Evaluation of the Effectiveness of the Activated Sludge Process in the Elimination Both ATB-Resistant and ATB-Susceptible E. coli Strains
Abstract
:1. Introduction
2. Materials and Methods
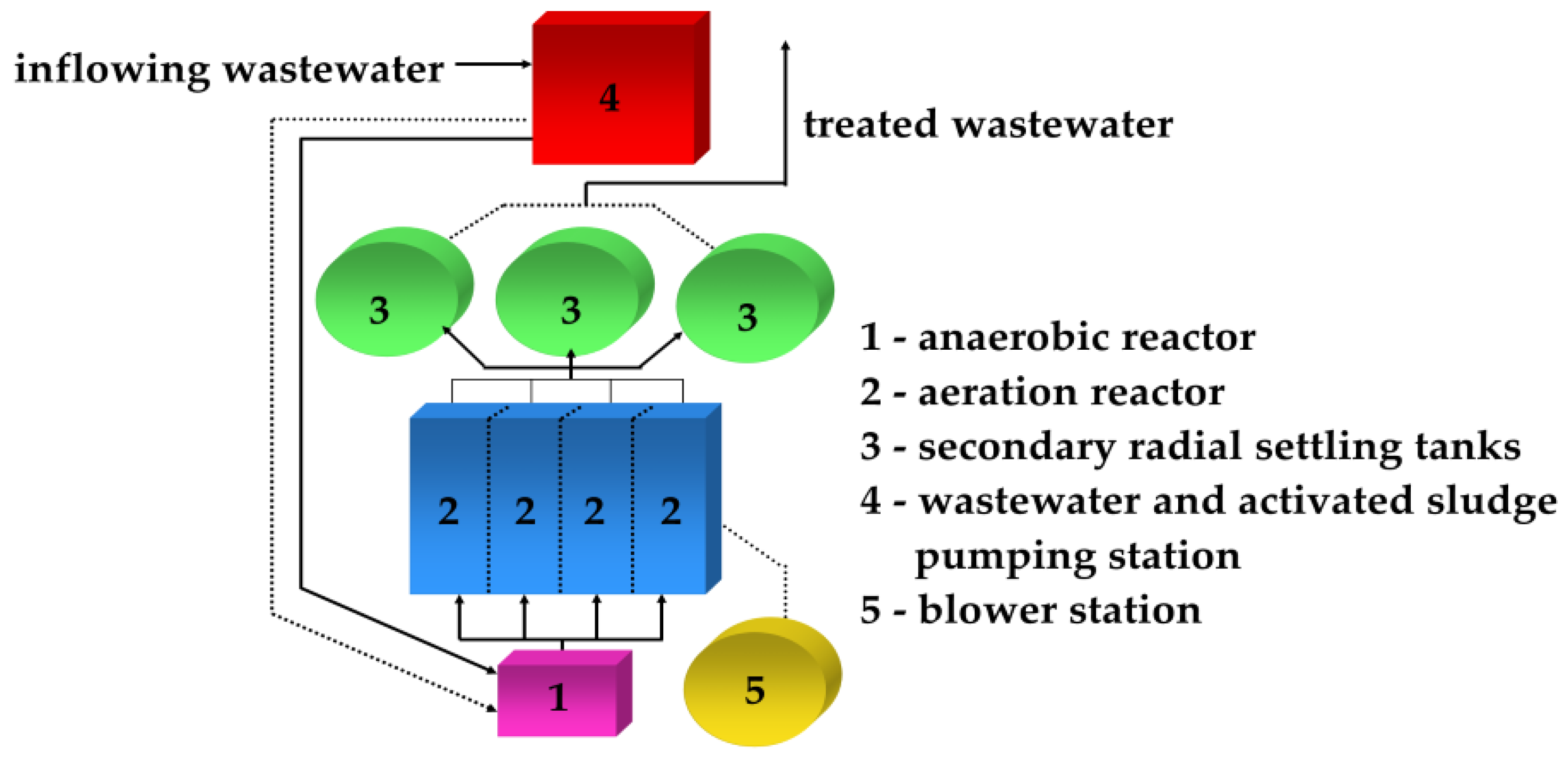
2.1. Treatment Plant Characteristics
- Domestic wastewater and wastewater from small production enterprises—11,320 m3 per day;
- Infiltration waters—2300 m3 per day;
- Wastewater delivered from septic tanks—380 m3 per day.
