COVID-19 Lessons for Climate Change and Sustainable Health
Abstract
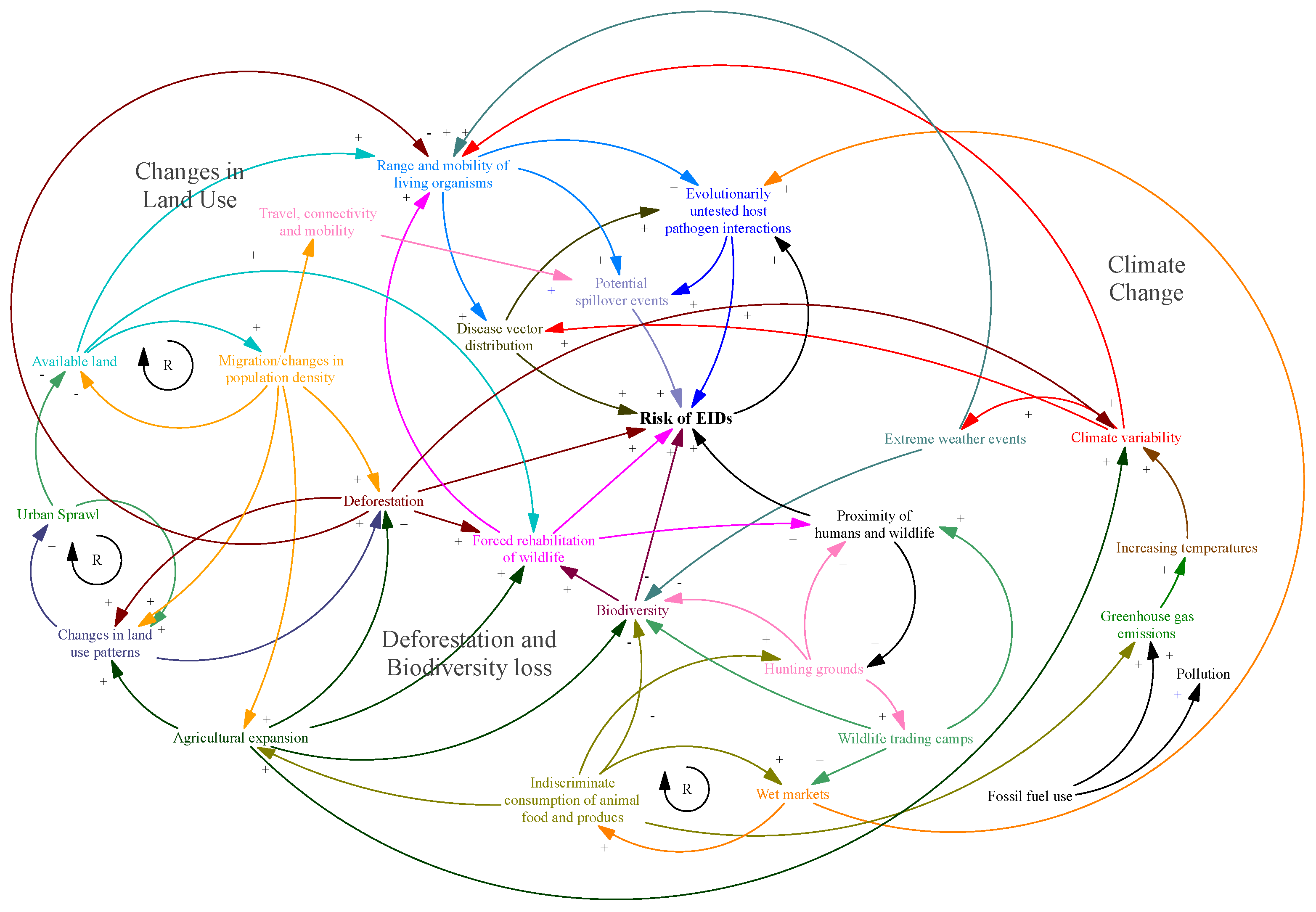
:1. Introduction
2. Disease Emergence in a Novel Geological Epoch
3. Mechanistic Drivers of an Interrelated Sustainability Crisis
3.1. Land Use Change
3.2. Deforestation and Biodiversity Loss
3.3. Travel Connectivity Ramifications
3.4. Energy Exploitation
3.5. Climate Change
4. Interrelationship between Climate Change, COVID-19, and AMR
5. A One Health Paradigm for Policy
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Glossary
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| COVID-19 | Coronavirus Disease 2019 |
| MERS-CoV | Middle Eastern Respiratory Syndrome |
| vCJD | Variant Creutzfeldt-Jakob disease |
| SARS | Severe Acute Respiratory Syndrome |
| HIV | Human Immunodeficiency Virus |
| AMR | Antimicrobial resistance |
| NCDs | Non-communicable diseases |
| AIDS | Acquired immune deficiency syndrome |
| EID | Emerging infectious disease |
| SARS-CoV-1 | Severe Acute Respiratory Syndrome |
References
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.-M.; Wang, W.; Song, Z.-G.; Hu, Y.; Tao, Z.-W.; Tian, J.-H.; Pei, Y.-Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johns Hopkins Coronavirus Resource Center. Global Map. Available online: https://coronavirus.jhu.edu/map.html. (accessed on 6 October 2020).
