Decomposition Characteristics of SF6 under Arc Discharge and the Effects of Trace H2O, O2, and PTFE Vapour on Its By-Products
Abstract
:1. Introduction
2. Methods
3. Results and Discussion
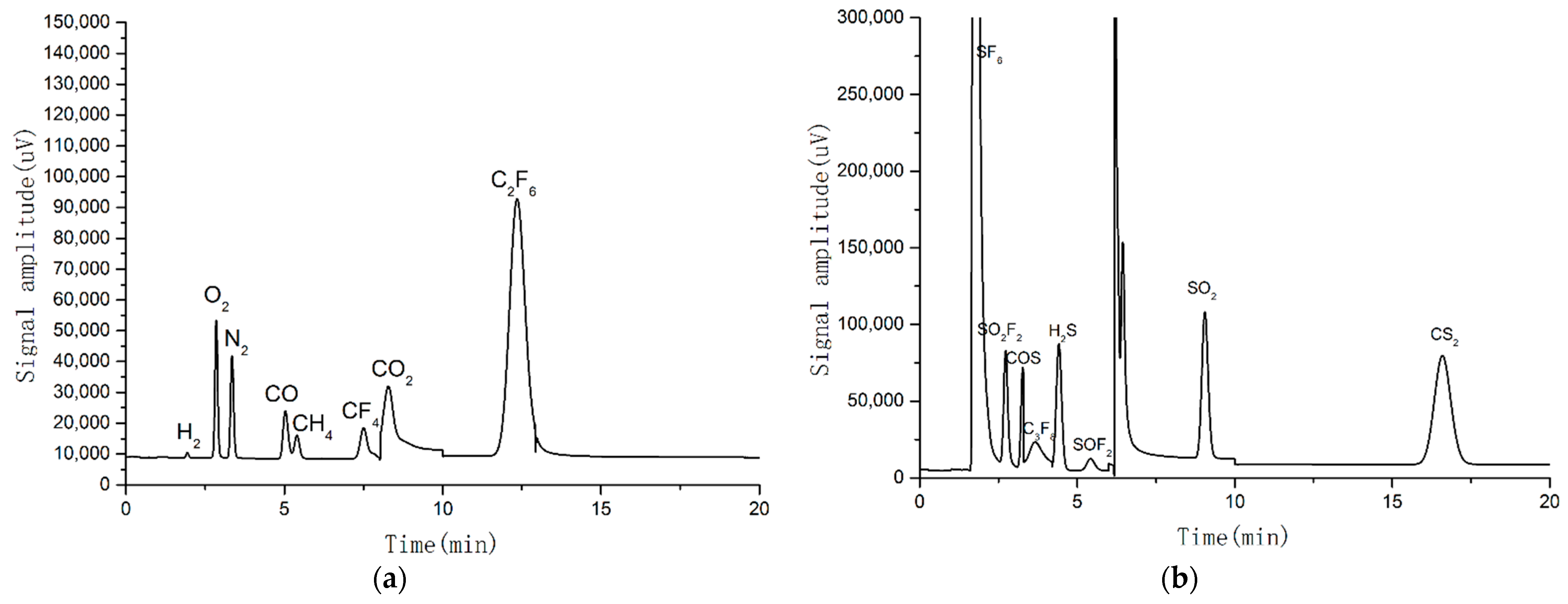
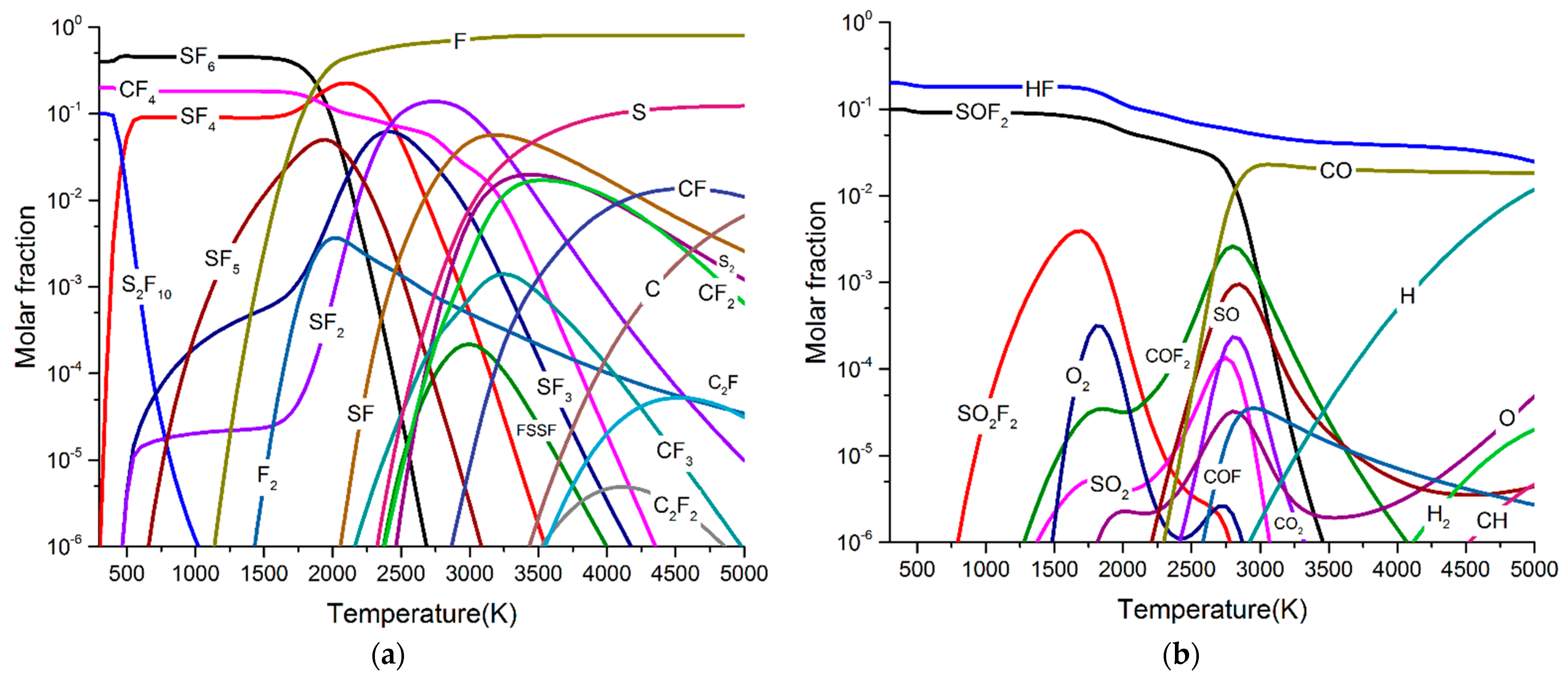
3.1. Particle Composition of SF6 and SF6/PTFE
3.2. Particle Composition of SF6 and SF6/PTFE/O2
3.3. Particle Composition of SF6 and SF6/PTFE/H2O
3.4. Particle Composition of SF6 and SF6/PTFE/O2/H2O
4. Conclusions
- (1)
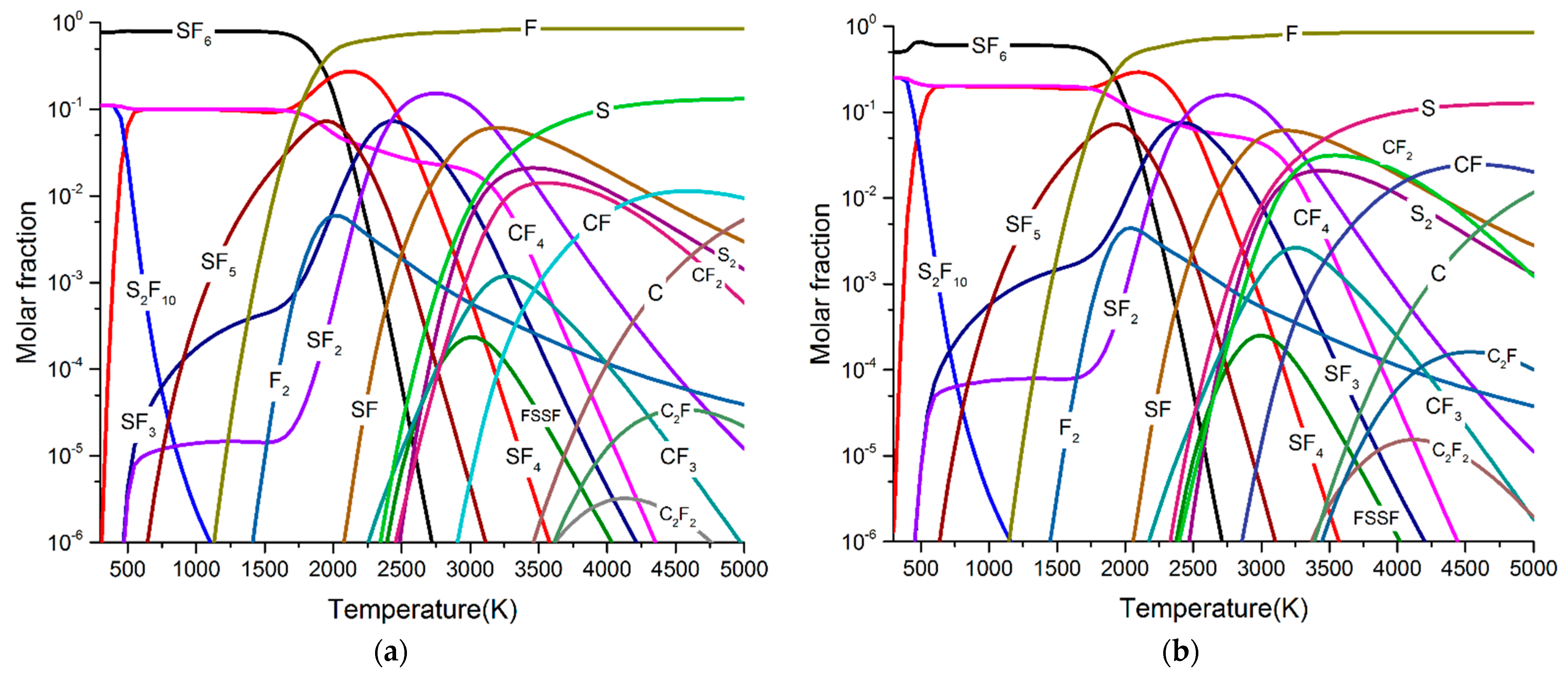
- SF6 molecules began to decompose at about 1000 K and form SF5, SF4, SF3, SF2, SF, S, and F with the increase of arc temperature. The maximum molar fractions of SF4 and SF2 were higher than SF5, SF3, and SF. As the arc temperature gradually decreases to room temperature, all low fluorine sulphides recombine into SF6.
- (2)
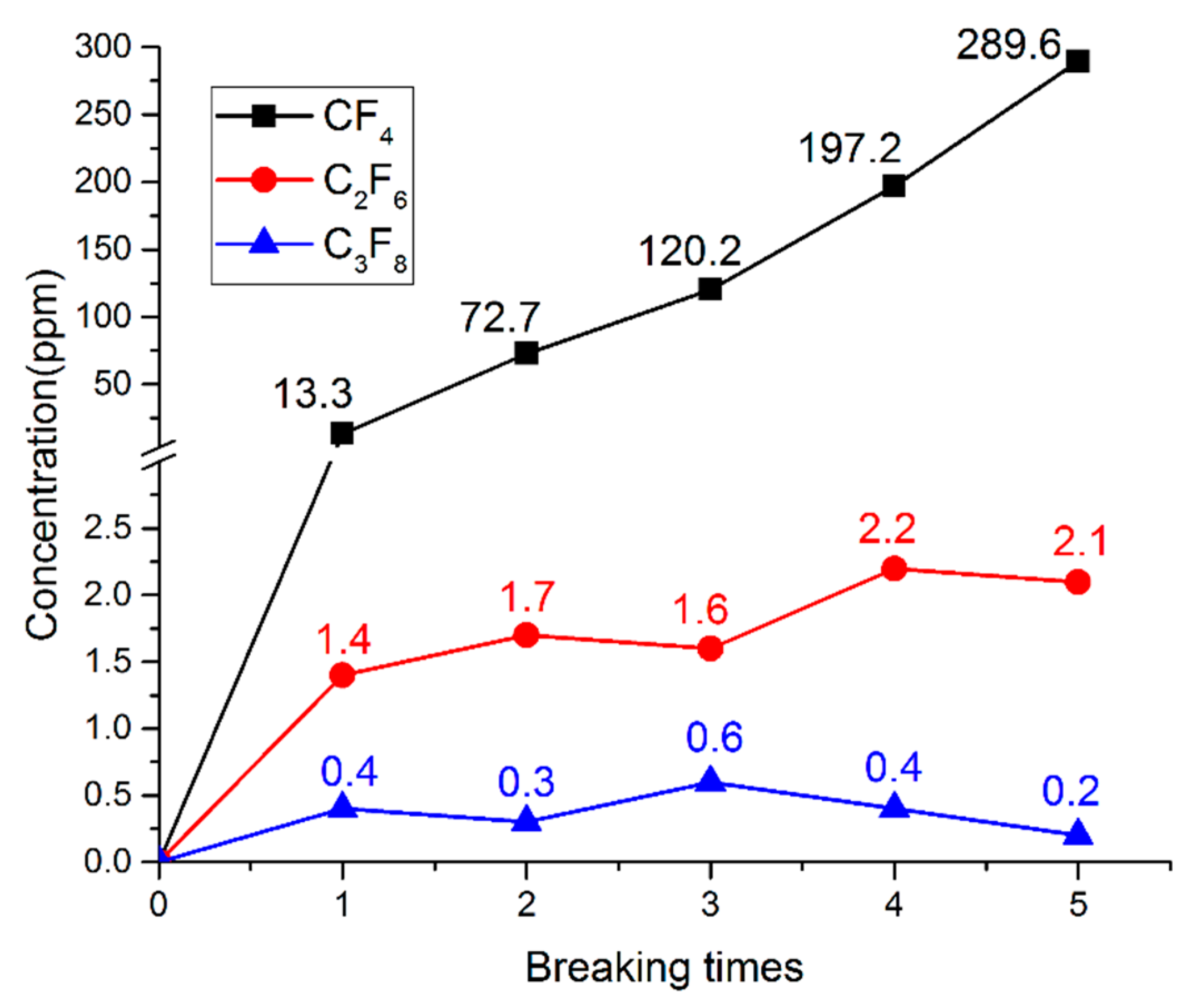
- When PTFE vapour was involved in the arc plasma, the main by-product after arc quenching was CF4, and the molar fractions of C2F6 and C3F8 were very low. After five breaking experiments, the concentration of CF4 can reach 289.6 ppm, while the concentration of C2F6 and C3F8 was only 2.1 ppm and 0.2 ppm, respectively. The simulation results were in good agreement with the experimental results.
