Hierarchical Auto-Ignition and Structure-Reactivity Trends of C2–C4 1-Alkenes
Abstract
:1. Introduction
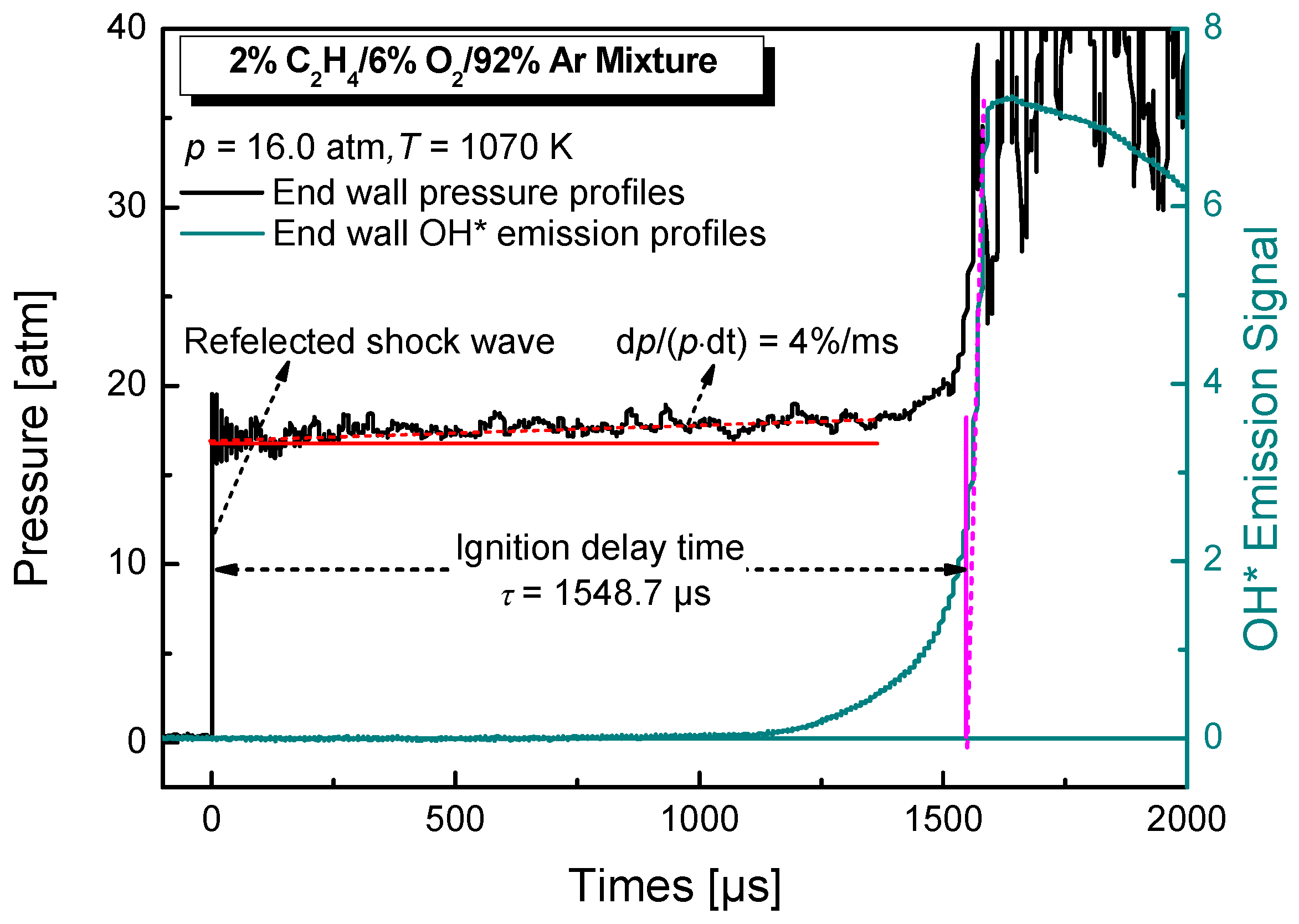
2. Experimental Details
3. Hierarchical Kinetics of 1–Alkenes
3.1. Comparison of Chemical Bond Energy
3.2. Reaction Scheme for Pyrolysis and Oxidation of 1-Alkenes
3.2.1. Unimolecular Reactions
3.2.2. H-Atom Abstraction Reactions
3.2.3. Fuel Radical Reactions
3.2.4. Radical Addition Reactions
4. Ignition Delay Times of C2–C4 1-Alkenes
4.1. Model Performance Comparison
4.2. Auto-Ignition of C2–C4 1-Alkenes
4.2.1. Pressure Dependent Behavior
4.2.2. Equivalence Ratio Dependence
4.2.3. Effect of Dilution
4.3. Arrhenius-Type Correlation of C2–C4 1-Alkenes
5. Reactivity Comparison at High Temperature
6. Concluding Remarks
- (1)
- Chemical kinetics scheme of 1-alkenes was highlighted according to the precious chemical studies on alkenes. Pressure-dependence of ethylene shows much more difference compared with propene and 1-butene due to different oxidation mechanisms at low temperatures. Nine generally accepted mechanisms, developed by different research groups, and published in recent years were used to simulate the ignition delay times from literature and the current study, only NUIGMech 1.1 was capable of representing the chemical reactivity for all of the three alkenes at all tested conditions.
- (2)
- A new type of Arrhenius correlation for the three alkenes was proposed against all the ignition data measured in the literature and this study, that can capture the various activation energy with temperature for propene and 1-butene due to essential difference chemistry at high and low-temperatures. The correlations can be used to predict IDTs in engineering with a wide range of pressure, temperature, equivalence ratio and dilution.