- 1918 Pandemic (H1N1 Virus)|Pandemic Influenza (Flu)|CDC. Available online: https://www.cdc.gov/flu/pandemic-resources/1918-pandemic-h1n1.html (accessed on 14 June 2021).
- Clouston, S.A.; Natale, G.; Link, B.G. Socioeconomic inequalities in the spread of coronavirus-19 in the United States: A examination of the emergence of social inequalities. Soc. Sci. Med. 2021, 268, 113554. [Google Scholar] [CrossRef]
- Mas-Coma, S.; Jones, M.K.; Marty, A.M. COVID-19 and globalization. One Health 2020, 9, 100132. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, N.; Shad, M.Y.; Ulvi, O.; Khan, M.H.; Karamehic-Muratovic, A.; Nguyen, U.-S.D.; Baghbanzadeh, M.; Wardrup, R.; Aghamohammadi, N.; Cervantes, D.; et al. The impact of COVID-19 on globalization. One Health 2020, 11, 100180. [Google Scholar] [CrossRef]
- Schwab, N.; Berger, E.; Sanjayan, M.; Gough, M.; President, K.R. Rodriguez: Policy brief, COVID 19 Response and Recovery Recommendations for Policymakers, National Capitals Coalition. 2020. Available online: https://capitalscoalition.org/covid-19-response-recovery-nature-based-solutions-for-people-planet-prosperity/CEO (accessed on 14 June 2021).
- McNeely, J.A. Nature and COVID-19: The pandemic, the environment, and the way ahead. Ambio 2021, 50, 767–781. [Google Scholar] [CrossRef]
- Akinsorotan, O.A.; Olaniyi, O.E.; Adeyemi, A.A.; Olasunkanmi, A.H. Corona Virus Pandemic: Implication on Biodiversity Conservation. Front. Water 2021, 3, 635529. [Google Scholar] [CrossRef]
- Platto, S.; Zhou, J.; Wang, Y.; Wang, H.; Carafoli, E. Biodiversity loss and COVID-19 pandemic: The role of bats in the origin and the spreading of the disease. Biochem. Biophys. Res. Commun. 2021, 538, 2–13. [Google Scholar] [CrossRef]
- Levy, D.L. COVID-19 and Global Governance. J. Manag. Stud. 2021, 58, 562–566. [Google Scholar] [CrossRef]
- Environmental Justice Foundation|Viral Diseases from Wildlife in China: Could SARS Happen Again? Available online: https://ejfoundation.org/reports/viral-diseases-from-wildlife-in-china-could-sars-happen-again (accessed on 28 June 2021).
