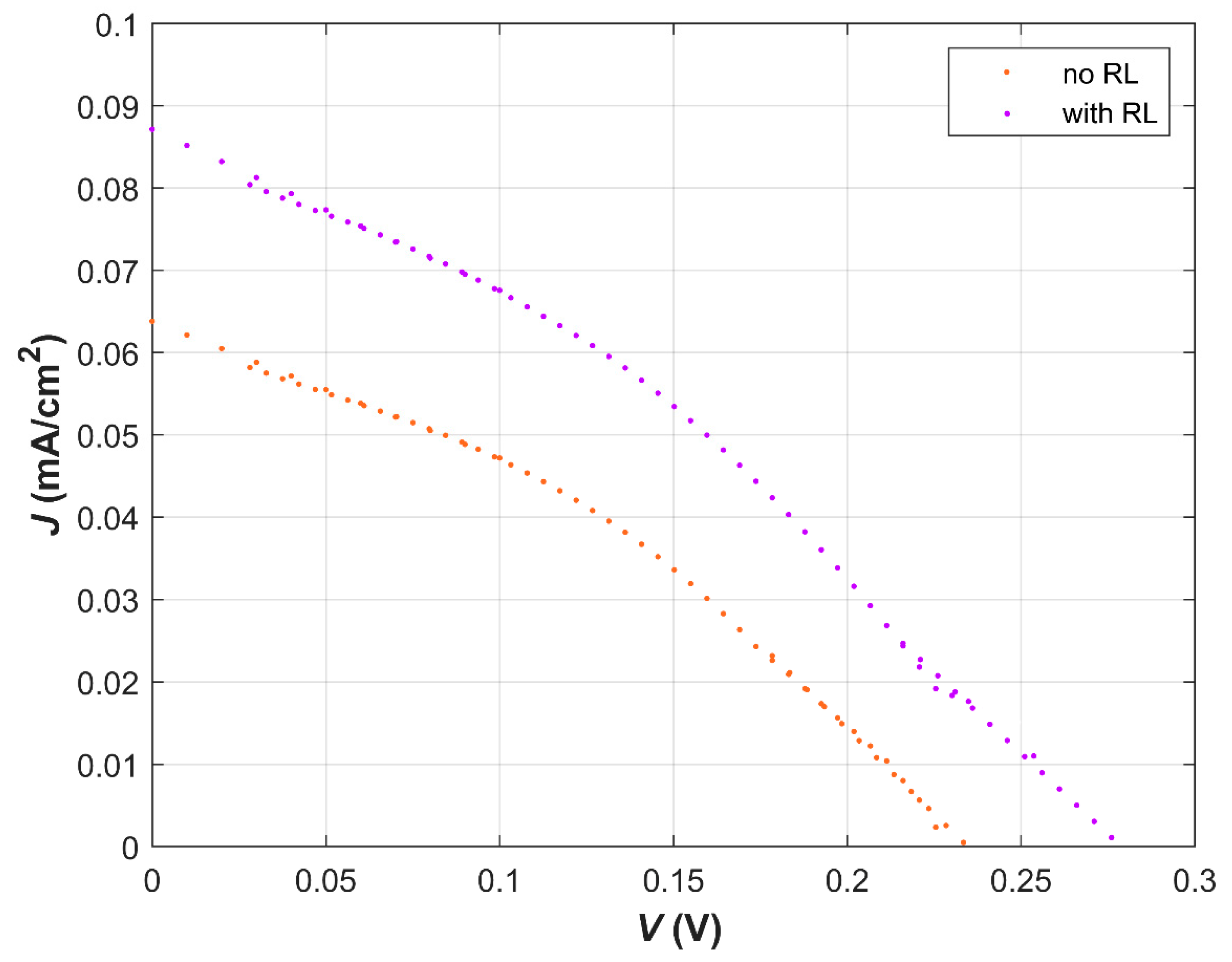
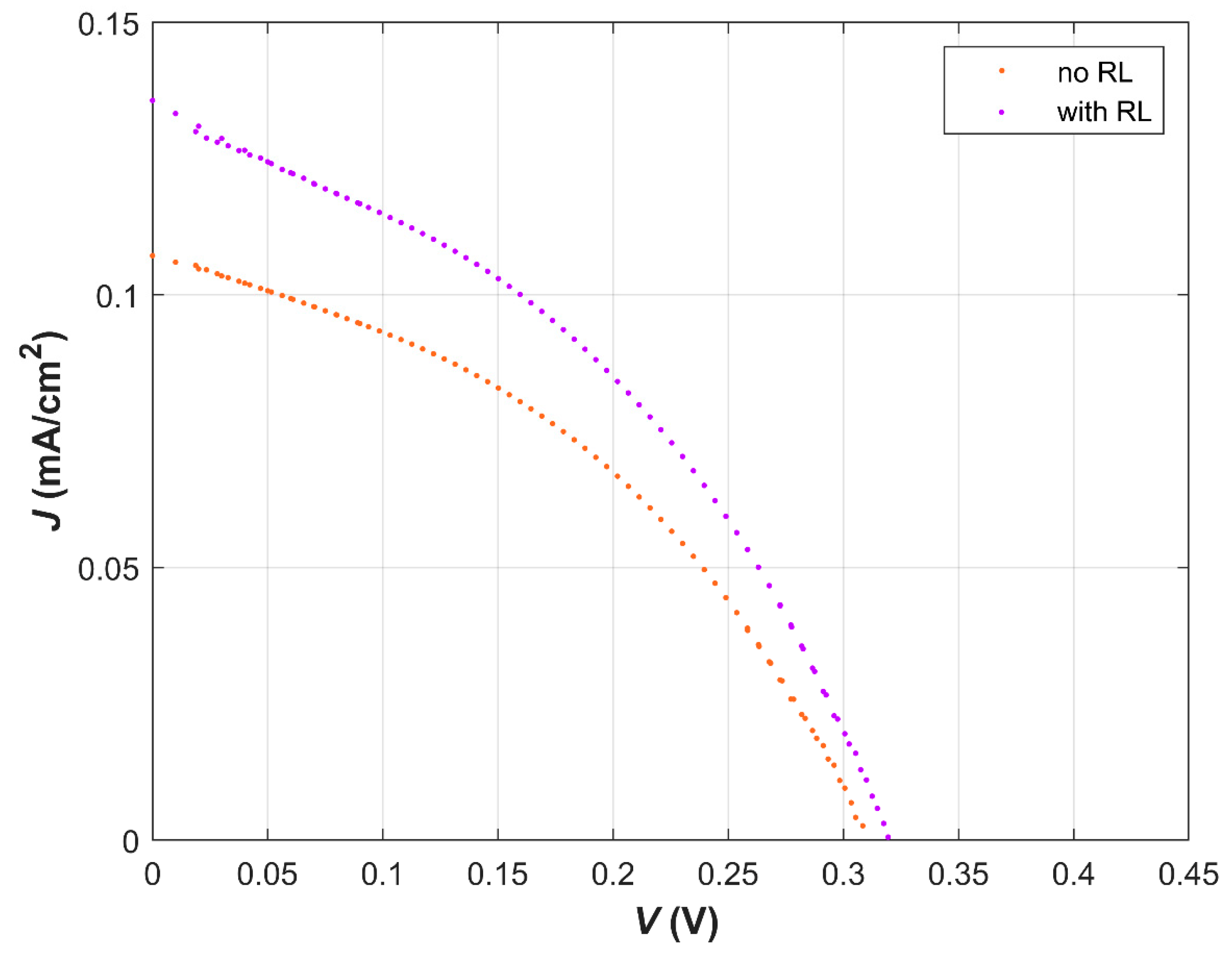
| Dye | λ (nm) at Max Abs. | E0−0 (eV) | EHOMO (eV) | ELUMO (eV) | ΔGinj (eV) | ΔGreg (eV) |
|---|---|---|---|---|---|---|
| PF | 498 | 2.49 | −5.58 | −3.09 | 1.21 | 0.78 |
| PCV | 511 | 2.426 | −5.5 | −3.074 | 1.23 | 0.70 |
| AL | 500 | 2.48 | −6.23 | −3.75 | 0.55 | 1.43 |
| Dye | μ (eV) | ηg (eV) | ω (eV) |
|---|---|---|---|
| PF | 4.335 | 1.245 | 7.545 |
| PCV | 4.287 | 1.213 | 7.575 |
| AL | 4.99 | 1.24 | 10.04 |
| PF | |||||
|---|---|---|---|---|---|
| Dipping Time (h) | JSC (mA/cm2) | VOC (mV) | Efficiency (%) | FF (%) | |
| No RL | 1 | 0.24 | 389.5 | 0.038 | 42.10 |
| With RL | 1 | 0.29 | 404.5 | 0.048 | 41.47 |
| No RL | 2 | 0.53 | 406.4 | 0.078 | 36.6 |
| With RL | 2 | 0.61 | 414.9 | 0.092 | 36.1 |
| No RL | 24 | 0.37 | 430.8 | 0.056 | 35 |
| With RL | 24 | 0.40 | 437.4 | 0.062 | 35.3 |