- (3)
- At high temperatures, ethylene shows the shortest ignition delay times, while propene shows the longest ones, with intermediate reactivity for 1-butene. The oxidation of ethylene depends on the Ḣ atom, Ö atom, and ȮH radical, and the consumption of vinylic radical accelerates the accumulation of the free radical pool, resulting in the highest reactivity of ethylene. The consumption of allylic radicals becomes a decisive step in propene and 1-butene by HȮ2 radicals. However, it has the efficient reaction pathways for HȮ2 formation in 1-butene (Ċ4H71-3 + O2 <=> C4H6 + HȮ2 and Ċ2H5 + O2 = C2H4 + HȮ2), but is not involved in propene.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cullen, J.M.; Allwood, J.M. The efficient use of energy: Tracing the global flow of energy from fuel to service. Energy Policy 2010, 38, 75–81. [Google Scholar] [CrossRef]
- Omid, M.; Ghojabeige, F.; Delshad, M.; Ahmadi, H. Energy use pattern and benchmarking of selected greenhouses in Iran using data envelopment analysis. Energy Convers. Manag. 2011, 52, 153–162. [Google Scholar] [CrossRef]
- Ranzi, E.; Cavallotti, C.; Cuoci, A.; Frassoldati, A.; Pelucchi, M.; Faravelli, T. New reaction classes in the kinetic modeling of low temperature oxidation of n-alkanes. Combust. Flame 2015, 162, 1679–1691. [Google Scholar] [CrossRef]
- Metcalfe, W.K.; Burke, S.M.; Ahmed, S.S.; Curran, H.J. A Hierarchical and Comparative Kinetic Modeling Study of C1–C2 Hydrocarbon and Oxygenated Fuels. Int. J. Chem. Kinet. 2013, 45, 638–675. [Google Scholar] [CrossRef]
- Healy, D.; Kopp, M.M.; Polley, N.L.; Petersen, E.L.; Bourque, G.; Curran, H.J. Methane/n-Butane Ignition Delay Measurements at High Pressure and Detailed Chemical Kinetic Simulations. Energy Fuels 2010, 24, 1617–1627. [Google Scholar] [CrossRef]
- Burke, U.; Metcalfe, W.K.; Burke, S.M.; Heufer, K.A.; Dagaut, P.; Curran, H.J. A detailed chemical kinetic modeling, ignition delay time and jet-stirred reactor study of methanol oxidation. Combust. Flame 2016, 165, 125–136. [Google Scholar] [CrossRef] [Green Version]
- Mittal, G.; Burke, S.M.; Davies, V.A.; Parajuli, B.; Metcalfe, W.K.; Curran, H.J. Autoignition of ethanol in a rapid compression machine. Combust. Flame 2014, 161, 1164–1171. [Google Scholar] [CrossRef] [Green Version]
- Dooley, S.; Curran, H.J.; Simmie, J.M. Autoignition measurements and a validated kinetic model for the biodiesel surrogate, methyl butanoate. Combust. Flame 2008, 153, 2–32. [Google Scholar] [CrossRef]
- Metcalfe, W.K.; Togbé, C.; Dagaut, P.; Curran, H.J.; Simmie, J.M. A jet-stirred reactor and kinetic modeling study of ethyl propanoate oxidation. Combust. Flame 2009, 156, 250–260. [Google Scholar] [CrossRef]
- Zhang, J.; Pan, L.; Mo, J.; Gong, J.; Huang, Z.; Law, C.K. A shock tube and kinetic modeling study of n-butanal oxidation. Combust. Flame 2013, 160, 1541–1549. [Google Scholar] [CrossRef]
- Richter, H.; Howard, J.B. Formation of polycyclic aromatic hydrocarbons and their growth to soot—a review of chemical reaction pathways. Prog. Energy Combust. Sci. 2000, 26, 565–608. [Google Scholar] [CrossRef]
- Grana, R.; Frassoldati, A.; Faravelli, T.; Niemann, U.; Ranzi, E.; Seiser, R.; Cattolica, R.; Seshadri, K. An experimental and kinetic modeling study of combustion of isomers of butanol. Combust. Flame 2010, 157, 2137–2154. [Google Scholar] [CrossRef]
- Yoshiaki, H.; Tsuyoshi, K.; Masao, S. A Shock-tube Investigation of Ignition in Ethylene–Oxygen–Argon Mixtures. Bull. Chem. Soc. Jpn. 1974, 47, 2166–2170. [Google Scholar]
- Brown, C.J.; Thomas, G.O. Experimental studies of shock-induced ignition and transition to detonation in ethylene and propane mixtures. Combust. Flame 1999, 117, 861–870. [Google Scholar] [CrossRef]
- Horning, D.C. A Study of the Hige-temperature Autoignition and Thermal Decomposition of Hydrocarbons; Stanford University: Stanford, CA, USA, 2001. [Google Scholar]
- Colket, M.B., III; Spadaccini, L.J. Scramjet Fuels Autoignition Study. J. Propuls. Power 2001, 17, 315–323. [Google Scholar] [CrossRef]
- Kalitan, D.M.; Hall, J.M.; Petersen, E.L. Ignition and Oxidation of Ethylene-Oxygen-Diluent Mixtures with and Without Silane. J. Propuls. Power 2005, 21, 1045–1056. [Google Scholar] [CrossRef]
- Kumar, K.; Mittal, G.; Sung, C.-J.; Law, C.K. An experimental investigation of ethylene/O2/diluent mixtures: Laminar flame speeds with preheat and ignition delays at high pressures. Combust. Flame 2008, 153, 343–354. [Google Scholar] [CrossRef]
