Design and Pinch Analysis of a GFT Process for Production of Biojet Fuel from Biomass and Plastics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Location and Feedstock
- The amount of waste generated per inhabitant is homogeneous throughout Spain;
- The average composition of plastic waste is based on the type of polymers demanded in Europe [8];
- A total of 90% of the polyethylene (PE) and polypropylene (PP) that would be exported from the provinces of Huesca, Zaragoza, Navarra, and La Rioja are fed into the chemical process.
2.2. Process Simulation and Thermodynamic Models
2.3. Heat Integration and Utilities
2.4. Economic Analysis
3. Results
3.1. Process Flow Diagram (PFD)
3.2. Chemical Process Design
3.2.1. Gasification (Unit 1)
| Cellulose + Hemicellulose + Lignin | → | Gas + Tar + Char |
| Plastic | → | Gas + Tar |
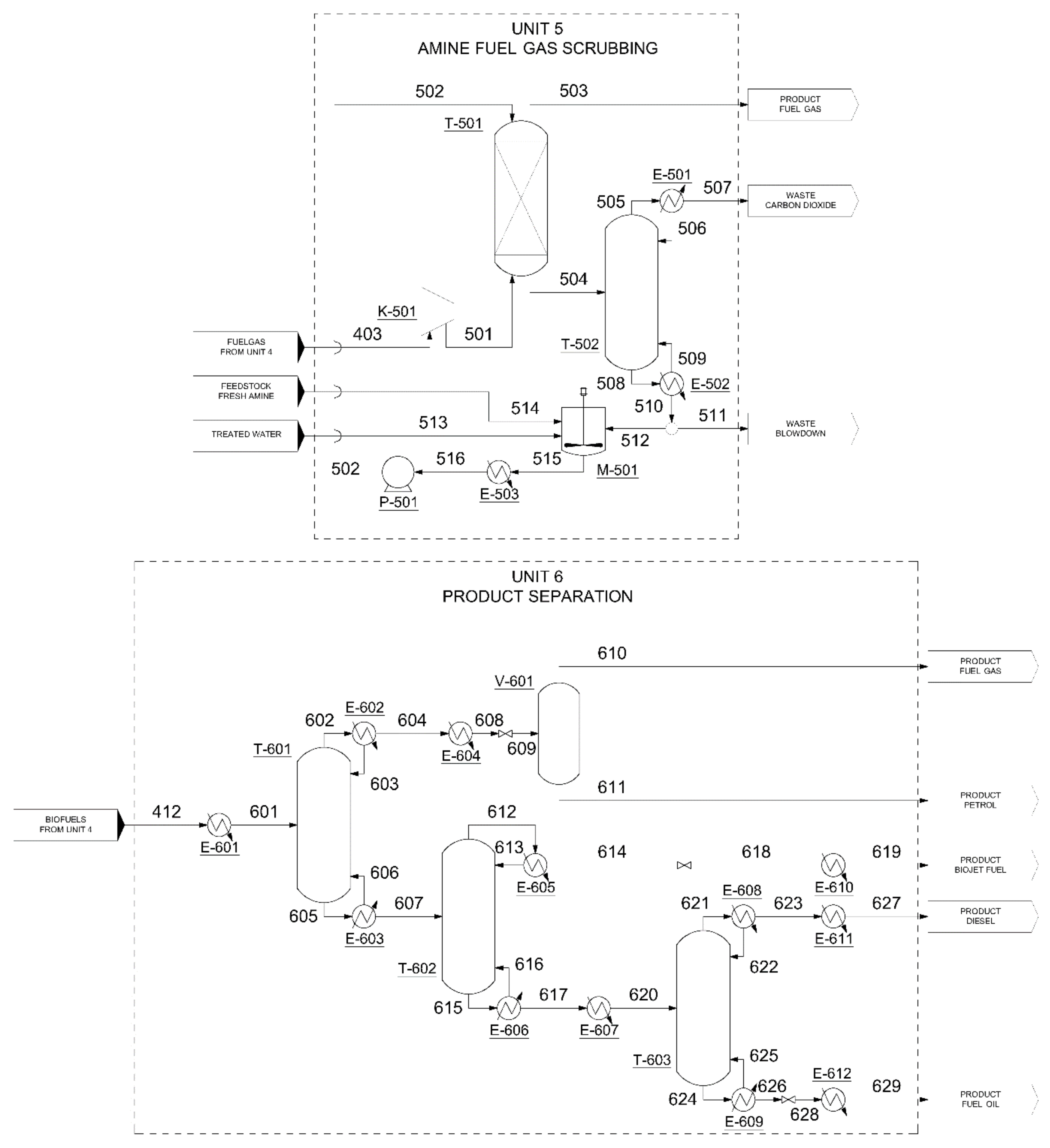
3.2.2. Amine Scrubbing (Units 2 and 5)
3.2.3. Fischer-Tropsch Process (Unit 3)
3.2.4. Upgrading (Unit 4)
- Hydrocracking:
- Isomerization:
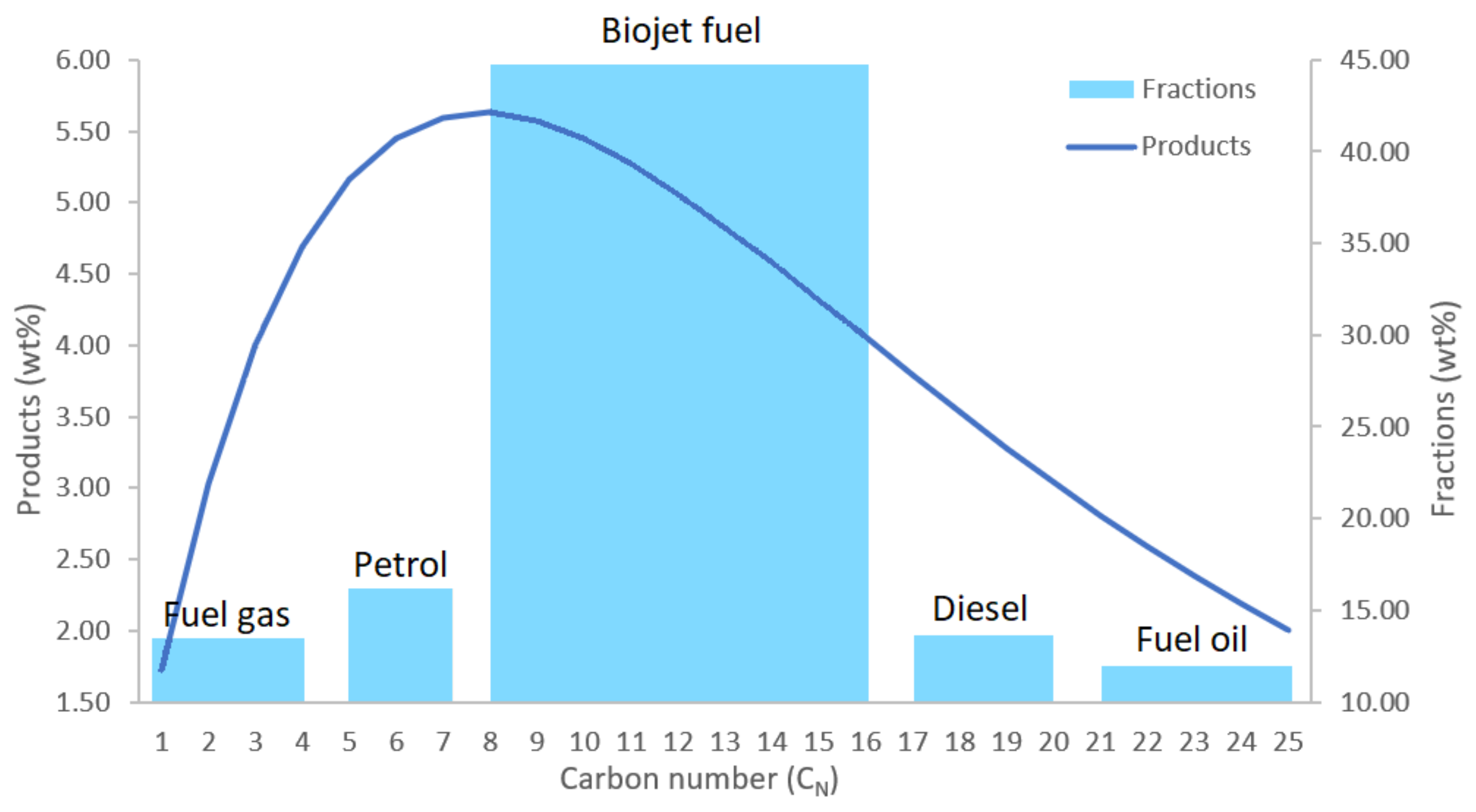
3.2.5. Product Separation (Unit 6)
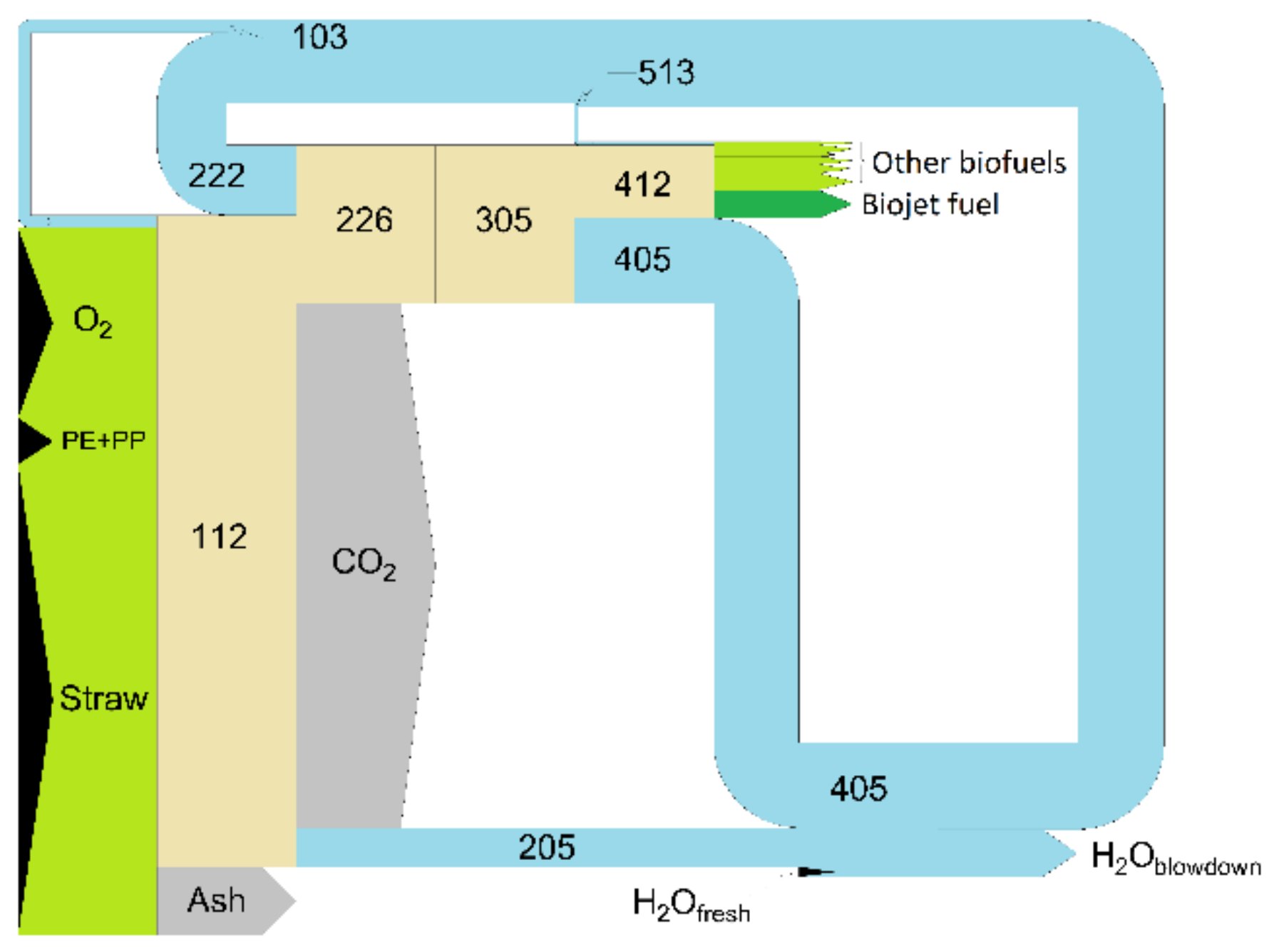
3.2.6. Overall Mass Balance
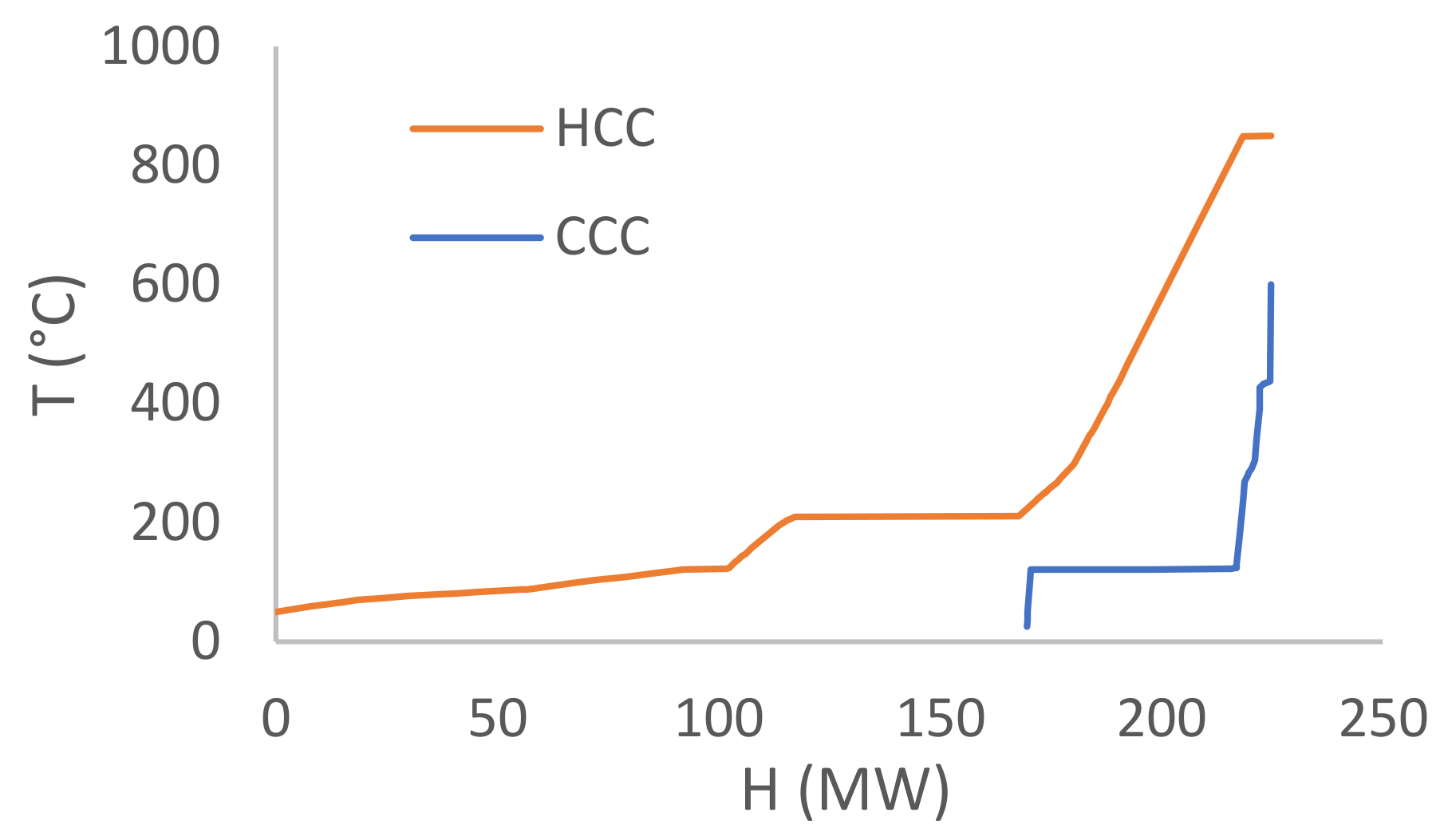
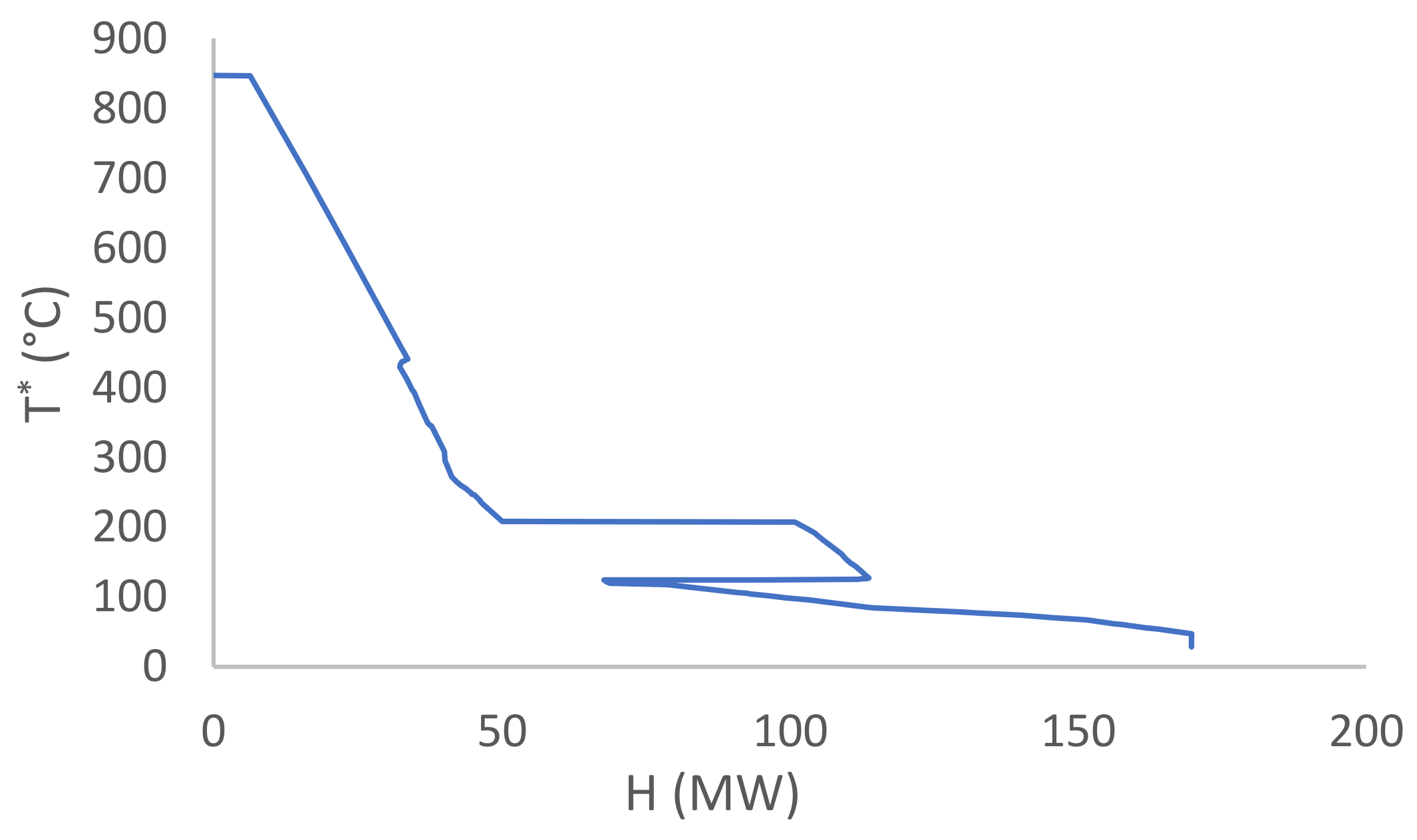
3.3. Heat Integration
3.4. Thermodynamic Cycles and Cooling Tower
3.4.1. Thermodynamic Cycles (Unit 7)
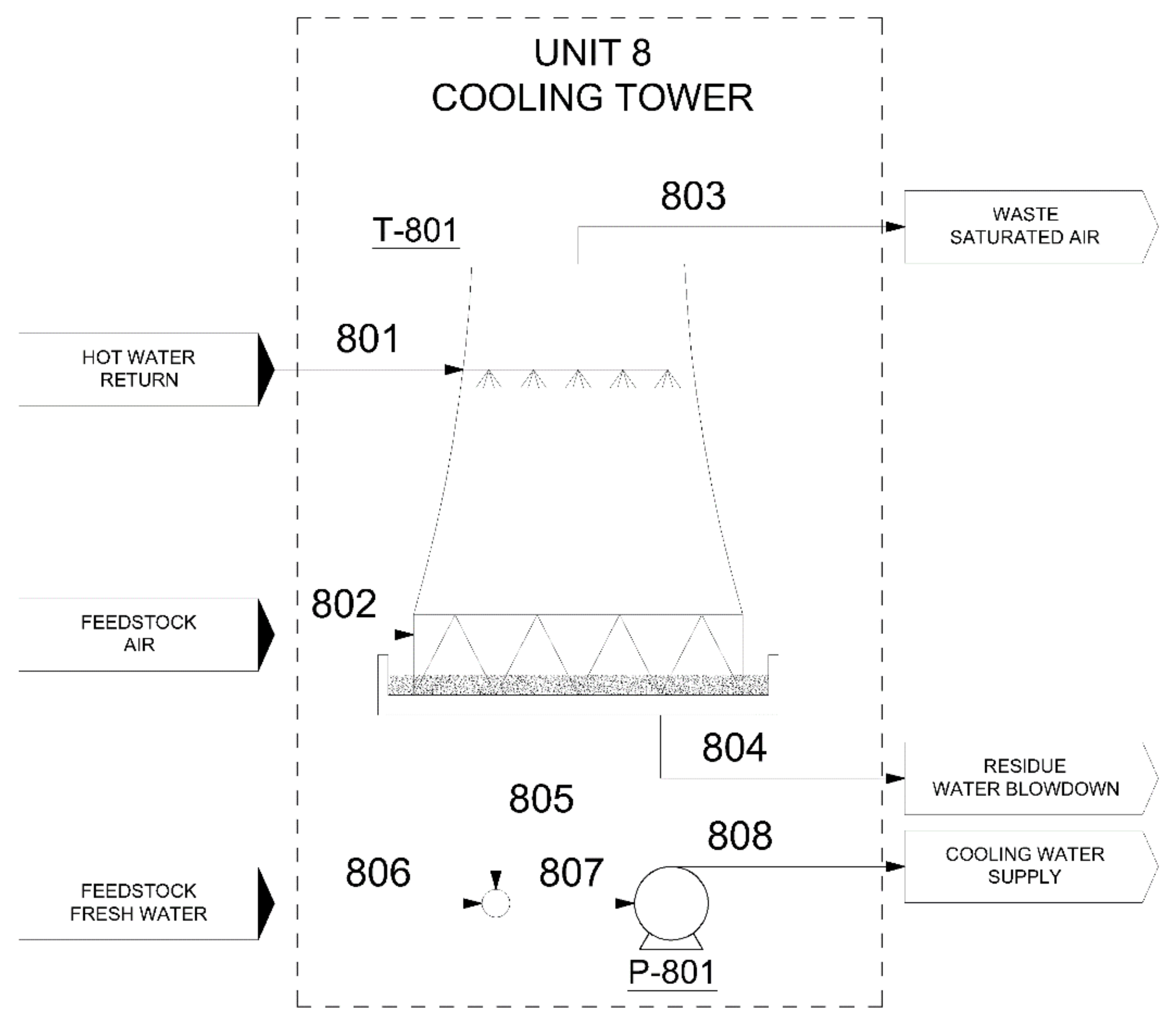
3.4.2. Cooling Tower (Unit 8)
3.5. CO2 Equivalent Emissions and Biojet Fuel Properties
3.6. Economic Analysis
3.6.1. Cost of Equipment
- Plastic and biomass feed hoppers and mixers:
- D is the diameter of the vessel (m);
- V, volume (m3);
- L, lenght (m);
- t, thickness (mm);
- C.A., corrosion allowance (mm);
- PD, the design pressure (kg/cm2);
- St, a dimensionless factor depending on the material, pressure and temperature conditions. This value is set to 1055 when using carbon steel and moderate conditions;
