Insights into CO2 Foaming Behavior of Ethoxylated Amines
Abstract
1. Introduction
- Investigate the CO2 foaming properties of EC12, such as initial foamability and foam stability at different surfactant concentrations, initial pH, and brine salinity and composition.
- Evaluate the surface tension of EC12 as a function of surfactant concentration in the presence of CO2 at 150 °F (65 °C) using the pendant drop method.
- Calculate the CMC and surface tension gradients from the surface tension versus surfactant concentration plot. The surface tension gradients were used to understand the foaming properties of EC12.
2. Materials
3. Experimental Tests and Procedures
3.1. Bottle Foam Test
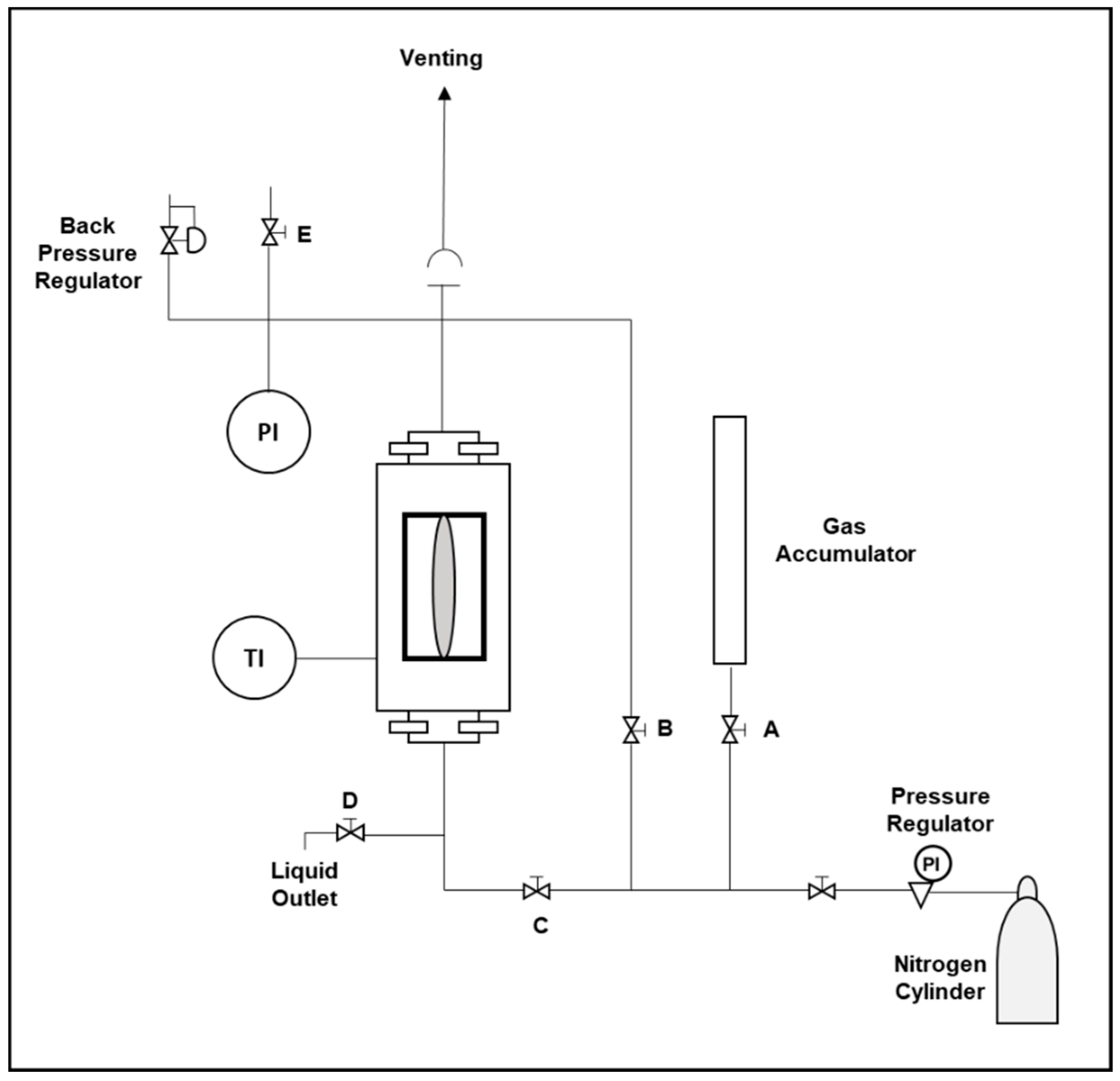
3.2. High-Pressure Foam Test
3.3. Surface Tension Study
4. Results and Discussion
4.1. Bottle Foam Tests at Ambient Conditions
4.2. HPVC Foam Tests
4.3. Surface Tension Study
5. Conclusions
- The initial foamability increased with surfactant concentration from 0.1–0.5 wt%. Solutions with pH 2.5 yielded better initial foam than those at pH 6.5.