- (3)
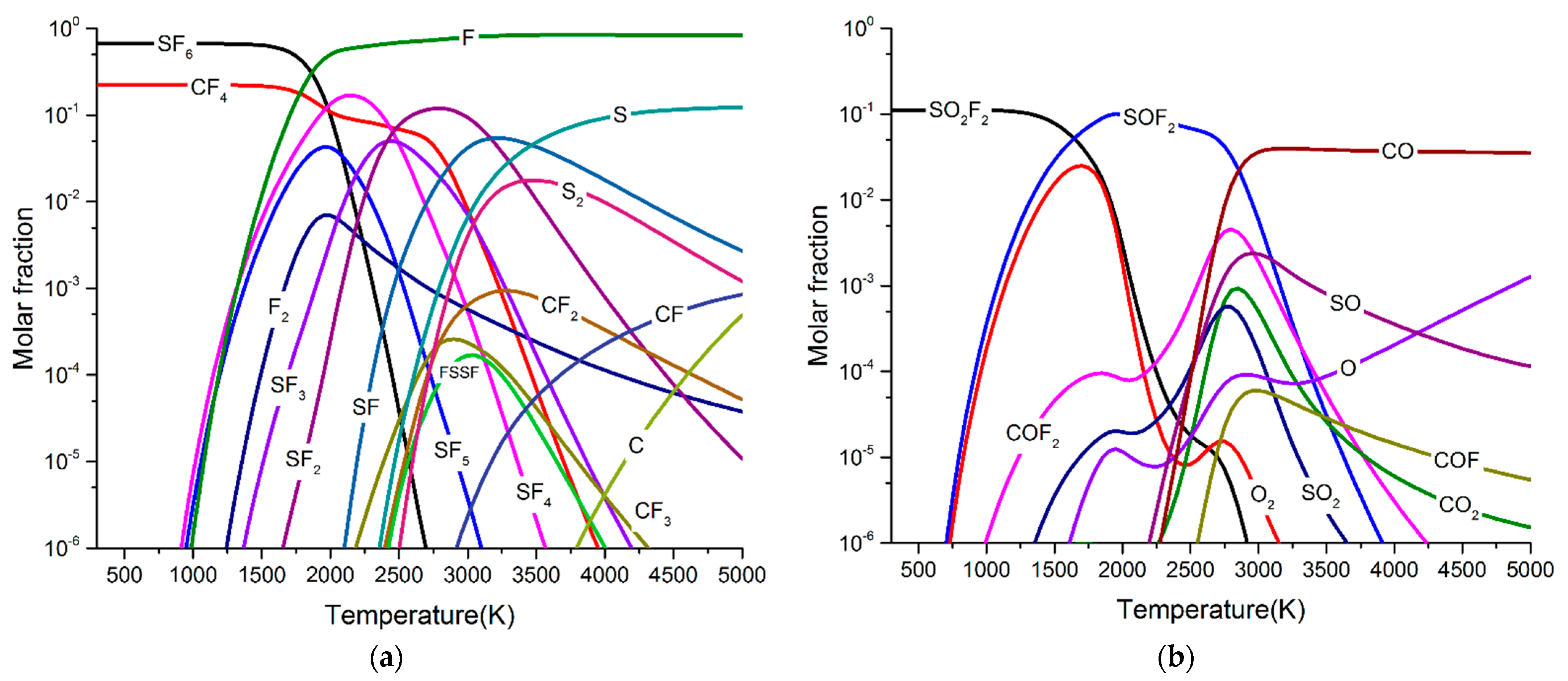
- When O2 was involved in the arc plasma, the main by-products were SOF2, SO2, and SO2F2. At the same time, a small amount of CO, and CO2 was produced. After five breaking experiments, the concentration of SOF2 can reach 1100 ppm, and the concentrations of SO2 and SO2F2 were both about 10 ppm, while the concentration of CO was lower than 2 ppm.
- (4)
- When H2O was involved in the arc plasma, the main by-products were SOF2, SO2, SO2F2, and HF. At the same time, a small amount of CO2, CO, and H2 was produced. After five breaking experiments, the concentration of SOF2 can reach 1200 ppm, and the concentration of SO2 and CO2 was lower than 20 ppm, while the concentration of CO was lower than 2 ppm. Contrary to the simulation results, SO2F2 and HF were hardly detected in this experiment.
- (5)
- When H2O and O2 impurities and PTFE vapour were involved in the arc plasma together, the main by-products were SOF2, CF4, SO2F2, and HF. At the same time, a small amount of SO2, CO2, CO, and H2 was be produced.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chu, F.Y. SF6 Decomposition in Gas-Insulated Equipment. IEEE Trans. Electr. Insul. 1986, 21, 693–725. [Google Scholar] [CrossRef]