- E, modulus of elasticity of carbon steel, which is 0.85 GPa;
- W, weight of the equipment (t);
- X, a complexity factor, considered 2 for simple equipment;
- Flash chambers:
- Distillation columns:
- For oxygen separation PSA, the air flow rate obtained (t/h);
- For hydrogen separation PSA, the flow rate of hydrogen separated (kg/h);
- For the Fischer-Tropsch reactor, the feed flow rate (kmol/h);
- For the upgrading reactor, the feed flow rate (kg/h).
3.6.2. Cost of Raw Materials and Products
3.6.3. Minimum Selling Price
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| α | Probability of chain growth |
| Δ | Variation, difference |
| ΔTmin | Pinch temperature of a heat exchanger |
| η | Yield |
| ν | Stoichiometric coefficient |
| ρ | Density |
| µ | Viscosity |
| [x] | Concentration of compound x |
| °C | Degree(s) centigrade(s) |
| bar | Bar(s) |
| BTU | British thermal unit(s) |
| C. | Cost(s) |
| C.A. | Corrosion allowance |
| C.F. | Cash flow |
| cal | Calorie(s) |
| Cap | Capacity |
| CCC | Cold composite curve |
| CEPCI | Chemical plant cost index(es) |
| cm | Centimetre |
| Con | Condenser |
| Con. | Construction |
| Con. S. | Construction supervision |
| cP | Centipoise |
| cp | Specific heat |
| CS | Cold stream |
| CW | Cooling water |
| Cx | Hydrocarbon of x number of carbons |
| Cx+ | Hydrocarbon with number of carbons greater than x |
| D. | Depreciation |
| D.E. | Detailed engineering |
| E | Modulus of elasticity, cost of equipment; as indicated |
| E- | Heat exchanger |
| EA | Activation energy |
| EUR | Euro(s) |
| F | Factor |
| F.C. | Fixed cost(s) |
| F.G. | Funds generated |
| F- | Furnace |
| F-T | Fischer-Tropsch |
| fC | Civil works factor |
| fEI | Electricity factor |
| fER | Equipment placement factor |
| fI | Instrumentation and control factor |