- Xu, R.-H.; He, J.-F.; Evans, M.R.; Peng, G.-W.; Field, H.E.; Yu, D.-W.; Lee, C.-K.; Luo, H.-M.; Lin, W.-S.; Lin, P.; et al. Epidemiologic Clues to SARS Origin in China. Emerg. Infect. Dis. 2004, 10, 1030–1037. [Google Scholar] [CrossRef]
- Middle East Respiratory Syndrome Coronavirus (MERS-CoV). Available online: https://www.who.int/health-topics/middle-east-respiratory-syndrome-coronavirus-mers#tab=tab_1 (accessed on 14 June 2021).
- Total Stimulus for the COVID-19 Crisis Already Triple That for the Entire 2008–2009 Recession|McKinsey & Company. Available online: https://www.mckinsey.com/featured-insights/coronavirus-leading-through-the-crisis/charting-the-path-to-the-next-normal/total-stimulus-for-the-covid-19-crisis-already-triple-that-for-the-entire-2008-09-recession# (accessed on 14 June 2021).
- Zoonotic Diseases|One Health|CDC. Available online: https://www.cdc.gov/onehealth/basics/zoonotic-diseases.html (accessed on 14 June 2021).
- Tarantola, A. Four Thousand Years of Concepts Relating to Rabies in Animals and Humans, Its Prevention and Its Cure. Trop. Med. Infect. Dis. 2017, 2, 5. [Google Scholar] [CrossRef] [Green Version]
- Pappaioanou, M.; Gomez, T.; Drenzek, C. New and Emerging Zoonoses. Emerg. Infect. Dis. 2004, 10, e28. [Google Scholar] [CrossRef]
- Sabin, N.S.; Calliope, A.S.; Simpson, S.V.; Arima, H.; Ito, H.; Nishimura, T.; Yamamoto, T. Implications of human activities for (re)emerging infectious diseases, including COVID-19. J. Physiol. Anthropol. 2020, 39, 1–12. [Google Scholar] [CrossRef]
- Smith, K.F.; Goldberg, M.; Rosenthal, S.; Carlson, L.; Chen, J.; Chen, C.; Ramachandran, S. Global rise in human infectious disease outbreaks. J. R. Soc. Interface 2014, 11, 20140950. [Google Scholar] [CrossRef] [PubMed]
- Steffen, W.; Rockström, J.; Richardson, K.; Lenton, T.M.; Folke, C.; Liverman, D.; Summerhayes, C.P.; Barnosky, A.D.; Cornell, S.E.; Crucifix, M.; et al. Trajectories of the Earth System in the Anthropocene. Proc. Natl. Acad. Sci. USA 2018, 115, 8252–8259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizumoto, K.; Kagaya, K.; Chowell, G. Effect of a wet market on coronavirus disease (COVID-19) transmission dynamics in China, 2019–2020. Int. J. Infect. Dis. 2020, 97, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-F.; Eaton, B.T. Bats, Civets and the Emergence of SARS. Curr. Top. Microbiol. Immunol. 2007, 315, 325–344. [Google Scholar] [CrossRef]
- Keatts, L.O.; Robards, M.; Olson, S.H.; Hueffer, K.; Insley, S.J.; Joly, D.O.; Kutz, S.; Lee, D.S.; Chetkiewicz, C.-L.B.; Lair, S.; et al. Implications of Zoonoses from Hunting and Use of Wildlife in North American Arctic and Boreal Biomes: Pandemic Potential, Monitoring, and Mitigation. Front. Public Health 2021, 9, 451. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (US); Committee on Achieving Sustainable Global Capacity for Surveillance and Response to Emerging Diseases of Zoonotic Origin. Sustaining Global Surveillance and Response to Emerging Zoonotic Diseases; Keusch, G.T., Pappaioanou, M., Gonzalez, M.C., Eds.; National Academies Press (US): Washington, DC, USA, 2009. Available online: https://www.ncbi.nlm.nih.gov/books/NBK215318/ (accessed on 14 June 2021).
- Winkler, K.; Fuchs, R.; Rounsevell, M.; Herold, M. Global land use changes are four times greater than previously estimated. Nat. Commun. 2021, 12, 1–10. [Google Scholar] [CrossRef]
- Update: Outbreak of Nipah Virus—Malaysia and Singapore. 1999. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/00057012.htm (accessed on 14 June 2021).