2.2. Sampling and Bacteriological Contamination Analysis
2.3. Escherichia coli Isolation and Identification
2.4. Antibiotic Resistance Testing
2.5. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, S.; Huang, J.; Zhao, Z.; Cao, Y.; Li, B. Hospital Wastewater as a Reservoir for Antibiotic Resistance Genes: A Meta-Analysis. Public Health Front. 2020, 8, 2296–2565. [Google Scholar] [CrossRef]
- Luo, L.; Yao, J.; Liu, W.; Yang, L.; Li, H.; Liang, M.; Ma, H.; Liu, Z.; Chen, Y. Comparison of bacterial communities and antibiotic resistance genes in oxidation ditches and membrane bioreactors. Sci. Rep. 2021, 11, 8955. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.; Hembach, N.; Schwartz, T. Evaluation of antibiotic resistance dissemination by wastewater treatment plant effluents with different catchment areas in Germany. Sci. Rep. 2020, 10, 8952. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, L.; Manaia, C.; Merlinc, C.; Schwartz, T.; Dagot, C.; Ploy, M.C.; Michael, I.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: A review. Sci. Total Environ. 2013, 447, 345–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majeed, H.J.; Riquelme, M.V.; Davis, B.C.; Gupta, S.; Angeles, L.; Aga, D.S.; Garner, E.; Pruden, A.; Vikesland, P.J. Evaluation of Metagenomic-Enabled Antibiotic Resistance Surveillance at a Conventional Wastewater Treatment Plant. Front. Microbiol. 2021, 12, 1048. [Google Scholar] [CrossRef]
- Barancheshme, F.; Munir, M. Strategies to Combat Antibiotic Resistance in the Wastewater Treatment Plants. Front. Microbiol. 2018, 17, 2603. [Google Scholar] [CrossRef] [Green Version]
- Adefisoye, M.A.; Okoh, A.I. Identification and antimicrobial resistance prevalence of pathogenic Escherichia coli strains from treated wastewater effluents in Eastern Cape, South Africa. Microbiologyopen 2016, 5, 143–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Praveenkumarreddy, Y.; Akiba, M.; Guruge, K.S.; Balakrishna, K.; Vandana, K.E.; Kumar, V. Occurrence of antimicrobial-resistant Escherichia coli in wastewater treatment plants of South India. J. Water Sanit. Hyg. Dev. 2020, 10, 48–55. [Google Scholar] [CrossRef] [Green Version]
- Fouz, N.; Pangesti, K.N.A.; Yasir, M.; Al-Malki, A.L.; Azhar, E.I.; Hill-Cawthorne, G.A.; Abd El Ghany, M. The Contribution of Wastewater to the Transmission of Antimicrobial Resistance in the Environment: Implications of Mass Gathering Settings. Trop. Med. Infect. Dis. 2020, 5, 33. [Google Scholar] [CrossRef] [Green Version]
- Karkman, A.; Do, T.T.; Walsh, F.; Virta, M.P.J. Antibiotic-Resistance Genes in Waste Water. Trends Microbiol. 2018, 26, 220–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watkinson, A.J.; Micalizzi, G.B.; Graham, G.M.; Bates, J.B.; Costanzo, S.D. Antibiotic-resistant Escherichia coli in wastewaters, surface waters, and oysters from an urban riverine system. Appl. Environ. Microbiol. 2007, 73, 5667–5670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakali, O.; Brooks, J.P.; Shahin, S.; Sherchan, S.P.; Haramoto, E. Removal of Antibiotic Resistance Genes at Two Conventional Wastewater Treatment Plants of Louisiana, USA. Water 2020, 12, 1729. [Google Scholar] [CrossRef]
- Nguyen, A.Q.; Vu, H.P.; Nguyen, L.N.; Wang, Q.; Djordjevic, S.P.; Donner, E.; Yin, H.; Nghiem, L.D. Monitoring antibiotic resistance genes in wastewater treatment: Current strategies and future challenges. Sci. Total Environ. 2021, 783, 146964. [Google Scholar] [CrossRef]
- Lucena, F.; Duran, A.; Morón, A.; Calderón, E.; Campos, C.; Gantzer, C.; Skraber, S.; Jofre, J. Reduction of bacterial indicators and bacteriophages infecting faecal bacteria in primary and secondary wastewater treatments. J. Appl. Microbiol. 2004, 97, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Aslan, A.; Cole, Z.; Bhattacharya, A.; Oyibo, O. Presence of Antibiotic-Resistant Escherichia coli in Wastewater Treatment Plant Effluents Utilized as Water Reuse for Irrigation. Water 2018, 10, 805. [Google Scholar] [CrossRef] [Green Version]
- Wen, Q.; Tutuka, C.; Keegan, A.; Jin, B. Fate of pathogenic microorganisms and indicators in secondary activated sludge wastewater treatment plants. J. Environ. Manag. 2009, 90, 1442–1447. [Google Scholar] [CrossRef] [PubMed]