| fL | Thermal insulation and painting factor |
| fM | Material factor |
| fP | Piping cost factor |
| fS | Buildings and structures factor |
| FT | Correction factor in a heat exchanger |
| g | Gravitational acceleration |
| Gen. | Generation |
| GFT-J | Gasification and Fischer-Tropsch synthesis to jet fuel |
| GPa | Gigapascals |
| h | Hour(s), specific enthalpy; as indicated |
| H | Enthalpy, heat |
| HCC | Hot composite curve |
| HHPS | Very high-pressure steam |
| HPS | High-pressure steam |
| HS | Hot stream |
| HTFT | High temperature Fischer-Tropsch |
| HX | Height of x |
| I | Inflation |
| i-Cx/i-CxHy | Isoparaffin of x number of carbons |
| Inv. | Invested |
| IRR | Internal rate of return |
| ISBL | Inside battery limits |
| J | Joule(s) |
| K | Kelvin(s) |
| K- | Compressor |
| kg | Kilogram(s) |
| kJ | Kilojoule(s) |
| kmol | Kilomol(es) |
| kWh | Kilowatt(s) hour |
| L | Litre(s) |
| LPS | Low-pressure steam |
| LTFT | Low temperature Fischer-Tropsch |
| m | Meter(s) |
| M | Material cost(s) |
| M.W. | Molecular weight |
| M- | Mixer |
| MEA | Monoethanolamine |
| min | Minimum |
| mm | Milimeter |
| MPS | Medium-pressure steam |
| MW | Megawatt(s) |
| MWh | Megawatt(s) hour |
| N | Number |
| N(x) | Molar flow rate of component x |
| n-Cx/n-CxHy | Paraffin of x number of carbons |
| n/n% | Mole distribution |
| NPV | Net present value |
| O.S. | Off-sites |
| OSBL | Outside battery limits |
| Overs. | Oversized |
| P | Pressure, power; as indicated |
| P.E. | Licenses and process engineering costs |
| P&ID | Piping and instrumentation diagram |
| P- | Pump |
| P.A.T. | Profit after tax |
| Pa | Pascal(s) |
| PD | Design pressure |
| PE | Polyethylene |
| PP | Polipropylene |
| PSA | Pressure swing adsorption |
| P.T.P. | Pre-tax profits |
| px | Partial pressure of compound x |
