Evaluation of Surfactant Mixture for Supercritical Carbon Dioxide Foamed Acid in Carbonate Matrix Acidizing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Apparatus
2.2. Material
2.3. Experimental Procedure
3. Results
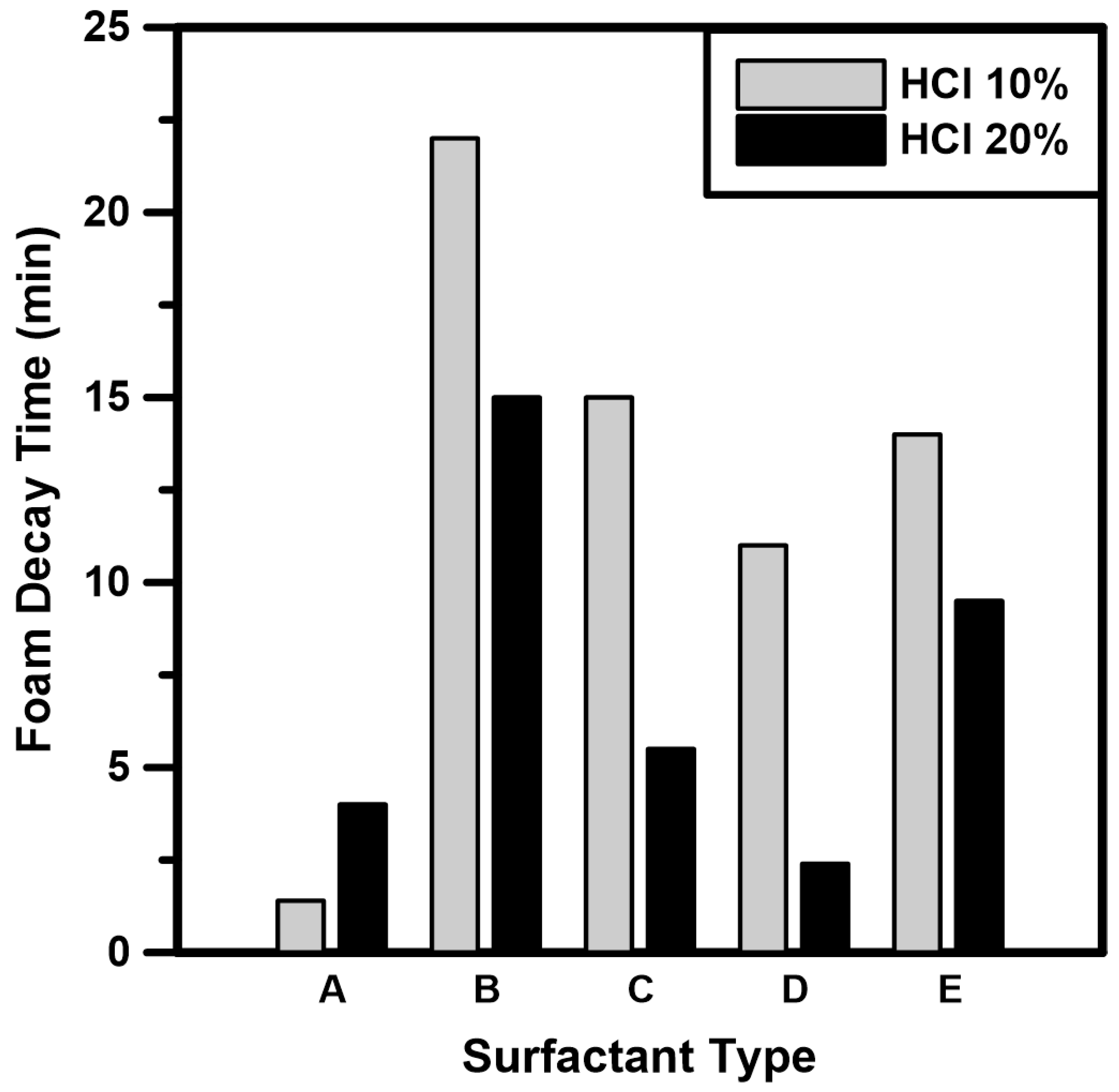