- Penyazkov, O.G.; Sevrouk, K.L.; Tangirala, V.; Joshi, N. High-pressure ethylene oxidation behind reflected shock waves. Proc. Combust. Inst. 2009, 32, 2421–2428. [Google Scholar] [CrossRef]
- Tereza, A.M.; Slutskii, V.G.; Severin, E.S. Autoignition of ethylene in shock waves. Russ. J. Phys. Chem. B 2010, 4, 475–485. [Google Scholar] [CrossRef]
- Saxena, S.; Kahandawala, M.S.P.; Sidhu, S.S. A shock tube study of ignition delay in the combustion of ethylene. Combust. Flame 2011, 158, 1019–1031. [Google Scholar] [CrossRef]
- Davidson, D.; Ren, W.; Hanson, R. Experimental Database for Development of a HiFiRE JP-7 Surrogate Fuel Mechanism. In Proceedings of the 50th AIAA Aerospace Sciences Meeting including the New Horizons Forum and Aerospace Exposition, Nashville, TN, USA, 9–12 January 2012; American Institute of Aeronautics and Astronautics: Reston, VA, USA, 2012. [Google Scholar]
- Kopp, M.M.; Donato, N.S.; Petersen, E.L.; Metcalfe, W.K.; Burke, S.M.; Curran, H.J. Oxidation of Ethylene–Air Mixtures at Elevated Pressures, Part 1: Experimental Results. J. Propuls. Power 2014, 30, 790–798. [Google Scholar] [CrossRef] [Green Version]
- Kopp, M.M.; Petersen, E.L.; Metcalfe, W.K.; Burke, S.M.; Curran, H.J. Oxidation of Ethylene—Air Mixtures at Elevated Pressures, Part 2: Chemical Kinetics. J. Propuls. Power 2014, 30, 799–811. [Google Scholar] [CrossRef] [Green Version]
- Mathieu, O.; Goulier, J.; Gourmel, F.; Mannan, M.S.; Chaumeix, N.; Petersen, E.L. Experimental study of the effect of CF3I addition on the ignition delay time and laminar flame speed of methane, ethylene, and propane. Proc. Combust. Inst. 2015, 35, 2731–2739. [Google Scholar] [CrossRef]
- Xiong, X.-H.; Ding, Y.-J.; Cao, X.-B.; Peng, Z.-M.; Li, Y.-H. Ethylene Oxidation Experimental Study and Kinetic Mechanism Analysis Based on Shock Tube. Acta Phys. Chim. Sin. 2016, 32, 1416–1423. [Google Scholar] [CrossRef]
- Shao, J.; Davidson, D.F.; Hanson, R.K. A shock tube study of ignition delay times in diluted methane, ethylene, propene and their blends at elevated pressures. Fuel 2018, 225, 370–380. [Google Scholar] [CrossRef]
- Baigmohammadi, M.; Patel, V.; Martinez, S.; Panigrahy, S.; Ramalingam, A.; Burke, U.; Somers, K.P.; Heufer, K.A.; Pekalski, A.; Curran, H.J. A Comprehensive Experimental and Simulation Study of Ignition Delay Time Characteristics of Single Fuel C1–C2 Hydrocarbons over a Wide Range of Temperatures, Pressures, Equivalence Ratios, and Dilutions. Energy Fuels 2020, 34, 3755–3771. [Google Scholar] [CrossRef]
- Dong, S.; Zhang, K.; Senecal, P.K.; Kukkadapu, G.; Wagnon, S.W.; Barrett, S.; Lokachari, N.; Panigaphy, S.; Pitz, W.J.; Curran, H.J. A comparative reactivity study of 1-alkene fuels from ethylene to 1-heptene. Proc. Combust. Inst. 2021, 38, 611–619. [Google Scholar] [CrossRef]
- Wan, Z.; Zheng, Z.; Wang, Y.; Zhang, D.; Li, P.; Zhang, C. A Shock Tube Study of Ethylene/Air Ignition Characteristics over a Wide Temperature Range. Combust. Sci. Technol. 2020, 192, 2297–2305. [Google Scholar] [CrossRef]
- Xiong, X.; Lv, Z.; Tan, H.; Peng, Z.; Ding, Y. Shock tube evaluation on C2H4 ignition delay differences among N2, Ar, He, CO2 diluent gases. J. Energy Inst. 2020, 93, 1271–1277. [Google Scholar] [CrossRef]
- Yang, M.; Wan, Z.; Tan, N.; Zhang, C.; Wang, J.; Li, X. An experimental and modeling study of ethylene–air combustion over a wide temperature range. Combust. Flame 2020, 221, 20–40. [Google Scholar] [CrossRef]
- Marinov, N.M.; Pitz, W.J.; Westbrook, C.K.; Vincitore, A.M.; Castaldi, M.J.; Senkan, S.M.; Melius, C.F. Aromatic and Polycyclic Aromatic Hydrocarbon Formation in a Laminar Premixed n-Butane Flame. Combust. Flame 1998, 114, 192–213. [Google Scholar] [CrossRef]
- Burcat, A.; Radhakrishnan, K. High temperature oxidation of propene. Combust. Flame 1985, 60, 157–169. [Google Scholar] [CrossRef]
- Qin, Z.; Yang, H.; Gardiner, W.C. Measurement and modeling of shock-tube ignition delay for propene. Combust. Flame 2001, 124, 246–254. [Google Scholar] [CrossRef]
- Heyberger, B.; Belmekki, N.; Conraud, V.; Glaude, P.-A.; Fournet, R.; Battin-Leclerc, F. Oxidation of small alkenes at high temperature. Int. J. Chem. Kinet. 2002, 34, 666–677. [Google Scholar] [CrossRef]
- Leon, L.; Goos, E.; Klauer, C.; Seidel, L.; Zeuch, T. Evaluation of the influence of thermodynamic data for propane and propene ignition delay time. In Proceedings of the 6th European Combustion Meeting (ECM), Lund, Sweden, 25–28 June 2013. [Google Scholar]
