Synthesis and Characterization of ZnO from Thermal Decomposition of Precipitated Zinc Oxalate Dihydrate as an Anode Material of Li-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
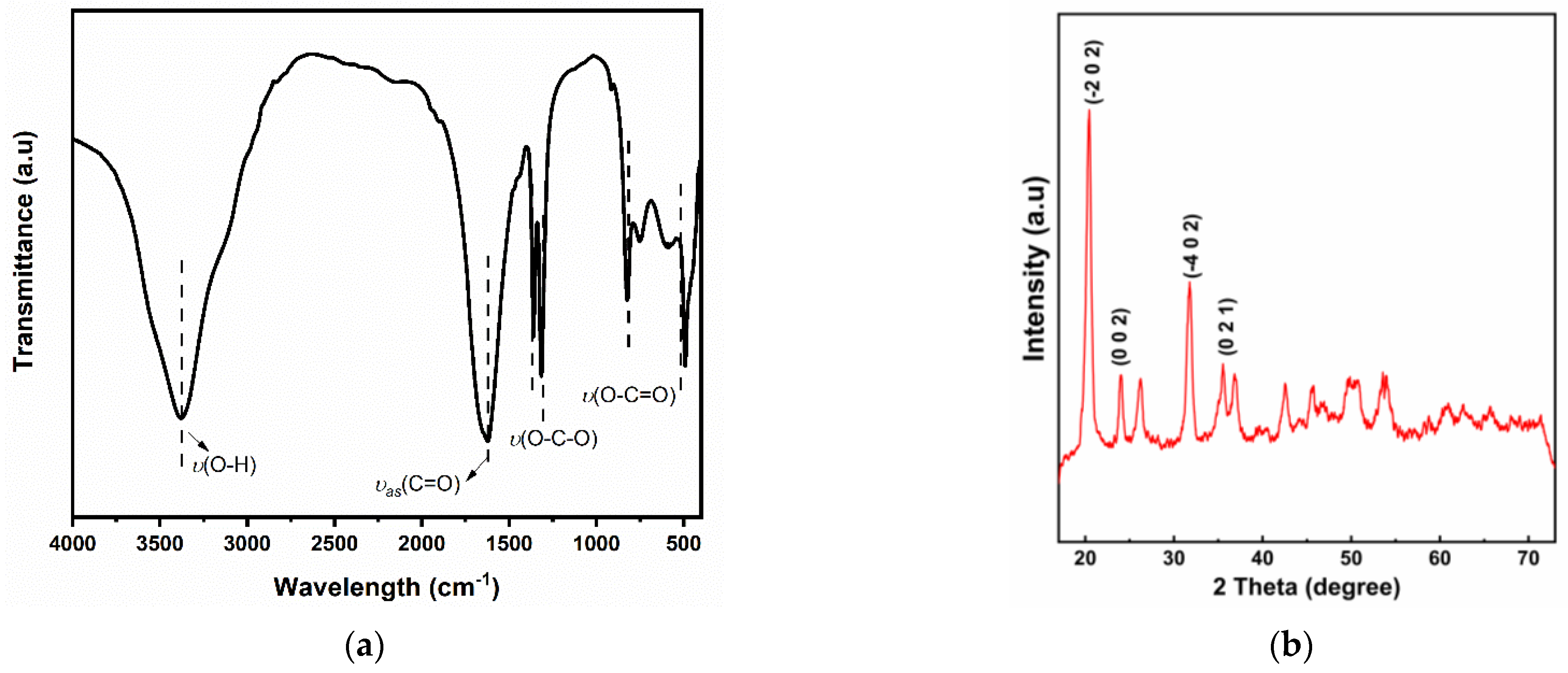
3.1. Zinc Oxalate Dihydrate Characterization
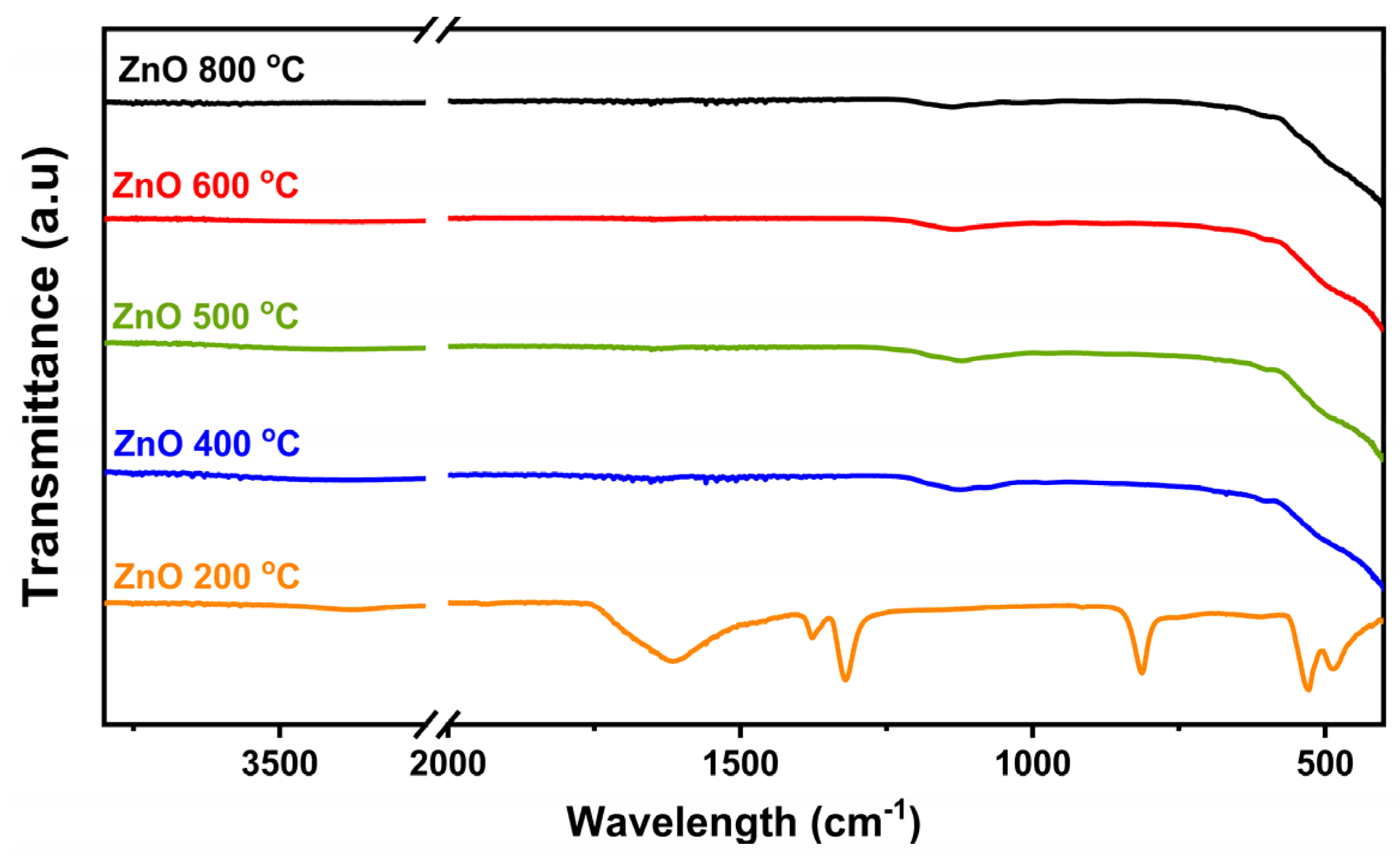
3.2. Zinc Oxide Characterization
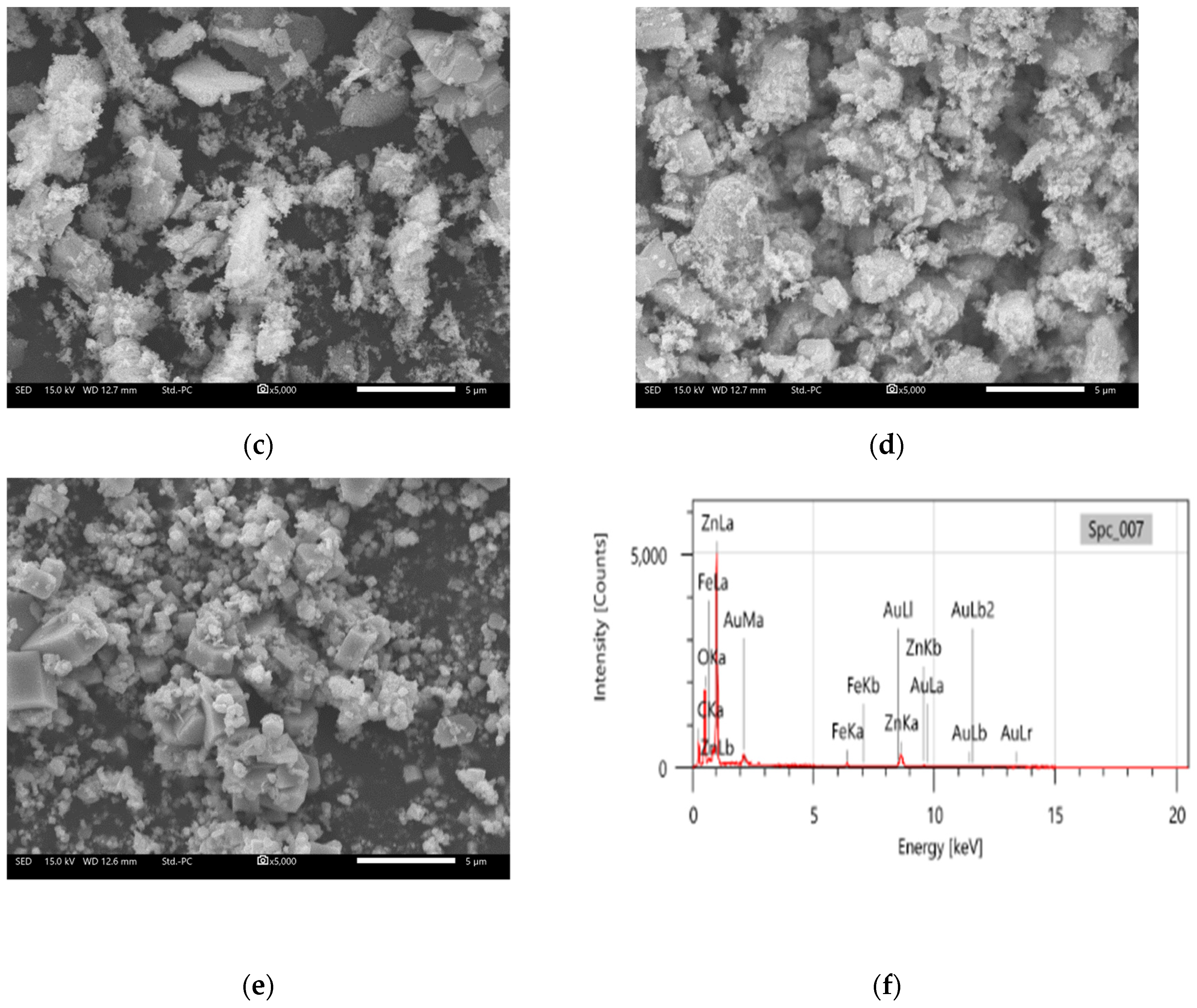
3.3. ZnO SEM Analysis
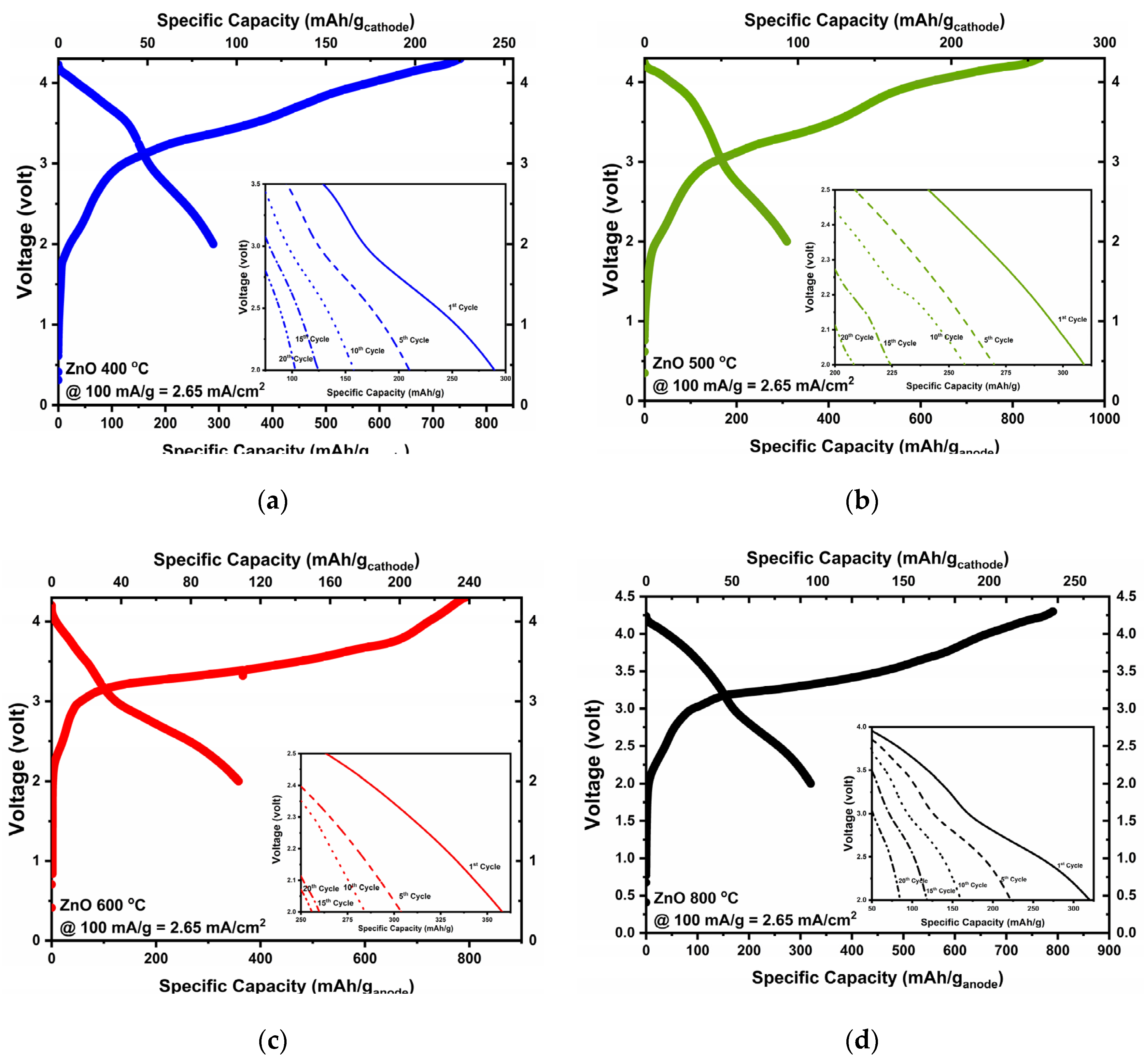
3.4. Electrochemical Performance of ZnO Samples
Li+ + Zn + e− → LiZn
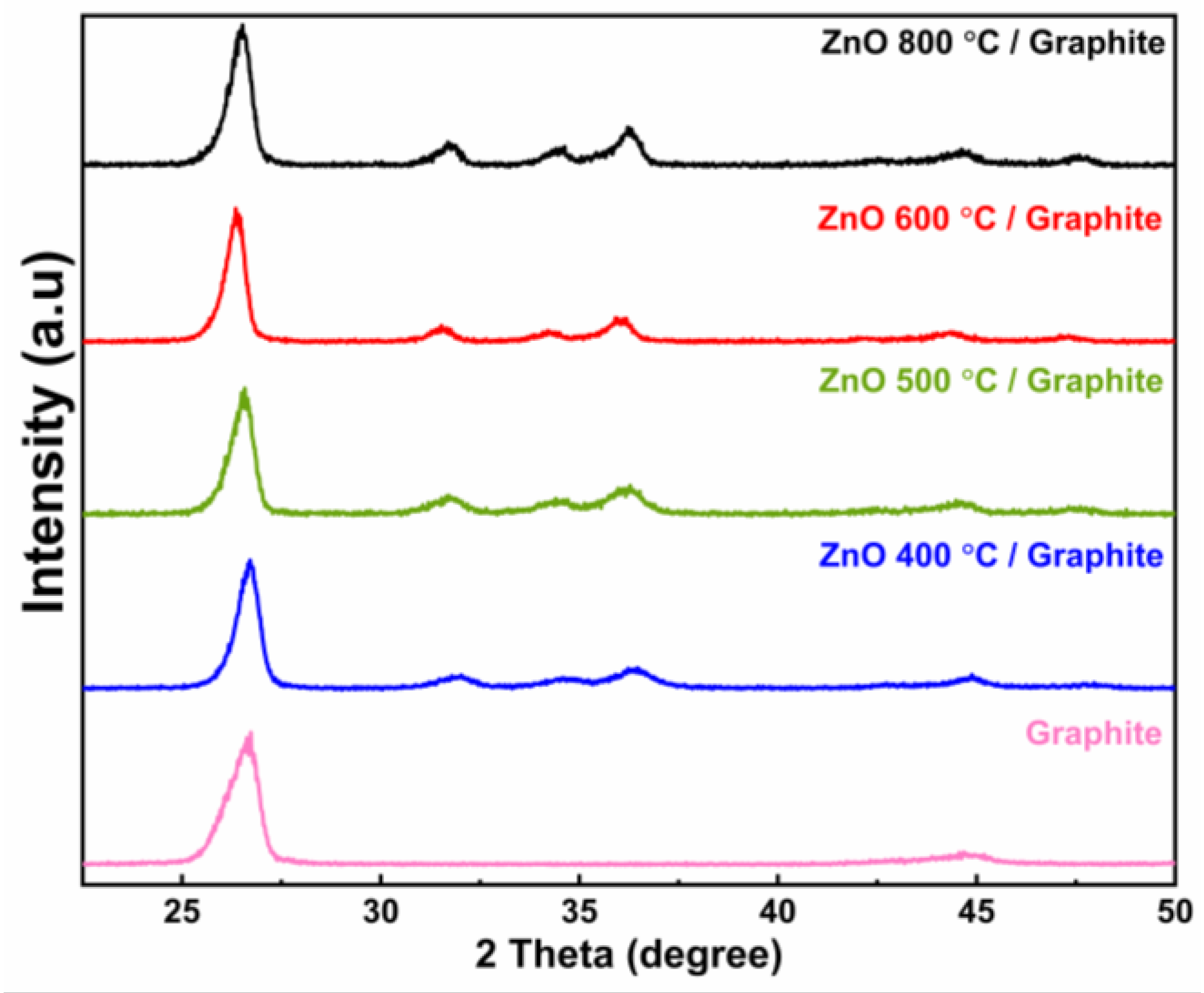
3.5. Zinc Oxide/Graphite Composite Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Berckmans, G.; Messagie, M.; Smekens, J.; Omar, N.; Vanhaverbeke, L.; Mierlo, J. Van Cost projection of state of the art lithium-ion batteries for electric vehicles up to 2030. Energies 2017, 10, 1314. [Google Scholar] [CrossRef] [Green Version]
- Tashenov, A.K.; Napolskiy, F.S.; Itkis, D.M.; Krivchenko, V.A.; Kaparova, B.T. Polyvinylidene fluoride based cathode produced by electrospinning of the lifepo4 based electrodes. Rasayan J. Chem. 2020, 13, 1598–1605. [Google Scholar] [CrossRef]
- Kim, H.; Kim, S.B.; Park, D.H.; Park, K.W. Fluorine-doped LiNi0.8Mn0.1Co0.1O2cathode for high-performance lithium-ion batteries. Energies 2020, 13, 4804. [Google Scholar]