- Shea, J.J. High voltage circuit breakers-design and applications. IEEE Electr. Insul. Mag. 1998, 14, 42. [Google Scholar] [CrossRef]
- Xu, M.; Yan, J. Theoretical study of the chemical reaction mechanisms and reaction rates of CFx + SFy (where x = 1–3 and y = 1–6) in SF6–polytetrafluoroethylene arc plasma. J. Appl. Phys. 2019, 126, 193304. [Google Scholar] [CrossRef]
- Xu, M.; Yan, J. Theoretical study of the chemical reaction mechanism and rate of SFn− + H2O (n = 3–6) under discharge. AIP Adv. 2020, 10, 095214. [Google Scholar] [CrossRef]
- Fu, Y.; Rong, M.; Yang, K. Calculated rate constants of the chemical reactions involving the main byproducts SO2F, SOF2, SO2F2 of SF6 decomposition in power equipment. J. Phys. D Appl. Phys. 2016, 49, 155502. [Google Scholar] [CrossRef]
- Fu, Y.; Yang, A.; Wang, X. Theoretical study of the neutral decomposition of SF6 in the presence of H2O and O2 in discharges in power equipment. J. Phys. D Appl. Phys. 2016, 49, 385203. [Google Scholar] [CrossRef]
- Tang, J.; Liu, J.; Zhang, X. Partial discharge recognition through an analysis of SF6 decomposition products part 1: Decomposition characteristics of SF6 under four different partial discharges. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 29–36. [Google Scholar] [CrossRef]