| No RL | 48 | 0.30 | 386.7 | 0.049 | 42.58 |
| With RL | 48 | 0.37 | 403.5 | 0.062 | 42.17 |
| PCV | |||||
| Dipping Time (h) | JSC(mA/cm2) | VOC(mV) | Efficiency (%) | FF(%) | |
| No RL | 0.5 | 0.063 | 208.9 | 0.002 | 18.84 |
| With RL | 0.5 | 0.067 | 227.0 | 0.004 | 25.23 |
| No RL | 1 | 0.069 | 228.4 | 0.005 | 33.44 |
| With RL | 1 | 0.092 | 266.7 | 0.008 | 32.6 |
| No RL | 2 | 0.077 | 216.4 | 0.004 | 27.14 |
| With RL | 2 | 0.089 | 251.6 | 0.007 | 31.12 |
| No RL | 24 | 0.078 | 182.9 | 0.003 | 22.26 |
| With RL | 24 | 0.084 | 206.4 | 0.005 | 26.66 |
| No RL | 48 | 0.087 | 119.1 | 0.002 | 18.64 |
| With RL | 48 | 0.1 | 151.5 | 0.003 | 19.67 |
| AL | |||||
| Dipping Time (h) | JSC(mA/cm2) | VOC(mV) | Efficiency (%) | FF(%) | |
| No RL | 1 | 0.076 | 279.8 | 0.008 | 37.24 |
| With RL | 1 | 0.086 | 289.4 | 0.009 | 37.13 |
| No RL | 2 | 0.112 | 308.2 | 0.014 | 39.14 |
| With RL | 2 | 0.138 | 318.1 | 0.017 | 38.79 |
| No RL | 24 | 0.078 | 284.6 | 0.008 | 37.34 |
| With RL | 24 | 0.083 | 288.1 | 0.009 | 37.33 |
| No RL | 48 | 0.105 | 128.7 | 0.002 | 16.31 |
| With RL | 48 | 0.11 | 130.2 | 0.003 | 18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zdyb, A.; Krawczak, E. Organic Dyes in Dye-Sensitized Solar Cells Featuring Back Reflector. Energies 2021, 14, 5529. https://doi.org/10.3390/en14175529
Zdyb A, Krawczak E. Organic Dyes in Dye-Sensitized Solar Cells Featuring Back Reflector. Energies. 2021; 14(17):5529. https://doi.org/10.3390/en14175529
Chicago/Turabian StyleZdyb, Agata, and Ewelina Krawczak. 2021. "Organic Dyes in Dye-Sensitized Solar Cells Featuring Back Reflector" Energies 14, no. 17: 5529. https://doi.org/10.3390/en14175529
APA StyleZdyb, A., & Krawczak, E. (2021). Organic Dyes in Dye-Sensitized Solar Cells Featuring Back Reflector. Energies, 14(17), 5529. https://doi.org/10.3390/en14175529