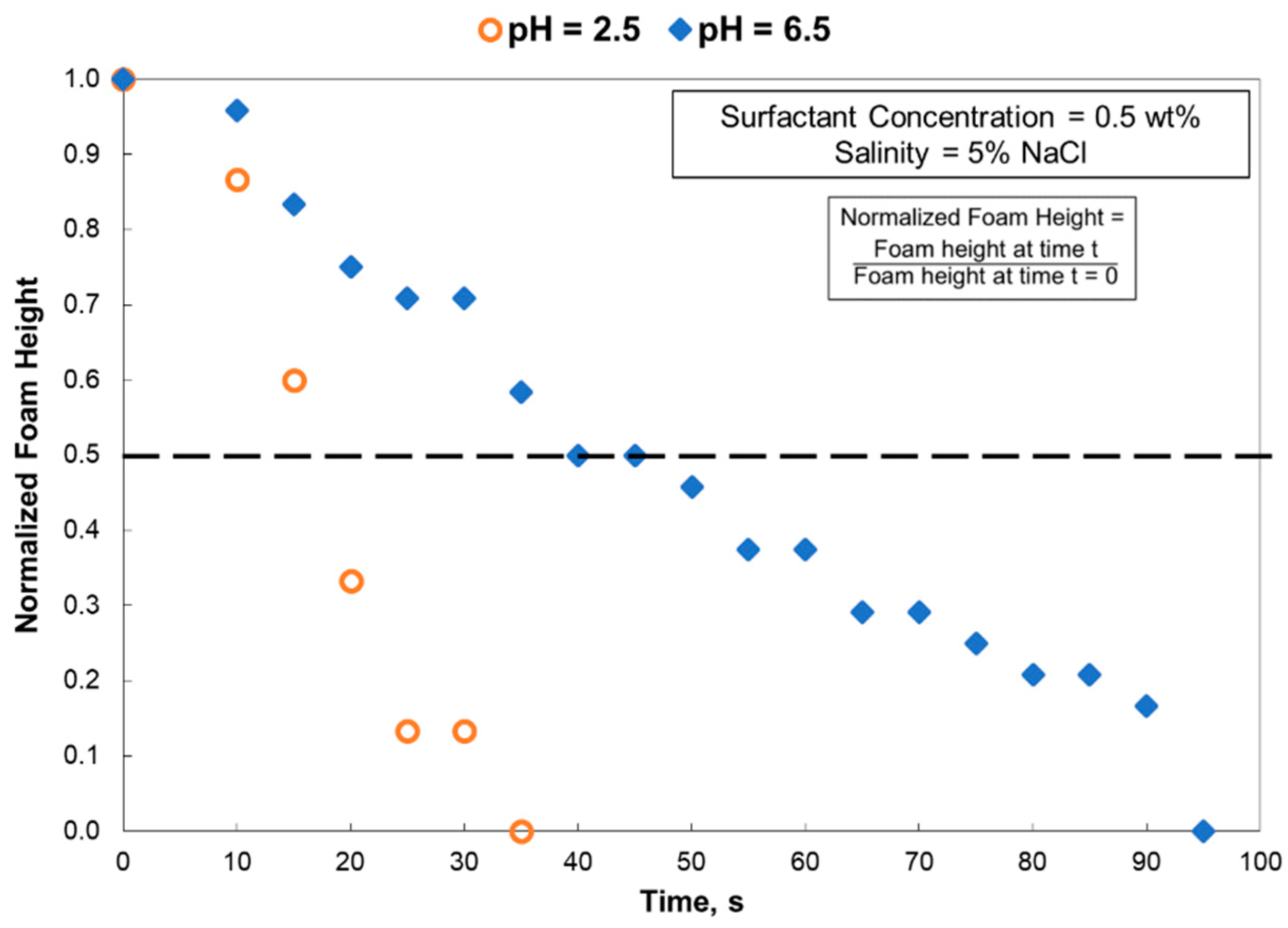
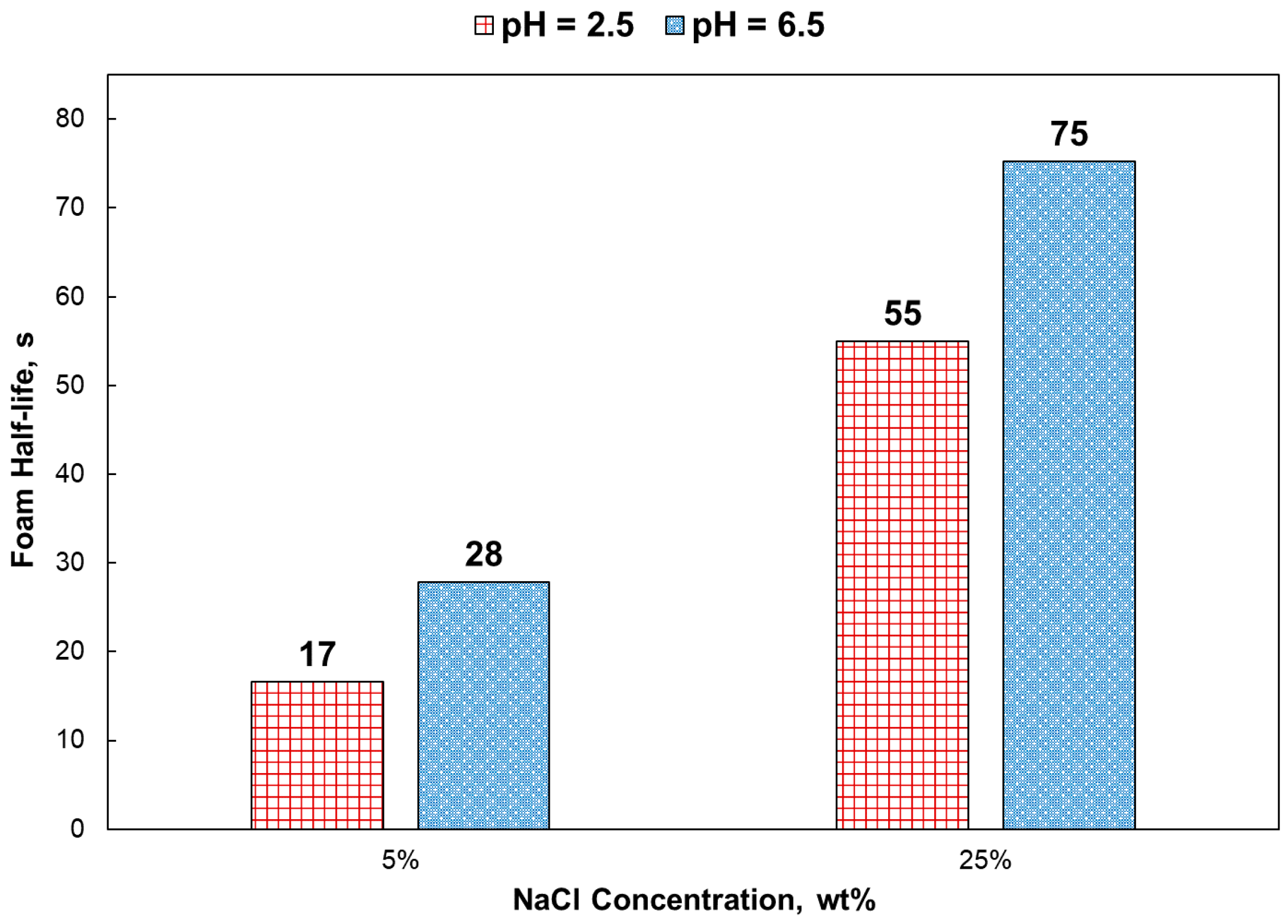
- Generally, the foam stability of the pH 6.5 solutions was better than that of the pH 2.5 solutions, especially at a low-salinity environment. The foam stability reached a maximum for 1.5% surfactant solutions.
