Types and Composition of Biomass in Biocoke Synthesis with the Coal Blending Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
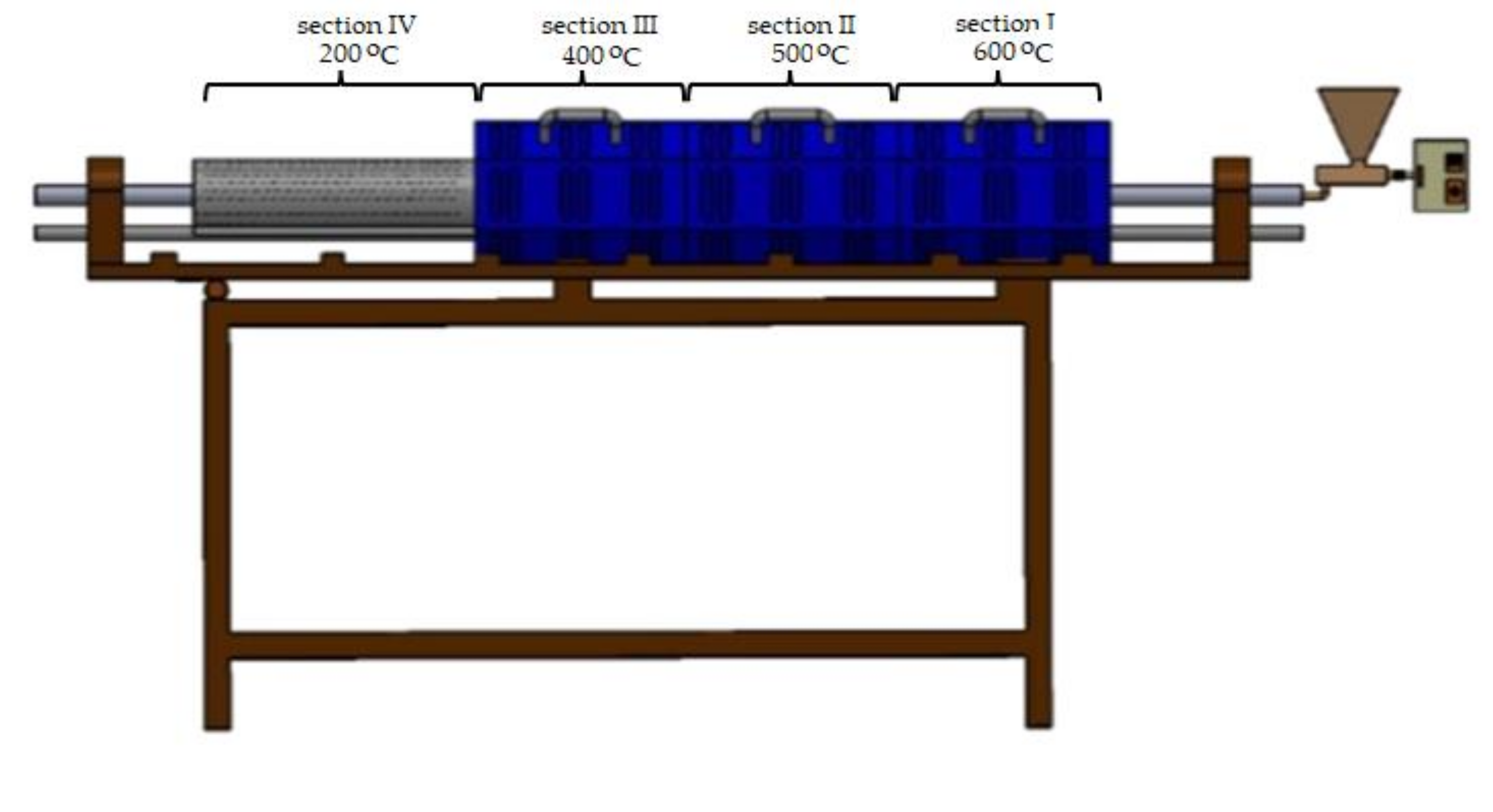
2.2.1. Biomass Carbonization
2.2.2. Coal Blending
2.3. Characterization
2.3.1. Proximate and Ultimate Analysis
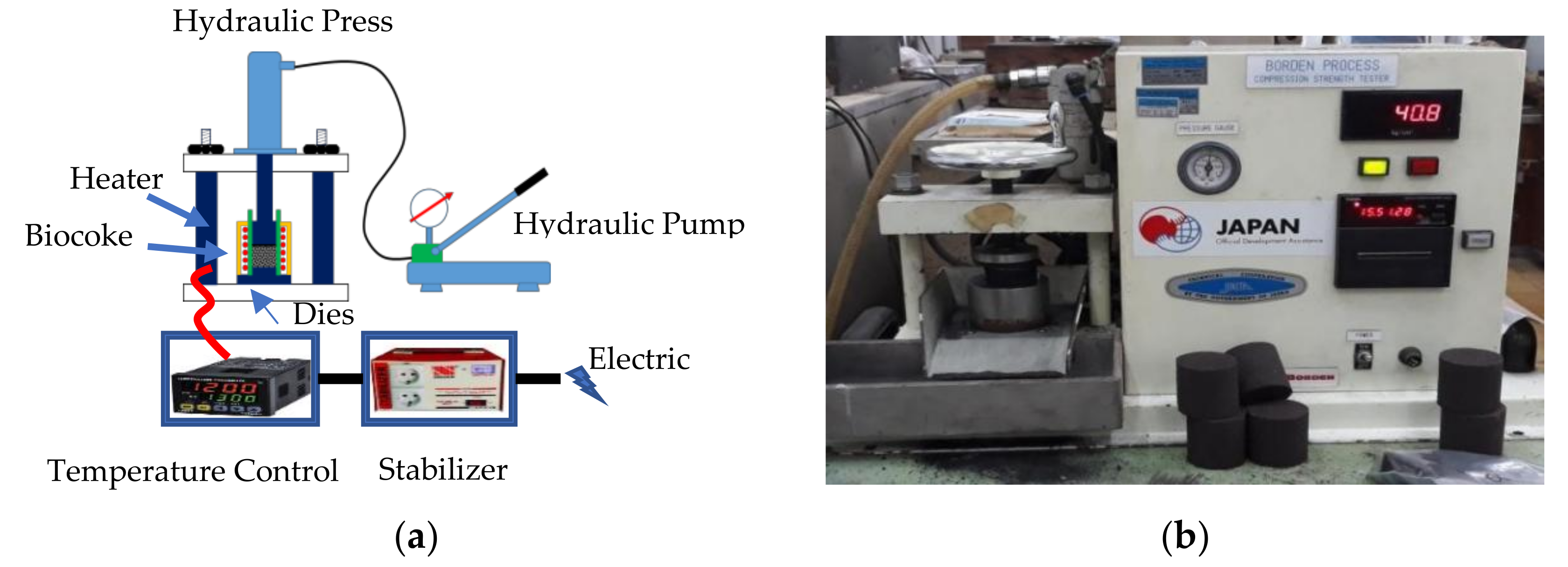
2.3.2. Mechanical Test
2.3.3. Chemical Structure
2.3.4. Morphology Analysis
3. Results and Discussion
3.1. Biochar Characteristics
3.1.1. Proximate and Ultimate Analyses
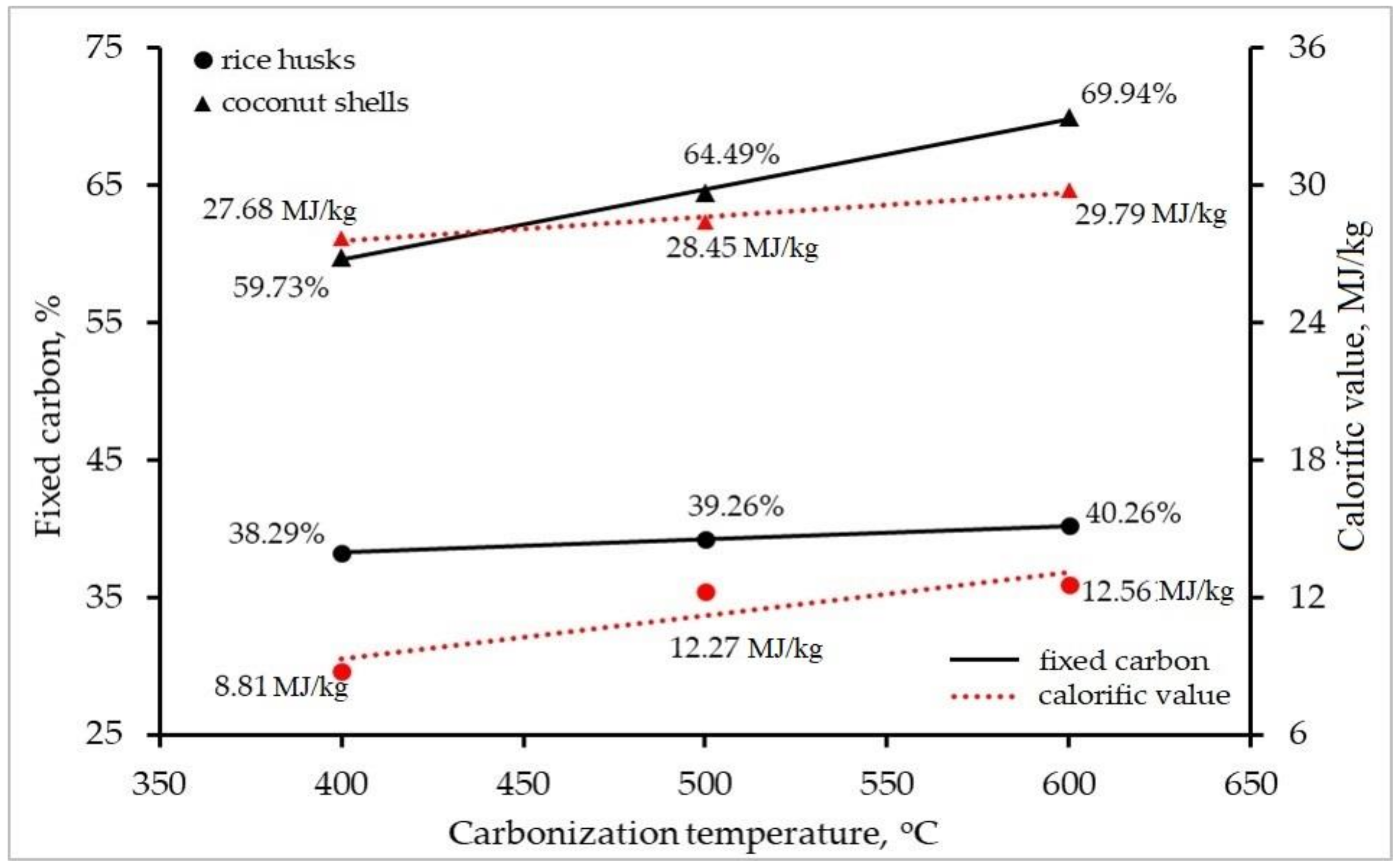
3.1.2. Carbonization
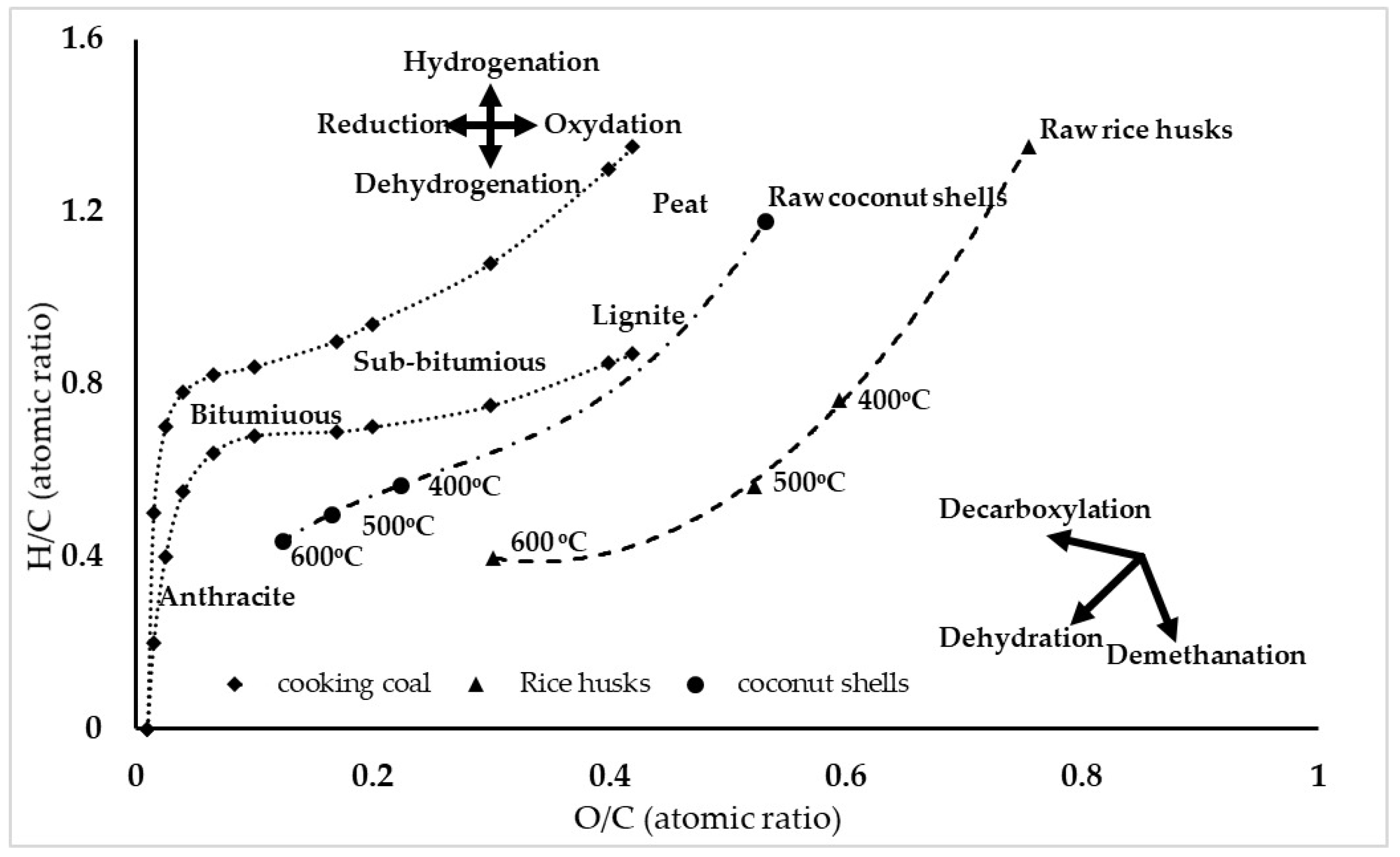
3.1.3. Coalification Band
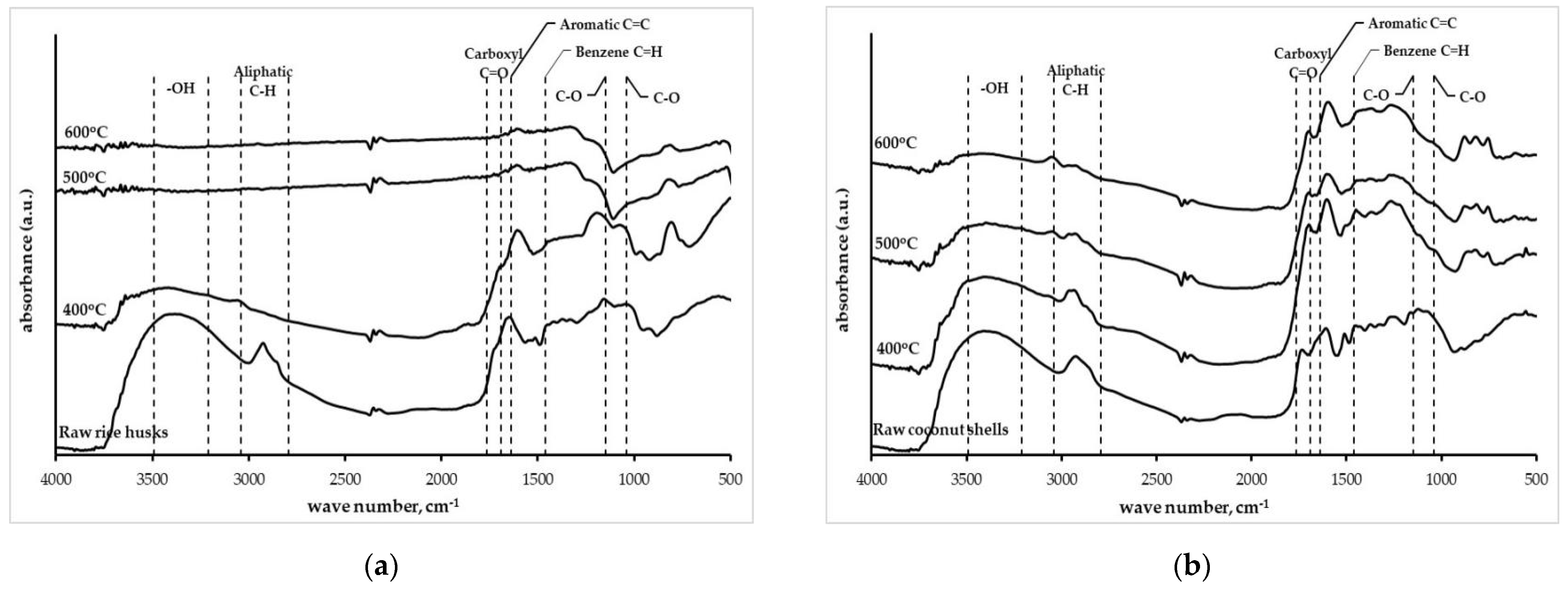
3.1.4. Functional Groups
3.2. Biocoke Characteristics
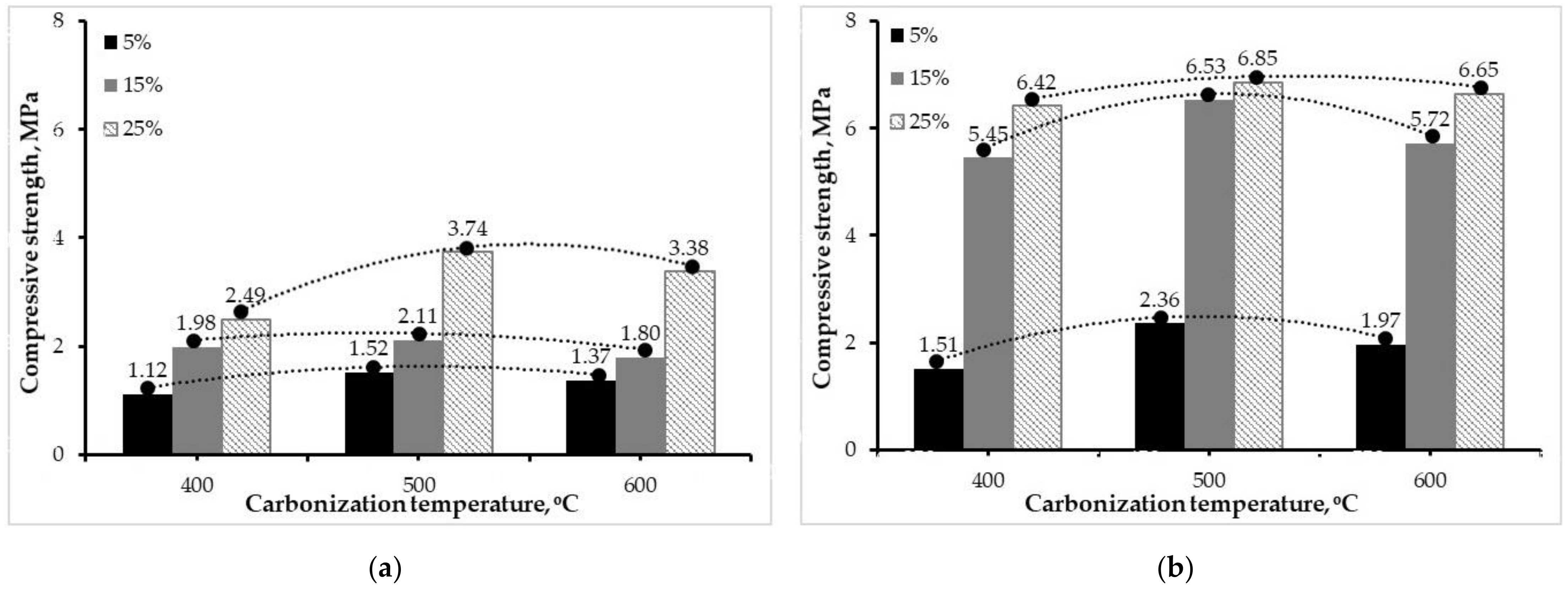
3.2.1. Compressive Strength
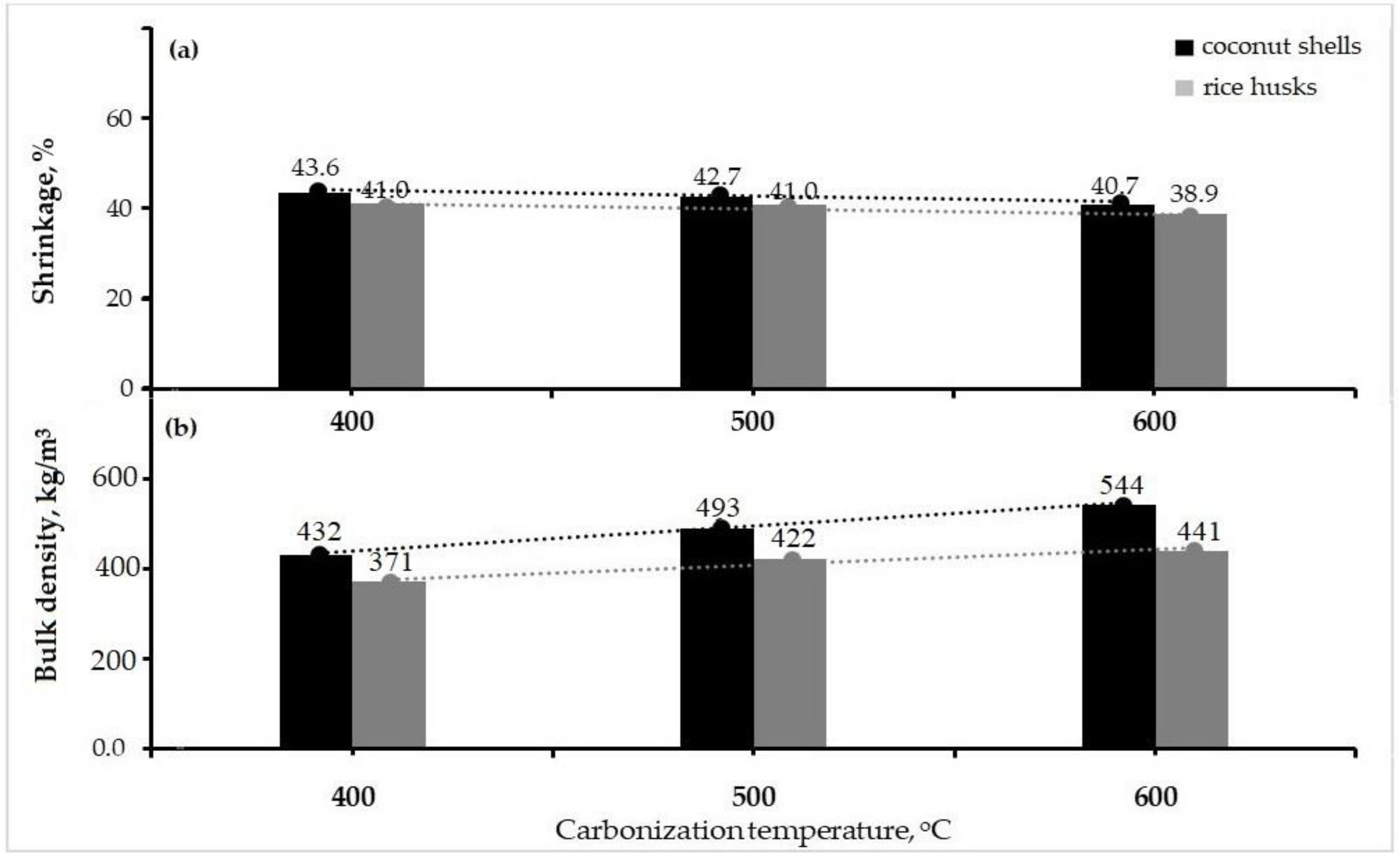
3.2.2. Mass Shrinkage and Bulk Density
3.2.3. CSR and CRI Tests
3.2.4. Thermal Rheology
3.2.5. Surface Morphology
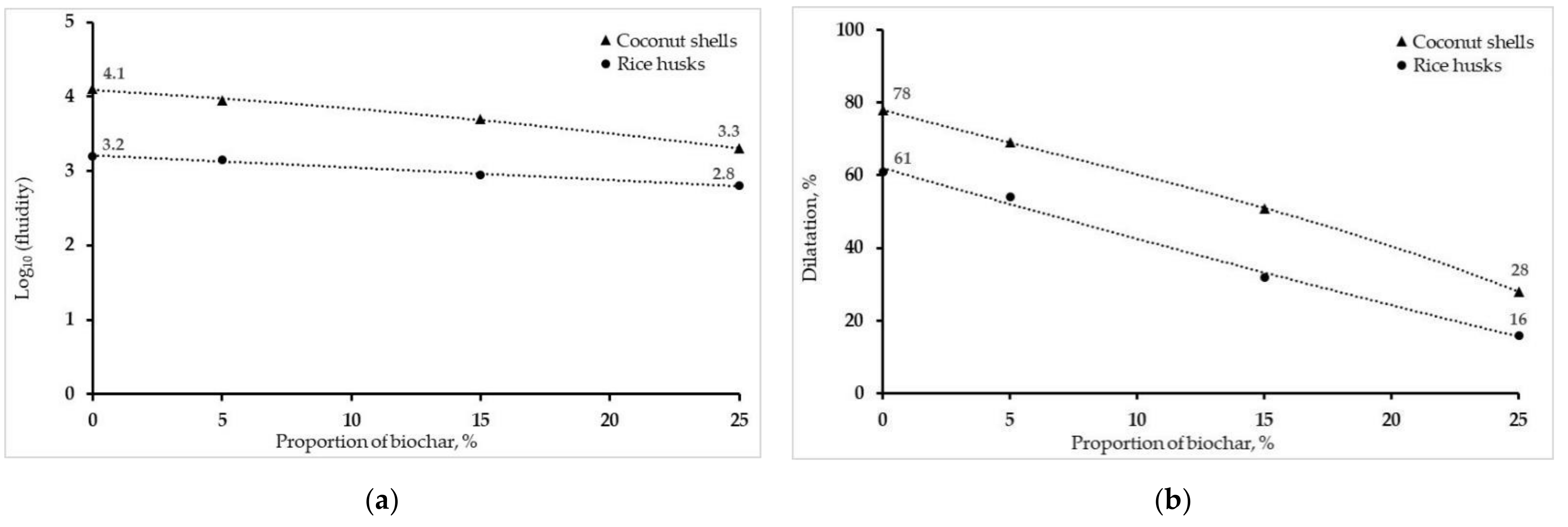
3.2.6. Rheological Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Montiano, M.G.; Diaz-Faes, E.; Barriocanal, C.; Alvarez, R. Influence of biomass on metallurgical coke quality. Fuel 2014, 116, 175–182. [Google Scholar] [CrossRef] [Green Version]
- Mousa, E.; Wang, C.; Riesbeck, J.; Larsson, M. Biomass applications in iron and steel industry: An overview of challenges and opportunities. Renew. Sustain. Energy Rev. 2016, 65, 1247–1266. [Google Scholar] [CrossRef]
- Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R. The role of renewable energy in the global energy transformation. Energy Strateg. Rev. 2019, 24, 38–50. [Google Scholar] [CrossRef]
- Perea-Moreno, M.A.; Samerón-Manzano, E.; Perea-Moreno, A.J. Biomass as renewable energy: Worldwide research trends. Sustainability 2019, 11, 863. [Google Scholar] [CrossRef] [Green Version]
- El Naggar, A.M.A.; El Sayed, H.A.; Elsalamony, R.A.; Elrazak, A.A. Biomass to fuel gas conversion through a low pyrolysis temperature induced by gamma radiation: An experimental and simulative study. RSC Adv. 2015, 5, 77897–77905. [Google Scholar] [CrossRef]
- Adrados, A.; Lopez-Urionabarrenechea, A.; Solar, J.; Requies, J.; De Marco, I.; Cambra, J.F. Upgrading of pyrolysis vapours from biomass carbonization. J. Anal. Appl. Pyrolysis 2013, 103, 293–299. [Google Scholar] [CrossRef]
- Fuchigami, Y.; Hara, K.; Kita, T.; Uwasu, M.; Kurimoto, S. Analysis of effect on CO2 emission reduction and cost estimation for the use of Bio-coke: A case study of Osaka, Japan. J. Wood Sci. 2016, 62, 93–100. [Google Scholar] [CrossRef]