3.1. Surfactant Screening Tests
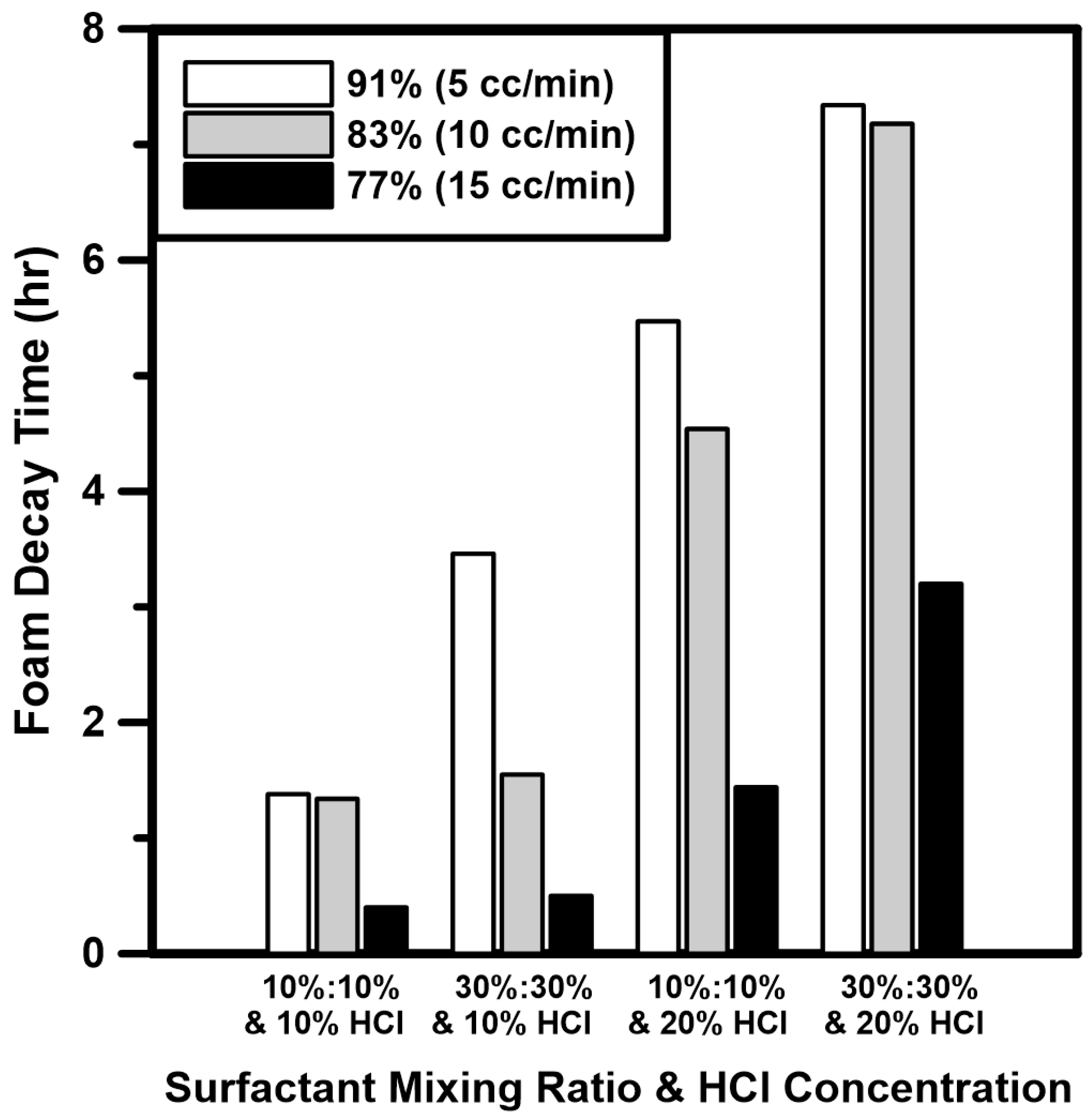
3.2. Foam Stability Analysis of Supercritical CO2 Foamed Acid
3.3. Wormhole-Propagation Tests
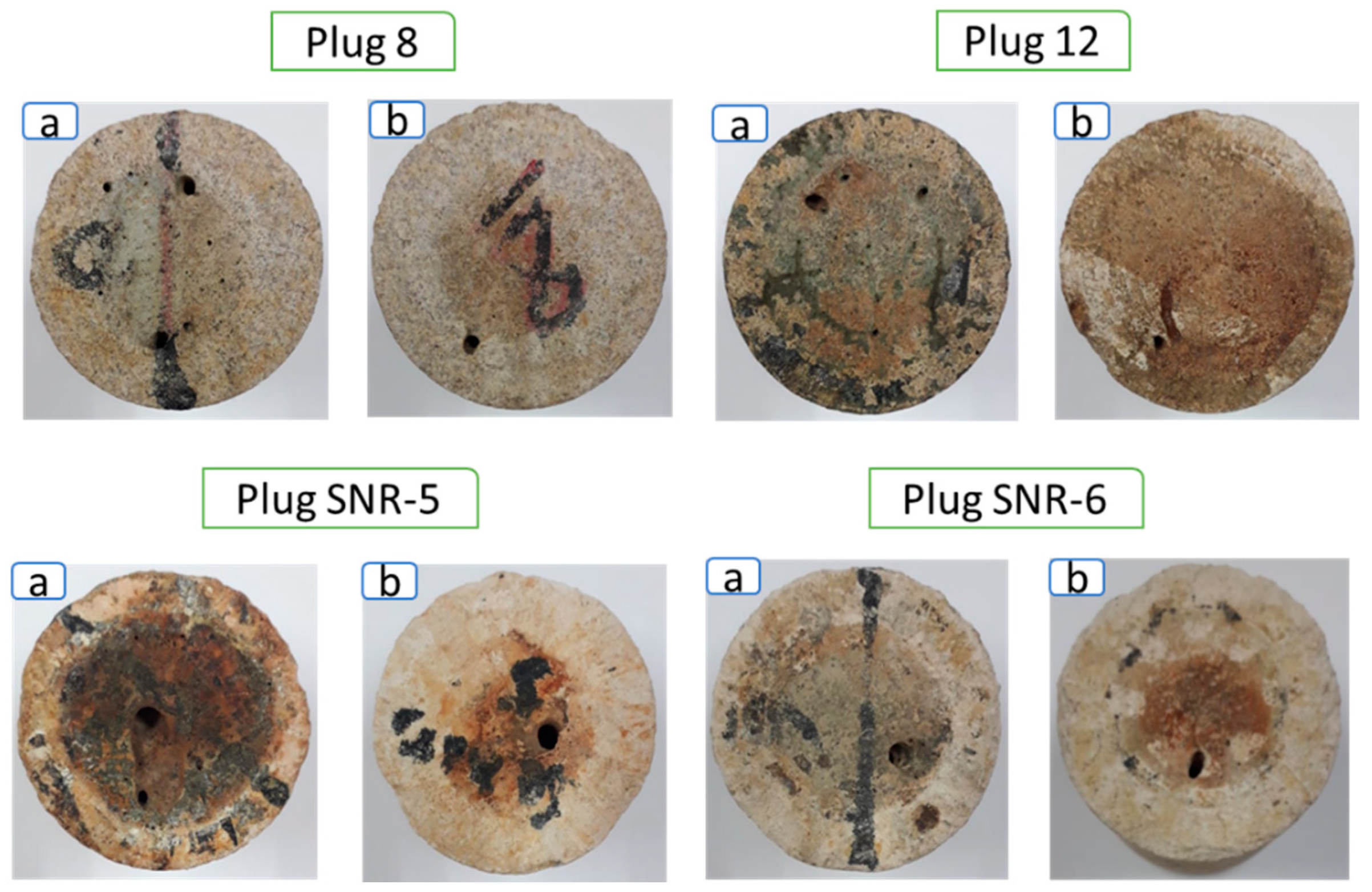
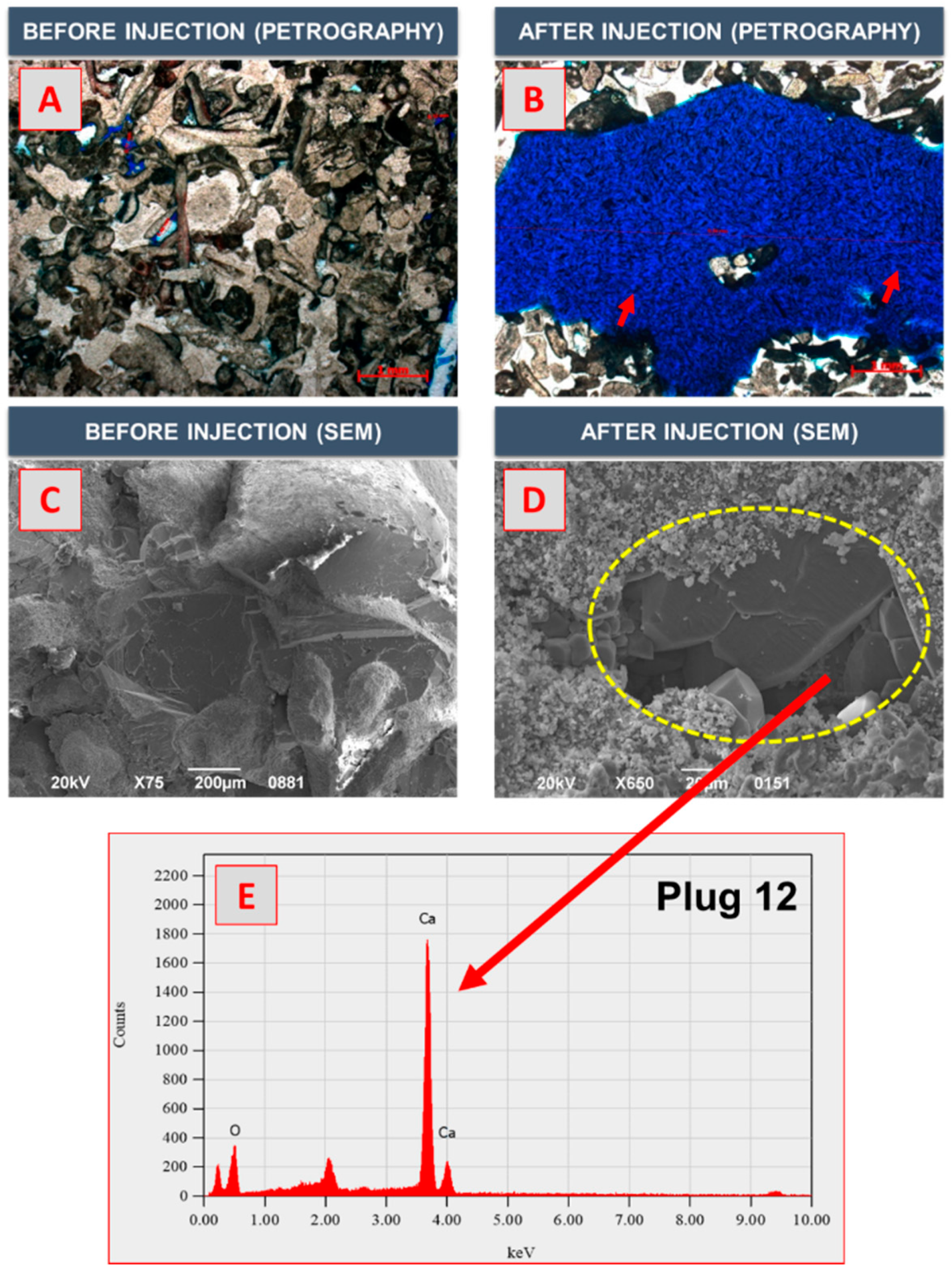