- Burke, S.M.; Metcalfe, W.; Herbinet, O.; Battin-Leclerc, F.; Haas, F.M.; Santner, J.; Dryer, F.L.; Curran, H.J. An experimental and modeling study of propene oxidation. Part 1: Speciation measurements in jet-stirred and flow reactors. Combust. Flame 2014, 161, 2765–2784. [Google Scholar] [CrossRef] [Green Version]
- Burke, S.M.; Burke, U.; Mc Donagh, R.; Mathieu, O.; Osorio, I.; Keesee, C.; Morones, A.; Petersen, E.L.; Wang, W.; DeVerter, T.A.; et al. An experimental and modeling study of propene oxidation. Part 2: Ignition delay time and flame speed measurements. Combust. Flame 2015, 162, 296–314. [Google Scholar] [CrossRef] [Green Version]
- Westbrook, C.K.; Pitz, W.J. A Comprehensive Chemical Kinetic Reaction Mechanism for Oxidation and Pyrolysis of Propane and Propene. Combust. Sci. Technol. 1984, 37, 117–152. [Google Scholar] [CrossRef]
- Dagaut, P.; Cathonnet, M.; Boettner, J.C. Experimental study and kinetic modeling of propene oxidation in a jet stirred flow reactor. J. Phys. Chem. 1988, 92, 661–671. [Google Scholar] [CrossRef]
- Pan, L.; Hu, E.; Zhang, J.; Tian, Z.; Li, X.; Huang, Z. A high pressure shock tube study of 1-butene oxidation and its comparison with n-butane and alkenes. Fuel 2015, 157, 21–27. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, C.-W.; Curran, H.J. An extensive experimental and modeling study of 1-butene oxidation. Combust. Flame 2017, 181, 198–213. [Google Scholar] [CrossRef] [Green Version]
- Kikui, S.; Nakamura, H.; Tezuka, T.; Hasegawa, S.; Maruta, K. Study on combustion and ignition characteristics of ethylene, propylene, 1-butene and 1-pentene in a micro flow reactor with a controlled temperature profile. Combust. Flame 2016, 163, 209–219. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhou, C.-W.; Somers, K.P.; Zhang, K.; Curran, H.J. The oxidation of 2-butene: A high pressure ignition delay, kinetic modeling study and reactivity comparison with isobutene and 1-butene. Proc. Combust. Inst. 2017, 36, 403–411. [Google Scholar] [CrossRef] [Green Version]
- Jach, A.; Rudy, W.; Pękalski, A.; Teodorczyk, A. Assessment of detailed reaction mechanisms for reproduction of ignition delay times of C2–C6 alkenes and acetylene. Combust. Flame 2019, 206, 37–50. [Google Scholar] [CrossRef]
- Nagaraja, S.S.; Liang, J.; Dong, S.; Panigrahy, S.; Sahu, A.; Kukkadapu, G.; Wagnon, S.W.; Pitz, W.J.; Curran, H.J. A hierarchical single-pulse shock tube pyrolysis study of C2–C6 1-alkenes. Combust. Flame 2020, 219, 456–466. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Z.; Wei, L.; Zhang, J.; Law, C.K. Experimental and modeling study on ignition delays of lean mixtures of methane, hydrogen, oxygen, and argon at elevated pressures. Combust. Flame 2012, 159, 918–931. [Google Scholar] [CrossRef]
- Chris, M. Gaseq Version 0.79. Available online: http://www.gaseq.co.uk (accessed on 21 January 2018).
- Deng, F.; Yang, F.; Zhang, P.; Pan, Y.; Bugler, J.; Curran, H.J.; Zhang, Y.; Huang, Z. Towards a kinetic understanding of the NOx promoting-effect on ignition of coalbed methane: A case study of methane/nitrogen dioxide mixtures. Fuel 2016, 181, 188–198. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Huang, W.; Qin, X.; Deng, Y.; Kang, Y.; Peng, W.; Zhang, Y.; Huang, Z. Water impact on the auto-ignition of kerosene/air mixtures under combustor relevant conditions. Fuel 2020, 267, 117184. [Google Scholar] [CrossRef]
- Petersen, E.L.; Rickard, M.J.A.; Crofton, M.W.; Abbey, E.D.; Traum, M.J.; Kalitan, D.M. A facility for gas- and condensed-phase measurements behind shock waves. Meas. Sci. Technol. 2005, 16, 1716–1729. [Google Scholar] [CrossRef]
- CHMEKIN-PRO; Reaction Design Inc.: San Diego, CA, USA; Available online: https://www.Ansys.com/ (accessed on 25 September 2017).
- Deng, F.; Yang, F.; Zhang, P.; Pan, Y.; Zhang, Y.; Huang, Z. Ignition delay time and chemical kinetic study of methane and nitrous oxide mixtures at high temperatures. Energy Fuels 2016, 30, 1415–1427. [Google Scholar] [CrossRef]
- Chaos, M.; Dryer, F.L. Chemical-kinetic modeling of ignition delay: Considerations in interpreting shock tube data. Int. J. Chem. Kinet. 2010, 42, 143–150. [Google Scholar] [CrossRef]
- Bross, B.R.D.H. Active Thermochemical Tables (ATcT) values based on ver. 1.122p of the Thermochemical Network. 2020. Available online: ATcT.anl.gov (accessed on 8 March 2020).