- The addition of chloride ions had both stabilization and destabilization effects on foam stability. Destabilization occurred by depressing the electrical double layer and stabilization by tighter packing of surfactant at the liquid films. For Ethomeen C12, the stabilization effect overcame the destabilization effect at 20–25 wt% NaCl.
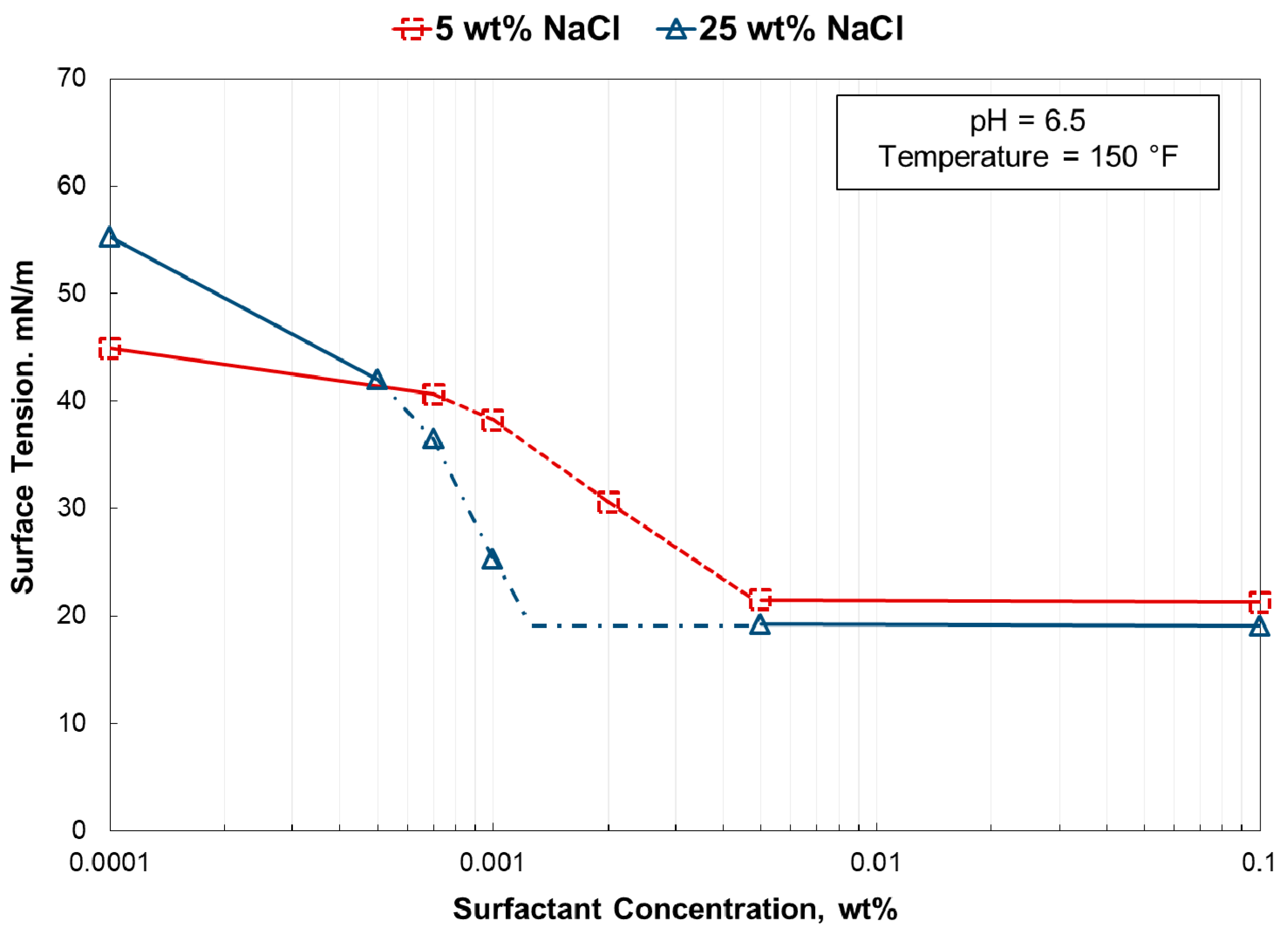
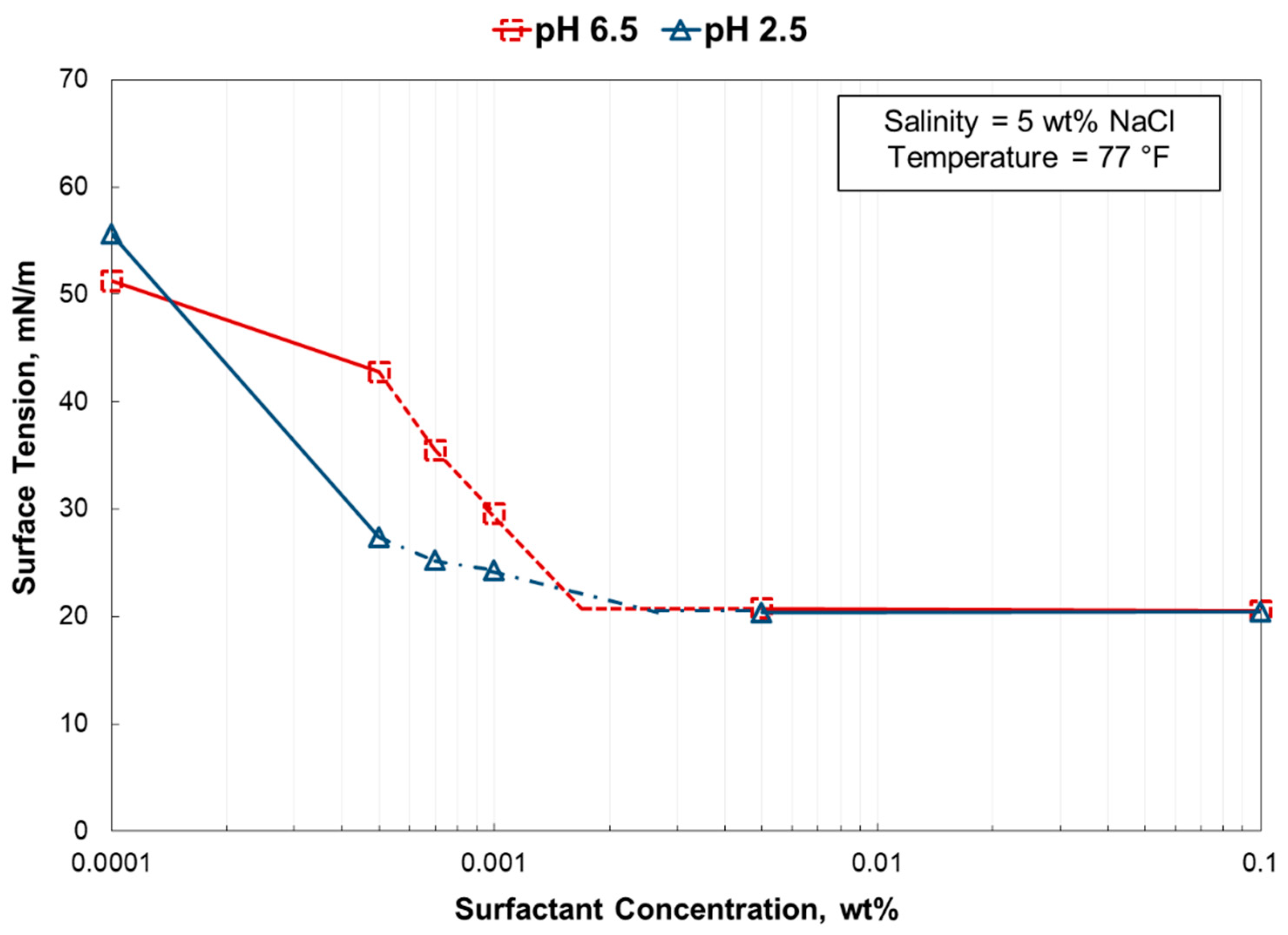
- The surface tension gradients calculated from a plot of surface tension vs. surfactant concentration yielded an excellent positive correlation to the foam stability.