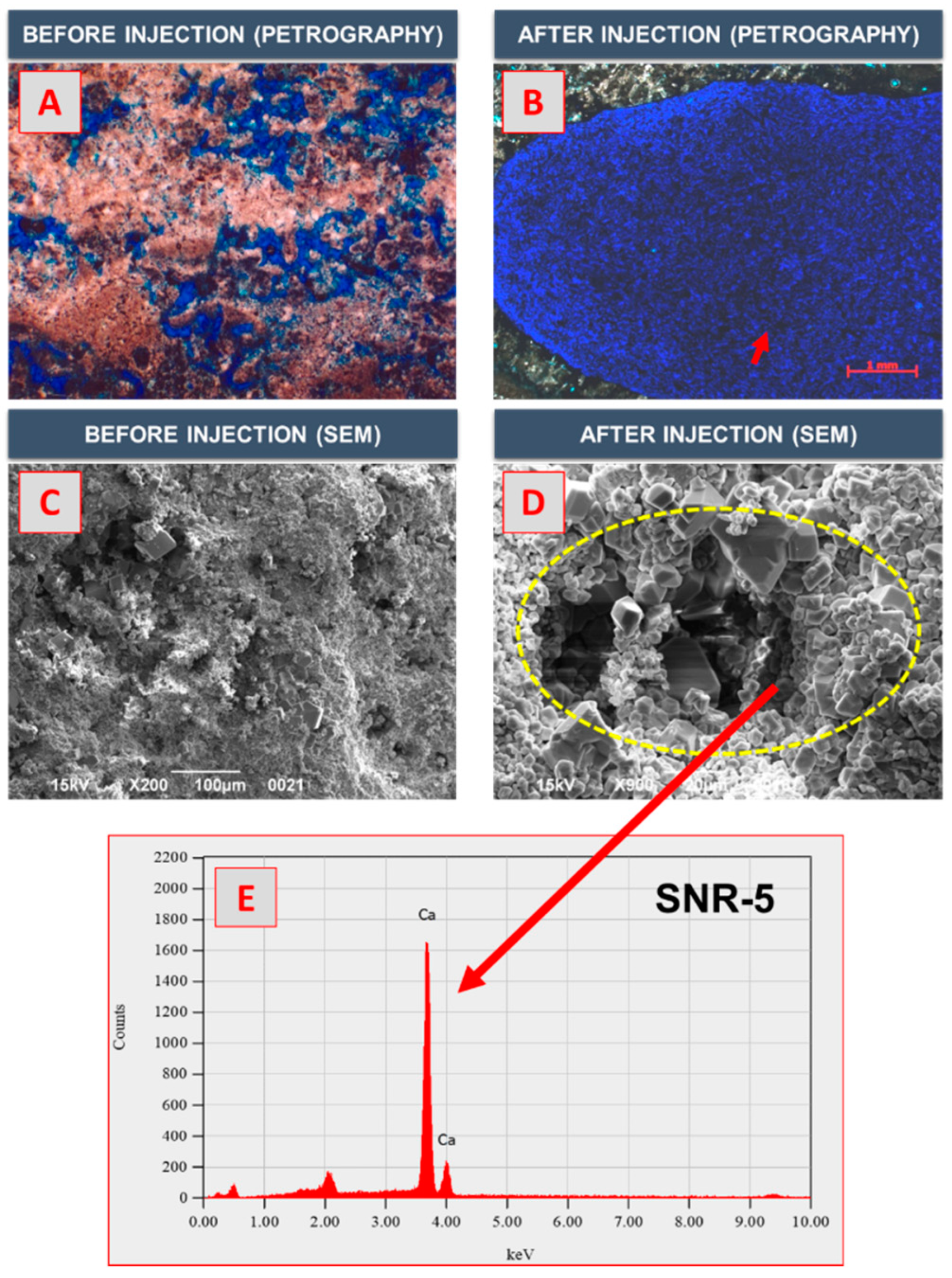
3.4. SEM–EDX and Thin-Section Analyses
4. Conclusions
- (1)
- The foam decay times of five types of surfactants were measured under atmospheric conditions. Based on the surfactant screening test results, Surfactant B (nonionic surfactant) and Surfactant E (nonionic-amphoteric surfactant) exhibited high stability because the nonionic surfactant had no charge on the hydrophilic end. Therefore, the surfactant mixture prepared by mixing two surfactants can improve the foam stability of CO2 foamed acid.