| r | Reaction speed |
| R | Ideal gas constant |
| R-yxx | Reactor |
| Reb | Reboiler |
| RoR | Rate of return |
| s | Second(s) |
| S | Surface |
| S. | Sales |
| S.C. | Start-up cost(s) |
| SI | Supplementary Information |
| SI X | Supplementary Information Section X |
| SI X-Y | Sections X to Y of Supplementary Information |
| ST- | Steam turbine |
| Sxxx | Stream xxx |
| t | Time, tonne(s), thickness; as indicated |
| T | Temperature |
| T. | Tax(es) |
| T* | Modified temperature |
| T- | Distillation tower |
| T.I. | Total invested |
| Tin | Temperature at inlet |
| Tout | Temperature at outlet |
| tR | Residence time |
| U | Overall heat transfer coefficient in a heat exchanger |
| U.C. | Unforeseen contingencies |
| USD | US dollar(s) |
| v | Velocity |
| V | Volume, volumetric flow; as indicated |
| V- | Vessel |
| vol% | Volumetric composition |
| W | Watt(s), weight; as indicated |
| W.C. | Working capital |
| wt% | Mass composition |
| x | Complexity factor |
References
- Viggiano, D.A. Simulation of Municipal Solid Waste Gasification Using Aspen Plus; Universidad Autónoma de Madrid; Universidad Rey Juan Carlos: Madrid, Spain, 2019. [Google Scholar]
- Dudley, B. BP Statistical Review of World Energy 2019; BP Statistical Review: London, UK, 2019. [Google Scholar]
- United Nations Department of Political Affairs; United Nations Environment Programme. Natural Resources and Conflict; United Nations Department of Political Affairs; United Nations Environment Programme: Nairobi, Kenya, 2015. [Google Scholar]
- Fernández, J.M. Guía Completa de la Biomasa y los Biocombustibles; Vicente, A.M., Ed.; AMV Ediciones: Madrid, Spain, 2010. [Google Scholar]
- U.S. Energy Information Administration. Available online: https://www.eia.gov/dnav/pet/hist/LeafHandler.ashx?n=pet&s=emm_epm0_pte_nus_dpg&f=a (accessed on 2 March 2021).