- Zeng, F.; Wu, S. SF6 fault decomposition feature component extraction and triangle fault diagnosis method. IEEE Trans. Dielectr. Electr. Insul. 2020, 27, 581–589. [Google Scholar] [CrossRef]
- Tang, J.; Rao, X.; Zeng, F. Influence Mechanisms of Trace H2O on the Generating Process of SF6 Spark Discharge Decomposition Components. Plasma Chem. Plasma Proc. 2017, 37, 325–340. [Google Scholar] [CrossRef]
- Boudene, C. Identification and study of some properties of compounds resulting from the decomposition of SF6 under the effect of electrical arcing in circuit-breakers. Rev. Gen. Electr. 1974, 1, 45–78. [Google Scholar]
- Belmadani, B.; Casanovas, J.; Casanovas, A.M.; Grob, R. SF6 decomposition under power arcs. I. Physical aspects. IEEE Trans. Electr. Insul. 1991, 26, 1163–1176. [Google Scholar] [CrossRef]
- Belmadani, B.; Casanovas, J.; Casanovas, A.M. SF6decomposition under power arcs. II. Chemical aspects. IEEE Trans. Electr. Insul. 1991, 26, 1177–1182. [Google Scholar] [CrossRef]
- Yang, R.; Xu, M. Influence of trace H2O and O2 on SF6 decomposition products under arcing conditions in electric power equipment. J. Eng. 2019, 16, 1774–1777. [Google Scholar] [CrossRef]
- Andrzej, P. Generation of negative ions from SF6 gas by means of hot surface ionization. Mass Spectrom. 2012, 26, 577–582. [Google Scholar]