- Samuel, A.J.; Deepi, A.; Srikesh, G.; Samson Nesaraj, A. Development of two-dimensional Mg doped ZnO nano hybrids as electrode materials for electrochemical supercapacitor applications. Rasayan J. Chem. 2020, 13, 562–569. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, G.; Liu, Q.; Duan, H. Recent progress in Zn-based anodes for advanced lithium ion batteries. Mater. Chem. Front. 2018, 2, 1414–1435. [Google Scholar] [CrossRef]
- Xiao, L.; Li, E.; Yi, J.; Meng, W.; Wang, S.; Deng, B.; Liu, J. Enhancing the performance of nanostructured ZnO as an anode material for lithium-ion batteries by polydopamine-derived carbon coating and confined crystallization. J. Alloys Compd. 2018, 764, 545–554. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Zhao, Y.; Tan, T. Synthesis of the ZnO@ZnS Nanorod for Lithium-Ion Batteries. Energies 2018, 11, 2117. [Google Scholar] [CrossRef] [Green Version]
- Guo, R.; Huang, X.; Wu, J.; Zhong, W.; Lin, Y.; Cao, Y. ZnO/C nanocomposite microspheres with capsule structure for anode materials of lithium ion batteries. Ceram. Int. 2020, 46, 19966–19972. [Google Scholar] [CrossRef]
- Song, J.; Kim, H.; Jae, W.; Kim, T.; Futalan, C.M.; Kim, J. Porous ZnO/C microspheres prepared with maleopimaric acid as an anode material for lithium-ion batteries. Carbon 2020, 165, 55–66. [Google Scholar] [CrossRef]