- Takeshi, U.; Keiichi, O.; Hajime, A. Reduction of coke consumption in high-temperature gasifying and direct melting system. JFE Tech. Rep. 2014, 19, 145–152. [Google Scholar]
- Hanrot, F.; Sert, D.; Delinchant, J.; Pietruck, R.; Burgler, T.; Babich, A.; Fernandez, M.; Alvarez, R.; Diez, M.A. CO2 Mitigation for Steelmaking Using Charcoal and Plastics Wastes as Reducing Agents and Secondary Raw Materials. 1st Spanish National Conference on Advances in Materials Recycling and Eco–Energy, Madrid, Spain, 12–13 November; 2009. [Google Scholar]
- Gil, M.V.; Oulego, P.; Casal, M.D.; Pevida, C.; Pis, J.J.; Rubiera, F. Mechanical durability and combustion characteristics of pellets from biomass blends. Bioresour. Technol. 2010, 101, 8859–8867. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Ma, X.; Peng, X.; Yu, Z.; Fang, S.; Lin, Y.; Fan, Y. Combustion, pyrolysis and char CO2-gasification characteristics of hydrothermal carbonization solid fuel from municipal solid wastes. Fuel 2016, 181, 905–915. [Google Scholar] [CrossRef]
- Zaini, I.N.; García López, C.; Pretz, T.; Yang, W.; Jönsson, P.G. Characterization of pyrolysis products of high-ash excavated-waste and its char gasification reactivity and kinetics under a steam atmosphere. Waste Manag. 2019, 97, 149–163. [Google Scholar] [CrossRef]
- Speight, J.G. The Chesmistry and Technology of Coal; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Florentino-Madiedo, L.; Díaz-Faes, E.; Barriocanal, C. Mechanical strength of bio-coke from briquettes. Renew. Energy 2020, 146, 1717–1724. [Google Scholar] [CrossRef]
- Amaya, A.; Corengia, M.; Cuña, A.; Vivo, J.D.; Sarachik, A.; Tancredi, N. Preparation of Charcoal Pellets from Eucalyptus Wood with Different Binders. J. Energy Nat. Resour. Resour. 2015, 4, 34–39. [Google Scholar] [CrossRef] [Green Version]
- Mansor, A.M.; Theo, W.L.; Lim, J.S.; Ani, F.N.; Hashim, H.; Ho, W.S. Potential commercialisation of biocoke production in Malaysia—A best evidence review. Renew. Sustain. Energy Rev. 2018, 90, 636–649. [Google Scholar] [CrossRef]
- Ito, H.; Sakai, Y.; Ida, T.; Nakamura, Y.; Fujita, O. Combustion of Bio-coke (Highly Densified Biomass Fuel) Block in High-Temperature Air Flow. In Proceedings of the ASME/JSME 2011 8th Thermal Engineering Joint Conference AJTEC2011, Honolulu, HI, USA, 13–17 March 2011; pp. 1–10. [Google Scholar]
- Mizuno, S.; Ida, T.; Fuchihata, M.; Namba, K. Effect of specimen size on ultimate compressive strength of Bio-coke produced from green tea grounds. Mech. Eng. J. 2016, 3, 15-00441. [Google Scholar] [CrossRef]
