Development and Validation of Mass Reduction Prediction Model and Analysis of Fuel Properties for Agro-Byproduct Torrefaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample
2.2. Experimental Method
2.3. Analysis Method
2.3.1. Bulk Density Analysis
2.3.2. Thermal Analysis
2.3.3. Fuel Property Analysis
3. Simulation Analysis
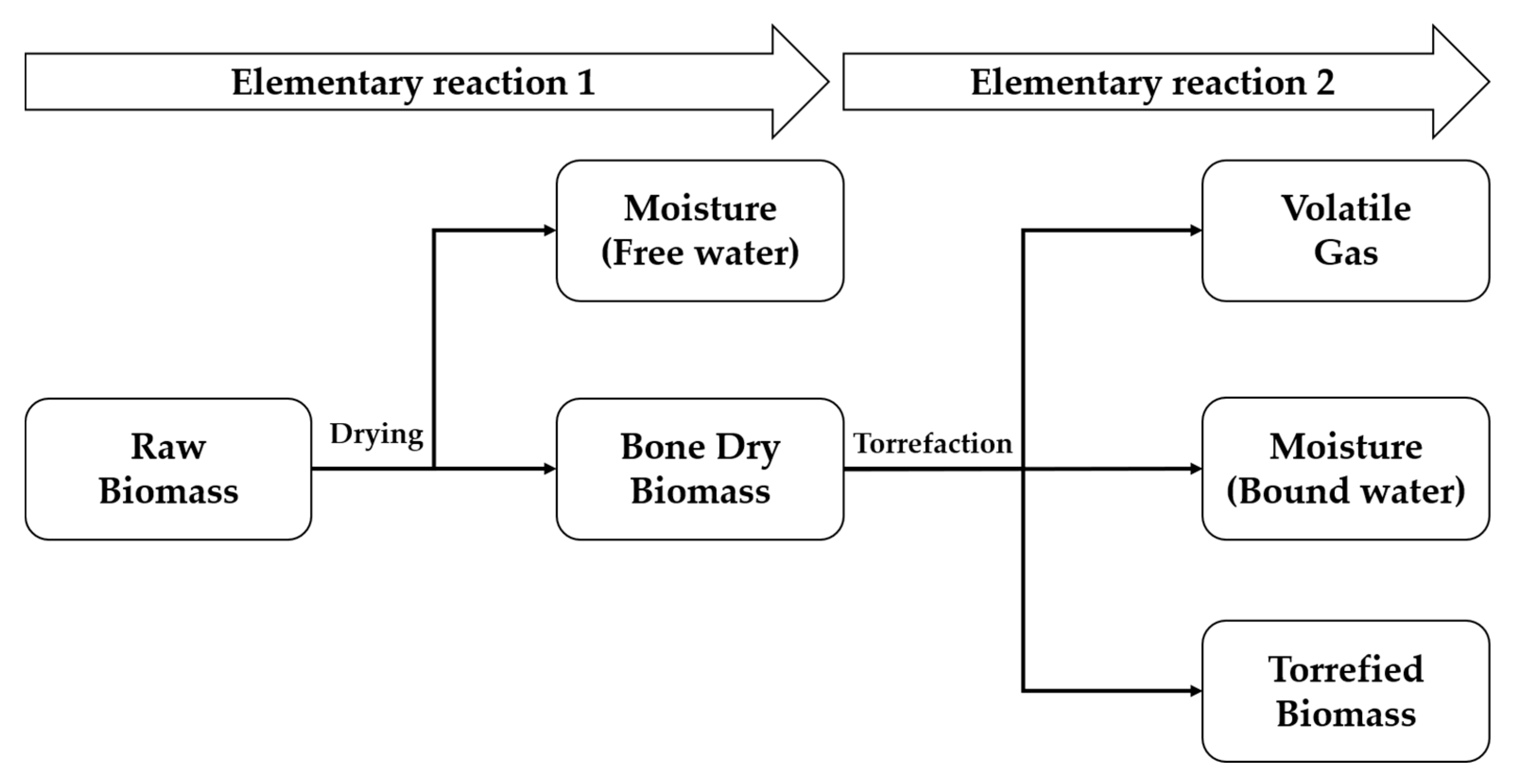
3.1. 1-D Mass Reduction Prediction Model
3.2. Thermal Change Analysis of Biomass
3.3. Mass Reduction Model
4. Results
4.1. Fuel Properties
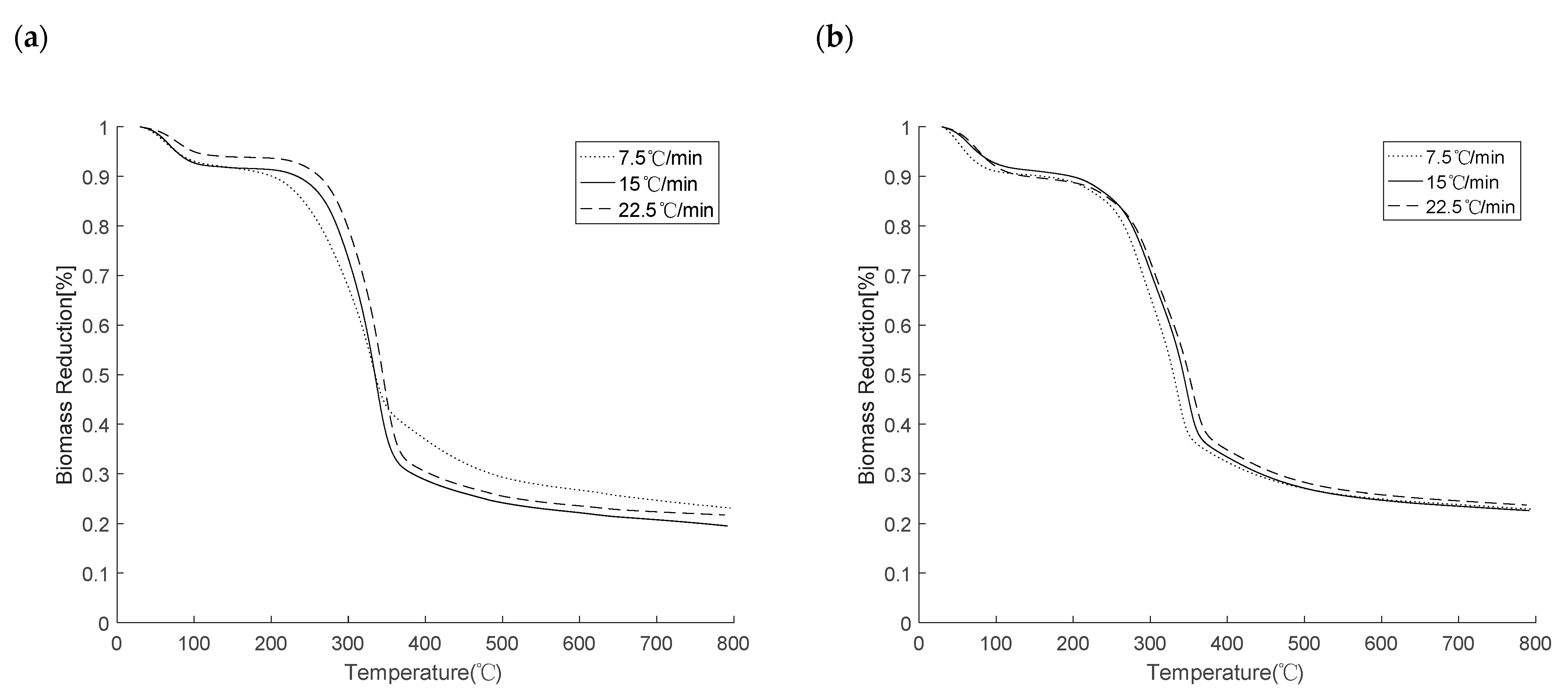
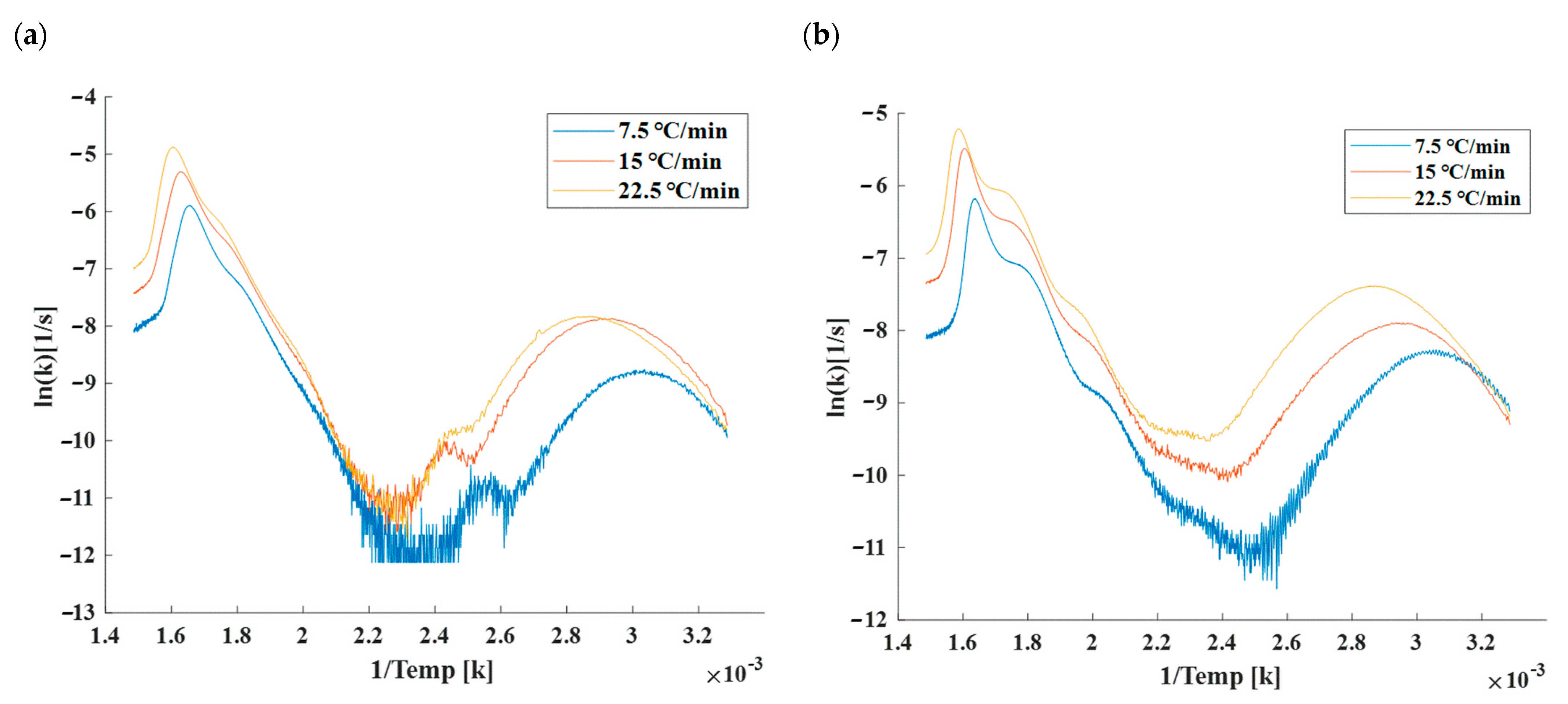
4.2. Thermogravimetric Analysis (TGA)
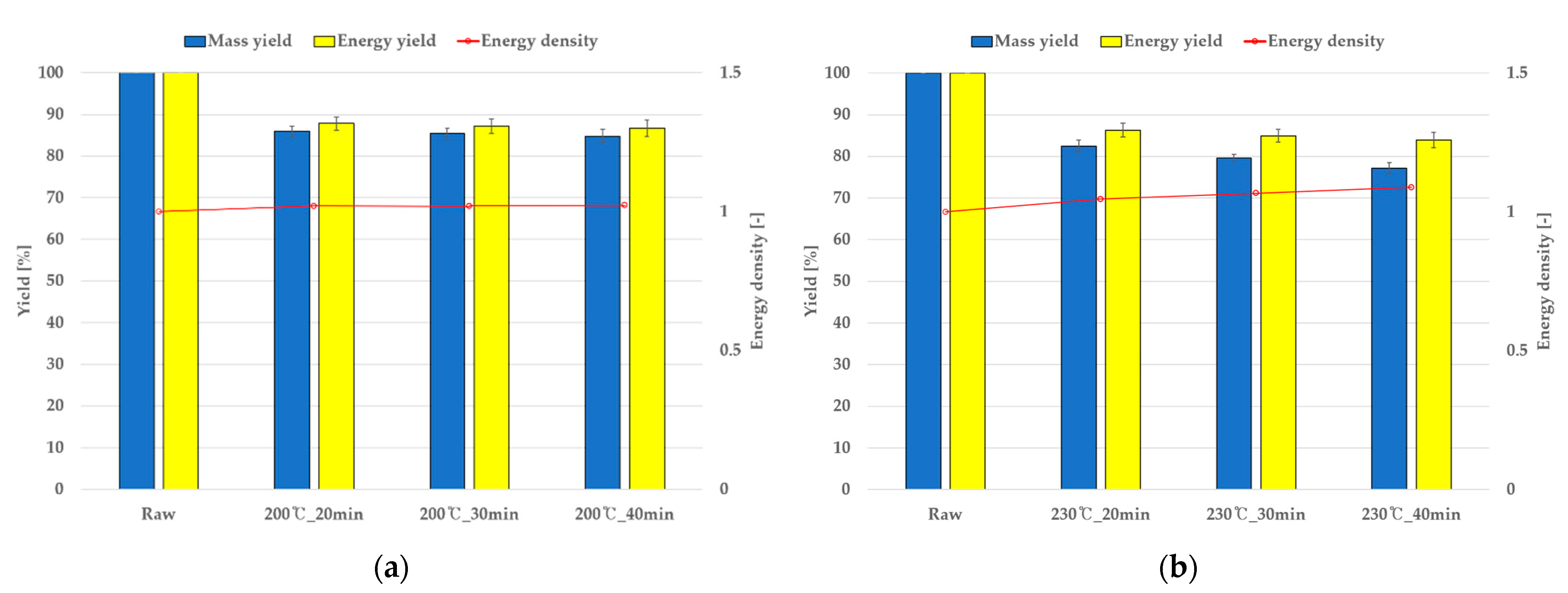