- Sachin Varma, U.; Gautham, P.; Ravi Kumar, D.V.; Sreekanth, K.M.; Sivasubramanian, G.; Sreedhar, K.M. Co-precipitation as a tool for effective doping of magnesium in zinc oxide: Studies on structural, optical and photocatalytic properties. Rasayan J. Chem. 2018, 11, 1491–1500. [Google Scholar] [CrossRef]
- Alam, M.N.; Potiyaraj, P. Synthesis of nano zinc hydroxide via sol–gel method on silica surface and its potential application in the reduction of cure activator level in the vulcanization of natural rubber. J. Sol-Gel Sci. Technol. 2017, 81, 903–911. [Google Scholar] [CrossRef]
- Muraleedharan, K.S.K. Exploration of the thermal decomposition of zinc oxalate by experimental and computational methods. J. Therm. Anal. Calorim. 2020, 142, 1315–1327. [Google Scholar]
- Cooper, N.D. Complete Kinetic and Mechanistic Decomposition of Zinc Oxalate with Characterization of Intermediates and Final Oxide. In Proceedings of the National Conference of Undergraduate Research, Washington, DC, USA, 16–18 April 2015. [Google Scholar]
- Yudha, C.S.; Rahmawati, M.; Jumari, A.; Hutama, A.P.; Purwanto, A. Synthesis of Zinc Oxide (ZnO) from Zinc Based-Fertilizer as Potential and Low-Cost Anode Material for Lithium Ion Batteries. In Proceedings of the ACM International Conference, Tangerang, Indonesia, 28 September 2020; Association for Computing Machinery: New York, NY, USA, 2020; pp. 1–6. [Google Scholar]