- Yustanti, E.; Kusumawati, H.; Partuti, T.; Mursito, A. The effects of hot briquetting on the coke strength in the biocoke making process with coal blending method. Mater. Sci. Eng. 2019, 478, 012025. [Google Scholar]
- Mursito, A.T.; Muharman, A.; Yustanti, E. Producing bio-coke by redwood charcoal blending for blast furnace application. In Proceedings of the 3rd International Seminar on Metallurgy and Materials Tangerang Selatan (ISMM2019), Tangerang Selatan, Indonesia, 23–24 October 2019; Darsono, N., Thaha, Y.N., Ridhova, A., Rhamdani, A., Utomo, M.S., Ridlo, F.M., Prasetyo, M.A., Eds.; America Institute of Physics AIP: Tangerang Selatan, Indonesia, 2020; Volume 2232, p. 060004-1. [Google Scholar]
- Rejdak, M.; Robak, J.; Czardybon, A.; Ignasiak, K.; Fudała, P. Research on the Production of Composite Fuel on the Basis of Fine-Grained Coal Fractions and Biomass—The Impact of Process Parameters and the Type of Binder on the Quality of Briquettes Produced. Minerals 2020, 10, 31. [Google Scholar] [CrossRef] [Green Version]
- Brittain, H. Particle-Size Distribution, Part II The Problem of Sampling Powdered Solids. Pharm. Technol. 2002, 26, 67–73. [Google Scholar]
- Campos-M, M.; Campos-C, R. Applications of quartering method in soils and foods. Int. J. Eng. Res. Appl. 2017, 7, 35–39. [Google Scholar] [CrossRef]
- Mori, A.; Yuniati, M.D.; Mursito, A.T.; Kudo, S.; Norinaga, K.; Nonaka, M.; Hirajima, T.; Kim, H.S.; Hayashi, J.I. Preparation of coke from Indonesian lignites by a sequence of hydrothermal treatment, hot briquetting, and carbonization. Energy Fuels 2013, 27, 6607–6616. [Google Scholar] [CrossRef]
- Grover, P.D.; Mishra, S.K. Biomass Briquetting: Technology and Pratices; FAO: Rome, Italy, 1996. [Google Scholar]
- Mursito, A.T.; Hirajima, T.; Sasaki, K. Upgrading and dewatering of raw tropical peat by hydrothermal treatment. Fuel 2010, 89, 635–641. [Google Scholar] [CrossRef]
- Mursito, A.T.; Hirajima, T.; Sasaki, K. Alkaline hydrothermal de-ashing and desulfurization of low quality coal and its application to hydrogen-rich gas generation. Energy Convers. Manag. 2011, 52, 762–769. [Google Scholar] [CrossRef]
- Mursito, A.T.; Hirajima, T.; Listiyowati, L.N.; Sudarsono, S. Surface physicochemical properties of semi-anthracitic coal from Painan-Sumatra during air oxidation. Int. J. Coal Sci. Technol. 2018, 5, 156–166. [Google Scholar] [CrossRef] [Green Version]