- The increase in temperature resulted in a lower surface tension gradient and thus poorer foam stability at higher temperatures. An increase in salinity resulted in higher surface tension gradients.
- In 5 wt% NaCl, the surface tension gradient was greatly affected by using a pH 2.5 solution. However, at 25 wt% NaCl, there was no impact by solution pH.
- This surfactant showed high resistance to the presence of divalent cations in terms of the interfacial properties and foam stability at 150 °F (65 °C).
6. Recommendation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| EC12 | ethomeen C12 |
| EOR | enhanced oil recovery |
| IFT | interfacial tension |
| PB | plateau borders |
| AOS | alpha-olefin sulfonate |
| EO | ethoxylated oxide |
| HLB | hydrophile-lipophile balance |
| CMC | critical micelle concentration |
| HPVC | high-pressure view chamber |
| DST | dynamic surface tension |
References
- Ghedan, S.G. Global Laboratory Experience of CO2-EOR Flooding. In Proceedings of the SPE/EAGE Reservoir Characterization and Simulation Conference, Abu Dhabi, UAE, 19–21 October 2009. [Google Scholar] [CrossRef]
- Patil, P.D.; Knight, T.; Katiyar, A.; Vanderwal, P.; Scherlin, J.; Rozowski, P.; Ibrahim, M.; Sridhar, G.B.; Nguyen, Q.P. CO2 Foam Field Pilot Test in Sandstone Reservoir. J. Pet. Technol. 2018, 70, 70–71. [Google Scholar] [CrossRef]
- Ramanathan, R.; Abdelwahab, O.; Nasr-El-Din, H.A. A New Effective Multiwalled Carbon Nanotube-Foam System for Mobility Control. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, UAE, 9–12 November 2020. [Google Scholar] [CrossRef]
- Talebian, S.H.; Masoudi, R.; Tan, I.M.; Zitha, P.L.J. Foam Assisted CO2-EOR; Concepts, Challenges and Applications. In Proceedings of the SPE Enhanced Oil Recovery Conference, Kuala Lumpur, Malaysia, 2–4 July 2013. [Google Scholar] [CrossRef]
- Rehm, B.; Haghshenas, A.; Paknejad, A.S.; Al-Yami, A.; Hughes, J. Underbalanced Drilling: Limits and Extremes, 1st ed.; Elsevier: Houston, TX, USA, 2013. [Google Scholar] [CrossRef]
- Almubarak, M.; AlYousef, Z.; Almajid, M.; Almubarak, T.; Ng, J.H. Enhancing Foam Stability through a Combination of Sufactant and Nanoparticles. In Proceedings of the Abu Dhabi International Petroleum Exhibition and Conference, Abu Dhabi, UAE, 9–12 November 2020. [Google Scholar] [CrossRef]
- Rio, E.; Biance, A. Thermodynamic and Mechanical Timescales Involved in Foam Film Rupture and Liquid Foam Coalescence. ChemPhysChem 2014, 15, 3692–3707. [Google Scholar] [CrossRef] [PubMed]