- Chen, X.; Liu, X.; Huang, K. Facile synthesis of flake-like dihydrate zinc oxalate particles. Int. J. Miner. Metall. Mater. 2019, 26, 234–240. [Google Scholar] [CrossRef]
- Thattil, P.P.; Leema Rose, A. Enhanced removal of crystal violet dye using zinc oxide nanorods and air oxidation under sunlight radiation. Rasayan J. Chem. 2020, 13, 1166–1173. [Google Scholar] [CrossRef]
- Saisa; Agusnar, H.; Alfian, Z.; Nainggolan, I. Chitosan-zinc oxide nanoparticle based highly sensitive electrochemical sensor for formaldehyde detection. Rasayan J. Chem. 2020, 13, 2530–2537. [Google Scholar] [CrossRef]
- Dey, S.; Bhattacharjee, S.; Chaudhuri, M.G.; Bose, R.S.; Halder, S.; Ghosh, C.K. Synthesis of pure nickel (III) oxide nanoparticles at room temperature for Cr(VI) ion removal. RSC Adv. 2015, 5, 54717–54726. [Google Scholar] [CrossRef]
- Yudha, C.S.; Muzayanha, S.U.; Rahmawati, M.; Widiyandari, H.; Sutopo, W.; Nizam, M.; Santosa, S.P.; Purwanto, A. Fast production of high performance LiNi0.815Co0.15Al0.035O2 cathode material via urea-assisted flame spray pyrolysis. Energies 2020, 13, 2757. [Google Scholar] [CrossRef]