- Rahman, M.; Chakraborty, A. Nipah virus outbreaks in Bangladesh: A deadly infectious disease. WHO South-East Asia J. Public Health 2012, 1, 208–212. [Google Scholar] [CrossRef] [Green Version]
- Chadha, M.S.; Comer, J.A.; Lowe, L.; Rota, P.A.; Rollin, P.; Bellini, W.J.; Ksiazek, T.G.; Mishra, A.C. Nipah Virus-associated Encephalitis Outbreak, Siliguri, India. Emerg. Infect. Dis. 2006, 12, 235–240. [Google Scholar] [CrossRef]
- Rulli, M.C.; D’Odorico, P.; Galli, N.; Hayman, D.T.S. Land-use change and the livestock revolution increase the risk of zoonotic coronavirus transmission from rhinolophid bats. Nat. Food 2021, 2, 409–416. [Google Scholar] [CrossRef]
- Living Planet Report 2020|Official Site|WWF. Available online: https://livingplanet.panda.org/en-us/ (accessed on 14 June 2021).
- Lecompte, E.; Fichet-Calvet, E.; Daffis, S.; Koulemou, K.; Sylla, O.; Kourouma, F.; Dore, A.; Soropogui, B.; Aniskin, V.; Allali, B.; et al. Mastomys natalensis and Lassa Fever, West Africa. Emerg. Infect. Dis. 2006, 12, 1971–1974. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.H.; Goba, A.; Chu, M.; Roth, C.; Healing, T.; Marx, A.; Fair, J.; Guttieri, M.C.; Ferro, P.; Imes, T.; et al. Mano River Union Lassa Fever Network. New opportunities for field research on the pathogenesis and treatment of Lassa fever. Antivir. Res. 2008, 78, 103–115. [Google Scholar] [CrossRef]
- Suk, J.E.; Van Cangh, T.; Beaute, J.; Bartels, C.; Tsolova, S.; Pharris, A.; Ciotti, M.; Semenza, J.C. The interconnected and cross-border nature of risks posed by infectious diseases. Glob. Health Action 2014, 10, 25287, Erratum in: 2015, 8, 27635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tynkkynen, K. Lontoon neljä 1800-luvun koleraepidemiaa [Four cholera epidemics in nineteenth-century London]. Hippokrates 1995, 12, 62–88. [Google Scholar] [PubMed]
- Alwan, A.; Armstrong, T.; Bettcher, D.; Branca, F.; Chisholm, D.; Ezzati, M.; Garfield, R.; MacLean, D.; Mendis, S.; Riley, L.; et al. Global Status Report on Noncommunicable Diseases; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Friel, S.; Bowen, K.; Campbell-Lendrum, D.; Frumkin, H.; McMichael, A.; Rasanathan, K. Climate Change, Noncommunicable Diseases, and Development: The Relationships and Common Policy Opportunities. Annu. Rev. Public Health 2011, 32, 133–147. [Google Scholar] [CrossRef] [Green Version]
- Roundtable on Environmental Health Sciences, Research, and Medicine; Board on Population Health and Public Health Practice; Institute of Medicine. Public Health Linkages with Sustainability: Workshop Summary; National Academies Press (US): Washington, DC, USA, 2013. Available online: https://www.ncbi.nlm.nih.gov/books/NBK202306/ (accessed on 14 June 2021).
- FAO. Technical Report: The State of Food and Agriculture 2019. Moving Forward on Food Loss and Waste Reduction. 2019. Available online: http://www.fao.org/3/ca6030en/ca6030en.pdf (accessed on 14 June 2021).
- Warren, C.J.; Sawyer, S.L. How host genetics dictates successful viral zoonosis. PLoS Biol. 2019, 17, e3000217. [Google Scholar] [CrossRef]
- Springmann, M.; Godfray, H.C.J.; Rayner, M.; Scarborough, P. Analysis and valuation of the health and climate change cobenefits of dietary change. Proc. Natl. Acad. Sci. USA 2016, 113, 4146–4151. [Google Scholar] [CrossRef] [Green Version]
- Why Earth Is Warming|UCAR Center for Science Education. Available online: https://scied.ucar.edu/learning-zone/how-climate-works/why-earth-warming (accessed on 14 June 2021).
