Progress in Development of Nanostructured Manganese Oxide as Catalyst for Oxygen Reduction and Evolution Reaction
Abstract
:1. Introduction
2. Oxygen Reduction Reaction
- i.
- The complete four-electron reduction pathway;
- ii.
- The two-electron hydrogen peroxide generation pathway.
3. Oxygen Evolution Reaction
4. Electrocatalytic Kinetics for ORR and OER
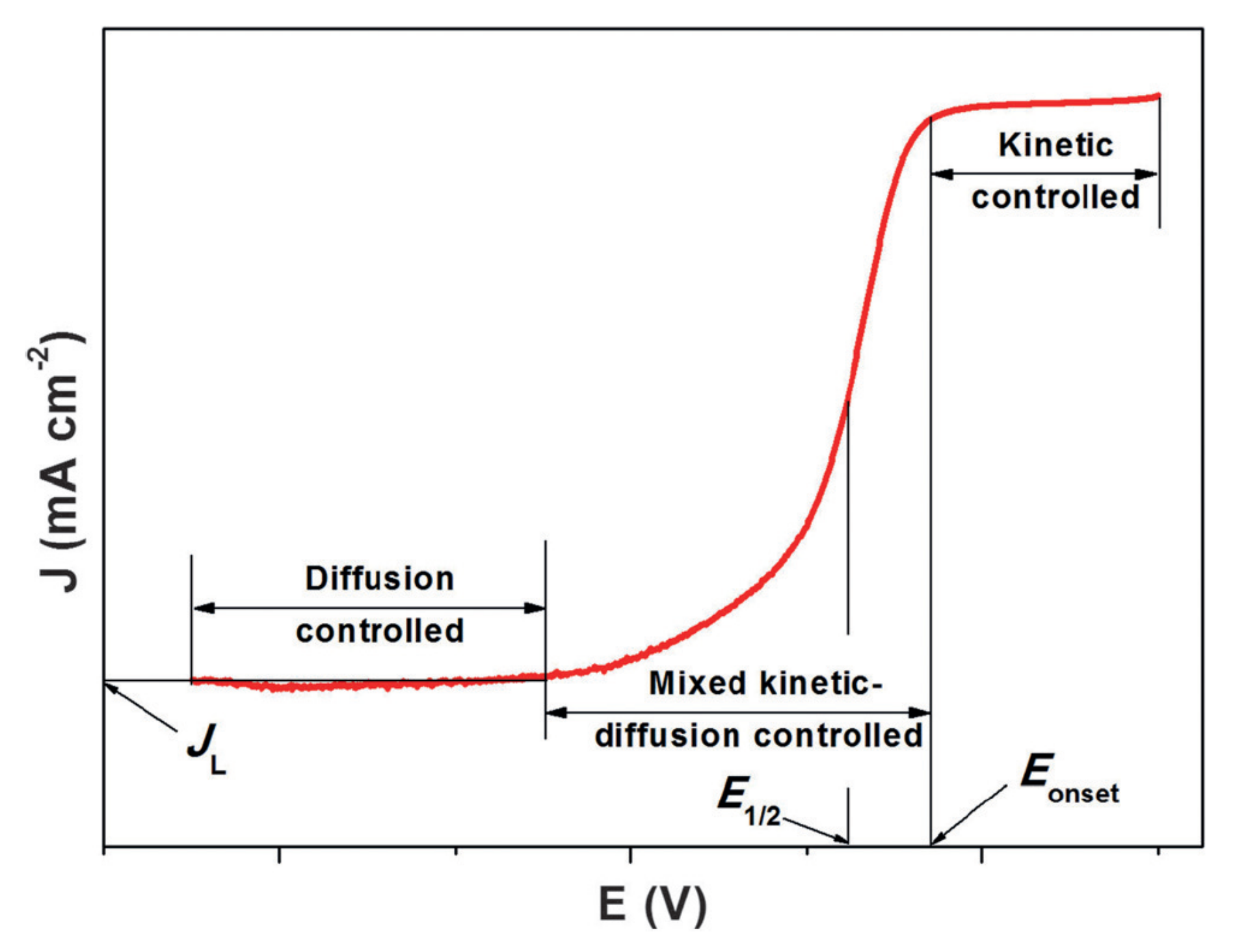
4.1. Onset Potential (Ei0)
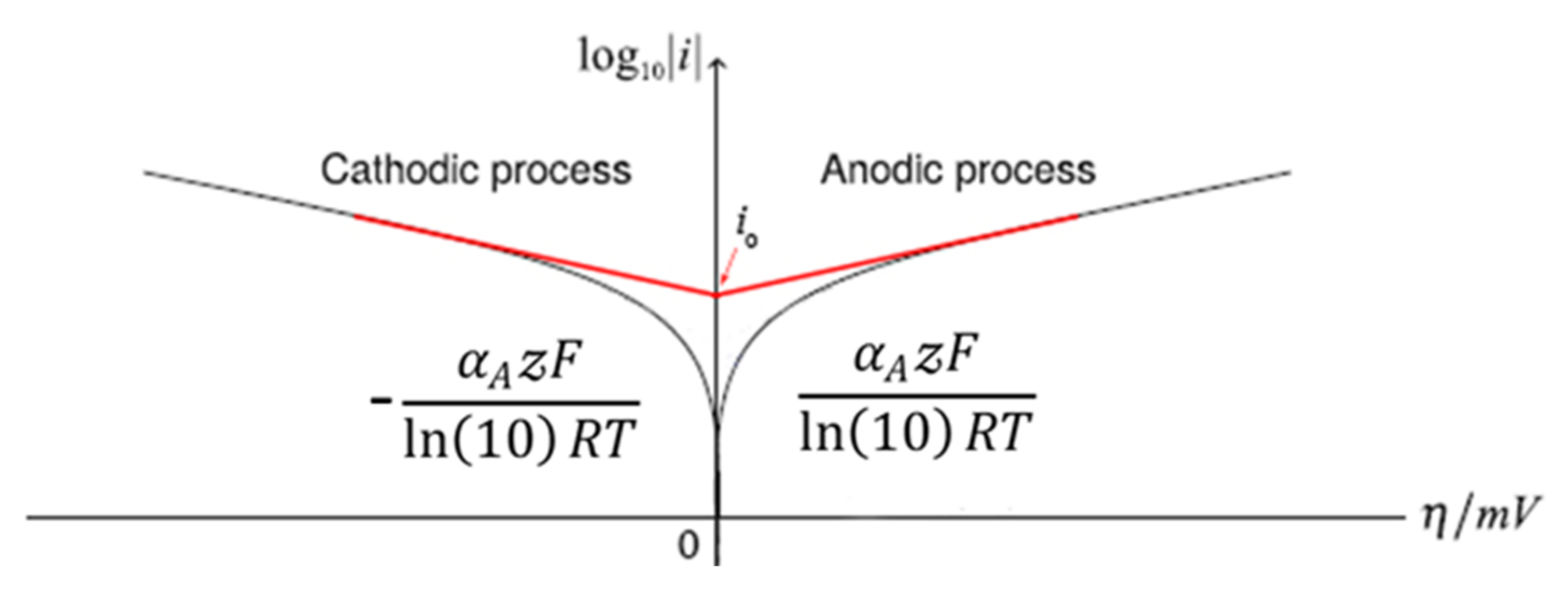
4.2. Overpotential (η)
4.3. Exchange Current Density (i0)
4.4. Tafel Slope
4.5. Number of Electrons Transferred for ORR
5. Effect of Phases of Manganese Oxide Catalyst
6. Effect of Nanosizing on Manganese Oxide
7. Electrocatalyst Performance of Nanostructured Manganese Oxide
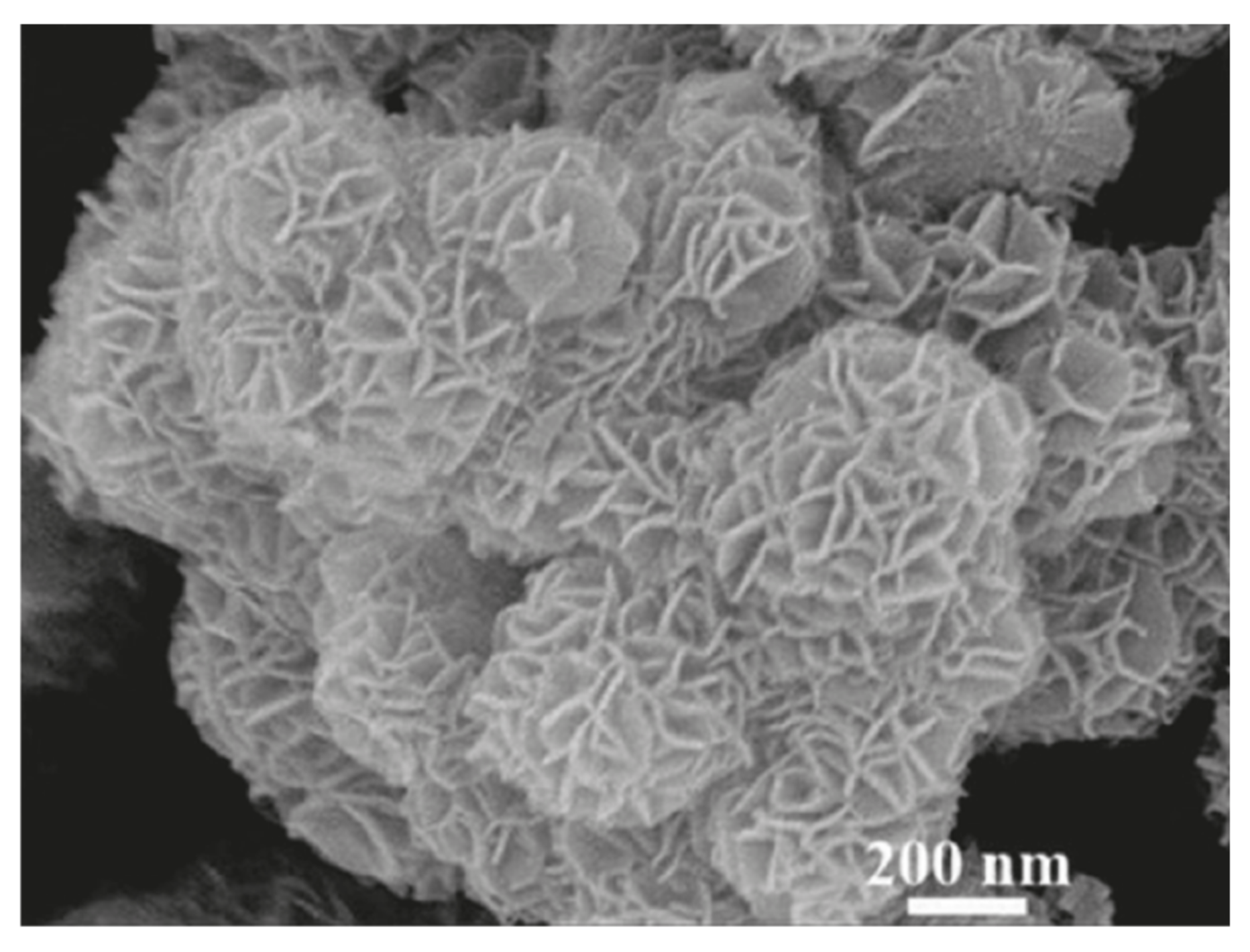
7.1. Nanoflower
7.2. Nanowires
7.3. Nanorod/Nanotube
| Catalyst | Nanostructure | Electrolyte and Reference Electrode | Synthesizing Method | BET Surface Area (m2/g) | Overpotential for ORR (mV) at −0.1 mA/cm2 | Electrons Transferred for ORR from K-L Plot | Cathodic Tafel Slope (mV dec−1) | Overpotential for OER (mV) at Specific Density | Anodic Tafel Slope (mV dec−1) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| α-MnO2 | Nanoflower | 0.1M KOH and Ag/AgCl | Hydrothermal | 52.4 | −0.570 | 3.68 | - | 0.89 at 5 mA/cm2 | - | [56] |
| α-MnO2 | Nanoflower/Nanowires | 34.9 | −0.590 | 3.31 | - | 0.91 at 5 mA/cm2 | - | |||
| α-MnO2 | Nanowires | 32.4 | −0.530 | 3.00 | - | 0.95 at 5 mA/cm2 | - | |||
| α-MnO2 | Nanoflower | 0.1M KOH and Ag/AgCl | Hydrothermal | 68.3 | −0.302 | 3.7 | - | - | - | [19] |
| α-MnO2 | Nanowires | 40.1 | −0.500 | 3.87 | - | - | - | |||
| α-MnO2 | Nanowires | 0.1M KOH and SCE | Hydrothermal | 27.7 | −0.616 | 3.5 | 65 | - | - | [30] |
| α-MnO2 | Nanotubes | 21.1 | −0.586 | 3.0 | 90 | - | - | |||
| α-MnO2 | Nanoparticles | 34.7 | −0.736 | 2.3 | 90 | - | - | |||
| α-MnO2 | Nanorod | 24.8 | −0.606 | 3.2 | 65 | - | - | |||
| α-MnO2 | Nanoflower | 32.4 | −0.876 | 1.9 | 115 | - | - | |||
| β-MnO2 | Nanorod | 0.1M KOH and Ag/AgCl | Hydrothermal | 37.9 | −0.75 | - | - | - | - | [57] |
| α-MnO2 | Nanorod | 0.1M KOH and Ag/AgCl | Electrosynthesis | 59.58 | −0.351 | 2.23 | - | - | - | [55] |
| α-MnO2 | Nanoflakes | 59.58 | −0.651 | 1.75 | - | - | - | |||