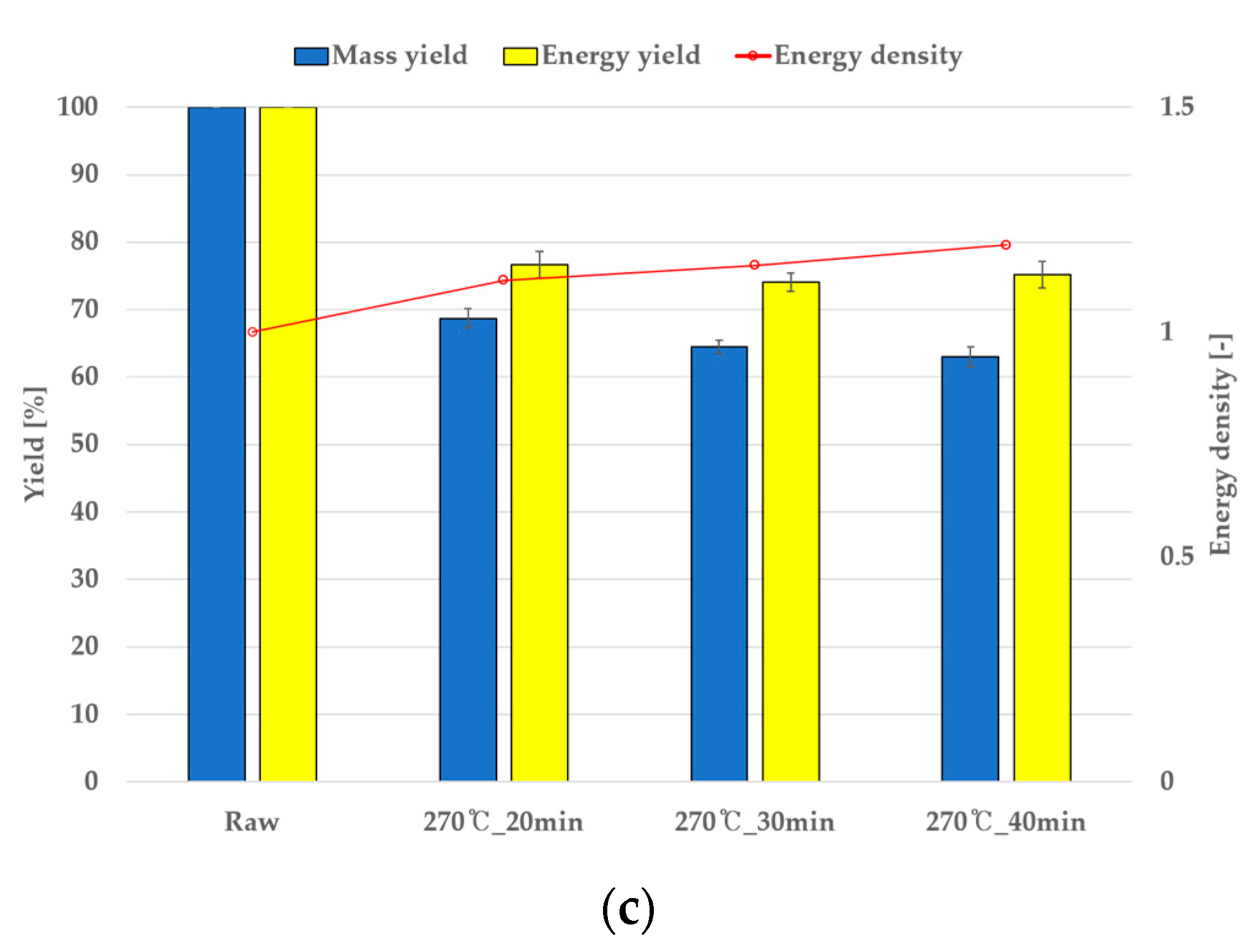
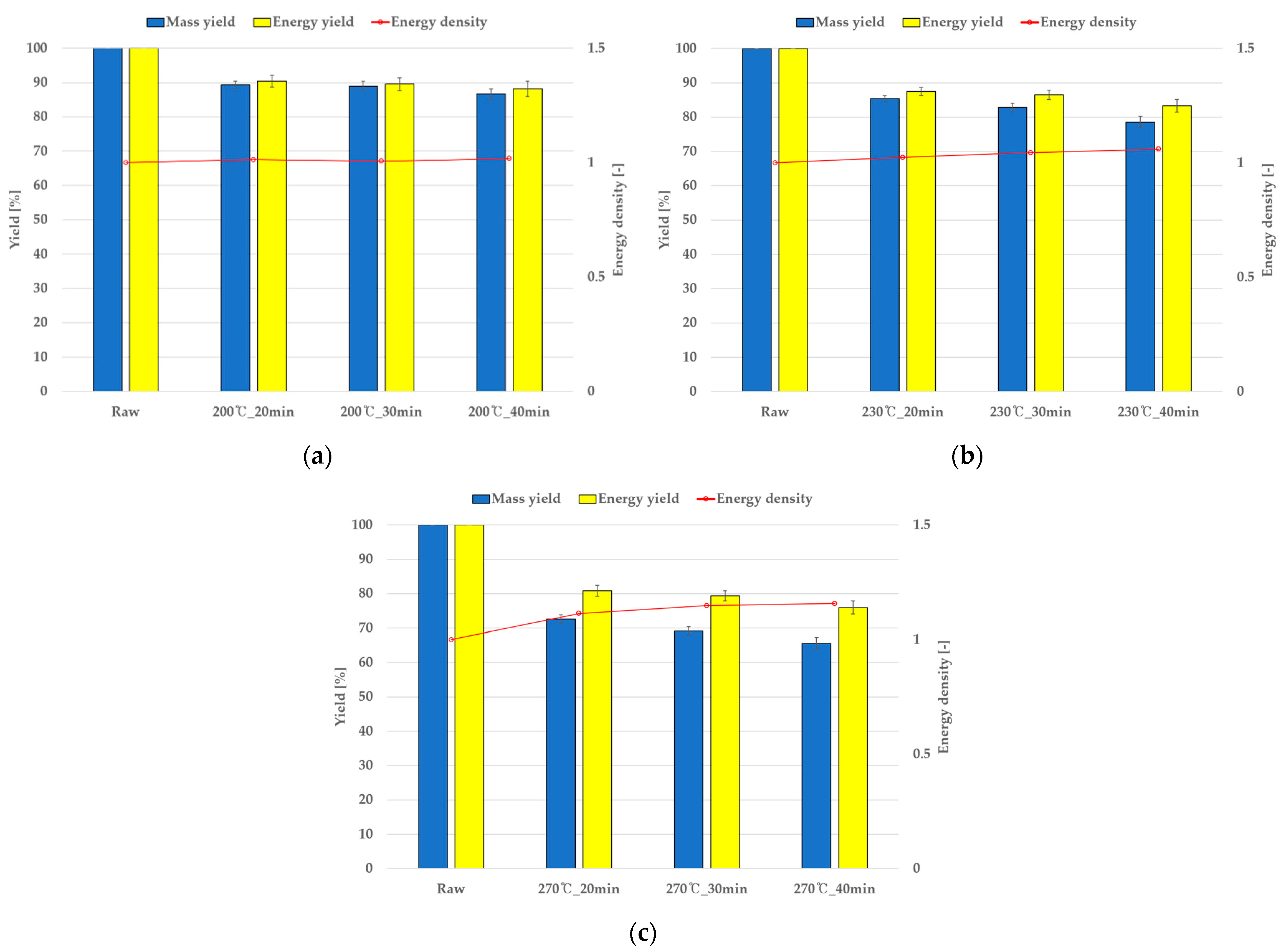
4.3. Torrefaction Mass Reduction and Comparison with Simulations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, J.P.; Park, S.C. Estimation of geographical and technical potential for biomass resources. New Renew. Energy 2016, 12, 53–58. [Google Scholar] [CrossRef]
- Oh, K.C.; Park, S.Y.; Kim, S.J.; Choi, Y.S.; Lee, C.G.; Cho, L.H.; Kim, D.H. Development and validation of mass reduction model to optimize torrefaction for agricultural byproduct biomass. Renew. Energy 2019, 139, 988–999. [Google Scholar] [CrossRef]