- (2)
- Foam quality tests of CO2 foamed acid using a surfactant mixture were performed under HPHT conditions. The HCl concentration and surfactant mixing ratio had a positive influence on the foam stability. In the case of foam quality, foam stability was high because the fine foam texture was produced at low injection rate of HCl. From the analysis results, the foam stability was considerably improved by adding the surfactant mixture to an HCl concentration of 20%.
- (3)
- The wormhole-propagation tests were conducted using Indiana and Indonesia limestones. After the injection of CO2 foamed acid, the permeabilities of the limestone cores significantly increased as wormholes were formed. While the CO2 foamed acid flowed from the inlet to the outlet of the core, the diameter of the wormhole gradually decreased due to the reduction of acid dissolution rate. The PVBT analysis results showed that the supercritical CO2 foamed acid was more efficient in cores with low permeabilities.
- (4)
- The results of the SEM–EDX and thin-section analyses revealed that as the number of large pores with pore sizes of ≥0.5 mm increased, the maximum pore size increased by a factor of 33 on average. Therefore, the supercritical CO2 foamed acid with surfactant mixture exhibited a high efficiency of matrix acidizing. Further research is required to optimize the injection rate of CO2 foamed acid by conducting experiments on the influencing factors of the PVBT by using the developed experimental apparatus.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yoo, H.; Kim, Y.; Lee, W.; Lee, J. An experimental study on acid-rock reaction kinetics using dolomite in carbonate acidizing. J. Pet. Sci. Eng. 2018, 168, 478–494. [Google Scholar] [CrossRef]
- Liu, X.; Ormond, A.; Bartko, K.; Li, Y.; Ortoleva, P. A geochemical reaction-transport simulator for matrix acidizing analysis and design. J. Pet. Sci. Eng. 1997, 17, 181–196. [Google Scholar] [CrossRef]
- Bernadiner, M.G.; Thompson, K.E.; Fogler, H.S. Effect of foams used during carbonate acidizing. SPE Prod. Eng. 1992, 7, 363–370. [Google Scholar] [CrossRef]
- Lund, K.; Fogler, H.S.; McCune, C.C.; Ault, J.W. Acidization-II. The dissolution of calcite in hydrochloric acid. Chem. Eng. Sci. 1975, 30, 825–835. [Google Scholar] [CrossRef] [Green Version]
- Gomaa, A.M.; Cutler, J.; Qu, Q.; Cawiezel, K.E. Acid placement: An effective VES system to stimulate high-temperature carbonate formations. In Proceedings of the SPE International Production and Operations Conference and Exhibition, Doha, Qatar, 14–16 May 2012. [Google Scholar]
- Zhang, L.; He, J.; Wang, H.; Li, Z.; Zhou, F.; Mou, J. Experimental investigation on wormhole propagation during foamed-VES acidizing. J. Pet. Sci. Eng. 2021, 198, 108139. [Google Scholar] [CrossRef]
- Yuan, H.; Chen, X.; Li, N.; Zhou, H.; Gong, Y.; Wang, Y. Numerical simulation of foam diversion acidizing in heterogeneous reservoirs. Petroleum 2021, in press. [Google Scholar] [CrossRef]
- Alawi, M.B.; Hassan, A.; Aljawad, M.S.; Kamal, M.S.; Mahmoud, M.; Al-Nakhli, A. A novel approach to improve acid diversion in carbonate rocks using thermochemical fluids: Experimental and numerical study. Molecules 2020, 25, 2976. [Google Scholar] [CrossRef]