- EU Policy Department for Citizens’ Rights and Constitutional Affairs. The Environmental Impacts of Plastics and Micro-Plastics Use, Waste and Pollution: EU and National Measures; European Union: Brussels, Belgium, 2020. [Google Scholar]
- United States Environmental Protection Agency. Available online: https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/plastics-material-specific-data (accessed on 15 March 2021).
- Plastics Europe. Available online: https://www.plasticseurope.org/application/files/5715/1717/4180/Plastics_the_facts_2017_FINAL_for_website_one_page.pdf (accessed on 23 November 2019).
- Greenpeace, “Maldito Plástico. Reciclar no es suficiente”. 2019. Available online: https://es.greenpeace.org/es/wp-content/uploads/sites/3/2019/03/reciclar_no_es_suficiente.pdf (accessed on 10 March 2019).
- National Geographic. 2011. Available online: https://www.ngenespanol.com/el-mundo/la-isla-de-basura-amenaza-vida-marina-pacifico/ (accessed on 5 March 2020).
- Gopinath, K.P.; Nagarajan, V.M.; Krishnan, A.; Malolan, R. A critical review on the influence of energy, environmental and economic factors on various processes used to handle and recycle plastic wastes: Development of a comprehensive index. J. Clean. Prod. 2020, 274, 12301. [Google Scholar] [CrossRef]
- Nanda, S.; Berruti, F. Thermochemical conversion of plastic waste to fuels: A review. Environ. Chem. Lett. 2020, 19, 123–148. [Google Scholar] [CrossRef]
- Morales, G. Valorización de la Biomasa Lignocelulósica; Universidad Rey Juan Carlos: Móstoles, Spain, 2013. [Google Scholar]
- Dudley, B. BP Energy Outlook 2019; BP Statistical Review: London, UK, 2019. [Google Scholar]
- U.S. Energy Information Administration. International Energy Outlook 2016; U.S. Energy Information Administration: Washington, DC, USA, 2016. [Google Scholar]
- Federal Aviation Administration. FAA Aerospace Forecast; Federal Aviation Administration: Los Angeles, CA, USA, 2020. [Google Scholar]
- Eurocontrol. European Aviation in 2040; European Organisation for the Safety of Air Navigation: Brussels, Belgium, 2018. [Google Scholar]
- Martín, D.B.; García, N.V.; Mosquera, A.M.; Ortega, M.F.; Matínez, M.J.G.; Canoira, L. Techno-economic and life cycle assessment of triisobutane production and its suitability as biojet fuel. Appl. Energy 2020, 268, 114897. [Google Scholar]
- Zhang, X.; Lei, H.; Zhu, L.; Zhu, X.; Qian, M. Enhancement of jet fuel range alkanes from co-feeding of lignocellulosic biomass with plastics via tandem catalytic conversions. Appl. Energy 2016, 173, 418–430. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Lei, H.; Zhu, L.; Zhu, X.; Qian, M.; Yadavalli, G.; Yan, D.; Wu, J.; Chen, S. Optimizing carbon efficiency of jet fuel range alkanes from cellulose cofed with polyethylene via catalytically combined processes. Bioresour. Technol. 2016, 214, 45–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diederichs, G.W.; Mohsen, A.; Farzad, M.; Görgens, J. Techno-economic comparison of biojet fuel production from lignocellulose, vegetable oil and sugar cane juice. Bioresour. Technol. 2016, 216, 331–339. [Google Scholar] [CrossRef] [PubMed]
- González, M.P.; Rubiera, F.; Pevida, C.; Pio, D.T.; Tarelho, L.A.C. Thermodynamic Analysis of Biomass Gasification Using Aspen Plus: Comparison of Stoichiometric and Non-Stoichiometric Models. Energies 2021, 14, 189. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, H.; Zhu, L.; Zhu, X. Thermal behavior and kinetic study for catalytic co-pyrolysis of biomass. Bioresour. Technol. 2016, 220, 233–238. [Google Scholar] [CrossRef]
- Burra, K.; Gupta, A. Kinetics of synergistic effects in co-pyrolysis of biomass with plastic wastes. Appl. Energy 2018, 220, 408–418. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, X.; Hildebrandt, D.; Glasser, D. The effect of CO2 on a cobalt-based catalyst for low temperature Fischer–Tropsch. Chem. Eng. J. 2012, 193, 318–327. [Google Scholar] [CrossRef]
- Gomes, J.; Santos, S.; Bordado, J. Choosing amine-based absorbents for CO2 capture. Environ. Technol. 2014, 36, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Marcon, J.; Colling, B.; Ferreira, M.; Barbosa, M.D.; de Mesquita, I.L.; Bonomi, A.; de Morais, E.R.; Cavalett, O. Techno-Economic and Environmental Assessment of Biomass Gasification and Fischer–Tropsch Synthesis Integrated to Sugarcane Biorefineries. Energies 2020, 13, 4576. [Google Scholar] [CrossRef]
- Diedrichs, G.W. Techno-Economic Assessment of Processes the Produce Jet Fuel from Plant-Derived Sources; Stellenbosch University: Stellenbosch, South Africa, 2015. [Google Scholar]
- Martínez-Hernández, E.; Ramírez-Verduzco, L.F.; Amezcua-Allieri, M.A.; Aburto, J. Process simulation and techno-economic analysis of bio-jet fuel and green diesel production—Minimum selling prices. Chem. Eng. Res. Des. 2019, 146, 60–70. [Google Scholar] [CrossRef]
- Thybaut, J.W.; Marin, G.B. Multiscale aspects inhydrocracking: From reaction mechanism over catalysts tokinetics and industrial application. Adv. Catal. 2016, 59, 109–238. [Google Scholar] [CrossRef]
- Weitkamp, J.; Jacobs, P.A.; Martens, J.A. Isomerization and hydrocracking of C9 through C16 n-alkanes on Pt/HZSM-5 zeolite. Appl. Catal. 1983, 8, 123–141. [Google Scholar] [CrossRef]
- Coonradt, H.L.; Garwood, W.E. Mechanism of Hydrocracking. Reactions of Paraffins and Olefins. Ind. Eng. Chem. Process. Des. Dev. 1964, 3, 38–45. [Google Scholar] [CrossRef]
- Flinn, R.A.; Larson, O.A.; Beuther, H. The Mechanism of Catalytic Hydrocracking. Chem. Eng. Res. Des. 1960, 52, 153–156. [Google Scholar] [CrossRef]
- Deane, J.P.; Pye, S. Europe’s ambition for biofuels in aviation—A strategic review of challenges and opportunities. Energy Strategy Rev. 2018, 20, 1–5. [Google Scholar] [CrossRef]
- European Commission. Statistical Factsheet; DG Agriculture and Rural Development: Brussels, Belgium; Farm Economics Unit: Brussels, Belgium, 2020. [Google Scholar]
- Ministerio de Agricultura, Pesca y Alimentación. Available online: https://www.mapa.gob.es/estadistica/pags/anuario/2018/CAPITULOS_TOTALES/AE18-C07.xlsx (accessed on 18 October 2019).