| β-MnO2 | Nanorod | 0.1M KOH and SCE | Solid state method | 5 | −0.551 | 2.4 | - | 0.6 at 10 mA/cm2 | 180.2 | [46] |
| δ-MnO2 | Nanoflower | Hydrothermal | 26 | −0.701 | 1.7 | - | 0.75 at 10 mA/cm2 | 188.6 | ||
| Porous Mn2O3 | Nanoplates | 0.1M KOH and Hg/HgO | Wet-chemical | - | - | - | - | - | 81 | [34] |
8. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Höök, M.; Tang, X. Depletion of Fossil Fuels and Anthropogenic Climate Change—A Review. Energy Policy 2013, 52, 797–809. [Google Scholar] [CrossRef] [Green Version]
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development; United Nations: New York, NY, USA, 2015; pp. 1–35. [Google Scholar]
- Saad, N.; Zainol Ariffin, Z. Implementation of Green Tax in Malaysia: An Exploratory Study. Growth 2019, 6, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Mahlia, T.M.I.; Syazmi, Z.A.H.S.; Mofijur, M.; Abas, A.E.P.; Bilad, M.R.; Ong, H.C.; Silitonga, A.S. Patent Landscape Review on Biodiesel Production: Technology Updates. Renew. Sustain. Energy Rev. 2020, 118, 109526. [Google Scholar] [CrossRef]
- Silitonga, A.S.; Shamsuddin, A.H.; Mahlia, T.M.I.; Milano, J.; Kusumo, F.; Siswantoro, J.; Dharma, S.; Sebayang, A.H.; Masjuki, H.H.; Ong, H.C. Biodiesel Synthesis from Ceiba Pentandra Oil by Microwave Irradiation-Assisted Transesterification: ELM Modeling and Optimization. Renew. Energy 2020, 146, 1278–1291. [Google Scholar] [CrossRef]
- Silitonga, A.S.; Masjuki, H.H.; Mahlia, T.M.I.; Ong, H.C.; Chong, W.T.; Boosroh, M.H. Overview Properties of Biodiesel Diesel Blends from Edible and Non-Edible Feedstock. Renew. Sustain. Energy Rev. 2013, 22, 346–360. [Google Scholar] [CrossRef]
- Ong, H.C.; Masjuki, H.H.; Mahlia, T.M.I.; Silitonga, A.S.; Chong, W.T.; Yusaf, T. Engine Performance and Emissions Using Jatropha Curcas, Ceiba Pentandra and Calophyllum Inophyllum Biodiesel in a CI Diesel Engine. Energy 2014, 69, 427–445. [Google Scholar] [CrossRef]
- Pei, P.; Wang, K.; Ma, Z. Technologies for Extending Zinc–Air Battery’s Cyclelife: A Review. Appl. Energy 2014, 128, 315–324. [Google Scholar] [CrossRef]
- Li, Y.; Dai, H. Recent Advances in Zinc–Air Batteries. Chem. Soc. Rev. 2014, 43, 5257–5275. [Google Scholar] [CrossRef] [Green Version]
- Ralph, T.R.; Hogarth, M.P. Catalysis for Low Temperature Fuel Cells. Platin. Met. Rev. 2002, 46, 117–135. [Google Scholar]