- Mursito, A.T.; Widodo, D.N.; Arifin, D.N. Characterization of bio-coal briquettes blended from low quality coal and biomass waste treated by Garant® bio-activator and its application for fuel combustion. Int. J. Coal Sci. Technol. 2020, 7, 796–806. [Google Scholar] [CrossRef] [Green Version]
- Seo, M.W.; Jeong, H.M.; Lee, W.J.; Yoon, S.J.; Ra, H.W.; Kim, Y.K.; Lee, D.; Han, S.W.; Kim, S.D.; Lee, J.G.; et al. Carbonization characteristics of biomass/coking coal blends for the application of bio-coke. Chem. Eng. J. 2020, 394, 124943. [Google Scholar] [CrossRef]
- Kokonya, S.; Castro-Díaz, M.; Barriocanal, C.; Snape, C.E. An investigation into the effect of fast heating on fluidity development and coke quality for blends of coal and biomass. Biomass Bioenergy 2013, 56, 295–306. [Google Scholar] [CrossRef]
- Riazi, M.R.; Gupta, R. Coal Production and Processing Technology, 1st ed.; Riazi, M., Gupta, R., Eds.; CRC Press: Boca Raton, FL, USA; Taylor and Francis Group LLC: Boca Raton, FL, USA, 2016; ISBN 9781482252187. [Google Scholar]
- Azeta, O.; Ayeni, A.O.; Agboola, O.; Elehinafe, F.B. A review on the sustainable energy generation from the pyrolysis of coconut biomass. Sci. Afr. 2021, 13, e00909. [Google Scholar] [CrossRef]
- Kabir Ahmad, R.; Anwar Sulaiman, S.; Yusup, S.; Sham Dol, S.; Inayat, M.; Aminu Umar, H. Exploring the potential of coconut shell biomass for charcoal production. Ain Shams Eng. J. in press. 2021. [Google Scholar] [CrossRef]
- Promdee, K.; Chanvidhwatanakit, J.; Satitkune, S.; Boonmee, C.; Kawichai, T.; Jarernprasert, S.; Vitidsant, T. Characterization of carbon materials and differences from activated carbon particle (ACP) and coal briquettes product (CBP) derived from coconut shell via rotary kiln. Renew. Sustain. Energy Rev. 2017, 75, 1175–1186. [Google Scholar] [CrossRef]
- Vieira, F.R.; Romero Luna, C.M.; Arce, G.L.A.F.; Ávila, I. Optimization of slow pyrolysis process parameters using a fixed bed reactor for biochar yield from rice husk. Biomass Bioenergy 2020, 132, 105412. [Google Scholar] [CrossRef]
- Jia, Y.; Shi, S.; Liu, J.; Su, S.; Liang, Q.; Zeng, X.; Li, T. Study of the effect of pyrolysis temperature on the Cd2+ adsorption characteristics of biochar. Appl. Sci. 2018, 8, 1019. [Google Scholar] [CrossRef] [Green Version]
- Singh Karam, D.; Nagabovanalli, P.; Sundara Rajoo, K.; Fauziah Ishak, C.; Abdu, A.; Rosli, Z.; Melissa Muharam, F.; Zulperi, D. An overview on the preparation of rice husk biochar, factors affecting its properties, and its agriculture application. J. Saudi Soc. Agric. Sci. 2021. [Google Scholar] [CrossRef]