- Baigmohammadi, M.; Patel, V.; Nagaraja, S.; Ramalingam, A.; Martinez, S.; Panigrahy, S.; Mohamed, A.A.E.-S.; Somers, K.P.; Burke, U.; Heufer, K.A.; et al. Comprehensive experimental and simulation study of the ignition delay time characteristics of binary blended methane, ethane, and ethylene over a wide range of temperature, pressure, equivalence ratio, and dilution. Energy Fuels 2020, 34, 8808–8823. [Google Scholar] [CrossRef]
- Lokachari, N.; Panigrahy, S.; Kukkadapu, G.; Kim, G.; Vasu, S.S.; Pitz, W.J.; Curran, H.J. The influence of iso-butene kinetics on the reactivity of di-isobutylene and iso-octane. Combust. Flame 2020, 222, 186–195. [Google Scholar] [CrossRef]
- Nagaraja, S.S.; Power, J.; Kukkadapu, G.; Dong, S.; Wagnon, S.W.; Pitz, W.J.; Curran, H.J. A single pulse shock tube study of pentene isomer pyrolysis. Proc. Combust. Inst. 2020, 38, 881–889. [Google Scholar] [CrossRef]
- Panigrahy, S.; Liang, J.; Nagaraja, S.S.; Zuo, Z.; Kim, G.; Dong, S.; Kukkadapu, G.; Pitz, W.J.; Vasu, S.S.; Curran, H.J. A comprehensive experimental and improved kinetic modeling study on the pyrolysis and oxidation of propyne. Proc. Combust. Inst. 2020, 38, 479–488. [Google Scholar] [CrossRef]
- Dong, S.; Zhang, K.; Ninnemann, E.M.; Najjar, A.; Kukkadapu, G.; Baker, J.; Arafin, F.; Wang, Z.; Pitz, W.J.; Vasu, S.S.; et al. A comprehensive experimental and kinetic modeling study of 1- and 2-pentene. Combust. Flame 2021, 223, 166–180. [Google Scholar] [CrossRef]
- Ramalingam, A.; Panigrahy, S.; Fenard, Y.; Curran, H.; Heufer, K.A. A chemical kinetic perspective on the low-temperature oxidation of propane/propene mixtures through experiments and kinetic analyses. Combust. Flame 2021, 223, 361–375. [Google Scholar] [CrossRef]
- Wu, Y.; Panigrahy, S.; Sahu, A.B.; Bariki, C.; Beeckmann, J.; Liang, J.; Mohamed, A.A.; Dong, S.; Tang, C.; Pitsch, H.; et al. Understanding the antagonistic effect of methanol as a component in surrogate fuel models: A case study of methanol/n-heptane mixtures. Combust. Flame 2021, 226, 229–242. [Google Scholar] [CrossRef]
- Kéromnès, A.; Metcalfe, W.K.; Heufer, K.A.; Donohoe, N.; Das, A.K.; Sung, C.-J.; Herzler, J.; Naumann, C.; Griebel, P.; Mathieu, O.; et al. An experimental and detailed chemical kinetic modeling study of hydrogen and syngas mixture oxidation at elevated pressures. Combust. Flame 2013, 160, 995–1011. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.-W.; Li, Y.; O’Connor, E.; Somers, K.P.; Thion, S.; Keesee, C.; Mathieu, O.; Petersen, E.L.; DeVerter, T.A.; Oehlschlaeger, M.A.; et al. A comprehensive experimental and modeling study of isobutene oxidation. Combust. Flame 2016, 167, 353–379. [Google Scholar] [CrossRef] [Green Version]
- Ranzi, E.; Frassoldati, A.; Stagni, A.; Pelucchi, M.; Cuoci, A.; Faravelli, T. Reduced Kinetic Schemes of Complex Reaction Systems: Fossil and Biomass-Derived Transportation Fuels. Int. J. Chem. Kinet. 2014, 46, 512–542. [Google Scholar] [CrossRef]
- Pejpichestakul, W.; Ranzi, E.; Pelucchi, M.; Frassoldati, A.; Cuoci, A.; Parente, A.; Parente, A.; Faravelli, T. Examination of a soot model in premixed laminar flames at fuel-rich conditions. Proc. Combust. Inst. 2019, 37, 1013–1021. [Google Scholar] [CrossRef]
- Chemical-Kinetic Mechanisms for Combustion Applications. Available online: http://combustion.ucsd.edu (accessed on 7 December 2020).
- Wang, H.; Dames, E.; Sirjean, B.; Sheen, D.A.; Tango, R.; Violi, A.; Lai, J.Y.W.; Egolfopoulos, F.N.; Davidson, D.F.; Hanson, R.K.; et al. A high-temperature chemical kinetic model of n-alkane (up to n-dodecane), cyclohexane, and methyl-, ethyl-, n-propyl and n-butyl-cyclohexane oxidation at high temperatures, JetSurF version 2.0. 19 September 2010. Available online: http://melchior.usc.edu/JetSurF/JetSurF2.0 (accessed on 15 November 2020).