- He, H.; Zhang, Y.; Li, Y.; Wang, P. Recent Innovations of Silk-Derived Electrocatalysts for Hydrogen Evolution Reaction, Oxygen Evolution Reaction and Oxygen Reduction Reaction. Int. J. Hydrog. Energy 2021, 46, 7848–7865. [Google Scholar] [CrossRef]
- Delaporte, N.; Rivard, E.; Natarajan, S.K.; Benard, P.; Trudeau, M.L.; Zaghib, K. Synthesis and Performance of MOF-Based Non-Noble Metal Catalysts for the Oxygen Reduction Reaction in Proton-Exchange Membrane Fuel Cells: A Review. Nanomaterials 2020, 10, 1947. [Google Scholar] [CrossRef]
- Yeager, E. Dioxygen Electrocatalysis: Mechanisms in Relation to Catalyst Structure. J. Mol. Catal. 1986, 38, 5–25. [Google Scholar] [CrossRef]
- Zhou, D.; Lü, X.; Liu, D. Electro-Catalytic Effect of Manganese Oxide on Oxygen Reduction at Teflonbonded Carbon Electrode. Trans. Nonferrous Met. Soc. China 2006, 16, 217–222. [Google Scholar] [CrossRef]
- Lima, F.H.B.; Calegaro, M.L.; Ticianelli, E.A. Electrocatalytic Activity of Manganese Oxides Prepared by Thermal Decomposition for Oxygen Reduction. Electrochim. Acta 2007, 52, 3732–3738. [Google Scholar] [CrossRef]
- Zhang, T.; Ge, X.; Zhang, Z.; Tham, N.N.; Liu, Z.; Fisher, A.; Lee, J.Y. Improving the Electrochemical Oxygen Reduction Activity of Manganese Oxide Nanosheets with Sulfurization-Induced Nanopores. ChemCatChem 2018, 10, 422–429. [Google Scholar] [CrossRef]
- Tang, Y.; Zheng, S.; Cao, S.; Xue, H.; Pang, H. Advances in the Application of Manganese Dioxide and Its Composites as Electrocatalysts for the Oxygen Evolution Reaction. J. Mater. Chem. A 2020, 8, 18492–18514. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, B.; Kim, H.; Kang, K. Recent Progress on Multimetal Oxide Catalysts for the Oxygen Evolution Reaction. Adv. Energy Mater. 2018, 8, 1702774. [Google Scholar] [CrossRef]
- Cheng, F.; Su, Y.; Liang, J.; Tao, Z.; Chen, J. MnO2-Based Nanostructures as Catalysts for Electrochemical Oxygen Reduction in Alkaline Media. Chem. Mater. 2010, 22, 898–905. [Google Scholar] [CrossRef]
- Julien, C.; Mauger, A. Nanostructured MnO2 as Electrode Materials for Energy Storage. Nanomaterials 2017, 7, 396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Chen, C.; Zhao, Y.; Jia, B. A Review on the Synthesis of Manganese Oxide Nanomaterials and Their Applications on Lithium-Ion Batteries. J. Nanomater. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, L.; Yan, X.; Yao, X. Recent Progress in Oxygen Electrocatalysts for Zinc-Air Batteries. Small Methods 2017, 1, 1700209. [Google Scholar] [CrossRef]