- MacPhee, J.A.; Gransden, J.F.; Giroux, L.; Price, J.T. Possible CO2 mitigation via addition of charcoal to coking coal blends. Fuel Process. Technol. 2009, 90, 16–20. [Google Scholar] [CrossRef]
- Liu, X.; Feng, X.; Huang, L.; He, Y. Rapid Determination of Wood and Rice Husk Pellets’ Proximate Analysis and Heating Value. Energies 2020, 13, 3741. [Google Scholar] [CrossRef]
- Muthukrishnan, S.; Gupta, S.; Kua, H.W. Application of rice husk biochar and thermally treated low silica rice husk ash to improve physical properties of cement mortar. Theor. Appl. Fract. Mech. 2019, 104, 102376. [Google Scholar] [CrossRef]
- Nazari, M.M.; San, C.P.; Atan, N.A. Combustion performance of biomass composite briquette from rice husk and banana residue. Int. J. Adv. Sci. Eng. Inf. Technol. 2019, 9, 455–460. [Google Scholar] [CrossRef]
- Yuliah, Y.; Kartawidjaja, M.; Suryaningsih, S.; Ulfi, K. Fabrication and characterization of rice husk and coconut shell charcoal based bio-briquettes as alternative energy source. In Proceedings of the International Conference on Biomass: Technology Application, and Sustainable Development, Bogor, Indonesia, 10–11 October 2016; IOP Publishing: Bristol, UK, 2017; Volume 65, p. 012021. [Google Scholar]
- Kim, D.; Lee, K.; Bae, D.; Park, K.Y. Characterizations of biochar from hydrothermal carbonization of exhausted coffee residue. J. Mater. Cycles Waste Manag. 2017, 19, 1036–1043. [Google Scholar] [CrossRef]
- Ibarra, J.V.; Muñoz, E.; Moliner, R. FTIR study of the evolution of coal structure during the coalification process. Org. Geochem. 1996, 24, 725–735. [Google Scholar] [CrossRef]
- Grant, M.G.K.; Chaklader, A.C.D.; Price, J.T. Factors affecting the strength of blast furnace coke. Fuel 1991, 70, 181–188. [Google Scholar] [CrossRef]
- Baharin, N.S.K.; Koesoemadinata, V.C.; Nakamura, S.; Azman, N.F.; Yuzir, M.A.M.; Akhir, F.N.M.; Iwamoto, K.; Yahya, W.J.; Othman, N.; Ida, T.; et al. Production of Bio-Coke from spent mushroom substrate for a sustainable solid fuel. Biomass Convers. Biorefinery 2020, 1–10. [Google Scholar] [CrossRef]
- Arman, M.A. An experimental study of the improvement of mechanical properties of blended bio-coke.; Kindai University: Higashiōsaka, Japan, 2019. [Google Scholar]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef] [Green Version]
- Stevens, C.; Verhe, R. Renewable Bioresources Scope and Modification for Non- Food Applications. Ind. Crops Prod. 2006, 23, 223–224. [Google Scholar] [CrossRef]