- Plazonić, I.; Barbarić-Mikočević, Ž.; Antonović, A. Chemical Composition of Straw as an Alternative Material to Wood Raw Material in Fibre Isolation. Drv. Ind. Znan. Časopis Pitanja Drv. Tehnol. 2016, 37, 119–125. [Google Scholar] [CrossRef]
- Summerell, B.A. Decomposition and chemical composition of cereal Straw. Soil Biol. Biochem. 1989, 21, 551–559. [Google Scholar] [CrossRef]
- Sunab, R.C.; Sunc, X.F.; Fowlerb, P.; Tomkinsonb, J. Structural and physico-chemical characterization of lignins solubilized during alkaline peroxide treatment of barley Straw. Eur. Polym. J. 2002, 38, 1399–1407. [Google Scholar] [CrossRef]
- Bilbao, R.; Millera, A.; Arauzo, J. Thermal decomposition of lignocellulosic materials: Influence of the thermal composition. Thermochim. Acta 1989, 143, 149–159. [Google Scholar] [CrossRef]
- Fackrhoseini, S.; Dastanian, M. Predicting pyrolysis products of PE, PP, and PET using NRTL activity. J. Chem. 2013, 3, 487676. [Google Scholar] [CrossRef]
- Yi, L.; Kate, A.T.; Mooijer, M. Comparison of Activity Coefficient Models for Electrolyte Systems. Am. Inst. Chem. Eng. 2010, 56, 1334–1351. [Google Scholar] [CrossRef]
- Aspentech, Acid Gas Cleaning Using Amine Solvents: Validation with Experimental and Plant Data. Available online: https://www.aspentech.com/en/-/media/aspentech/home/resources/white-papers/pdfs/at-03942-wp-acid-gas-cleaning-using-amine-solvents.pdf (accessed on 16 August 2021).
- Lombard, J.E. Thermodynamic Modelling of Hydrocarbon-Chains and Light-Weight Supercritical Solvents; Stellenbosch University: Stellenbosch, South Africa, 2015. [Google Scholar]
- White, M.T.; Sayma, A.I. Simultaneous Cycle Optimization and Fluid Selection for ORC Systems Accounting for the Effect of the Operating Conditions on Turbine Efficiency. Front. Energy Res. 2019, 7, 50. [Google Scholar] [CrossRef]
- Towler, G.; Sinnott, R. Chemical Engineering Design; Butterworth-Heinemann: San Diego, CA, USA, 2008. [Google Scholar]
- Vázquez, M.; Montero, G.; Sosa, J.F.; Coronado, M.; García, C. Economic analysis of biodiesel production from waste vegetable oil in Mexicali, Baja California. Energy Sci. Technol. 2011, 1, 87–93. [Google Scholar]
- Mart, S. Modelado y Análisis de Sistemas de CO-Gasificación de Biomasa y Residuos Plásticos. Ph.D. Thesis, Universidad de Zaragoza, Zaragoza, Spain, 2015. [Google Scholar]
- Marcantonio, V.; Monarca, D.; Villarini, M.; di Carlo, A.; del Zotto, L.; Bocci, E. Biomass Steam Gasification, High-Temperature Gas Cleaning, and SOFC Model: A Parametric Analysis. Energies 2020, 13, 5936. [Google Scholar] [CrossRef]
- Ruan, R.; Zhang, Y.; Chen, P.; Liu, S. Biofuels: Introduction. In Biofuels: Alternative Feedstocks and Conversion Processes for the Production of Liquid and Gaseous Biofuels, 2nd ed.; Academic Press: Harbin, China, 2019; pp. 3–43. [Google Scholar]
- Lacaze, G. Arrhenius Kinetic Parameters for a Reversed Equilibrium Reaction; Centre Européen de Recherche et de Formation Avancée en Calcul Scientifique: Toulouse, France, 2006. [Google Scholar]
- Martín, M.M. Chapter 5—Syngas. In Industrial Chemical Process Analysis and Design; Elsevier: Salamanca, Spain, 2016. [Google Scholar]
- Dujjanutat, P.; Neramittagapong, A.; Kaewkannetra, P. Optimization of Bio-Hydrogenated Kerosene from Refined Palm Oil by Catalytic Hydrocracking. Energies 2019, 12, 3196. [Google Scholar] [CrossRef] [Green Version]
- Hancsók, J.; Kasza, T.; Visnyei, O. Isomerization of n-C5/C6 Bioparaffins to Gasoline Components with High Octane Number. Energies 2020, 13, 1672. [Google Scholar] [CrossRef] [Green Version]
- Pleyer, O.; Vrtiška, D.; Straka, P.; Vráblík, A.; Jenčík, J.; Šimáček, P. Hydrocracking of a Heavy Vacuum Gas Oil with Fischer–Tropsch Wax. Energies 2020, 13, 5497. [Google Scholar] [CrossRef]
- Bou, C.; Zoughalib, A.; Rivera, R. Schuhler.Hybrid Optimization Methodology (Exergy/Pinch) and Application on a Simple Process. Energies 2019, 12, 3324. [Google Scholar] [CrossRef] [Green Version]
- Canmet Energy Technology Centre. Pinch Analysis: For the Efficient Use of Energy, Water and Hydrogen; Canadian Government: Ottawa, ON, Canada, 2003. [Google Scholar]
- Jamaluddin, K.; Alwi, S.W.; Hamzah, K.; Klemeš, J. A Numerical Pinch Analysis Methodology for Optimal Sizing of a Centralized Trigeneration System with Variable Energy Demands. Energies 2020, 13, 2038. [Google Scholar] [CrossRef] [Green Version]
- Diffor, F.J.C.; del Pozo, J.L.M. Diseño de un Sistema Industrial de Enfriamiento con Agua de Refrigeración para un Complejo Industrial en Lima; ICAI: Madrid, Spain, 2012. [Google Scholar]
- Meteosolana. Available online: https://es.meteosolana.net/estacion/9491X (accessed on 20 May 2020).
- Regufe, M.J.; Pereira, A.; Pereira, A.F.P.; Ribeiro, A.M.; Rodrigues, A.E. Current Developments of Carbon Capture Storage and/or Utilization–Looking for Net-Zero Emissions Defined in the Paris Agreement. Energies 2021, 14, 2406. [Google Scholar] [CrossRef]
- Red Eléctrica Española. Available online: https://www.ree.es/es/datos/generacion/no-renovables-detalle-emisiones-CO2 (accessed on 25 May 2020).
- Croezen, H.; Kampman, B. Calculating Greenhouse Gas Emissions of EU Biofuels: An Assessment of the EU Methodology Proposal for Biofuels CO2 Calculations; CE Delft: Delft, The Netherlands, 2008. [Google Scholar]
- Juhrich, K. CO2 Emission Factors for Fossil Fuels; German Environment Agency: Dessau, Germany, 2016. [Google Scholar]
- European Parliament. Directive (EU) 2018/2001 of the European Parliament and of the Council of 11 December 2018; European Parliament: Brussels, Belgium, 2018. [Google Scholar]
- American Society for Testing and Materials. Standard Specification for Aviation Turbine Fuel Containing Synthesized Hydrocarbons; American Society for Testing and Materials: West Conshohocken, PA, USA, 2020. [Google Scholar]
- Santos, B.G.; King, F. Corrosion Of Carbon Steel in Petrochemical Environments. In Proceedings of the CORROSION, New Orleans, LA, USA, 16 March 2008. [Google Scholar]
- Chemical Engineering Online. Available online: https://www.chemengonline.com/ (accessed on 15 May 2020).