- Saadi, H.; Rhouma, F.I.H.; Benzarti, Z.; Bougrioua, Z.; Guermazi, S.; Khirouni, K. Electrical conductivity improvement of Fe doped ZnO nanopowders. Mater. Res. Bull. 2020, 129, 110884. [Google Scholar] [CrossRef]
- Khera, S.; Chand, P. Influence of different solvents on the structural, optical, impedance and dielectric properties of ZnO nanoflakes. Chin. J. Phys. 2019, 57, 28–46. [Google Scholar] [CrossRef]
- Zhang, W.; Du, L.; Chen, Z.; Hong, J.; Yue, L. ZnO Nanocrystals as Anode Electrodes for Lithium-Ion Batteries. J. Nanomater. 2016, 2016, 8056302. [Google Scholar] [CrossRef]
- Xu, G.L.; Li, Y.; Ma, T.; Ren, Y.; Wang, H.H.; Wang, L.; Wen, J.; Miller, D.; Amine, K.; Chen, Z. PEDOT-PSS coated ZnO/C hierarchical porous nanorods as ultralong-life anode material for lithium ion batteries. Nano Energy 2015, 18, 253–264. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Liu, X.; Lu, Z. Systematic investigation of prelithiated SiO2 particles for high-performance anodes in lithium-ion battery. Appl. Sci. 2018, 8, 1245. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Wu, J.; Zhang, Q.; Su, X. Recent advancement of SiOx based anodes for lithium-ion batteries. J. Power Sources 2017, 363, 126–144. [Google Scholar] [CrossRef]