- Buijse, M.A.; Van Domelen, M.S. Novel application of emulsified acids to matrix stimulation of heterogeneous formations. SPE Prod. Oper. 2000, 15, 208–213. [Google Scholar] [CrossRef]
- Eoff, L.; Dalrymple, D.; Reddy, B.R. Development of associative polymer technology for acid diversion in sandstone and carbonate lithology. SPE Prod. Oper. 2004, 20, 250–256. [Google Scholar] [CrossRef]
- Lungwitz, B.; Fredd, C.; Brady, M.; Miller, M.; Ali, S.; Hughes, K. Diversion and Cleanup Studies of Viscoelastic Surfactant-Based Self-Diverting Acid. SPE Prod. Oper. 2007, 22, 121–127. [Google Scholar] [CrossRef]
- Nasr-El-Din, H.A.; Al-ghamdi, A.H.; Al-Qahtani, A.A.; Samuel, M.M. Impact of acid additives on the rheological properties of viscoelastic surfactants and their influence on field application. SPE J. 2008, 13, 35–47. [Google Scholar] [CrossRef]
- Farajzadeh, R.; Andrianov, A.; Bruining, H.; Zitha, P.L.J. New insights into application of foam for acid diversion. In Proceedings of the SPE European Formation Damage Conference, Scheveningen, The Netherlands, 27–29 May 2009. [Google Scholar]
- Ford, W.G.F. Foamed Acid, an Effective Stimulation Fluid. J. Pet. Technol. 1981, 33, 1203–1210. [Google Scholar] [CrossRef]
- Rahmani, O. Mobility control in carbon dioxide-enhanced oil recovery process using nanoparticle-stabilized foam for carbonate reservoirs. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 550, 245–255. [Google Scholar] [CrossRef]
- Czupski, M.; Kasza, P.; Lesniakl, U. Development of selective acidizing technology for an oil field in the zechstein main dolomite. Energies 2020, 13, 5940. [Google Scholar] [CrossRef]
- Cheng, L.; Kam, S.I.; Delshad, M.; Rossen, W.R. Simulation of Dynamic Foam-Acid Diversion Processes. SPE J. 2001, 7, 316–324. [Google Scholar] [CrossRef]
- Bybee, K. Acid placement and diversion. J. Pet. Technol. 2010, 62, 53–54. [Google Scholar] [CrossRef]
- Al-Ghamdi, A.H.; Mahmoud, M.A.; Wang, G.; Hill, A.D.; Nasr-El-Din, H.A. Acid diversion by use of viscoelastic surfactants: The effects of flow rate and initial permeability contrast. SPE J. 2014, 19, 1203–1216. [Google Scholar] [CrossRef]
- Hematpur, H.; Mahmood, S.M.; Nasr, N.H.; Elraies, K.A. Foam flow in porous media: Concepts, models and challenges. J. Nat. Gas Sci. Eng. 2018, 53, 163–180. [Google Scholar] [CrossRef]
- Yan, Y.L.; Xi, Q.; Una, C.C.; He, B.C.; Wu, C.S.; Dou, L.L. A novel acidizing technology in carbonate reservoir: In-Situ formation of CO2 foamed acid and its self-diversion. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 580, 123787. [Google Scholar] [CrossRef]
- Solbakken, J.S.; Skauge, A.; Aarra, M.G. Supercritical CO2-foam—The importance of CO2 density on foams performance. In Proceedings of the SPE Enhanced Oil Recovery Conference, Kuala Lumpur, Malaysia, 2–4 July 2013; pp. 1–16. [Google Scholar]
- Karadkar, P.; Bataweel, M.; Bulekbay, A.; Alshaikh, A.A. Energized fluids for upstream production enhancement: A review. In Proceedings of the SPE Kingdom of Saudi Arabia Annual Technical Symposium and Exhibition 2018, Dammam, Saudi Arabia, 23–26 April 2018; pp. 1–26. [Google Scholar]
- Schramm, L.L.; Wassmuth, F. Foams: Basic Principles. In Foams: Fundamentals and Applications in the Petroleum Industry; American Chemical Society: Washington, DC, USA, 1994; Volume 242, pp. 3–45. [Google Scholar]