- Brand, K.P.; Kopainsky, J. Particle densities in a decaying SF6 plasma. Appl. Phys. 1978, 16, 425–432. [Google Scholar] [CrossRef]
- Chervy, B.; Gleizes, A.; Razafinimanana, M. Thermodynamic properties and transport coefficients in SF6-Cu mixtures at temperatures of 300–30000 K and pressures of 0.1–1 MPa. J. Phys. D Appl. Phys. 1994, 27, 1193–1206. [Google Scholar] [CrossRef]
- Coufal, O. Composition and thermodynamic properties of SF6-C-(C2F4)n-CaF2-Cu-W reacting mixtures. J. Phys. D Appl. Phys. 1998, 31, 2025–2039. [Google Scholar] [CrossRef]
- Weizong, W.; Murphy, A.B.; Mingzhe, R. Investigation on critical breakdown electric field of hot sulfur hexafluoride/carbon tetrafluoride mixtures for high voltage circuit breaker applications. J. Appl. Phys. 2013, 114, 103301. [Google Scholar]
- Chase, M.W.; Davies, M.C.A.; Downey, J.R. JANAF thermochemical tables third edition. J. Phys. Chem. Ref. Data 1985, 14 (Suppl. 1). [Google Scholar]
- Wang, X.; Gao, Q.; Fu, Y. Dominant particles and reactions in a two-temperature chemical kinetic model of a decaying SF6 arc. J. Phys. D Appl. Phys. 2016, 49, 105502–105519. [Google Scholar] [CrossRef]








| Phase | H2O Concentration | Breaking Current | Breaking Times |
|---|---|---|---|
| A | 106 ppm | ~10 kA | 5 |
| B | 748 ppm | ~10 kA | 5 |