- Mao, L.; Sotomura, T.; Nakatsu, K.; Koshiba, N.; Zhang, D.; Ohsaka, T. Electrochemical Characterization of Catalytic Activities of Manganese Oxides to Oxygen Reduction in Alkaline Aqueous Solution. J. Electrochem. Soc. 2002, 149, A504. [Google Scholar] [CrossRef]
- Cheng, F.; Chen, J. Metal–Air Batteries: From Oxygen Reduction Electrochemistry to Cathode Catalysts. Chem. Soc. Rev. 2012, 41, 2172. [Google Scholar] [CrossRef] [PubMed]
- Spendelow, J.S.; Wieckowski, A. Electrocatalysis of Oxygen Reduction and Small Alcohol Oxidation in Alkaline Media. Phys. Chem. Chem. Phys. 2007, 9, 2654. [Google Scholar] [CrossRef]
- Jo, H.-G.; Ahn, H.-J. Accelerating the Oxygen Reduction Reaction and Oxygen Evolution Reaction Activities of N and P Co-Doped Porous Activated Carbon for Li-O2 Batteries. Catalysts 2020, 10, 1316. [Google Scholar] [CrossRef]
- Speck, F.D.; Santori, P.G.; Jaouen, F.; Cherevko, S. Mechanisms of Manganese Oxide Electrocatalysts Degradation during Oxygen Reduction and Oxygen Evolution Reactions. J. Phys. Chem. C 2019, 123, 25267–25277. [Google Scholar] [CrossRef]
- Valim, R.B.; Santos, M.C.; Lanza, M.R.V.; Machado, S.A.S.; Lima, F.H.B.; Calegaro, M.L. Oxygen Reduction Reaction Catalyzed by Ɛ-MnO2: Influence of the Crystalline Structure on the Reaction Mechanism. Electrochim. Acta 2012, 85, 423–431. [Google Scholar] [CrossRef]
- Calegaro, M.L.; Lima, F.H.B.; Ticianelli, E.A. Oxygen Reduction Reaction on Nanosized Manganese Oxide Particles Dispersed on Carbon in Alkaline Solutions. J. Power Sources 2006, 158, 735–739. [Google Scholar] [CrossRef]
- Selvakumar, K.; Kumar, S.M.S.; Thangamuthu, R.; Ganesan, K.; Murugan, P.; Rajput, P.; Nath Jha, S.; Bhattacharyya, D. Physiochemical Investigation of Shape-Designed MnO2 Nanostructures and Their Influence on Oxygen Reduction Reaction Activity in Alkaline Solution. J. Phys. Chem. 2015, 119, 6604–6618. [Google Scholar] [CrossRef]
- Tahir, M.; Pan, L.; Idrees, F.; Zhang, X.; Wang, L.; Zou, J.-J.; Wang, Z.L. Electrocatalytic Oxygen Evolution Reaction for Energy Conversion and Storage: A Comprehensive Review. Nano Energy 2017, 37, 136–157. [Google Scholar] [CrossRef]