- Barancheshme, F.; Munir, M. Development of Antibiotic Resistance in Wastewater Treatment Plants. In Antimicrobial Resistance-A Global Threat, 1st ed.; Kumar, Y., Ed.; IntechOpen Limited: London, UK, 2019; Available online: https://www.intechopen.com/books/antimicrobial-resistance-a-global-threat/development-of-antibiotic-resistance-in-wastewater-treatment-plants (accessed on 17 April 2021). [CrossRef] [Green Version]
- EN ISO 9308-1:2000. Part 1: Membrane filtration method. In Water Quality—Detection and Enumeration of Escherichia coli and coli form Bacteria; American National Standards Institute (ANSI): New York, NY, USA, 2007. [Google Scholar]
- Pepper, I.L.; Gerba, C.G. Environmental Microbiology. A laboratory Manual, 2nd ed.; Elsevier AP: Burlington, VT, USA, 2008; pp. 123–139. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Disk Susceptibility Tests, 13th ed.; CLSI Standard M02; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018; Available online: https://kaldur.landspitali.is/focal/gaedahandbaekur/gnhsykla.nsf/5e27f2e5a88c898e00256500003c98c2/94e1f81249a5e5560025756d005a560f/$FILE/M02Ed13E%20Performance%20Standards%20for%20Antimicrobial%20Disk%20Susceptibility%20Tests.pdf (accessed on 13 December 2019).
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 10.0. 2020. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_10.0_Breakpoint_Tables.pdf (accessed on 13 December 2019).
- Paśmionka, I.B. Evaluation of the efficiency of removing sanitation indicators in the process of biological wastewater treatment. Acta Sci. Pol. Formatio Circumiectus 2020, 19, 15–22. [Google Scholar] [CrossRef]
- Delanka-Pedige, H.M.K.; Munasinghe-Arachchige, S.P.; Zhang, Y.; Nirmalakhandan, N. Bacteria and virus reduction in secondary treatment: Potential for minimizing post disinfectant demand. Water Res. 2020, 177, 115802. [Google Scholar] [CrossRef]
- Maffettone, R.; Manoli, K.; Santoro, D.; Passalacqua, K.D.; Wobus, C.E.; Sarathy, S. Performic Acid Disinfection of Municipal Secondary Effluent Wastewater: Inactivation of Murine Norovirus, Fecal Coliforms, and Enterococci. Environ. Sci. Technol. 2020, 54, 12761–12770. [Google Scholar] [CrossRef]
- Boni, W.; Parrish, K.; Patil, S.; Fahrenfeld, N.L. Total coliform and Escherichia coli in microplastic biofilms grown in wastewater and inactivation by peracetic acid. Water Environ. Res. 2021, 93, 334–342. [Google Scholar] [CrossRef]
- Fars, S.; Oufdou, K.; Nejmeddine, A.; Hassani, L.; Melloul, A.A.; Bousselhaj, K.; Amahmid, O.; Bouhoum, K.; Lakmichi, H.; Mezrioui, N. Antibiotic resistance and survival of faecal coliforms in activated sludge system in a semi-arid region (Beni Mellal, Morocco). World J. Microbiol. Biotechnol. 2005, 21, 493–500. [Google Scholar] [CrossRef]
- Pignato, S.; Coniglio, M.A.; Faro, G.; Weill, F.X.; Giammanco, G. Plasmid-mediated multiple antibiotic resistances of Escherichia coli in crude and treated wastewaters used in agriculture. J. Water Health 2009, 7, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Patoli, A.A.; Patoli, B.B.; Mehraj, V. High prevalence of multi-drug resistant Escherichia coli in drinking water samples from Hyderabad. Gomal J. Med. Sci. 2010, 8, 23–26. [Google Scholar]
- Barbosa, V.; Morais, M.; Silva, A.; Delerue-Matos, C.; Figueiredo, S.A.; Domingues, V.F. Comparison of antibiotic resistance in the influent and effluent of two wastewater treatment plants. AIMS Environ. Sci. 2021, 8, 101–116. [Google Scholar] [CrossRef]
- Schages, L.; Wichern, F.; Geisen, S.; Kalscheuer, R.; Bockmühl, D. Distinct Resistomes and Microbial Communities of Soils, Wastewater Treatment Plants and Households Suggest Development of Antibiotic Resistances Due to Distinct Environmental Conditions in Each Environment. Antibiotics 2021, 10, 514. [Google Scholar] [CrossRef]
- Cycoń, M.; Mrozik, A.; Piotrowska-Seget, Z. Antibiotics in the Soil Environment—Degradation and Their Impact on Microbial Activity and Diversity. Front. Microbiol. 2019, 10, 338. [Google Scholar] [CrossRef] [PubMed]
- Pazda, M.; Kumirska, J.; Stepnowski, P.; Mulkiewicz, E. Antibiotic resistance genes identified in wastewater treatment plant systems—A review. Sci. Total Environ. 2019, 697, 134023. Available online: https://doi.org/10.1016/j.scitotenv.2019.134023 (accessed on 9 February 2021).