- Konnov, A.A. Implementation of the NCN pathway of prompt-NO formation in the detailed reaction mechanism. Combust. Flame 2009, 156, 2093–2105. [Google Scholar] [CrossRef]
- Wang, H.; You, X.; Joshi, A.V.; Davis, S.G.; Laskin, A.; Egolfopoulos, F.; Law, C.K. USC Mech Version II. High-Temperature Combustion Reaction Model of H2/CO/C1-C4 Compounds. May 2007. Available online: http://ignis.usc.edu/USC_Mech_II.htm (accessed on 14 December 2020).
- Lopez, J.G.; Rasmussen, C.L.; Alzueta, M.U.; Gao, Y.; Marshall, P.; Glarborg, P. Experimental and kinetic modeling study of C2H4 oxidation at high pressure. Proc. Combust. Inst. 2009, 32, 367–375. [Google Scholar] [CrossRef]





















| Mixture | Fuel | Xfuel (%) | XO2 (%) | XAR (%) |
|---|---|---|---|---|
| 1 | ethylene | 2.0 | 6 | 92.0 |
| 2 | propene | 1.33 | 6 | 92.67 |
| 2 | 1-butene | 1.0 | 6 | 93.0 |
| Experimental Device | Experimental Conditions | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Type | Diameter (cm) | Diagnostic | Mixture | Dilution | φ | T (K) | p (atm) | IDT (us) Range | |
| ST | 4.3 | CH* oneset at sidewall | C2H4/O2/Ar | 96% | 1.0 | 1422–2042 | 1.77–3.12 | <108 | Hidaka et al. [13] |
| 97% | 1.5 | 1469–2081 | 1.86–3.12 | <152 | |||||
| 98% | 3.0 | 1596–2077 | 2.42–5.00 | <62 | |||||
| ST | 7.6 × 3.8 cross section | CH* oneset at endwall | C2H4/O2/Ar | 75%/96% | 1.0 | 1102–2236 | 1.26–4.09 | <765.44 | Brown and Thomas [14] |
| C2H4/O2/N2 | 75% | 1.0 | 1073–1566 | 2.22–4.74 | <755.82 | ||||
| ST | 15.24 | d[CH*]/dt max at endwall | C2H4/O2/Ar | 84%/92%/96% | 1.0 | 1253–1572 | 1,2 and 4 | <248 | Horning et al. [15] |
| ST | 3.8 | OH* oneset at sidewall | C2H4/O2/Ar | 95.10% | 0.5 | 1125–1308 | 4.83–7.89 | <948 | Collect and Spadaccini [16] |
| 96.50% | 0.75 | 1182–1350 | 5.76–7.53 | <524 | |||||
| 97.20% | 1.0 | 1380–1414 | 6.58–7.64 | <136 | |||||
| ST | 16.2 | d[OH*]/dt max at endwall | C2H4/O2/Ar | 96%/98% | 1.0 | 1223–1746 | 0.9–3.3 | <1780 | Kalitan et al. [17] |
| 96%/98% | 0.5 | 1115–1754 | 1–1.38 | <3397 | |||||
| ST | 5.08 | CH*/OH* oneset, Visibel light at endwall | C2H4/O2/Ar | 93%/96%/98% | 1.0 | 1034–1828 | 2,10 and 18 | <4200 | Saxena et al. [21] |
| 93% | 3.0 | 1000–1592 | 2,10 and 18 | <4404 | |||||
| ST | 15.24 | d[CH*]/dt max at endwall | C2H4/O2/Ar | 95.33% | 0.5 | 1113–1244 | 15 | <1708 | Davidson et al. [22] |
| 94.67% | 1.0 | 1130–1267 | 15,35 | <1119 | |||||
| 93.33% | 2.0 | 1099–1216 | 15 | <1325 | |||||
| ST | 15.24 | d[OH*]/dt max at sidewall | C2H4/O2/Ar | 98% | 0.5, 1.0 and 2.0 | 1181–1808 | 0.9 and 1.7 | <1512 | Mathieu et al. [25] |
| ST | 7.5 | d[CH*]/dt max at sidewall | C2H4/O2Ar | 75% and 96% | 1.0 | 1092–1743 | 1.3–3.0 | <3257 | Xiong et al. [26] |
| ST | 5(diver insert) | dp/dt max and d[OH*]/dt max at sidewall | C2H4/O2/Ar | 94.73% | 1.0 | 1090–1317 | 16 and 60 | <1120 | Shao et al. [27] |
| 93.39% | 2.0 | 1122–1268 | 16 | <1070 | |||||
| ST | 7.5 | d[CH*]/dt max at sidewall | C2H4/O2/Ar | 94.92% | 1.0 | 1132–1745 | 2 | <1679 | Xiong et al. [31] |
| C2H4/O2/Ar/N2 | 94.92%(75.94%AR+18.98%N2) | 1.0 | 1074–1710 | 2 | <2467 | ||||
| ST | 6.35 | dp/dt max at end wall and d[OH*]/dt max at sidewall | C2H4/O2/Ar | 90% | 0.5 | 1017–1503 | 40 | <2258 | Baigmohammadi et al. [28] |
| 75% | 1.0 | 987–1113 | 20 | <1782 | |||||
| 85% | 1.0 | 998–1349 | 40 | <1620 | |||||
| 75% | 2.0 | 945–1349 | 40 | <1686 | |||||