- Eftekhari, A. Tuning the Electrocatalysts for Oxygen Evolution Reaction. Mater. Today Energy 2017, 5, 37–57. [Google Scholar] [CrossRef]
- Su, H.-Y.; Gorlin, Y.; Man, I.C.; Calle-Vallejo, F.; Nørskov, J.K.; Jaramillo, T.F.; Rossmeisl, J. Identifying Active Surface Phases for Metal Oxide Electrocatalysts: A Study of Manganese Oxide Bi-Functional Catalysts for Oxygen Reduction and Water Oxidation Catalysis. Phys. Chem. Chem. Phys. 2012, 14, 14010. [Google Scholar] [CrossRef] [PubMed]
- Sim, H.; Lee, J.; Yu, T.; Lim, B. Manganese Oxide with Different Composition and Morphology as Electrocatalyst for Oxygen Evolution Reaction. Korean J. Chem. Eng. 2018, 35, 257–262. [Google Scholar] [CrossRef]
- Mainar, A.R.; Colmenares, L.C.; Leonet, O.; Alcaide, F.; Iruin, J.J.; Weinberger, S.; Hacker, V.; Iruin, E.; Urdanpilleta, I.; Blazquez, J.A. Manganese Oxide Catalysts for Secondary Zinc Air Batteries: From Electrocatalytic Activity to Bifunctional Air Electrode Performance. Electrochim. Acta 2016, 217, 80–91. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Solis, C.D.; Vimalanandan, A.; Altin, A.; Mondragón-Ochoa, J.S.; Kreth, K.; Keil, P.; Erbe, A. Fundamentals of Electrochemistry, Corrosion and Corrosion Protection. In Soft Matter at Aqueous Interfaces; Lang, P., Liu, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2016; Volume 917, pp. 29–70. ISBN 978-3-319-24500-3. [Google Scholar]
- Khezri, R.; Hosseini, S.; Lahiri, A.; Motlagh, S.R.; Nguyen, M.T.; Yonezawa, T.; Kheawhom, S. Enhanced Cycling Performance of Rechargeable Zinc–Air Flow Batteries Using Potassium Persulfate as Electrolyte Additive. IJMS 2020, 21, 7303. [Google Scholar] [CrossRef]
- Suen, N.-T.; Hung, S.-F.; Quan, Q.; Zhang, N.; Xu, Y.-J.; Chen, H.M. Electrocatalysis for the Oxygen Evolution Reaction: Recent Development and Future Perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Mahmood, A.; Liang, Z.; Zou, R.; Guo, S. Earth-Abundant Nanomaterials for Oxygen Reduction. Angew. Chem. Int. Ed. 2016, 55, 2650–2676. [Google Scholar] [CrossRef] [PubMed]
- Garsany, Y.; Ge, J.; St-Pierre, J.; Rocheleau, R.; Swider-Lyons, K.E. Analytical Procedure for Accurate Comparison of Rotating Disk Electrode Results for the Oxygen Reduction Activity of Pt/C. J. Electrochem. Soc. 2014, 161, F628–F640. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, S.; Sun, S. Tuning Nanoparticle Catalysis for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2013, 52, 8526–8544. [Google Scholar] [CrossRef]