- Lucassen, R.; Rehberg, L.; Heyden, M.; Bockmühl, D.P. Strong correlation of total phenotypic resistance of samples from household environments and the prevalence of class 1 integrons suggests for the use of the relative prevalence of intI1 as a screening tool for multi-resistance. PLoS ONE 2019, 14, e0218277. [Google Scholar]
- Onderdonk, A.B.; Louie, T.J.; Tally, F.P.; Bartlett, J.G. Activity of metronidazole against escherichia coli in experimental infra-abdominal sepsis. J. Antimicrob. Chemother. 1979, 5, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Rabbia, V.; Bello-Toledo, H.; Jiménez, S.; Quezada, M.; Domínguez, M.; Vergara, L.; Gómez-Fuentes, C.; Calisto-Ulloa, N.; González-Acuña, D.; López, J.; et al. Antibiotic resistance in Escherichia coli strains isolated from Antarctic bird feces, water from inside a wastewater treatment plant, and seawater samples collected in the Antarctic Treaty area. Polar Sci. 2016, 10, 123–131. [Google Scholar] [CrossRef]
- Frąc, M.; Jezierska-Tys, S.; Oszust, K.; Gryta, A.; Pastor, M. Assessment of microbiological and biochemical properties of dairy wastewater sludge. Int. J. Environ. Sci. Technol. 2017, 14, 679–688. [Google Scholar] [CrossRef] [Green Version]
- Sahm, D.F.; Thornsberry, C.; Mayfield, D.C.; Jones, M.E.; Karlowsky, J.A. Multidrugresistant urinary tract isolates of Escherichia coli: Prevalence and patient demographics in the United States in 2000. Antimicrob Agents Chemother. 2001, 45, 1402–1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aghalari, Z.; Dahms, H.U.; Sillanpää, M.; Sosa-Hernandez, J.E.; Parra-Saldívar, R. Effectiveness of wastewater treatment systems in removing microbial agents: A systematic review. Glob. Health 2020, 16, 13. [Google Scholar] [CrossRef]
- Barrios-Hernández, M.L.; Pronk, M.; Garcia, H.; Boersma, A.; Brdjanovic, D.; van Loosdrecht, M.C.M.; Hooijmans, C.M. Removal of bacterial and viral indicator organisms in full-scale aerobic granular sludge and conventional activated sludge systems. Water Res. X 2020, 6, 100040. [Google Scholar] [CrossRef]
- Pazda, M.; Rybicka, M.; Stolte, S.; Piotr Bielawski, K.; Stepnowski, P.; Kumirska, J.; Wolecki, D.; Mulkiewicz, E. Identification of Selected Antibiotic Resistance Genes in Two Different Wastewater Treatment Plant Systems in Poland: A Preliminary Study. Molecules 2020, 25, 2851. [Google Scholar] [CrossRef]
- Bengtsson-Palme, J.; Kristiansson, E.; Larsson, D.G.J. Environmental factors influencing the development and spread of antibiotic resistance. FEMS Microbiol. Rev. 2018, 42, fux053. [Google Scholar] [CrossRef] [PubMed]
- Müller, H.; Sib, E.; Gajdiss, M.; Klanke, U.; Lenz-Plet, F.; Barabasch, V.; Albert, C.; Schallenberg, A.; Timm, C.; Zacharias, N.; et al. Dissemination of multi-resistant Gram-negative bacteria into German wastewater and surface waters. FEMS Microbiol. Ecol. 2018, 94, fiy057. [Google Scholar] [CrossRef]
- World Health Organization. Briefing Note: Antimicrobial Resistance: An Emerging Water, Sanitation and Hygiene Issue. 2014. Available online: https://apps.who.int/iris/bitstream/handle/10665/204948/WHO_FWC_WSH_14.7_eng.pdf?sequence=1&isAllowed=y (accessed on 11 April 2021).