| ST | 3.8 | p increased by 10% at sidewall | C2H4/O2/Ar | Ar=N2 in air | 0.33,1.0 and 3.0 | 1090–1520 | 6.5 | <318 | Tereza et al. [20] |
| ST | 7.6 | CH/OH/C2/p oneset at end wall | C2H4/Air | / | 0.5,1.0 and 2.0 | 1060–1520 | 5.9–16.5 | <1112 | Penyazkov et al. [19] |
| ST | 16.2/15.24 | dp/dt max at sidewall | C2H4/Air | / | 0.3,0.5,1.0 and 2.0 | 1003–1401 | 1.0–24.9 | <2228 | Kopp et al. [23] |
| ST | 10 | d[CH*]/dt max at sidewall | C2H4/Air | / | 0.5,1.0 and 2.0 | 721–1320 | 1, 4, 10 and 19 | <8664 | Yang et al. [32] |
| ST | 6.3 | dp/dt max at endwall | C2H4/Air | / | 1.0 | 1055–1250 | 30 | <500 | Dong et al. [29] |
| RCM | / | dp/dt max | C2H4/O2/Ar/N2 | 89.429%(15.856% N2, 73.573% Ar) | 1.0 | 850–1050 | 15, 30 and 50 | 572–273,000 | Kumar et al. [18] |
| RCM | / | dp/dt max | C2H4/O2/Ar/N2 | 85%(48% N2,37%Ar) | 0.5 | 915–1008 | 20 | 4748–142,200 | Baigmohammadi et al. [28] |
| 85%(75%N2,15%Ar) | 0.5 | 882–958 | 40 | 9700–92,250 | |||||
| 75%(30%N2,45%Ar) | 1.0 | 886–947 | 20 | 8443–87,890 | |||||
| 85%(55%N2,30%Ar) | 1.0 | 838–935 | 40 | 10,700–191,000 | |||||
| 90%(45%N2,45%Ar) | 2.0 | 881–980 | 20 | 10,230–418,900 | |||||
| RCM | / | dp/dt max | C2H4/O2/“Air” | “Air”(16.93%O2,40%Ar,33.83N2) | 1.0 | 800–920 | 30 | 7196–310,000 | Dong et al. [29] |
| ST | 5.4 | p onset at endwall | C3H6/O2/Ar | 84% and 92% | 0.5 | 1272–1772 | ≈4 | <932 | Burcat and Radhakrishnan [34] |
| 91.2% and 96.7% | 1 | 1366–1725 | 2.19–6.473 | <922 | |||||
| 94.8% and 89.6% | 2 | 1443–1936 | 4.19–7.018 | <737 | |||||
| ST | 7.62 | dp/dt max at endwall | C3H6/O2/Ar | 84% and 92% | 0.5 | 1270–1705 | 3.89 | <1535 | Qin et al. [35] |
| 82.10% | 0.8 | 1285–1505 | 3.71 | <1260 | |||||
| 91.20% | 1 | 1530–1820 | 1.14 | <265 | |||||
| 89% | 1.8 | 1320–1565 | 3.92 | <1285 | |||||
| 94.80% | 2 | 1415–1770 | 4.05 | <1305 | |||||
| ST | 6.35 | dp/dt max at sidewall | C3H6/O2/Ar | 95.11% | 1 | 1175–1500 | 40 | <1754 | Burke et al. [39] |
| ST | 15.2 | dp/dt max at end endwall or d[OH*]/dt max at sidewall | C3H6/O2/Ar | 85.33% and 95.11% | 1 | 1222–1645 | 2 and 10 | <2046 | |
| 94.22% | 2 | 1313–1714 | 2 and 10 | <2096 | |||||
| ST | 5.7 | d[OH*]/dt max at endwall | C3H6/O2/Ar | 85.33% | 1 | 1220–1462 | 10 | <1476 | |
| C3H6/O2/N2 | 85.33% | 1 | 1253–1422 | 10 | <1059 | ||||
| ST | 14.13/15.34 | d[OH*]/dt max at endwall | C3H6/O2/Ar | 95.55% | 0.5 | 1360–1689 | 2 | <2002 | |
| 91.13% and 95.11% | 1 | 1333–1720 | 2 and 4.5 | <2472 | |||||
| 94.22% | 2 | 1388–1756 | 2 | <2324 | |||||
| ST | 5 (diver insert) | d[OH*]/dt max at endwall | C3H6/O2/Ar | 95.11% | 1 | 1195–1302 | 40 | <1877 | |
| 94.22% | 2 | 1200–1432 | 40 | <1543 | |||||
| ST | 5 (diver insert) | dp/dt max and d[OH*]/dt max at sidewall | C3H6/O2/Ar | 95.16% | 1 | 1255–1488 | 15.11 | <3022 | Shao et al. [27] |
| ST | 6.35 | dp/dt max at sidewall | C3H6/Air | / | 0.5 and 1.0 | 1106–1364 | 10 | <1736 | Burke et al. [39] |
| ST | 15.2 | dp/dt max at end endwall or d[OH*]/dt max at sidewall | C3H6/Air | / | 0.5 and 1.0 | 1112–1535 | 2 and 10 | <1660 | |
| ST | 5.7 | d[OH*]/dt max at endwall | C3H6/Air | / | 0.5, 1.0 and 2.0 | 1036–1406 | 10 and 40 | <1642 | |
| ST | 10 | p/OH* onset at both sidewall and endwall | C3H6/Air | / | 0.5, 1.0 and 2.0 | 1024–1332 | 40 | <1319 | |
| RCM | / | dp/dt max | C3H6//Air | / | 0.5 and 1.0 | 722–1108 | 10 and 40 | 2000–325,000 | Burke et al. [39] |
| C3H6/O2/Ar | 95.55% | 0.5 | 941–1220 | 10 and 40 | 5800–212,000 | ||||