- Global Climate Report—Annual 2020|2020 Year-To-Date Temperatures versus Previous Years|State of the Climate|National Centers for Environmental Information (NCEI). Available online: https://www.ncdc.noaa.gov/sotc/global/202013/supplemental/page-1 (accessed on 15 June 2021).
- Harvey, J.A.; Malcicka, M. Climate Change, Range Shifts and Multitrophic Interactions. Biodivers. Ecosyst. 2015, 1, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Ruscio, B.A.; Brubaker, M.; Glasser, J.; Hueston, W.; Hennessy, T.W. One Health—A strategy for resilience in a changing arctic. Int. J. Circumpolar Health 2015, 74. [Google Scholar] [CrossRef] [PubMed]
- Semenza, J.C.; Suk, J.E. Vector-borne diseases and climate change: A European perspective. FEMS Microbiol. Lett. 2018, 365, fnx244. [Google Scholar] [CrossRef]
- Githeko, A.K.; Lindsay, S.W.; Confalonieri, U.E.; Patz, J.A. Climate change and vector-borne diseases: A regional analysis. Bull. World Health Organ. 2000, 78, 1136–1147. [Google Scholar] [PubMed]
- Brooks, D.R.; Hoberg, E.P. How will global climate change affect parasite–host assemblages? Trends Parasitol. 2007, 23, 571–574. [Google Scholar] [CrossRef] [Green Version]
- Asadgol, Z.; Mohammadi, H.; Kermani, M.; Badirzadeh, A.; Gholami, M. The effect of climate change on cholera disease: The road ahead using artificial neural network. PLoS ONE 2019, 14, e0224813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Climate Change: Regional Impacts|UCAR Center for Science Education. Available online: https://scied.ucar.edu/learning-zone/climate-change-impacts/regional (accessed on 15 June 2021).
- Colombo, E.; Masera, D.; Bologna, S. Renewable Energies to Promote Local Development. Renew. Energy Unleashing Sustain. Dev. 2013, 1, 3–25. [Google Scholar] [CrossRef]
- Ogwu, M.C. Towards Sustainable Development in Africa: The Challenge of Urbanization and Climate Change Adaptation. Geogr. Clim. Change Adapt. Urban Afr. 2019, 29–55. [Google Scholar] [CrossRef]
- Energy and Climate Change—European Environment Agency. Available online: https://www.eea.europa.eu/signals/signals-2017/articles/energy-and-climate-change (accessed on 7 August 2021).
- Walton, D.; Van Aalst, M. Climate-Related Extreme Weather Events and COVID-19 A First Look at the Number of People Affected by Intersecting Disasters: Assessment Report. 2020. Available online: https://reliefweb.int/report/world/climate-related-extreme-weather-events-and-covid-19-first-look-number-people-affected (accessed on 14 June 2021).
- Jan van Oldenborgh, G.; Krikken, F.; Lewis, S.; Leach, N.J.; Lehner, F.; Saunders, K.R.; van Weele, M.; Haustein, K.; Li, S.; Wallom, D. Attribution of the Australian bushfire risk to anthropogenic climate change. Hazards Earth Syst. Sci 2021, 21, 941–960. [Google Scholar] [CrossRef]
- Arriagada, N.B.; Palmer, A.J.; Bowman, D.M.; Morgan, G.; Jalaludin, B.B.; Johnston, F.H. Unprecedented smoke-related health burden associated with the 2019–20 bushfires in eastern Australia. Med. J. Aust. 2020, 213, 282. [Google Scholar] [CrossRef]
- The Impact of Coronavirus (COVID-19) on Forcibly Displaced Persons in Developing Countries. Available online: https://www.oecd.org/coronavirus/policy-responses/the-impact-of-coronavirus-covid-19-on-forcibly-displaced-persons-in-developing-countries-88ad26de/ (accessed on 15 June 2021).