- Zhang, L.; Shen, K.; He, W.; Liu, Y.; Guo, S. SiO2@graphite composite generated from sewage sludge as anode material for lithium ion batteries. Int. J. Electrochem. Sci. 2017, 12, 10221–10229. [Google Scholar] [CrossRef]
- Abe, Y.; Kumagai, S. Effect of negative/positive capacity ratio on the rate and cycling performances of LiFePO4/graphite lithium-ion batteries. J. Energy Storage 2018, 19, 96–102. [Google Scholar] [CrossRef]
- Wu, X.; Song, K.; Zhang, X.; Hu, N.; Li, L.; Li, W.; Zhang, L.; Zhang, H. Safety issues in lithium ion batteries: Materials and cell design. Front. Energy Res. 2019, 7, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Li, H. Practical Evaluation of Li-Ion Batteries. Joule 2019, 3, 911–914. [Google Scholar] [CrossRef] [Green Version]




| Samples | Peak Location (°) | FWHM (°) | Crystallite Size (nm) |
|---|---|---|---|
| ZnO 800 °C | 36.01 | 0.488 | 17.89 |
| ZnO 600 °C | 36.18 | 0.721 | 15.11 |
| ZnO 500 °C | 35.94 | 1.202 | 13.16 |
| ZnO 400 °C | 36.46 | 0.77 | 11.35 |
| Samples | a (Å) | c (Å) | c/a | V | L | APF |
|---|---|---|---|---|---|---|
| ZnO 400 °C | 3.235 | 5.170 | 1.598 | 140.614 | 1.967 | 75.617 |
| ZnO 500 °C | 3.275 | 5.191 | 1.584 | 144.726 | 1.986 | 76.252 |
| ZnO 600 °C | 3.297 | 5.254 | 1.651 | 148.444 | 2.003 | 75.848 |
| ZnO 800 °C | 3.274 | 5.229 | 1.597 | 145.646 | 1.990 | 75.673 |