- Andrianov, A.; Farajzadeh, R.; Mahmoodi Nick, M.; Talanana, M.; Zitha, P.L.J. Immiscible foam for enhancing oil recovery: Bulk and porous media experiments. Ind. Eng. Chem. Res. 2012, 51, 2214–2226. [Google Scholar] [CrossRef]
- Almobarky, M.A.; Yousef, Z.A.I.; Schechter, D. Comparing two anionic surfactants for mobility control with Supercritical CO2 in miscible EOR. In Proceedings of the Carbon Management Technology Conference, Houston, TX, USA, 17–20 July 2017. [Google Scholar]
- Alyousef, Z.A.; Almobarky, M.A.; Schechter, D.S. Surfactant and a mixture of surfactant and nanoparticles stabilized-CO2/brine foam for gas mobility control and enhanced oil recovery. In Proceedings of the Carbon Management Technology Conference, Houston, TX, USA, 17–20 July 2017. [Google Scholar]
- Almobarky, M.; AlYousef, Z.; Schechter, D. Enhancing the foam stability using surfactants mixtures. In Proceedings of the SPE Kingdom of Saudi Arabia Annual Technical Symposium and Exhibition 2018, Dammam, Saudi Arabia, 23–26 April 2018. [Google Scholar]
- Alli, Y.F.; Damayantri, D.; Irawan, Y. The Effect of Anionic and Nonionic Co-Surfactant for Improving Solubility of Polyoxy-Based Surfactant for Chemical Flooding. Sci. Contrib. Oil Gas 2017, 40, 117–123. [Google Scholar] [CrossRef]
- Curbelo, F.D.S.; Garnica, A.I.C.; Castro Dantas, T.N.; Barros Neto, E.L. Synergism Study of Mixtures of Ionic and Nonionic Surfactants in Enhanced Oil Recovery Adsorption. Braz. J. Pet. Gas 2017, 11, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Kesarwani, H.; Saxena, A.; Mandal, A.; Sharma, S. Anionic/nonionic surfactant mixture for enhanced oil recovery through the investigation of adsorption, interfacial, rheological, and rock wetting characteristics. Energy Fuels 2021, 35, 3065–3078. [Google Scholar] [CrossRef]
- Kumari, R.; Kakati, A.; Nagarajan, R.; Sangwai, J.S. Synergistic effect of mixed anionic and cationic surfactant systems on the interfacial tension of crude oil-water and enhanced oil recovery. J. Dispers. Sci. Technol. 2019, 40, 969–981. [Google Scholar] [CrossRef]
- Osei-Bonsu, K.; Shokri, N.; Grassia, P. Foam stability in the presence and absence of hydrocarbons: From bubble- to bulk-scale. Colloids Surfaces A Physicochem. Eng. Asp. 2015, 481, 514–526. [Google Scholar] [CrossRef]
- He, K.; Xu, L. Unique mixtures of anionic/cationic surfactants: A new approach to enhance surfactant performance in liquids-rich shale reservoirs. SPE Prod. Oper. 2017, 33, 363–370. [Google Scholar] [CrossRef]
- Aveyard, R.; Binks, B.P.; Fletcher, P.D.I.; Peck, T.G.; Rutherford, C.E. Aspects of aqueous foam stability in the presence of hydrocarbon oils and solid particles. Adv. Colloid Interface Sci. 1994, 48, 93–120. [Google Scholar] [CrossRef]
- Simjoo, M.; Rezaei, T.; Andrianov, A.; Zitha, P.L.J. Foam stability in the presence of oil: Effect of surfactant concentration and oil type. Colloids Surfaces A Physicochem. Eng. Asp. 2013, 438, 148–158. [Google Scholar] [CrossRef]
- Hutchins, R.D.; Miller, M.J. A circulating-foam loop for evaluating foam at conditions of use. SPE Prod. Facil. 2005, 20, 286–294. [Google Scholar] [CrossRef]