- Bhakta, A.; Ruckenstein, E. Drainage and Coalescence in Standing Foams. J. Colloid Interface Sci. 1997, 191, 184–201. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, V.; Radke, C.J. Equilibrium Measurements of Oscillatory Disjoning Pressure in Aqueous Foam Films. Langmuir 1992, 8, 3020–3026. [Google Scholar] [CrossRef]
- Emrani, A.S.; Nasr-El-Din, H.A. Stabilizing CO2 Foam by Use of Nanoparticles. SPE J. 2017, 22, 494–504. [Google Scholar] [CrossRef]
- Ibrahim, A.F.; Emrani, A.; Nasr-El-Din, H.A. Stabilized CO2 Foam for EOR Applications. In Proceedings of the Carbon Management Technology Conference, Houston, TX, USA, 17–20 July 2017. [Google Scholar] [CrossRef]
- Belhaj, A.F.; Elraies, K.A.; Mahmood, S.M.; Zulkifli, N.N.; Akbari, S.; Hussien, O.S. The Effect of Surfactant Concentration, Salinity, Temperature, and pH on Surfactant Adsorption for Chemical Enhanced Oil Recovery: A Review. J. Pet. Explor. Prod. Technol. 2019, 9, 125–137. [Google Scholar] [CrossRef]
- Chen, Y.; Elhag, A.S.; Poon, B.M.; Cui, L.; Ma, K.; Liao, S.Y.; Reddy, P.P.; Worthen, A.J.; Hirasaki, G.J.; Nguyen, Q.P.; et al. Switchable Nonionic to Cationic Ethoxylated Amine Surfactants for CO2 Enhanced Oil Recovery in High-Temperature, High-Salinity Carbonate Reservoirs. SPE J. 2014, 19, 249–259. [Google Scholar] [CrossRef]
- Cui, L.; Kun, M.; Puerto, M.; Chen, H.; Cui, L.; Worthen, A.J.; Ma, K.; Quintanilla, H.; Noguera, J.A.; Hirasaki, G.J. Mobility of Ethomeen C12 and Carbon Dioxide (CO2) Foam at High Temperature/high Salinity and in Carbonate Cores. SPE J. 2016, 21, 1151–1163. [Google Scholar] [CrossRef]
- Chen, Y.; Elhag, A.S.; Reddy, P.P.; Chen, H.; Cui, L.; Worthen, A.J.; Ma, K.; Quintanilla, H.; Noguera, J.A.; Hirasaki, G.J.; et al. Phase Behavior and Interfacial Properties of a Switchable Ethoxylated Amine Surfactant at High Temperature and Effects on CO2-in-Water Foams. J. Colloid Interface Sci. 2016, 470, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Joseph, D.D. Questions in Fluid Mechanics: Understanding Foams and Foaming. J. Fluids Eng. 1997, 119, 497–498. [Google Scholar] [CrossRef]
- Velarde, M. Drops, Liquid Layers and the Marangoni Effect. Philos. Trans. R. Soc. Lond. 1998, 356, 829–844. [Google Scholar] [CrossRef]
- Pilling, M. Foaming in Fractionation Columns. 2015. Available online: https://www.digitalrefining.com/article/1001169,Foaming_in_fractionation_columns.html (accessed on 13 November 2019).