- Seider, W.; Lewin, D.; Seader, J.; Widagdo, S.; Gani, R.; Ming, K. Product and Process Design Principles: Synthesis, Analysis and Evaluation; Wiley: New York, NY, USA, 2004. [Google Scholar]
- United States Department of Labor. U.S. Bureau of Labor Statistics. 2020. Available online: https://www.bls.gov/oes/current/oes518091.htm (accessed on 21 June 2021).
- Twidale, S. Analysts Raise EU Carbon Price Forecasts as Tougher Climate Targets Loom; Reuters: New York, NY, USA, 2021. [Google Scholar]
- Deane, J.P.; Shea, R.O.; Gallachóir, B.Ó. Biofuels for Aviation. Rapid Response Energy Brief 2015, 20, 1–5. [Google Scholar]




| t/year | t/h | wt% | wt% | ||
|---|---|---|---|---|---|
| Barley straw | 579,448.00 | 72,431 | 70 | 91 | Biomass |
| Wheat straw | 178,002.00 | 22,250 | 21 | ||
| Polyethylene | 48,869.67 | 6109 | 6 | 9 | Plastic |
| Polypropylene | 25,630.02 | 3204 | 3 |
| a0 | a1 | a2 | a3 | Result (wt%) (1) | ||
|---|---|---|---|---|---|---|
| TarBiomass | C6H10O3 | 19 | −0.1735 | 7.4 × 10−5 | 0 | 0.10 |
| C10H10 | 19 | −0.1735 | 7.4 × 10−5 | 0 | 0.10 | |
| TarPlastic | -- | 2850 | −11.1 | 1.44 × 10−2 | −6.22·10−6 | 30.48 |
| TarPrimary (2) | C6H12 | 87.037 | −0.52287 | 6.1991·10−4 | 0 | 0.00 |
| TarSecondary (3) | C10H20 | -- | -- | -- | -- | 30.48 |
| CharBiomass | C | 209 | −0.347 | 1.48 × 10−4 | 0 | 0.19 |
| Gas (4) | H2 | 26 | −4.47 × 10−2 | 4.54· × 10−5 | 0 | 1.68 (5) |
| −49.816 | 0.13572 | −8.6478 × 10−5 | 0 | 0.31 (6) | ||
| CO (5) | 26 | 5.44 × 10−2 | −3.88 × 10−5 | 0 | 48.59 | |
| CO2 (5) | 214 | −0.353 | 1.55 × 10−4 | 0 | 42.27 | |
| CH4 | −45 | 0.112 | −5.47 × 10−5 | 0 | 7.08 (5) | |
| 1440.6 | −3.75 | 2.4997 × 10−3 | 0 | 54.74 (6) | ||
| C2H4 | −132 | 0.251 | −1.15 × 10−4 | 0 | 0.00 (5) | |
| −748.92 | 2.13 | −1.4122 × 10−3 | 0 | 14.47 (6) | ||
| C4H8 (6) | −529.15 | 1.4567 | −9.7835 × 10−4 | 0 | 0.00 |
| kg/s | cp (kJ/kg·K) | h600°C (kJ/kg) | |
|---|---|---|---|
| Feedstock | 28.9 | 0.35 | -- |
| Reactants | 26.0 | -- | −224.7 |
| Products | -- | −4088.3 |
| T-202 | T-502 | |
|---|---|---|
| Stages | 30 | 15 |
| TCondenser (°C) | 70 | 102 |
| TReboiler (°C) | 122 | 124 |
| PCondenser (bar) | 1.013 | 1.71 |
| PReboiler (bar) | 2.00 | 2.00 |
| Reflux ratio | 0.75 | 1.00 |
| Compound | wt% | Compound | wt% | Compound | wt% |
|---|---|---|---|---|---|
| H2O | 53.93 | C8H18 | 1.62 | C17H36 | 2.29 |
| CO2 | 3.63 | C9H20 | 1.80 | C18H38 | 2.26 |
| CH4 | 0.07 | C10H22 | 1.95 | C19H40 | 2.21 |
| C2H4 | 0.12 | C11H24 | 2.08 | C20H42 | 2.15 |
| C2H6 | 0.23 | C12H26 | 2.17 | C21H44 | 2.09 |
| C3H8 | 0.45 | C13H28 | 2.24 | C22H46 | 2.02 |
| C4H10 | 0.69 | C14H30 | 2.28 | C23H48 | 1.94 |
| C5H12 | 0.94 | C15H32 | 2.31 | C24H50 | 1.86 |
| C6H14 | 1.19 | C16H34 | 2.31 | C25H52 | 1.77 |
| C7H16 | 1.41 |
| T-601 | T-602 | T-603 | |
|---|---|---|---|
| Stages | 25 | 25 | 30 |
| TCondenser (°C) | 135 | 252 | 349 |
| TReboiler (°C) | 305 | 438 | 438 |
| PCondenser (bar) | 4.00 | 5.00 | 1.71 |
| PReboiler (bar) | 6.00 | 2.50 | 3.50 |
| Reflux ratio | 1.15 | 1.30 | 2.90 |
| Stream | kg/h | Stream | kg/h |
|---|---|---|---|
| Straw | 94,681 | S405 | 17,160 |
| PE + PP | 9312 | S408 | 24 |
| S103 | 2600 | S412 | 12,940 |
| O2 | 38,200 | S503 | 646 |
| Ash | 13,753 | S507 | 1936 |
| S112 | 130,959 | S511 | 0 |
| S205 | 7843 | S513 | 780 |
| CO2 | 105,337 | S514 | 0 |
| S219 | 2 | Other Biofuels (1) | 561 |
| S221 | 1 | 1590 | |
| S222 | 14,094 | 1697 | |
| S225 | 45 | 3416 | |
| S226 | 31,831 | Biojet fuel | 5676 |
| S305 | 31,880 | H2Ofresh | 1991 |
| S403 | 1802 | H2Oblowdown | 7742 |
| Utility | Conditions at Heat Exchanger Inlet | Conditions at Heat Exchanger Outlet | |
|---|---|---|---|
| For heating | Very high-pressure steam (HHPS) | Saturated steam at 260 °C | Saturated liquid at 259 °C |
| High-pressure steam (HPS) | Saturated steam at 250 °C | Saturated liquid at 249 °C | |
| For cooling (1) | Very high-pressure steam generation | Saturated liquid at 259 °C | Superheated steam at 270 °C |
| High-pressure steam generation | Saturated liquid at 249 °C | Superheated steam at 260 °C | |
| Medium-pressure steam (MPS) generation | Saturated liquid at 174 °C | Superheated steam at 185 °C | |
| Low-pressure steam (LPS) generation | Saturated liquid at 124 °C | Superheated steam at 135 °C | |
| Water from cooling tower | Subcooled liquid at 28 °C | Subcooled liquid at 42 °C |
| Cycle | Heat Exchanged (kW) | Evaporators |
|---|---|---|
| Very high-pressure steam | 311 | E-950 |
| High-pressure steam | 40,487 | E-927, E-928, E-944, E-945 and E-951 (E-GHPS) |
| Medium-pressure steam | 38,641 | E-953 and E-954 (E-GMPS) |
| Low-pressure steam | 312 | E-949 |
| Exchanger | HS (1) | TinHS (°C) | ToutHS (°C) | CS (2) | TinCS (°C) | ToutCS (°C) | H (kW) |
|---|---|---|---|---|---|---|---|
| E-701 | HHPS | 164 | 164 | CW | 28 | 42 | 254 |
| E-702 | HPS | 153 | 152 | CW | 28 | 42 | 31,823 |
| E-703 | MPS | 90 | 40 | CW | 28 | 42 | 33,191 |