- Alvarez, J.; Rivas, H.; Navarro, G. An Optimal Foam Quality for Diversion in Matrix-Acidizing Projects. In Proceedings of the SPE International Symposium on Formation Damage Control, Lafayette, Louisiana, 23–27 February 2000. [Google Scholar]
- Kovisuith, P. Effects of pH on Surfactant Solutions and Gas on IFT, Foaming, and Adsorption on Rock; New Mexico Institute of Mining and Technology: Socorro, NM, USA, 2009. [Google Scholar]
- Liu, Y.; Grigg, R.B.; Bai, B. Salinity, pH, and surfactant concentration effects on CO2-foam. In Proceedings of the SPE International Symposium on Oilfield Chemistry, The Woodlands, TX, USA, 2–4 February 2005; pp. 1–11. [Google Scholar]
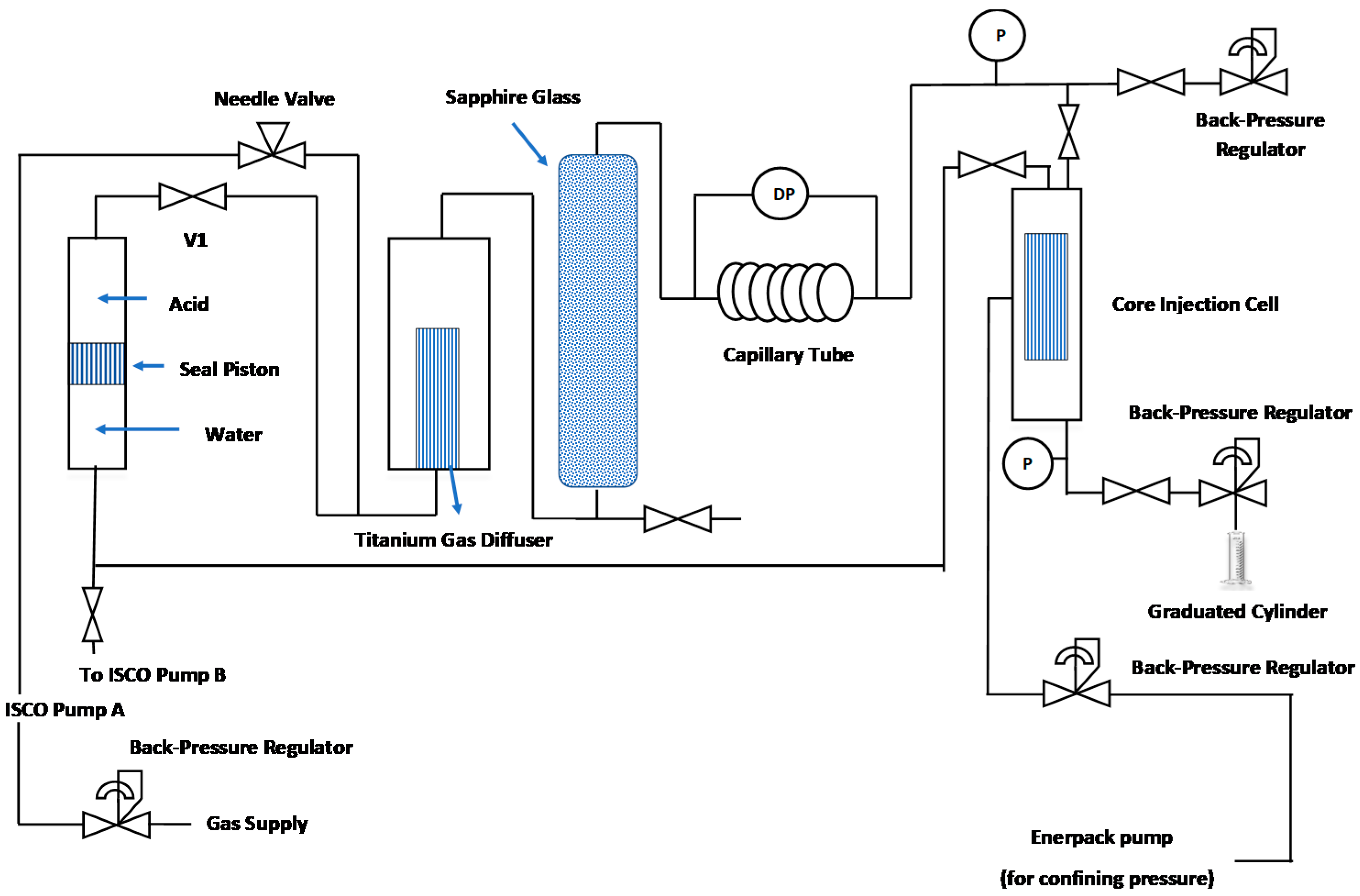




| Symbol | Model | Surfactant | Type |
|---|---|---|---|
| A | Amphitol 20 HD | Lauryl hydroxysultaine | Amphoteric |
| B | Aromox C/12 W | Coco bis-(2-hydroxyethyl) amine oxide | Nonionic |
| C | Neopelex FS | Alkyl (C10-16) benzenesulfonic acid | Anionic |
| D | Ampholak YJH 40 | Octyl iminodipropionate | Amphoteric |
| E | Trimax | Cocamidopropyl betaine, disodium cocoamphodiacetate, and amine oxide | Nonionic-Amphoteric |
| Plug. No | Location of Sampling | Diameter, cm | Length, cm | Calcite Composition, % |
|---|---|---|---|---|
| Plug 8 | Mississippian formation, USA | 3.799 | 4.697 | 99 |
| Plug 12 | Mississippian formation, USA | 3.726 | 4.246 | 99 |
| SNR-5 | Banggai Basin, Indonesia | 3.773 | 3.042 | 92 |
| SNR-6 | Banggai Basin, Indonesia | 3.792 | 4.167 | 96 |
| Parameters | Values |
|---|---|
| Pressure, psi | 1100 |
| Temperature, °C | 176 |
| HCl concentration, wt% | 10, 20 |
| Surfactant mixing ratio, %:% | 10:10, 30:30 |
| Injection rate of HCl, cc/min | 5, 10, 15 |
| Plug. No | Before Treatment | After Treatment | PVBT | ||
|---|---|---|---|---|---|
| Porosity, % | Permeability, md | Porosity, % | Permeability, md | ||
| Plug 8 | 15.40 | 9.63 | 16.40 | 38,123.00 | 7.32 |
| Plug 12 | 15.07 | 4.96 | 16.11 | 29,009.00 | 7.15 |
| SNR-5 | 24.75 | 22.04 | 26.42 | 36,032.00 | 8.12 |
| SNR-6 | 23.86 | 4.11 | 26.21 | 32,436.00 | 7.53 |
| Plug. No | Pore Size Distribution, mm | Maximum Pore Size, mm | ||||
|---|---|---|---|---|---|---|
| <0.1 | 0.1–0.25 | 0.25–0.5 | 0.5–1.0 | >1.0 | ||
| Before treatment | ||||||
| Plug 8 | 18 | 41 | 16 | 3 | 0 | 0.62 |
| Plug 12 | 36 | 19 | 12 | 1 | 0 | 0.54 |
| SNR-5 | 16 | 21 | 15 | 8 | 0 | 0.50 |
| SNR-6 | 36 | 26 | 3 | 1 | 0 | 0.25 |
| After treatment | ||||||
| Plug 8 | 18 | 46 | 18 | 4 | 3 | 22.10 |
| Plug 12 | 35 | 18 | 13 | 6 | 1 | 16.35 |
| SNR-5 | 16 | 23 | 18 | 12 | 3 | 14.2 |
| SNR-6 | 38 | 29 | 4 | 2 | 5 | 9.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kartini, R.; Kim, Y.; Lee, W. Evaluation of Surfactant Mixture for Supercritical Carbon Dioxide Foamed Acid in Carbonate Matrix Acidizing. Energies 2021, 14, 6567. https://doi.org/10.3390/en14206567
Kartini R, Kim Y, Lee W. Evaluation of Surfactant Mixture for Supercritical Carbon Dioxide Foamed Acid in Carbonate Matrix Acidizing. Energies. 2021; 14(20):6567. https://doi.org/10.3390/en14206567
Chicago/Turabian StyleKartini, Rachmi, Youngmin Kim, and Wonsuk Lee. 2021. "Evaluation of Surfactant Mixture for Supercritical Carbon Dioxide Foamed Acid in Carbonate Matrix Acidizing" Energies 14, no. 20: 6567. https://doi.org/10.3390/en14206567