- Garret, P.R. Recent Development in the Understanding of Foam Generation and Stability. J. Chem. Eng. Sci. 1993, 48, 367–392. [Google Scholar] [CrossRef]
- Gallego-Juárez, J.A.; Rodríguez, G.; Riera, E.; Cardoni, A. Ultrasonic Defoaming and Debubbling in Food Processing and Other Applications. In Power Ultrasonics; Elsevier BV: Cambridge, UK, 2015; Chapter 26; pp. 793–814. [Google Scholar] [CrossRef]
- AlMatouq, H.; Almubarak, M.; Algadrah, A.; Alhodaythi, W. A Study on the Adsorption Behavior of Different Surfactants in Carbonate Using Different Techniques. In Proceedings of the SPE Europec Featured at 82nd EAGE Conference and Exhibition, Amsterdam, The Netherlands, 8–11 December 2020. [Google Scholar] [CrossRef]
- Hull, K.L.; Sayed, M.; Al-Muntasheri, G.A. Recent Advances in Viscoelastic Surfactants for Improved Production from Hydrocarbon Reservoirs. SPE J. 2016, 21, 1340–1357. [Google Scholar] [CrossRef]
- Rushing, J.A.; Newsham, K.E.; Van Fraassen, K.C.; Mehta, S.A.; Moore, G.R. Laboratory Measurements of Gas-Water Interfacial Tension at HP/HT Reservoir Conditions. In Proceedings of the CIPC/SPE Gas Technology Symposium Joint Conference, Calgary, AB, Canada, 16–19 June 2008. [Google Scholar] [CrossRef]
- Shariat, A.; Moore, R.G.; Mehta, S.A.; Van Fraassen, K.C.; Rushing, J.A. Gas/Water IFT Measurements Using the Pendant Drop Method at HP/HT Conditions: The Selected Plane vs. Computerized Image Processing Methods. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 8–10 October 2012. [Google Scholar] [CrossRef]
- Franses, E.I.; Basaran, O.A.; Chang, C. Techniques to Measure Dynamic Surface Tension. Curr. Opin. Colloid Interface Sci. 1996, 1, 296–303. [Google Scholar] [CrossRef]
- Almubarak, M.; Almubarak, T.; Ng, J.H.; Hernandez, J.; Nasr-El-Din, H. Recent Advances in Waterless Fracturing Fluids: A Review. In Proceedings of the Abu Dhabi International Petroleum Exhibition and Conference, Abu Dhabi, UAE, 9–12 November 2020. [Google Scholar] [CrossRef]
- Tcholakova, S.; Mitrinova, Z.; Golemanov, K.; Denkov, N.D.; Vethamuthu, M.; Ananthapadmanabhan, K.P. Control of Ostwald Ripening by Using Surfactants with High Surface Modulus. Langmuir 2011, 27, 14807–14819. [Google Scholar] [CrossRef] [PubMed]
- Elhag, A.S.; Chen, Y.; Chen, H.; Reddy, P.P.; Cui, L.; Worthen, A.J.; Ma, K.; Hirasaki, G.J.; Nguyen, Q.P.; Biswal, S.L.; et al. Switchable Amine Surfactants for Stable CO2/Brine Foams in High Temperature High Salinity Reservoirs. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 12–16 April 2014. [Google Scholar] [CrossRef]