| C3H6/O2/Ar/N2 | 95.11%(47.555%Ar,47.555%N2) | 1 | 1109–1238 | 10 | 9517–162,800 | ||||
| C3H6/O2/N2 | 95.11% | 1 | 898–1008 | 40 | 20,000–146,700 | ||||
| C3H6/O2/Ar/N2 | 85.33%(21.33%Ar, 64%N2) | 1 | 988–1129 | 10 | 6450–128,000 | ||||
| C3H6/O2/Ar | 85.33% | 1 | 781–957 | 40 | 6225–296,700 | ||||
| RCM | / | dp/dt max | C3H6/‘Air’ | Air’(21O2:37.5AR:37.5N2) | 1 and 2 | 859–1009 | 10 | 10,220–157,200 | |
| C3H6/O2/Ar/N2 | 85.33%(42.665%Ar, 42.665%N2) | 1 | 813–1062 | 10 and 40 | 9900–105,740 | ||||
| C3H6/O2/Ar/N2 | 94.22%(47.11%Ar,47.11%N2) | 2 | 892–1178 | 10 and 40 | 12,000–114,560 | ||||
| C3H6/O2/N2 | 95.11% | 1 | 912–1021 | 40 | 15,900–80,500 | ||||
| ST | 7.8 | OH* increased by 10% at sidewall | C4H8-1/O2/Ar | 87% | 0.5 | 1248–1538 | ~7.89 | <932 | Heyberger et al. [36] |
| 86% and 93% | 1 | 1202–1568 | ~7.94 | <1907 | |||||
| 96% | 2 | 1442–1664 | ~7.44 | <148 | |||||
| ST | 11.5 | d[OH*]/dt max at endwall | C4H8-1/O2/Ar | 87% | 0.5 | 974–1585 | 1.2, 4.0 and 16.0 | <3948 | Pan et al. [42] |
| 94.75% and 96.5% | 1 | 1142–1705 | 4 | <3431 | |||||
| 96% | 2 | 1082–1835 | 1.2, 4.0 and 16.0 | <3518 | |||||
| ST | 6.35 | dp/dt max at endwall | C4H8-1/Air | / | 0.5 | 929–1289 | 10, 30 and 50 | <2008 | Li et al. [43] |
| 1 | 940–1285 | 10, 30 and 50 | <1945 | ||||||
| 2 | 899–1301 | 10, 30 and 50 | <1960 | ||||||
| RCM | / | dp/dt max | C4H8-1/Air | / | 0.5 | 765–1012 | 10 and 30 | <92,290 | |
| 1 | 688–941 | 10 and 30 | <231,700 | ||||||
| 2 | 676–940 | 10 and 30 | <201,500 | ||||||
| Mechanism | No. of Species | No. of Reactions | Application of Present | Year of Released | Ref |
|---|---|---|---|---|---|
| NUIGMech 1.1 | 2845 | 112,60 | C2–C4 | 2021 | [29,47,57,58,59,60,61,62,63] |
| AramcoMech 2.0 | 493 | 2716 | C2–C4 | 2013 | [4,6,38,39,45,64,65] |
| Creck | 621 | 27,369 | C2–C4 | 2020 | [3,66,67] |
| UCSD | 55 | 245 | C2–C4 | 2016 | [68] |
| Jetsurf 2.0 | 344 | 2163 | C2–C4 | 2010 | [69] |
| Konnov 0.6 | 131 | 1256 | C2–C4 | 2009 | [70] |
| USC 2.0 | 113 | 809 | C2–C4 | 2007 | [71] |
| ChuanDa | 146 | 567 | C2 | 2020 | [32] |
| Glarborg 2009 | 118 | 987 | C2 | 2009 | [72] |
| Fuel | Data Points | A | a | b | c | d | e | E0 | R2 |
|---|---|---|---|---|---|---|---|---|---|
| Ethylene | 1039 | 1.79 × 103 | −0.28 ±0.02 | −1.94 ±0.68 | −1.38 ±0.07 | 0.58 ±0.07 | 0.53 ±0.07 | 24.76 ±1.69 | 0.92 |
| Propene | 595 | 4.02 × 1050 | −0.80 ±0.02 | −15.34 ±0.66 | −1.19 ±0.04 | 0.07 ±0.04 | 0.12 ±0.05 | −2.29 ±1.41 | 0.98 |
| 1-Butene | 315 | 1.16 × 1073 | −0.94 ±0.02 | −22.00 ±0.66 | 0.57 ±0.04 | −2.53 ±0.04 | −3.12 ±0.05 | −19.48 ±1.6 | 0.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, W.; Zhang, Y.; Li, Y.; Huang, Z. Hierarchical Auto-Ignition and Structure-Reactivity Trends of C2–C4 1-Alkenes. Energies 2021, 14, 5797. https://doi.org/10.3390/en14185797
Sun W, Zhang Y, Li Y, Huang Z. Hierarchical Auto-Ignition and Structure-Reactivity Trends of C2–C4 1-Alkenes. Energies. 2021; 14(18):5797. https://doi.org/10.3390/en14185797
Chicago/Turabian StyleSun, Wuchuan, Yingjia Zhang, Yang Li, and Zuohua Huang. 2021. "Hierarchical Auto-Ignition and Structure-Reactivity Trends of C2–C4 1-Alkenes" Energies 14, no. 18: 5797. https://doi.org/10.3390/en14185797
APA StyleSun, W., Zhang, Y., Li, Y., & Huang, Z. (2021). Hierarchical Auto-Ignition and Structure-Reactivity Trends of C2–C4 1-Alkenes. Energies, 14(18), 5797. https://doi.org/10.3390/en14185797