- Song, Y.; Tumuluru, J.S.; Iroba, K.L.; Tabil, L.G.; Xin, M.; Meda, V. Material and operating variables affecting the physical quality of biomass briquettes. In XVIIth World Congress of the International Commission of Agricultural and Biosystems Engineering; Canadian Society for Bioengineering: Québec City, QC, Canada, 2010; pp. 13–17. [Google Scholar]
- Gendek, A.; Aniszewska, M.; Malaťák, J.; Velebil, J. Evaluation of selected physical and mechanical properties of briquettes produced from cones of three coniferous tree species. Biomass Bioenergy 2018, 117, 173–179. [Google Scholar] [CrossRef]
- Diez, M.; Alvarez, R.; Barriocanal, C. Coal for metallurgical coke production: Predictions of coke quality and future requirements for cokemaking. Coal Geol. 2002, 50, 389–412. [Google Scholar] [CrossRef]
- Sundqvist Ökvist, L.; Lundgren, M. Experiences of bio-coal applications in the blast furnace process—opportunities and limitations. Minerals 2021, 11, 863. [Google Scholar] [CrossRef]
- Zhong, Q.; Yang, Y.; Li, Q.; Xu, B.; Jiang, T. Coal tar pitch and molasses blended binder for production of formed coal briquettes from high volatile coal. Fuel Process. Technol. 2017, 157, 12–19. [Google Scholar] [CrossRef]
- Nomura, S.; Arima, T. Influence of binder (coal tar and pitch) addition on coal caking property and coke strength. Fuel Process. Technol. 2017, 159, 369–375. [Google Scholar] [CrossRef]
- Prachethan Kumar, P.; Barman, S.C.; Singh, S.; Ranjan, M. Influence of coal fluidity on coal blend and coke quality. Ironmak. Steelmak. 2008, 35, 416–420. [Google Scholar] [CrossRef]
- Diez, M.A.; Alvarez, R.; Fernández, M. Biomass derived products as modifiers of the rheological properties of coking coals. Fuel 2012, 96, 306–313. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, D.R.; Mahoney, M.R. A mechanistic model for the softening of coking coal and its use for predicting the dilatation of blends. Fuel 2015, 153, 585–594. [Google Scholar] [CrossRef]
- Ketov, A.; Rudakova, L.; Vaisman, I.; Ketov, I.; Haritonovs, V.; Sahmenko, G. Recycling of rice husks ash for the preparation of resistant, lightweight and environment-friendly fired bricks. Constr. Build. Mater. 2021, 302, 124385. [Google Scholar] [CrossRef]
- Xing, X.; Rogers, H.; Zhang, G.; Hockings, K.; Zulli, P.; Deev, A.; Mathieson, J.; Ostrovski, O. Effect of charcoal addition on the properties of a coke subjected to simulated blast furnace conditions. Fuel Process. Technol. 2017, 157, 42–51. [Google Scholar] [CrossRef]