- Zhou, R.; Zheng, Y.; Jaroniec, M.; Qiao, S.-Z. Determination of the Electron Transfer Number for the Oxygen Reduction Reaction: From Theory to Experiment. ACS Catal. 2016, 6, 4720–4728. [Google Scholar] [CrossRef]
- Rossouw, M.H.; Liles, D.C.; Thackeray, M.M. Alpha manganese dioxide for lithium batteries: A structural and electrochemical study. Mater. Res. Bull. 1992, 27, 10. [Google Scholar] [CrossRef]
- Yang, J.; Xu, J.J. Nanoporous Amorphous Manganese Oxide as Electrocatalyst for Oxygen Reduction in Alkaline Solutions. Electrochem. Commun. 2003, 5, 306–311. [Google Scholar] [CrossRef]
- Aveiro, L.R.; da Silva, A.G.M.; Antonin, V.S.; Candido, E.G.; Parreira, L.S.; Geonmonond, R.S.; de Freitas, I.C.; Lanza, M.R.V.; Camargo, P.H.C.; Santos, M.C. Carbon-Supported MnO2 Nanoflowers: Introducing Oxygen Vacancies for Optimized Volcano-Type Electrocatalytic Activities towards H2O2 Generation. Electrochim. Acta 2018, 268, 101–110. [Google Scholar] [CrossRef]
- Meng, Y.; Song, W.; Huang, H.; Ren, Z.; Chen, S.-Y.; Suib, S.L. Structure–Property Relationship of Bifunctional MnO2 Nanostructures: Highly Efficient, Ultra-Stable Electrochemical Water Oxidation and Oxygen Reduction Reaction Catalysts Identified in Alkaline Media. J. Am. Chem. Soc. 2014, 136, 11452–11464. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Lin, Y.L.; Li, W.S. Manganese Dioxide with High Specific Surface Area for Alkaline Battery. Chem. Res. Chin. Univ. 2012, 28, 874–877. [Google Scholar]
- Shende, P.; Kasture, P.; Gaud, R.S. Nanoflowers: The Future Trend of Nanotechnology for Multi-Applications. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. 1), 1–10. [Google Scholar] [CrossRef]
- Yan, D.; Yan, P.X.; Yue, G.H.; Liu, J.Z.; Chang, J.B.; Yang, Q.; Qu, D.M.; Geng, Z.R.; Chen, J.T.; Zhang, G.A.; et al. Self-Assembled Flower-like Hierarchical Spheres and Nanobelts of Manganese Oxide by Hydrothermal Method and Morphology Control of Them. Chem. Phys. Lett. 2007, 440, 134–138. [Google Scholar] [CrossRef]
- Zhu, G.; Deng, L.; Wang, J.; Kang, L.; Liu, Z.-H. Hydrothermal Preparation and the Capacitance of Hierarchical MnO2 Nanoflower. Colloids Surf. A Physicochem. Eng. Asp. 2013, 434, 42–48. [Google Scholar] [CrossRef]