- Almubarak, T.; AlKhaldi, M.; Ng, J.H.; Nasr-El-Din, H.A. Design and Application of High-Temperature Raw-Seawater-Based Fracturing Fluids. SPE J. 2019, 24, 1929–1946. [Google Scholar] [CrossRef]



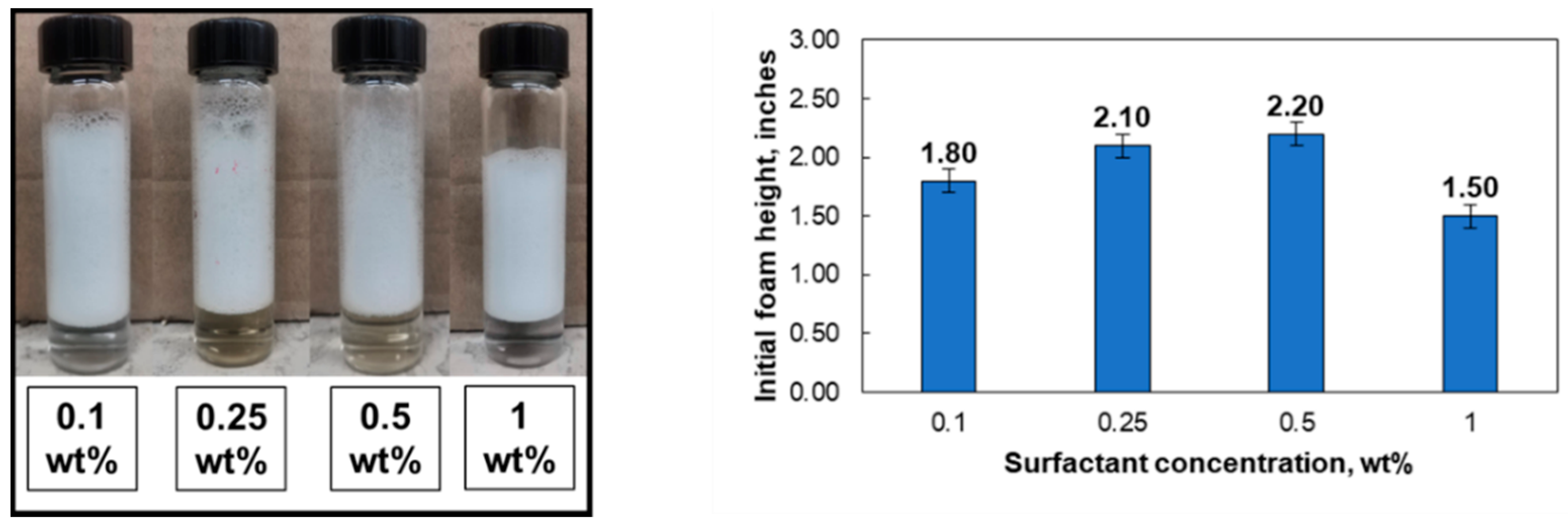

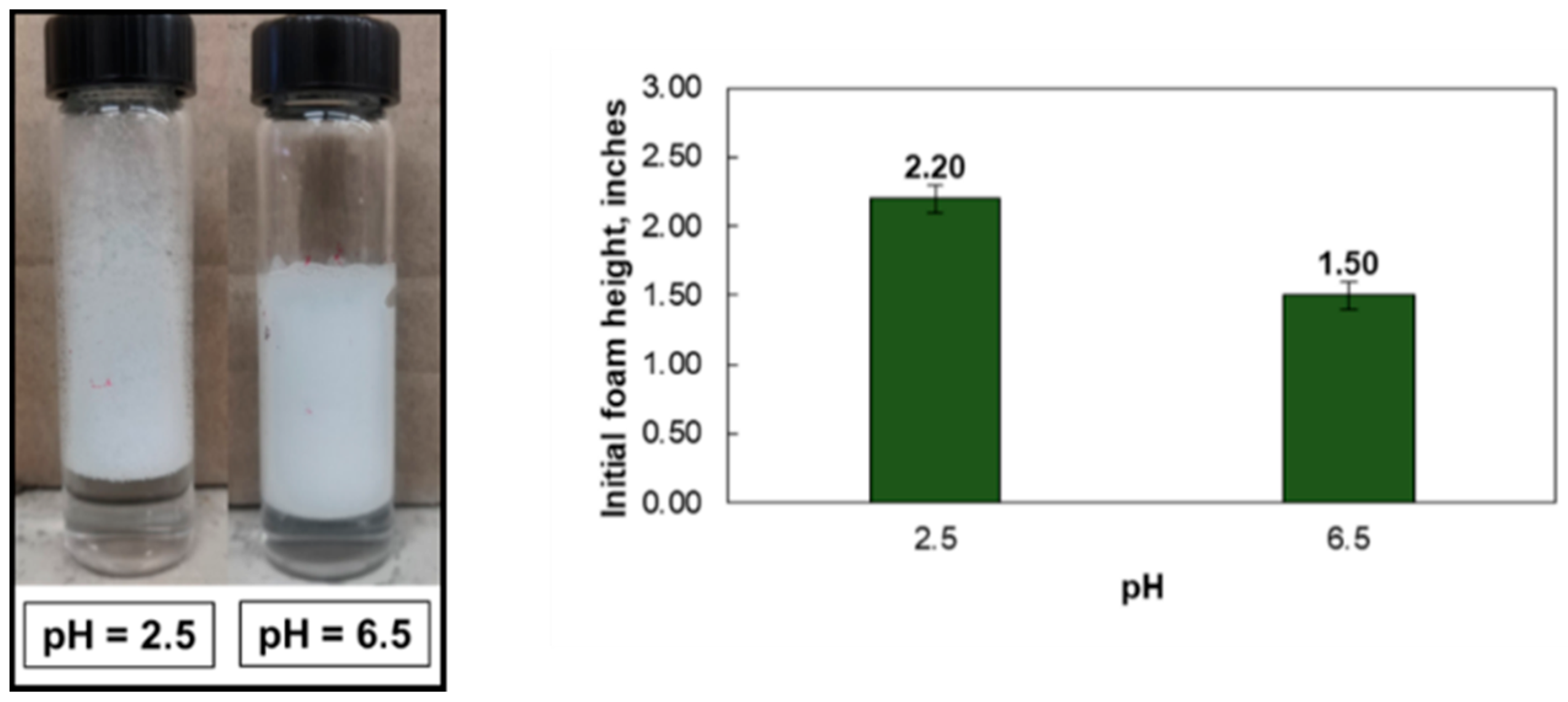








| Surfactant Concentration (wt%) | Salinity (wt%) | Initial Foam Height (Inches) | Foam Half-Life (h) |
|---|---|---|---|
| 0.1 | 5 | 1.80 | 6 |
| 0.25 | 5 | 2.10 | 10 |
| 0.5 | 5 | 2.20 | 14 |
| 1.0 | 5 | 1.50 | 22 |
| 0.1 | 10 | 1.35 | 10 |
| 0.25 | 10 | 1.50 | 14 |
| 0.5 | 10 | 1.60 | 20 |
| 1.0 | 10 | 1.30 | 24 |
| 0.1 | 15 | 1.35 | 14 |
| 0.25 | 15 | 1.35 | 22 |
| 0.5 | 15 | 1.45 | 24 |
| 1.0 | 15 | 1.30 | 16 |
| 0.1 | 20 | 1.35 | 18 |
| 0.25 | 20 | 1.40 | 42 |
| 0.5 | 20 | 1.45 | 42 |
| 1.0 | 20 | 1.40 | 18 |
| 0.1 | 25 | 1.30 | 22 |
| 0.25 | 25 | 1.30 | 46 |
| 0.5 | 25 | 1.30 | 42 |
| 1.0 | 25 | 1.30 | 22 |
| Surfactant Concentration (wt%) | Salinity (wt%) | Initial Foam Height (Inches) | Foam Half-Life (h) |
|---|---|---|---|
| 0.1 | 5 | 2.10 | 1 |
| 0.25 | 5 | 2.10 | 0.5 |
| 0.5 | 5 | 2.10 | 0.5 |
| 1.0 | 5 | 2.20 | <0.5 |
| 0.1 | 10 | 2.00 | 1 |
| 0.25 | 10 | 2.20 | 0.5 |
| 0.5 | 10 | 2.20 | 0.5 |
| 1.0 | 10 | 2.20 | 0.5 |
| 0.1 | 15 | 1.70 | 10 |
| 0.25 | 15 | 1.75 | 6 |
| 0.5 | 15 | 1.85 | 6 |
| 1.0 | 15 | 2.00 | 3 |
| 0.1 | 20 | 1.50 | 15 |
| 0.25 | 20 | 1.60 | 22 |
| 0.5 | 20 | 1.70 | 26 |
| 1.0 | 20 | 1.80 | 10 |
| 0.1 | 25 | 1.30 | 22 |
| 0.25 | 25 | 1.40 | 42 |
| 0.5 | 25 | 1.45 | 38 |
| 1.0 | 25 | 1.50 | 18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, L.; Ramanathan, R.; Almubarak, T.; Nasr-El-Din, H.A. Insights into CO2 Foaming Behavior of Ethoxylated Amines. Energies 2021, 14, 290. https://doi.org/10.3390/en14020290
Le L, Ramanathan R, Almubarak T, Nasr-El-Din HA. Insights into CO2 Foaming Behavior of Ethoxylated Amines. Energies. 2021; 14(2):290. https://doi.org/10.3390/en14020290
Chicago/Turabian StyleLe, Linh, Raja Ramanathan, Tariq Almubarak, and Hisham A. Nasr-El-Din. 2021. "Insights into CO2 Foaming Behavior of Ethoxylated Amines" Energies 14, no. 2: 290. https://doi.org/10.3390/en14020290
APA StyleLe, L., Ramanathan, R., Almubarak, T., & Nasr-El-Din, H. A. (2021). Insights into CO2 Foaming Behavior of Ethoxylated Amines. Energies, 14(2), 290. https://doi.org/10.3390/en14020290