- Jóźwiakowski, K.; Bugajski, P.; Mucha, Z.; Wójcik, W.; Jucherski, A.; Nastawny, M.; Siwiec, T.; Mazur, A.; Obroślak, R.; Gajewska, M. Reliability and efficiency of pollution removal during long-term operation of a one-stage constructed wetland system with horizontal flow. Sep. Purif. Technol. 2017, 187, 60–66. [Google Scholar] [CrossRef]

| Sampling Date | pH | BOD5 (mg·dm−3) | COD (mg·dm−3) | Total Suspended Solids (mg·dm−3) | Total Nitrogen (mg·dm−3) | Total Phosphorus (mg·dm−3) |
|---|---|---|---|---|---|---|
| Spring | 7.7 | 406.64 | 663 | 337 | 46.9 | 8.44 |
| Summer | 7.7 | 454.71 | 733 | 253 | 43.9 | 8.98 |
| Autumn | 7.6 | 232.14 | 956 | 174 | 52.5 | 8.84 |
| Winter | 7.8 | 199.66 | 883 | 388 | 50.1 | 6.18 |
| Sampling Date | Faecal Coliform Bacteria (FC) | The Degree of Reduction FC (%) | Average Temperature (°C) | |||||
|---|---|---|---|---|---|---|---|---|
| (CFU·100 cm−3) | ||||||||
| Inflowing Wastewater | Treated Wastewater | |||||||
| The Amount of FC | Average Number of FC with SD | The Amount of FC | Average Number of FC with SD | Inflowing Wastewater | Treated Wastewater | |||
| Spring | Mar | 3.7 × 105 | 4.73 ± 1.1 × 105 ab * | 5.3 × 103 | 7.13 ± 2.1 × 103 a | 98.5 | 13.70 | 14.00 |
| Apr | 4.6 × 105 | 6.7 × 103 | ||||||
| May | 5.9 × 105 | 9.4 × 103 | ||||||
| Summer | Jun | 6.1 × 105 | 6.43 ± 3.1 × 105 b | 11.1 × 103 | 13.53 ± 2.6 × 103 b | 97.9 | 18.30 | 18.30 |
| Jul | 6.5 × 105 | 13.2 × 103 | ||||||
| Aug | 6.7 × 105 | 16.3 × 103 | ||||||
| Autumn | Sep | 5.1 × 105 | 4.70 ± 4.6 × 105 ab | 8.6 × 103 | 5.57 ± 2.6 × 103 a | 98.8 | 14.70 | 15.30 |
| Oct | 4.8 × 105 | 4.2 × 103 | ||||||
| Nov | 4.2 × 105 | 3.9 × 103 | ||||||
| Winter | Dec | 3.8 × 105 | 4.03 ± 5.9 × 105 a | 3.1 × 103 | 3.03 ± 0.1 × 103 a | 99.3 | 9.70 | 10.00 |
| Jan | 4.7 × 105 | 3.1 × 103 | ||||||
| Feb | 3.6 × 105 | 2.9 × 103 | ||||||
| Sampling Date | Escherichia coli | The Degree of Reduction E. coli (%) | Average Temperature (°C) | |||||
|---|---|---|---|---|---|---|---|---|
| (CFU·100 cm−3) | ||||||||
| Inflowing Wastewater | Treated Wastewater | |||||||
| The Amount of E. coli | Average Number of E. coli with SD | The Amount of E. coli | Average Number of E. coli with SD | Inflowing Wastewater | Treated Wastewater | |||
| Spring | Mar | 3.0 × 104 | 3.77 ± 0.9 × 104 a | 44 | 59 ± 19.30 a | 99.84 | 13.70 | 14.00 |
| Apr | 3.5 × 104 | 53 | ||||||
| May | 4.8 × 104 | 81 | ||||||
| Summer | Jun | 5.2 × 104 | 5.23 ± 0.2 × 104 b | 94 | 111 ± 19.43 b | 99.79 | 18.30 | 18.30 |
| Jul | 5.1 × 104 | 106 | ||||||
| Aug | 5.4 × 104 | 132 | ||||||
| Autumn | Sep | 4.2 × 104 | 3.63 ± 0.6 × 104 a | 71 | 44 ± 24.27 a | 99.88 | 14.70 | 15.30 |
| Oct | 3.6 × 104 | 37 | ||||||
| Nov | 3.1 × 104 | 24 | ||||||