| C | 1131 ppm | ~10 kA | 5 |
| Phase | O2 Concentration | Breaking Current | Breaking Times |
| A | 59 ppm | ~10 kA | 5 |
| B | 736 ppm | ~10 kA | 5 |
| C | 1202 ppm | ~10 kA | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, R.; Xu, M.; Yan, J.; Yang, M.; Geng, Y.; Liu, Z.; Wang, J. Decomposition Characteristics of SF6 under Arc Discharge and the Effects of Trace H2O, O2, and PTFE Vapour on Its By-Products. Energies 2021, 14, 414. https://doi.org/10.3390/en14020414
Yang R, Xu M, Yan J, Yang M, Geng Y, Liu Z, Wang J. Decomposition Characteristics of SF6 under Arc Discharge and the Effects of Trace H2O, O2, and PTFE Vapour on Its By-Products. Energies. 2021; 14(2):414. https://doi.org/10.3390/en14020414
Chicago/Turabian StyleYang, Ren, Mengyuan Xu, Jing Yan, Minghao Yang, Yingsan Geng, Zhiyuan Liu, and Jianhua Wang. 2021. "Decomposition Characteristics of SF6 under Arc Discharge and the Effects of Trace H2O, O2, and PTFE Vapour on Its By-Products" Energies 14, no. 2: 414. https://doi.org/10.3390/en14020414
APA StyleYang, R., Xu, M., Yan, J., Yang, M., Geng, Y., Liu, Z., & Wang, J. (2021). Decomposition Characteristics of SF6 under Arc Discharge and the Effects of Trace H2O, O2, and PTFE Vapour on Its By-Products. Energies, 14(2), 414. https://doi.org/10.3390/en14020414