- Rejdak, M.; Pawłowski, P.; Rodź, A.; Ignasiak, K.; Helt, I. The Study of Influence of Fine Coal Fraction Addition to Coking Blend and Its Partial Briquetting on Coke Quality Parameters. In Proceedings of the Mineral Engineering Conference (MEC 2019), Kocierz, Beskid Mały, Poland, 16–19 September 2019; IOP Publisher: Bristol, UK, 2019; Volume 641. [Google Scholar]
- Ueki, Y.; Nunome, Y.; Yoshiie, R.; Naruse, I.; Nishibata, Y.; Aizawa, S. Effect of Woody Biomass Addition on Coke Properties. ISIJ Int. 2014, 54, 2454–2460. [Google Scholar] [CrossRef] [Green Version]
- Matsumura, T.; Ichida, M.; Nagasaka, T.; Kato, K. Carbonization Behaviour of Woody Biomass and Resulting Metallurgical Coke Properties. Iron Steel Inst. Jpn. Int. 2008, 48, 572–577. [Google Scholar] [CrossRef] [Green Version]
- Kaur, A.; Roy, M.; Kundu, K. Densification of Biomass by Briquetting: A Review. Int. J. Recent Sci. Res. 2017, 8, 20561–20568. [Google Scholar] [CrossRef]


| Parameter | Value |
|---|---|
| pH (25 °C) | 7.91 |
| Density, kg/m3 | 1221 |
| Brix number, % | 81.50 |
| Polarization, % | 48.86 |
| Parameter | Raw Materials | ||
|---|---|---|---|
| Coconut Shells | Rice Husk | Coking Coal | |
| Proximate (db) | |||
| Equil. moisture (ar), % | 8.35 ± 0.79 | 9.54 ± 0.50 | 1.23 ± 0.20 |
| Volatile matter, % | 69.47 ± 2.54 | 63.75 ± 1.75 | 30.02 ± 1.16 |
| Ash, % | 12.48 ± 0.92 | 22.16 ± 0.84 | 2.28 ± 0.29 |
| Fixed carbon, % | 18.05 ± 0.96 | 14.09 ± 0.56 | 67.70 ± 1.10 |
| Gross calorific, MJ/kg | 19.05 ± 0.31 | 12.89 ± 0.26 | 33.75 ± 0.99 |
| Eff. calorific, MJ/kg | 18.87 ± 0.27 | 12.69 ± 0.36 | - |
| Ultimate (db) | |||
| Carbon, % | 53.01 ± 0.24 | 41.18 ± 0.37 | 81.07 ± 0.5 |
| Hydrogen, % | 6.25 ± 0.31 | 5.56 ± 0.38 | 5.10 ± 0.26 |
| Oxygen, % | 28.23 ± 0.32 | 31.08 ± 0.31 | 9.11 ± 0.33 |
| Nitrogen, % | ** | ** | 1.75 ± 0.08 |
| Sulfur | 0.03 ± 0.01 | 0.02 ± 0.01 | 0.69 ± 0.1 |
| Parameter | Biochar | |||||
|---|---|---|---|---|---|---|
| Rice Husks (°C) | Coconut Shells (°C) | |||||
| 400 | 500 | 600 | 400 | 500 | 600 | |
| Proximate (db) | ||||||
| Eq. moisture (ar), % | 7.71 ± 0.86 | 7.39 ± 0.84 | 7.12 ± 0.85 | 5.57 ± 0.81 | 5.6 ± 0.73 | 5.54 ± 0.66 |
| Volatile matter, % | 16.48 ± 0.45 | 15.31 ± 0.37 | 13.5 ± 0.33 | 39.08 ± 1.9 | 34.09 ± 1.5 | 28.37 ± 1.2 |
| Ash, % | 45.23 ± 1.81 | 45.43 ± 1.89 | 46.24 ± 1.4 | 1.19 ± 0.20 | 1.42 ± 0.18 | 1.69 ± 0.18 |
| Fixed carbon, % | 38.29 ± 1.26 | 39.26 ± 1.27 | 40.26 ± 1.2 | 59.73 ± 2.9 | 64.49 ± 2.1 | 69.94 ± 2.0 |