| Winter | Dec | 2.9 × 104 | 2.93 ± 0.2 × 104 a | 15 | 15 ± 1.53 a | 99.95 | 9.70 | 10.00 |
| Jan | 3.1 × 104 | 16 | ||||||
| Feb | 2.8 × 104 | 13 | ||||||
| Sampling Date | Total Amount of E. coli Isolates | Number of E. coli Isolates Resistant to: | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ERY | AZM | CLR | OFX | CIP | AMP | TMP | MTR | |||
| Spring | March | 44 | 17 | 11 | 6 | 7 | 8 | 23 | 27 | 0 |
| April | 53 | 19 | 14 | 6 | 8 | 10 | 28 | 33 | 0 | |
| May | 81 | 31 | 20 | 9 | 12 | 16 | 44 | 50 | 0 | |
| Summer | June | 94 | 45 | 48 | 34 | 28 | 27 | 64 | 58 | 0 |
| July | 106 | 51 | 54 | 38 | 31 | 31 | 71 | 68 | 0 | |
| August | 132 | 64 | 68 | 48 | 39 | 39 | 89 | 116 | 0 | |
| Autumn | September | 71 | 15 | 13 | 10 | 8 | 14 | 33 | 42 | 0 |
| October | 37 | 8 | 8 | 6 | 4 | 7 | 18 | 22 | 0 | |
| November | 24 | 5 | 4 | 4 | 3 | 4 | 11 | 15 | 0 | |
| Winter | December | 15 | 2 | 2 | 2 | 0 | 2 | 2 | 10 | 0 |
| January | 16 | 3 | 3 | 3 | 5 | 3 | 3 | 14 | 0 | |
| February | 13 | 2 | 2 | 2 | 2 | 2 | 2 | 13 | 0 | |
| Antibiotic | Number of Susceptible Strains | Number of Resistant Strains | Resistance (%) |
|---|---|---|---|
| ERY | 424 | 262 | 38 |
| AZM | 439 | 247 | 36 |
| CLR | 518 | 168 | 24 |
| OFX | 539 | 147 | 21 |
| CIP | 523 | 163 | 23 |
| AMP | 298 | 388 | 56 |
| TMP | 218 | 468 | 68 |
| MTR | 686 | 0 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paśmionka, I.B.; Bulski, K.; Herbut, P.; Boligłowa, E.; Vieira, F.M.C.; Bonassa, G.; Prá, M.C.D.; Bortoli, M. Evaluation of the Effectiveness of the Activated Sludge Process in the Elimination Both ATB-Resistant and ATB-Susceptible E. coli Strains. Energies 2021, 14, 5868. https://doi.org/10.3390/en14185868
Paśmionka IB, Bulski K, Herbut P, Boligłowa E, Vieira FMC, Bonassa G, Prá MCD, Bortoli M. Evaluation of the Effectiveness of the Activated Sludge Process in the Elimination Both ATB-Resistant and ATB-Susceptible E. coli Strains. Energies. 2021; 14(18):5868. https://doi.org/10.3390/en14185868
Chicago/Turabian StylePaśmionka, Iwona Beata, Karol Bulski, Piotr Herbut, Elżbieta Boligłowa, Frederico Márcio C. Vieira, Gabriela Bonassa, Marina Celant De Prá, and Marcelo Bortoli. 2021. "Evaluation of the Effectiveness of the Activated Sludge Process in the Elimination Both ATB-Resistant and ATB-Susceptible E. coli Strains" Energies 14, no. 18: 5868. https://doi.org/10.3390/en14185868
APA StylePaśmionka, I. B., Bulski, K., Herbut, P., Boligłowa, E., Vieira, F. M. C., Bonassa, G., Prá, M. C. D., & Bortoli, M. (2021). Evaluation of the Effectiveness of the Activated Sludge Process in the Elimination Both ATB-Resistant and ATB-Susceptible E. coli Strains. Energies, 14(18), 5868. https://doi.org/10.3390/en14185868