- Hamawand, I.; da Silva, W.; Seneweera, S.; Bundschuh, J. Value Proposition of Different Methods for Utilisation of Sugarcane Wastes. Energies 2021, 14, 5483. [Google Scholar] [CrossRef]
- D’Adamo, I.; Falcone, P.M.; Huisingh, D.; Morone, P. A circular economy model based on biomethane: What are the opportunities for the municipality of Rome and beyond? Renew. Energy 2021, 163, 1660–1672. [Google Scholar] [CrossRef]
- D’Adamo, I.; Falcone, P.M.; Morone, P. A New Socio-economic Indicator to Measure the Performance of Bioeconomy Sectors in Europe. Ecol. Econ. 2020, 176, 1–12. [Google Scholar] [CrossRef]
- De Toro, A.; Gunnarsson, C.; Jonsson, N.; Sundberg, M. Effects of Variable Weather Conditions on Baled Proportion of Varied Amounts of Harvestable Cereal Straw, Based on Simulations. Sustainability 2021, 13, 9449. [Google Scholar] [CrossRef]
- Holmgren, P.; Wagner, D.R.; Strandberg, A.; Molinder, R.; Wiinikka, H.; Umeki, K.; Broström, M. Size, shape, and density changes of biomass particles during rapid devolatilization. Fuel 2017, 206, 342–351. [Google Scholar] [CrossRef]
- Nonaka, H. Change of chemical composition during wood pelletization: Whole wood pellet as a raw material for biorefinery. J. Jpn. Inst. Energy 2014, 93, 1005–1009. [Google Scholar] [CrossRef] [Green Version]
- Basu, P. Biomass Gasification, Pyrolysis and Torrefaction: Practical Design and Theory; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Granados, D.A.; Chejne, F.; Basu, P. A two dimensional model for torrefaction of large biomass particles. J. Anal. Appl. Pyrol. 2016, 120, 1–14. [Google Scholar] [CrossRef]
- Park, S.W.; Yang, J.K.; Baek, K.R. Fuel ratio and combustion characteristics of torrefied biomass. J. Korea Soc. Waste Manag. 2013, 30, 376–382. [Google Scholar] [CrossRef]
- Chansaem, P. Modeling and Optimal Design of Biomass Torrefaction Process. Ph.D. Thesis, The Graduate School of Seoul National University, Seoul, Korea, 2014. Available online: http://hdl.handle.net/10371/119714 (accessed on 30 August 2014).