- Haoran, Y.; Lifang, D.; Tao, L.; Yong, C. Hydrothermal Synthesis of Nanostructured Manganese Oxide as Cathodic Catalyst in a Microbial Fuel Cell Fed with Leachate. Sci. World J. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Yuan, H.; Deng, L.; Qi, Y.; Kobayashi, N.; Hasatani, M. Morphology-Dependent Performance of Nanostructured MnO2 as an Oxygen Reduction Catalyst in Microbial Fuel Cells. Int. J. Electrochem. Sci. 2015, 10, 14. [Google Scholar]
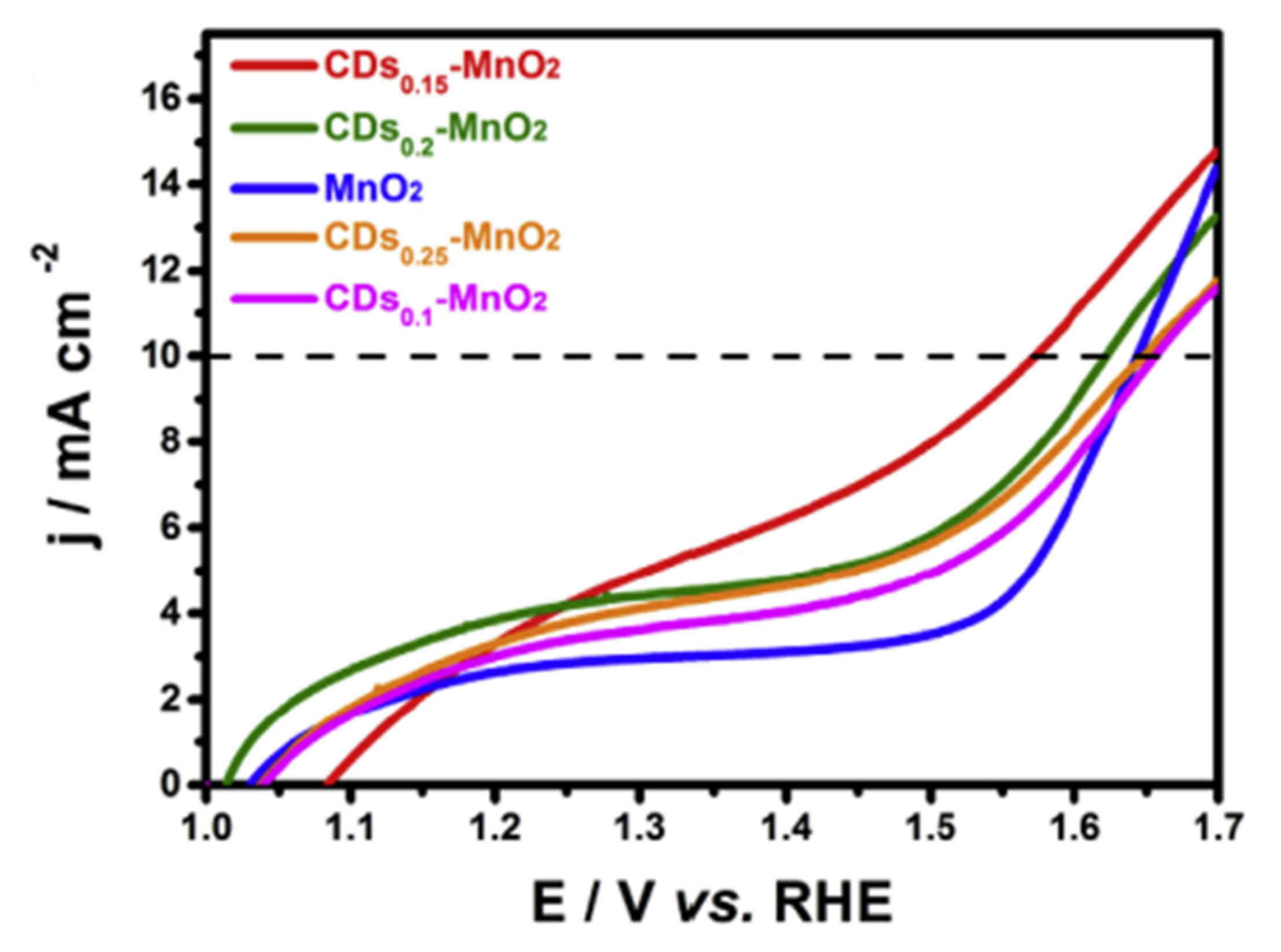
- Tian, L.; Wang, J.; Wang, K.; Wo, H.; Wang, X.; Zhuang, W.; Li, T.; Du, X. Carbon-Quantum-Dots-Embedded MnO2 Nanoflower as an Efficient Electrocatalyst for Oxygen Evolution in Alkaline Media. Carbon 2019, 143, 457–466. [Google Scholar] [CrossRef]
- Wang, L.; Duan, G.; Chen, S.-M.; Liu, X. Hydrothermally Controlled Synthesis of α-MnO2, γ-MnOOH, and Mn3O4 Nanomaterials with Enhanced Electrochemical Properties. J. Alloy. Compd. 2018, 752, 123–132. [Google Scholar] [CrossRef]
- Mahmudi, M.; Widiyastuti, W.; Nurlilasari, P.; Affandi, S.; Setyawan, H. Manganese Dioxide Nanoparticles Synthesized by Electrochemical Method and Its Catalytic Activity towards Oxygen Reduction Reaction. J. Ceram. Soc. Jpn. 2018, 126, 906–913. [Google Scholar] [CrossRef] [Green Version]
- Jing Han, S.; Ameen, M.; Hanifah, M.F.R.; Aqsha, A.; Bilad, M.R.; Jaafar, J.; Kheawhom, S. Catalytic Evaluation of Nanoflower Structured Manganese Oxide Electrocatalyst for Oxygen Reduction in Alkaline Media. Catalysts 2020, 10, 822. [Google Scholar] [CrossRef]
- Kumar, N.; Dineshkumar, P.; Rameshbabu, R.; Sen, A. Facile Size-Controllable Synthesis of Single Crystalline β-MnO2 Nanorods under Varying Acidic Strengths. RSC Adv. 2016, 6, 7448–7454. [Google Scholar] [CrossRef] [Green Version]
- Osman, A.; Mat Nawi, N.I.; Samsuri, S.; Bilad, M.R.; Shamsuddin, N.; Khan, A.L.; Jaafar, J.; Nordin, N.A.H. Patterned Membrane in an Energy-Efficient Tilted Panel Filtration System for Fouling Control in Activated Sludge Filtration. Polymers 2020, 12, 432. [Google Scholar] [CrossRef] [Green Version]
- Wan Osman, W.N.A.; Mat Nawi, N.I.; Samsuri, S.; Bilad, M.R.; Khan, A.L.; Hunaepi, H.; Jaafar, J.; Lam, M.K. Ultra Low-Pressure Filtration System for Energy Efficient Microalgae Filtration. Heliyon 2021, 7, e07367. [Google Scholar] [CrossRef] [PubMed]