| Component | % Mass (%) | % Atom (%) |
|---|---|---|
| Zn | 43.7 | 16.5 |
| O | 30.9 | 47.4 |
| C | 16.8 | 34.3 |
| Fe | 2.5 | 1.1 |
| Anode | Cathode | Specific Charge Capacity (mAh/g) | Specific Discharge Capacity (mAh/g) | Initial Columbic Efficiency | Specific Discharge Energy (mWh/g) |
|---|---|---|---|---|---|
| ZnO 400 °C | NMC622 | 751.2 | 289.7 | 38% | 932.7 |
| ZnO 500 °C | NMC622 | 858.3 | 309.0 | 36.0% | 989.8 |
| ZnO 600 °C | NMC622 | 792.3 | 356.7 | 45.0% | 1034.1 |
| ZnO 800 °C | NMC622 | 790.8 | 318.7 | 40.3% | 1009.6 |
| Samples | Peak Location (°) | FWHM (°) | Crystallite Size (nm) |
|---|---|---|---|
| ZnO 800 °C/Graphite | 31.68 | 0.657 | 13.13 |
| ZnO 600 °C/Graphite | 31.06 | 0.879 | 11.64 |
| ZnO 500 °C/Graphite | 31.49 | 0.972 | 8.87 |
| ZnO 400 °C/Graphite | 32.52 | 1.067 | 9.39 |
| Samples | Specific Discharge Capacity (mAh/g) | Specific Discharge Energy (mWh/g) |
|---|---|---|
| ZnO 400 °C/Graphite | 327.3 | 1162.8 |
| ZnO 500 °C/Graphite | 397.4 | 1443.9 |
| ZnO 600 °C/Graphite | 450.2 | 1668.9 |
| ZnO 800 °C/Graphite | 447.2 | 1648.0 |