- Tumuluru, J.S.; Sokhansanj, S.; Hess, J.R.; Wright, C.T.; Boardman, R.D. A review on biomass torrefaction process and product properties for energy applications. Ind. Biotechnol. 2011, 7, 384–401. [Google Scholar] [CrossRef] [Green Version]
- Pentananunt, R.; Rahman, A.M.; Bhattacharya, S.C. Upgrading of biomass by means of torrefaction. Energy 1990, 15, 1175–1179. [Google Scholar] [CrossRef]
- Li, J.; Brzdekiewicz, A.; Yang, W.; Blasiak, W. Co-firing based on biomass torrefaction in a pulverized coal boiler with aim of 100% fuel switching. Appl. Energy 2012, 99, 344–354. [Google Scholar] [CrossRef]
- Fisher, E.M.; Dupont, C.; Darvell, L.I.; Commandré, J.M.; Saddawi, A.; Jones, J.M.; Grateau, M.; Nocquet, T.; Salvador, S. Combustion and gasification characteristics of chars from raw and torrefied biomass. Bioresour. Technol. 2012, 119, 157–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broström, M.; Nordin, A.; Pommer, L.; Branca, C.; Di Blasi, C. Influence of torrefaction on the devolatilization and oxidation kinetics of wood. J. Anal. Appl. Pyrol. 2012, 96, 100–109. [Google Scholar] [CrossRef]
- Berrueco, C.; Recari, J.; Güell, B.M.; Del Alamo, G. Pressurized gasification of torrefied woody biomass in a lab scale fluidized bed. Energy 2014, 70, 68–78. [Google Scholar] [CrossRef]
- Deng, J.; Wang, G.J.; Kuang, J.H.; Zhang, Y.L.; Luo, Y.H. Pretreatment of agricultural residues for co-gasification via torrefaction. J. Anal. Appl. Pyrol. 2009, 86, 331–337. [Google Scholar] [CrossRef]
- Pittman, C.U., Jr.; Mohan, D.; Eseyin, A.; Li, Q.; Ingram, L.; Hassan, E.B.; Mitchell, B.; Guo, H.; Steele, P.H. Characterization of bio-oils produced from fast pyrolysis of corn stalks in an auger reactor. Energy Fuels 2012, 26, 3816–3825. [Google Scholar] [CrossRef]
- Zheng, A.; Zhao, Z.; Chang, S.; Huang, Z.; Wang, X.; He, F.; Li, H. Effect of torrefaction on structure and fast pyrolysis behavior of corncobs. Bioresour. Technol. 2013, 128, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Granados, D.A.; Velásquez, H.I.; Chejne, F. Energetic and exergetic evaluation of residual biomass in a torrefaction process. Energy 2014, 74, 181–189. [Google Scholar] [CrossRef]
- Bates, R.B.; Ghoniem, A.F. Biomass torrefaction: Modeling of volatile and solid product evolution kinetics. Bioresour. Technol. 2012, 124, 46–49. [Google Scholar] [CrossRef]
- Bates, R.B.; Ghoniem, A.F. Modeling kinetics-transport interactions during biomass torrefaction: The effects of temperature, particle size, and moisture content. Fuel 2014, 137, 216–229. [Google Scholar] [CrossRef]
- Sullivan, A.L.; Ball, R. Thermal decomposition and combustion chemistry of cellulosic biomass. Atmos. Environ. 2012, 47, 133–141. [Google Scholar] [CrossRef]
- Van der Stelt, M.J.C.; Gerhauser, H.; Kiel, J.H.A.; Ptasinski, K.J. Biomass upgrading by torrefaction for the production of biofuels: A review. Biomass Bioenergy 2011, 35, 3748–3762. [Google Scholar] [CrossRef]
- Sarvaramini, A.; Assima, G.P.; Larachi, F. Dry torrefaction of biomass–Torrefied products and torrefaction kinetics using the distributed activation energy model. Chem. Eng. J. 2013, 229, 498–507. [Google Scholar] [CrossRef]
- Hankalin, V.; Ahonen, T.; Raiko, R. On thermal properties of a pyrolysing wood particle. Finn.-Swed. Flame Days 2009, 16, 1–16. [Google Scholar]
- Grønli, M.G. A Theoretical and Experimental Study of the Thermal Degradation of Biomass. 1996. Available online: https://www.osti.gov/etdeweb/servlets/purl/642467 (accessed on 10 August 2021).
- Koch, P. Specific heat of ovendry spruce pine wood and bark. Wood Sci. 1968, 1, 203–214. [Google Scholar]
- Gupta, M.; Yang, J.; Roy, C. Specific heat and thermal conductivity of softwood bark and softwood char particles. Fuel 2003, 82, 919–927. [Google Scholar] [CrossRef]
- Spearpoint, M.J.; Quintiere, J.G. Predicting the burning of wood using an integral model. Combust. Flame 2000, 123, 308–325. [Google Scholar] [CrossRef]