| Compound | Mineral | Crystal Symmetry | Lattice Parameters (Å) | Features |
|---|---|---|---|---|
| Hollandite | Tetragonal (I4/m) | a = 9.96; c = 2.85 | (2 × 2) tunnel | |
| Ramsdellite | Orthorhombic (Pbnm) | a = 4.53; b = 9.27; c = 2.87 | (1 × 2) tunnel | |
| Pyrolusite | Tetragonal (P42/mnm) | a = 4.39; c = 2.87 | (1 × 1) tunnel | |
| Nsutite | Complex tunnel (hex.) | a = 9.65; c = 4.43 | (1 × 1)/ (1 × 2) | |
| Birnessite | Rhombohedral(R-3m) | ahex = 2.94; chex = 21.86 | (1 × ) layer | |
| Mg-birnessite | Monoclinic (C2/m) | a = 5.18; b = 2.84; c = 7.33 | (1 × ) layer | |
| Na-birnessite | Monoclinic (C2/m) | a = 5.17; b = 2.85; c = 7.32 | (1 × ) layer | |
| Akhtenkite | Hexagonal (P63/mmc) | a = 2.85; c = 4.65 | Dense stack | |
| Spinel | Cubic (Fd3m) | a = 8.04 | (1 × 1) tunnel | |
| Psilomelane | Monoclinic (P2/m) | a = 9.56; b = 2.88; c = 13.85 | (2 × 3) tunnel | |
| Todorokite | Monoclinic (P2/m) | a = 9.75; b = 2.85; c = 9.59 | (3 × 3) tunnel |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siow, J.H.; Bilad, M.R.; Caesarendra, W.; Leam, J.J.; Bustam, M.A.; Sambudi, N.S.; Wibisono, Y.; Mahlia, T.M.I. Progress in Development of Nanostructured Manganese Oxide as Catalyst for Oxygen Reduction and Evolution Reaction. Energies 2021, 14, 6385. https://doi.org/10.3390/en14196385
Siow JH, Bilad MR, Caesarendra W, Leam JJ, Bustam MA, Sambudi NS, Wibisono Y, Mahlia TMI. Progress in Development of Nanostructured Manganese Oxide as Catalyst for Oxygen Reduction and Evolution Reaction. Energies. 2021; 14(19):6385. https://doi.org/10.3390/en14196385
Chicago/Turabian StyleSiow, Jing Han, Muhammad Roil Bilad, Wahyu Caesarendra, Jia Jia Leam, Mohammad Azmi Bustam, Nonni Soraya Sambudi, Yusuf Wibisono, and Teuku Meurah Indra Mahlia. 2021. "Progress in Development of Nanostructured Manganese Oxide as Catalyst for Oxygen Reduction and Evolution Reaction" Energies 14, no. 19: 6385. https://doi.org/10.3390/en14196385
APA StyleSiow, J. H., Bilad, M. R., Caesarendra, W., Leam, J. J., Bustam, M. A., Sambudi, N. S., Wibisono, Y., & Mahlia, T. M. I. (2021). Progress in Development of Nanostructured Manganese Oxide as Catalyst for Oxygen Reduction and Evolution Reaction. Energies, 14(19), 6385. https://doi.org/10.3390/en14196385