| Gross calorific, MJ/kg | 8.81 ± 0.34 | 12.27 ± 0.53 | 12.56 ± 0.2 | 27.68 ± 0.9 | 28.45 ± 0.8 | 29.79 ± 0.4 |
| Eff. calorific, MJ/kg | 12.17 ± 0.5 | 12.23 ± 0.4 | 12.53 ± 0.4 | 27.54 ± 0.9 | 28.33 ± 0.8 | 29.72 ± 0.3 |
| Ultimate (db) | ||||||
| Carbon, % | 32.44 ± 0.28 | 34.24 ± 0.26 | 39.71 ± 0.3 | 76.98 ± 0.9 | 80.91 ± 0.3 | 83.98 ± 0.3 |
| Hydrogen, % | 2.48 ± 0.37 | 1.93 ± 0.29 | 1.58 ± 0.23 | 4.35 ± 0.3 | 4.04 ± 0.29 | 3.66 ± 0.28 |
| Oxygen, % | 19.28 ± 1.4 | 17.91 ± 1.57 | 12.01 ± 0.9 | 17.22 ± 1.5 | 13.42 ± 0.8 | 10.41 ± 0.8 |
| Nitrogen, % | 0.54 ± 0.07 | 0.47 ± 0.07 | 0.45 ± 0.06 | 0.24 ± 0.0 | 0.2 ± 0.02 | 0.25 ± 0.03 |
| Sulfur, % | 0.03 ± 0.01 | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.01 |
| Analysis | Rice Husk Charcoal * | Coconut Shell Charcoal * | Unit | ||||
|---|---|---|---|---|---|---|---|
| RHB 4 | RHB 5 | RHB 6 | CSB 4 | CSB 5 | CSB 6 | ||
| Moisture (ar) | 1.21 ± 0.16 | 1.03 ± 0.14 | 1.12 ± 0.16 | 1.26 ± 0.18 | 1.01 ± 0.12 | 1.13 ± 0.11 | % |
| Volatile Matter (db) | 3.54 ± 0.28 | 3.46 ± 0.28 | 3.36 ± 0.28 | 1.79 ± 0.29 | 1.56 ± 0.26 | 1.64 ± 0.22 | % |
| Ash (db) | 29.12 ± 0.54 | 26.21 ± 0.42 | 23.20 ± 0.49 | 9.41 ± 0.25 | 9.42 ± 0.17 | 9.13 ± 0.12 | % |
| Fixed Carbon (db) | 67.34 ± 0.57 | 70.33 ± 0.60 | 73.44 ± 0.65 | 88.80 ± 0.12 | 89.02 ± 0.11 | 89.23 ± 0.14 | % |
| Gross Calorific (db) | 22.506 ± 0.54 | 23.029 ± 0.57 | 24.970 ± 0.51 | 29.648 ± 0.53 | 29.681 ± 0.46 | 30.033 ± 0.49 | MJ/kg |
| Sulfur (db) | 0.62 ± 0.02 | 0.62 ± 0.02 | 0.62 ± 0.02 | 0.68 ± 0.03 | 0.68 ± 0.02 | 0.68 ± 0.02 | % |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yustanti, E.; Wardhono, E.Y.; Mursito, A.T.; Alhamidi, A. Types and Composition of Biomass in Biocoke Synthesis with the Coal Blending Method. Energies 2021, 14, 6570. https://doi.org/10.3390/en14206570
Yustanti E, Wardhono EY, Mursito AT, Alhamidi A. Types and Composition of Biomass in Biocoke Synthesis with the Coal Blending Method. Energies. 2021; 14(20):6570. https://doi.org/10.3390/en14206570
Chicago/Turabian StyleYustanti, Erlina, Endarto Yudo Wardhono, Anggoro Tri Mursito, and Ali Alhamidi. 2021. "Types and Composition of Biomass in Biocoke Synthesis with the Coal Blending Method" Energies 14, no. 20: 6570. https://doi.org/10.3390/en14206570
APA StyleYustanti, E., Wardhono, E. Y., Mursito, A. T., & Alhamidi, A. (2021). Types and Composition of Biomass in Biocoke Synthesis with the Coal Blending Method. Energies, 14(20), 6570. https://doi.org/10.3390/en14206570