- Park, S.W.; Jang, C.H.; Baek, K.R.; Yang, J.K. Torrefaction and low-temperature carbonization of woody biomass: Evaluation of fuel characteristics of the products. Energy 2012, 45, 676–685. [Google Scholar] [CrossRef]
- Kök, M.V.; Pamir, M.R. Comparative pyrolysis and combustion kinetics of oil shales. J. Anal. Appl. Pyrol. 2000, 55, 185–194. [Google Scholar] [CrossRef]
- Martín-Lara, M.A.; Blázquez, G.; Zamora, M.C.; Calero, M. Kinetic modelling of torrefaction of olive tree pruning. Appl. Therm. Eng. 2017, 113, 1410–1418. [Google Scholar] [CrossRef]
- Kang, S.H.; Ryu, J.H.; Park, S.N.; Byun, Y.S.; Seo, S.J.; Yun, Y.S.; Lee, J.W.; Kim, Y.J.; Kim, J.H.; Park, S.R. Kinetic studies of pyrolysis and Char-CO2 gasification on low rank coals. Korean Chem Eng. Res. 2011, 49, 114–119. [Google Scholar] [CrossRef]
- Tzou, D.Y. On the wave theory in heat conduction. J. Heat Trans. 1994, 116, 526–535. [Google Scholar]
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J. Torrefaction of wood: Part 1. Weight loss kinetics. J. Anal. Appl. Pyrol. 2006, 77, 28–34. [Google Scholar] [CrossRef]
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J. Torrefaction of wood: Part 2. Analysis of products. J. Anal. Appl. Pyrol. 2006, 77, 35–40. [Google Scholar] [CrossRef]
- Bach, Q.V.; Chen, W.H.; Chu, Y.S.; Skreiberg, Ø. Predictions of biochar yield and elemental composition during torrefaction of forest residues. Bioresour. Technol. 2016, 215, 239–246. [Google Scholar] [CrossRef]
- Ozisik, M.N. Heat Transfer: A Basic Approach; McGraw-Hill: New York, NY, USA, 1985. [Google Scholar]
- Kreith, F.; Black, W.Z. Basic Heat Transfer; Harper & Row: New York, NY, USA, 1980. [Google Scholar]
- Kaviany, M. Principles of Heat Transfer in Porous Media; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Grieco, E.; Baldi, G. Analysis and modelling of wood pyrolysis. Chem. Eng. Sci. 2011, 66, 650–660. [Google Scholar] [CrossRef]
- El-Sayed, S.A.; Mostafa, M.E. Pyrolysis characteristics and kinetic parameters determination of biomass fuel powders by differential thermal gravimetric analysis (TGA/DTG). Energy Convers. Manag. 2014, 85, 1–8. [Google Scholar] [CrossRef]
- Lu, J.J.; Chen, W.H. Investigation on the ignition and burnout temperatures of bamboo and sugarcane bagasse by thermogravimetric analysis. Appl. Energy 2015, 160, 49–57. [Google Scholar] [CrossRef]





| Bulk Density (kg/m3) | Higher Heating Value (MJ/kg) | Proximate Analysis (%) | Element Analysis (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Moisture | Ash | Volatile | Fixed Carbon | C | H | N | O | |||
| Grape | 290 | 19.1 | 11.4 | 3.55 | 76 | 9.05 | 44.41 | 6.07 | 0.97 | 40.2 |
| Perilla | 150 | 18.9 | 7.77 | 5.83 | 71.2 | 15.21 | 41.19 | 5.68 | 1.15 | 31.49 |
| Grape (MJ/kg) | Perilla (MJ/kg) | |||||
|---|---|---|---|---|---|---|
| 200 °C | 230 °C | 270 °C | 200 °C | 230 °C | 270 °C | |
| 20 min | 19.46 | 19.56 | 21.27 | 19.06 | 19.27 | 20.95 |
| 30 min | 19.46 | 20.36 | 21.90 | 19.10 | 19.65 | 21.59 |
| 40 min | 19.51 | 20.75 | 22.77 | 19.14 | 19.95 | 21.77 |
| Grape (%) | Perilla (%) | |||||
|---|---|---|---|---|---|---|
| 200 °C | 230 °C | 270 °C | 200 °C | 230 °C | 270 °C | |
| 20 min | 87.8 | 86.3 | 76.5 | 90.5 | 87.5 | 80.8 |
| 30 min | 87.2 | 85.0 | 74.1 | 89.5 | 86.5 | 79.3 |
| 40 min | 86.7 | 84.0 | 75.1 | 88.2 | 83.3 | 76.0 |
| Material | Heating Rate (°C/min) | Experiment Time (min) | Peak Temperature (°C) | Burnout Temperature (°C) | Purge Gas (mL/min) | Particle Size (mm) |
|---|---|---|---|---|---|---|
| Perilla | 7.5 | 102.9 | 337.0 | 370.0 | N2 (100) | <0.154 |
| 15 | 52.2 | 350.4 | 390.0 | |||
| 22.5 | 34.7 | 357.0 | 432.0 | |||
| Grape | 7.5 | 102.9 | 331.5 | 363.3 | ||
| 15 | 52.2 | 341.0 | 380.5 | |||
| 22.5 | 34.7 | 349.5 | 402.5 |
| 7.5 °C/min | Temperature range (°C) | 30–55 | 65–130 | 130–350 |