- Impact of COVID-19 on Migrants and Refugees in the Arab Region, Technical Paper, United Nations Economic and Social Commission for Western Asia (ESCWA), the United Nations High Commissioner for Refugees (UNHCR) and the International Labour Organization (ILO). 2020. Available online: https://www.ilo.org/beirut/publications/WCMS_764756/lang--en/index.htm (accessed on 14 June 2021).
- A Virus That Respects no Borders: Protecting Refugees and Migrants during COVID-19. Available online: https://www.who.int/news-room/feature-stories/detail/a-virus-that-respects-no-borders-protecting-refugees-and-migrants-during-covid-19 (accessed on 15 June 2021).
- Rahman, M.R.; Islam, K. Massive diphtheria outbreak among Rohingya refugees: Lessons learnt. J. Travel Med. 2019, 26, 1–3. [Google Scholar] [CrossRef]
- Dureab, F.; Müller, O.; Jahn, A. Resurgence of diphtheria in Yemen due to population movement. J. Travel Med. 2018, 25, tay094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Addressing Climate Change Post-Coronavirus|McKinsey. Available online: https://www.mckinsey.com/business-functions/sustainability/our-insights/addressing-climate-change-in-a-post-pandemic-world (accessed on 15 June 2021).
- Nadimpalli, M.L.; Chan, C.W.; Doron, S. Antibiotic resistance: A call to action to prevent the next epidemic of inequality. Nat. Med. 2021, 27, 187–188. [Google Scholar] [CrossRef]
- Pokharel, S.; Raut, S.; Adhikari, B. Tackling antimicrobial resistance in low-income and middle-income countries. BMJ Glob. Health 2019, 4, e002104. [Google Scholar] [CrossRef] [PubMed]
- Godman, B.; Egwuenu, A.; Haque, M.; Malande, O.; Schellack, N.; Kumar, S.; Saleem, Z.; Sneddon, J.; Hoxha, I.; Islam, S.; et al. Strategies to Improve Antimicrobial Utilization with a Special Focus on Developing Countries. Life 2021, 11, 528. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Soucy, J.R.; Westwood, D.; Daneman, N.; MacFadden, D.R. Antibiotic prescribing in patients with COVID-19: Rapid review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Rawson, T.M.; Ming, D.; Ahmad, R.; Moore, L.S.P.; Holmes, A.H. Antimicrobial use, drug-resistant infections and COVID-19. Nat. Rev. Microbiol. 2020, 18, 409–410. [Google Scholar] [CrossRef]
- Burnham, J.P. Climate change and antibiotic resistance: A deadly combination. Ther. Adv. Infect. Dis. 2021, 8. [Google Scholar] [CrossRef]
- MacFadden, D.R.; McGough, S.F.; Fisman, D.; Santillana, M.; Brownstein, J.S. Antibiotic resistance increases with local temperature. Nat. Clim. Chang. 2018, 8, 510–514. [Google Scholar] [CrossRef]
- Arias-Andres, M.; Klümper, U.; Rojas-Jimenez, K.; Grossart, H. Microplastic pollution increases gene exchange in aquatic ecosystems. Environ. Pollut. 2018, 237, 253–261. [Google Scholar] [CrossRef] [Green Version]
- Ramirez, J.; Guarner, F.; Fernandez, L.B.; Maruy, A.; Sdepanian, V.L.; Cohen, H. Antibiotics as Major Disruptors of Gut Microbiota. Front. Cell. Infect. Microbiol. 2020, 10, 572912. [Google Scholar] [CrossRef]
- Clapp, M.; Aurora, N.; Herrera, L.; Bhatia, M.; Wilen, E.; Wakefield, S. Gut Microbiota’s Effect on Mental Health: The Gut-Brain Axis. Clin. Pract. 2017, 7, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Collignon, P.; Beggs, J.J.; Walsh, T.; Gandra, S.; Laxminarayan, R. Anthropological and socioeconomic factors contributing to global antimicrobial resistance: A univariate and multivariable analysis. Lancet Planet. Health 2018, 2, e398–e405. [Google Scholar] [CrossRef]