| E-704 | LPS | 76 | 71 | CW | 28 | 42 | 279 |
| Exchanger | U (kW/(m2·K)) | LMTD (°C) | FT | S (m2) | SOvers. 10% (m2) | mCW (kg/h) |
|---|---|---|---|---|---|---|
| E-701 | 0.17 | 129 | 1.00 | 11.9 | 8.3 | 15,662 |
| E-702 | 1.25 | 117 | 1.00 | 217.9 | 677.7 | 1,963,629 |
| E-703 | 1.34 | 26 | 1.00 | 969.5 | 1066.5 | 2,048,032 |
| E-704 | 0.15 | 38 | 1.00 | 48.9 | 24.1 | 17,210 |
| Water Flow to be cooled (kg/h) | 11,279,099 |
| Blowdown flow (kg/h) | 53,932 |
| Water makeup flow (kg/h) | 270,230 |
| TIn hot water (°C) | 42 |
| TOut cooled water (°C) | 28 |
| POvers. 10% (P-801) (kW) | 343 |
| POvers. 10% (fan) (kW) | 641 |
| Parameter | GFT Biojet Fuel | ASTM D7566 |
|---|---|---|
| Density at 15 °C (kg/m3) | 742 | 730 to 770 |
| Kinematic viscosity at −20 °C (cSt) | 4 | lower than 8 |
| Pour point (°C) | −66 | lower than −40 |
| Flammability point (°C) | 97 | greater than 38 |
| Lower calorific value (MJ/kg) | 43.1 | greater than 42.8 |
| Equipment | Cost Equation (EUR) | Equation Number |
|---|---|---|
| Pumps (1) | (21) | |
| Compressors (2) | (22) | |
| Heat exchangers (3) | (23) | |
| Furnace (4) | (24) | |
| Cooling tower (5) | (25) | |
| Steam turbines (6) | (26) |
| Equipment | CapBase | CostBase (USD) | Year | Exp | Cap | FInstallation | CostInstalled (EUR) |
|---|---|---|---|---|---|---|---|
| R-301 | 3186 | 10,500,000 | 2003 | 0.72 | 2955.4 | 1 | 9,151,283 |
| R-401 | 4355 | 5,021,074 | 2011 | 0.65 | 12,940.0 | 3 | 28,126,803 |
| PSAAir | 24 | 24,552,000 | 2002 | 0.75 | 38.2 | 1 | 32,008,387 |
| V-202A/B | 8665 | 4,855,471 | 2002 | 0.6 | 44.8 | 2.47 | 468,393 |
| Equipment | CostPurchase2007 (EUR) | CostInstalled2007 (EUR) | CostInstalled2018 (EUR) |
|---|---|---|---|
| Trays | 485,804 | 485,804 | 557,649 |
| Pumps | 5,544,448 | 17,742,233 | 20,366,084 |
| Compressors | 10,840,115 | 34,688,369 | 39,818,339 |
| Heat exchangers | 2,384,730 | 7,631,136 | 8,759,684 |
| Furnace | 17,014,877 | 54,447,606 | 62,499,717 |
| Hoppers and mixers | 243,213 | 778,283 | 893,381 |
| Flash chambers | 296,036 | 947,314 | 1,087,410 |
| Tower vessels | 2,791,047 | 8,931,351 | 10,252,184 |
| Cooling tower | 2,071,034 | 6,627,309 | 7,607,404 |
| Steam turbines | 1,537,455 | 4,919,856 | 5,647,441 |
| Total | 253,468,920 | ||
| Quantity | CostUpdated | ||
|---|---|---|---|
| Purchase | WaterProcess | 1991 kg/h | 2.06 × 10−4 EUR/kg |
| WaterCooling | 270,230 kg/h | 2.06 × 10−5 EUR/kg | |
| Electricity | 16,698 kWh | 0.098 EUR/kWh | |
| Wheat straw | 22,250 kg/h | 0.037 EUR/kg | |
| Barley straw | 72,431 kg/h | 0.037 EUR/kg | |
| Poliethylene | 6109 kg/h | 0.02 EUR/kg | |
| Polipropylene | 3204 kg/h | 0.02 EUR/kg | |
| Amine | 0.97 kg/h | 1.40 EUR/kg | |
| Sale | Fuel gas | 1 m3/h | 0.16 EUR/L (1) |
| Petrol | 2 m3/h | 0.34 EUR/L (1) | |
| Biojet fuel | 8 m3/h | 0.39 EUR/L (1) | |
| Diesel | 2 m3/h | 0.42 EUR/L (1) | |
| Fuel oil | 4 m3/h | 0.24 EUR/L (1) |
| Cost of Equipment (E) | 253,468,921 EUR | |
|---|---|---|
| % of E | Cost (EUR) | |
| Materials (M) | 65 | 164,754,798 |
| % of E + M | Cost (EUR) | |
| Detailed engineering (D.E.) | 17.5 | 73,189,150 |
| Licenses, process engineering… (P.E.) | -- | 1,500,000 |
| Construction (Con.) | 60 | 250,934,231 |
| Construction supervision (Con.S.) | 10 | 41,822,372 |
| Inside battery limits (ISBL) | 785,669,471 EUR | |
| % of ISBL | Cost (EUR) | |
| Utilities (Ut.) | 4 | 31,426,779 |
| Off-sites (O.S.) | 8 | 62,853,558 |
| Start-up costs (S.C.) | 3.5 | 27,498,431 |
| Unforeseen contingencies (U.C.) | 10 | 78,566,947 |
| Fixed capital (F.C.) | 986,015,186 EUR | |
| Working capital (W.C.) | 197,203,037 EUR | |
| Product | MSP | |
|---|---|---|
| EUR/kg | EUR/L | |
| Natural gas | 0.51 | 0.54 |
| Petrol | 1.71 | 1.16 |
| Biojet fuel | 1.85 | 1.37 |
| Diesel | 1.90 | 1.45 |
| Fuel oil | 1.42 | 1.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Fernández, A.; Bolonio, D.; Amez, I.; Castells, B.; Ortega, M.F.; García-Martínez, M.-J. Design and Pinch Analysis of a GFT Process for Production of Biojet Fuel from Biomass and Plastics. Energies 2021, 14, 6035. https://doi.org/10.3390/en14196035
López-Fernández A, Bolonio D, Amez I, Castells B, Ortega MF, García-Martínez M-J. Design and Pinch Analysis of a GFT Process for Production of Biojet Fuel from Biomass and Plastics. Energies. 2021; 14(19):6035. https://doi.org/10.3390/en14196035
Chicago/Turabian StyleLópez-Fernández, Alejandro, David Bolonio, Isabel Amez, Blanca Castells, Marcelo F. Ortega, and María-Jesús García-Martínez. 2021. "Design and Pinch Analysis of a GFT Process for Production of Biojet Fuel from Biomass and Plastics" Energies 14, no. 19: 6035. https://doi.org/10.3390/en14196035
APA StyleLópez-Fernández, A., Bolonio, D., Amez, I., Castells, B., Ortega, M. F., & García-Martínez, M.-J. (2021). Design and Pinch Analysis of a GFT Process for Production of Biojet Fuel from Biomass and Plastics. Energies, 14(19), 6035. https://doi.org/10.3390/en14196035