| A (L/s) | 1.99 × 102 | 2.14 × 10−11 | 5.72 × 103 | |
| Ea (L/Jmol) | 3.8 × 104 | −4.39 × 104 | 7.38 × 104 | |
| r2 | 0.944 | 0.918 | 0.972 | |
| 15 °C/min | Temperature range (°C) | 30–70 | 70–140 | 140–350 |
| A (L/s) | 412.08 | 1.88 × 10−11 | 3.01 × 104 | |
| Ea (L/Jmol) | 3.8892 × 104 | −4.89 × 104 | 7.95 × 104 | |
| r2 | 0.924 | 0.955 | 0.993 | |
| 22.5 °C/min | Temperature range (°C) | 30–75 | 75–165 | 165–350 |
| A (L/s) | 1.16 × 103 | 2.19 × 10−11 | 1.57 × 103 | |
| Ea (L/Jmol) | 4.22 × 104 | −4.96 × 104 | 6.55 × 104 | |
| r2 | 0.956 | 0.944 | 0.903 |
| 7.5 °C/min | Temperature range (°C) | 30–55 | 55–120 | 130–350 |
| A (L/s) | 8.156 | 1.07 × 10−11 | 2.30 × 10 | |
| Ea (L/Jmol) | 2.801 × 104 | −4.746 × 104 | 4.89 × 104 | |
| r2 | 0.934 | 0.950 | 0.973 | |
| 15 °C/min | Temperature range (°C) | 30–60 | 60–140 | 140–350 |
| A (L/s) | 9.63 × 102 | 2.15 × 10−9 | 4.83 × 102 | |
| Ea (L/Jmol) | 4.060 × 104 | −3.457 × 104 | 5.00 × 104 | |
| r2 | 0.975 | 0.950 | 0.978 | |
| 22.5 °C/min | Temperature range (°C) | 30–75 | 75–150 | 150–350 |
| A (L/s) | 2.958 × 102 | 1.020 × 10−9 | 8.790 × 10 | |
| Ea (L/Jmol) | 3.714 × 103 | −3.952 × 104 | 5.17 × 104 | |
| r2 | 0.967 | 0.972 | 0.978 |
| Temp. (°C) | Time (min) | Experiment | Simulation | Simulation | Simulation | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 7.5 °C/min | 15 °C/min | 22.5 °C/min | |||||||||
| Mass Reduction (%) | Mass Reduction (%) | r2 | RMSE | Mass Reduction (%) | r2 | RMSE | Mass Reduction (%) | r2 | RMSE | ||
| 200 | 20 | 13.98 | 4.07 | 0.996 | 0.0847 | 8.30 | 0.996 | 0.0356 | 8.44 | 0.996 | 0.0604 |
| 30 | 14.57 | 6.25 | 12.61 | 12.93 | |||||||
| 40 | 15.26 | 8.35 | 16.68 | 17.17 | |||||||
| 230 | 20 | 17.52 | 11.30 | 0.997 | 0.0411 | 19.26 | 0.993 | 0.0929 | 20.48 | 0.995 | 0.1109 |
| 30 | 20.41 | 16.96 | 28.34 | 30.25 | |||||||
| 40 | 22.84 | 22.70 | 36.73 | 39.06 | |||||||
| 270 | 20 | 31.28 | 28.0 | 0.964 | 0.0956 | 39.48 | 0.976 | 0.2057 | 43.12 | 0.981 | 0.2408 |
| 30 | 35.48 | 41.50 | 54.98 | 59.01 | |||||||
| 40 | 37.03 | 52.10 | 65.70 | 69.37 | |||||||
| Temp. (°C) | Time (min) | Experiment | Simulation | Simulation | Simulation | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 7.5 °C/min | 15 °C/min | 22.5 °C/min | |||||||||
| Mass Reduction (%) | Mass Reduction (%) | r2 | RMSE | Mass Reduction (%) | r2 | RMSE | Mass Reduction (%) | r2 | RMSE | ||
| 200 | 20 | 10.69 | 5.93 | 0.858 | 0.0285 | 7.60 | 0.854 | 0.0368 | 9.86 | 0.851 | 0.0964 |
| 30 | 11.07 | 9.95 | 13.08 | 16.95 | |||||||
| 40 | 13.31 | 14.02 | 18.51 | 23.87 | |||||||
| 230 | 20 | 14.61 | 10.80 | 0.979 | 0.0379 | 14.46 | 0.975 | 0.0910 | 18.95 | 0.969 | 0.1604 |
| 30 | 17.16 | 18.67 | 25.0 | 32.27 | |||||||
| 40 | 21.46 | 26.59 | 35.14 | 44.36 | |||||||
| 270 | 20 | 27.43 | 20.12 | 0.985 | 0.0866 | 27.29 | 0.977 | 0.1670 | 35.42 | 0.963 | 0.2578 |
| 30 | 30.48 | 34.64 | 45.52 | 56.36 | |||||||
| 40 | 34.36 | 46.78 | 59.07 | 69.87 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-J.; Oh, K.-C.; Park, S.-Y.; Ju, Y.-M.; Cho, L.-H.; Lee, C.-G.; Kim, M.-J.; Jeong, I.-S.; Kim, D.-H. Development and Validation of Mass Reduction Prediction Model and Analysis of Fuel Properties for Agro-Byproduct Torrefaction. Energies 2021, 14, 6125. https://doi.org/10.3390/en14196125
Kim S-J, Oh K-C, Park S-Y, Ju Y-M, Cho L-H, Lee C-G, Kim M-J, Jeong I-S, Kim D-H. Development and Validation of Mass Reduction Prediction Model and Analysis of Fuel Properties for Agro-Byproduct Torrefaction. Energies. 2021; 14(19):6125. https://doi.org/10.3390/en14196125
Chicago/Turabian StyleKim, Seok-Jun, Kwang-Cheol Oh, Sun-Yong Park, Young-Min Ju, La-Hoon Cho, Chung-Geon Lee, Min-Jun Kim, In-Seon Jeong, and Dae-Hyun Kim. 2021. "Development and Validation of Mass Reduction Prediction Model and Analysis of Fuel Properties for Agro-Byproduct Torrefaction" Energies 14, no. 19: 6125. https://doi.org/10.3390/en14196125