- Zinsstag, J.; Crump, L.; Schelling, E.; Hattendorf, J.; Maidane, Y.O.; Ali, K.O.; Muhummed, A.; Umer, A.; Aliyi, F.; Nooh, F.; et al. Climate change and One Health. FEMS Microbiol. Lett. 2018, 365, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, S.N.; Osburn, B.I.; Jay-Russell, M.T. One Health for Food Safety, Food Security, and Sustainable Food Production. Front. Sustain. Food Syst. 2020, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Thumbi, S.M.; Njenga, M.K.; Marsh, T.L.; Noh, S.; Otiang, E.; Munyua, P.; Ochieng, L.; Ogola, E.; Yoder, J.; Audi, A.; et al. Linking Human Health and Livestock Health: A “One-Health” Platform for Integrated Analysis of Human Health, Livestock Health, and Economic Welfare in Livestock Dependent Communities. PLoS ONE 2015, 10, e0120761. [Google Scholar] [CrossRef] [Green Version]
- Musoke, D.; Ndejjo, R.; Atusingwize, E.; Halage, A.A. The role of environmental health in One Health: A Uganda perspective. One Health 2016, 2, 157–160. [Google Scholar] [CrossRef] [Green Version]
- Gardy, J.L.; Loman, N.J. Towards a genomics-informed, real-time, global pathogen surveillance system. Nat. Rev. Genet. 2018, 19, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Tanaka, K.; Matsuoka, S. Environmental and economic effectiveness of the Kyoto Protocol. PLoS ONE 2020, 15, e0236299. [Google Scholar] [CrossRef]
- World Health Organization. COP24 Special Report: Health and Climate Change. 2014. Available online: https://apps.who.int/iris/handle/10665/276405 (accessed on 15 June 2021).
- Amuasi, J.H.; Walzer, C.; Heymann, D.; Carabin, H.; Huong, L.T.; Haines, A.; Winkler, A.S. Calling for a COVID-19 One Health Research Coalition. Lancet 2020, 395, 1543–1544. [Google Scholar] [CrossRef]
- Queenan, K. Roadmap to a One Health agenda 2030. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2017, 12. [Google Scholar] [CrossRef] [Green Version]
- Ogden, N.H.; Wilson, J.R.U.; Richardson, D.M.; Hui, C.; Davies, S.; Kumschick, S.; Le Roux, J.; Measey, J.; Saul, W.-C.; Pulliam, J.R.C. Emerging infectious diseases and biological invasions: A call for a One Health collaboration in science and management. R. Soc. Open Sci. 2019, 6, 181577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ståhl, T. Health in All Policies: From rhetoric to implementation and evaluation—The Finnish experience. Scand. J. Public Heal. 2018, 46, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Implementing Health in All Policies|The Health Foundation. Available online: https://www.health.org.uk/publications/reports/implementing-health-in-all-policies (accessed on 23 August 2021).
- Bhaumik, S.; Dutta, R.; Sen, B. A call for increased collaboration between environmental health scientists and lawyers. Lancet Planet. Health 2019, 3, e60–e61. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srivastava, S.; Khokhar, F.; Madhav, A.; Pembroke, B.; Shetty, V.; Mutreja, A. COVID-19 Lessons for Climate Change and Sustainable Health. Energies 2021, 14, 5938. https://doi.org/10.3390/en14185938
Srivastava S, Khokhar F, Madhav A, Pembroke B, Shetty V, Mutreja A. COVID-19 Lessons for Climate Change and Sustainable Health. Energies. 2021; 14(18):5938. https://doi.org/10.3390/en14185938
Chicago/Turabian StyleSrivastava, Siddharth, Fahad Khokhar, Archana Madhav, Billy Pembroke, Vignesh Shetty, and Ankur Mutreja. 2021. "COVID-19 Lessons for Climate Change and Sustainable Health" Energies 14, no. 18: 5938. https://doi.org/10.3390/en14185938
APA StyleSrivastava, S., Khokhar, F., Madhav, A., Pembroke, B., Shetty, V., & Mutreja, A. (2021). COVID-19 Lessons for Climate Change and Sustainable Health. Energies, 14(18), 5938. https://doi.org/10.3390/en14185938