| Graphite | 307.85 | 889.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yudha, C.S.; Hutama, A.P.; Rahmawati, M.; Widiyandari, H.; Nursukatmo, H.; Nilasary, H.; Oktaviano, H.S.; Purwanto, A. Synthesis and Characterization of ZnO from Thermal Decomposition of Precipitated Zinc Oxalate Dihydrate as an Anode Material of Li-Ion Batteries. Energies 2021, 14, 5980. https://doi.org/10.3390/en14185980
Yudha CS, Hutama AP, Rahmawati M, Widiyandari H, Nursukatmo H, Nilasary H, Oktaviano HS, Purwanto A. Synthesis and Characterization of ZnO from Thermal Decomposition of Precipitated Zinc Oxalate Dihydrate as an Anode Material of Li-Ion Batteries. Energies. 2021; 14(18):5980. https://doi.org/10.3390/en14185980
Chicago/Turabian StyleYudha, Cornelius Satria, Anjas Prasetya Hutama, Mintarsih Rahmawati, Hendri Widiyandari, Hartoto Nursukatmo, Hanida Nilasary, Haryo Satriya Oktaviano, and Agus Purwanto. 2021. "Synthesis and Characterization of ZnO from Thermal Decomposition of Precipitated Zinc Oxalate Dihydrate as an Anode Material of Li-Ion Batteries" Energies 14, no. 18: 5980. https://doi.org/10.3390/en14185980
APA StyleYudha, C. S., Hutama, A. P., Rahmawati, M., Widiyandari, H., Nursukatmo, H., Nilasary, H., Oktaviano, H. S., & Purwanto, A. (2021). Synthesis and Characterization of ZnO from Thermal Decomposition of Precipitated Zinc Oxalate Dihydrate as an Anode Material of Li-Ion Batteries. Energies, 14(18), 5980. https://doi.org/10.3390/en14185980







