Methane/Ammonia Radical Formation during High Temperature Reactions in Swirl Burners
Abstract
:1. Introduction
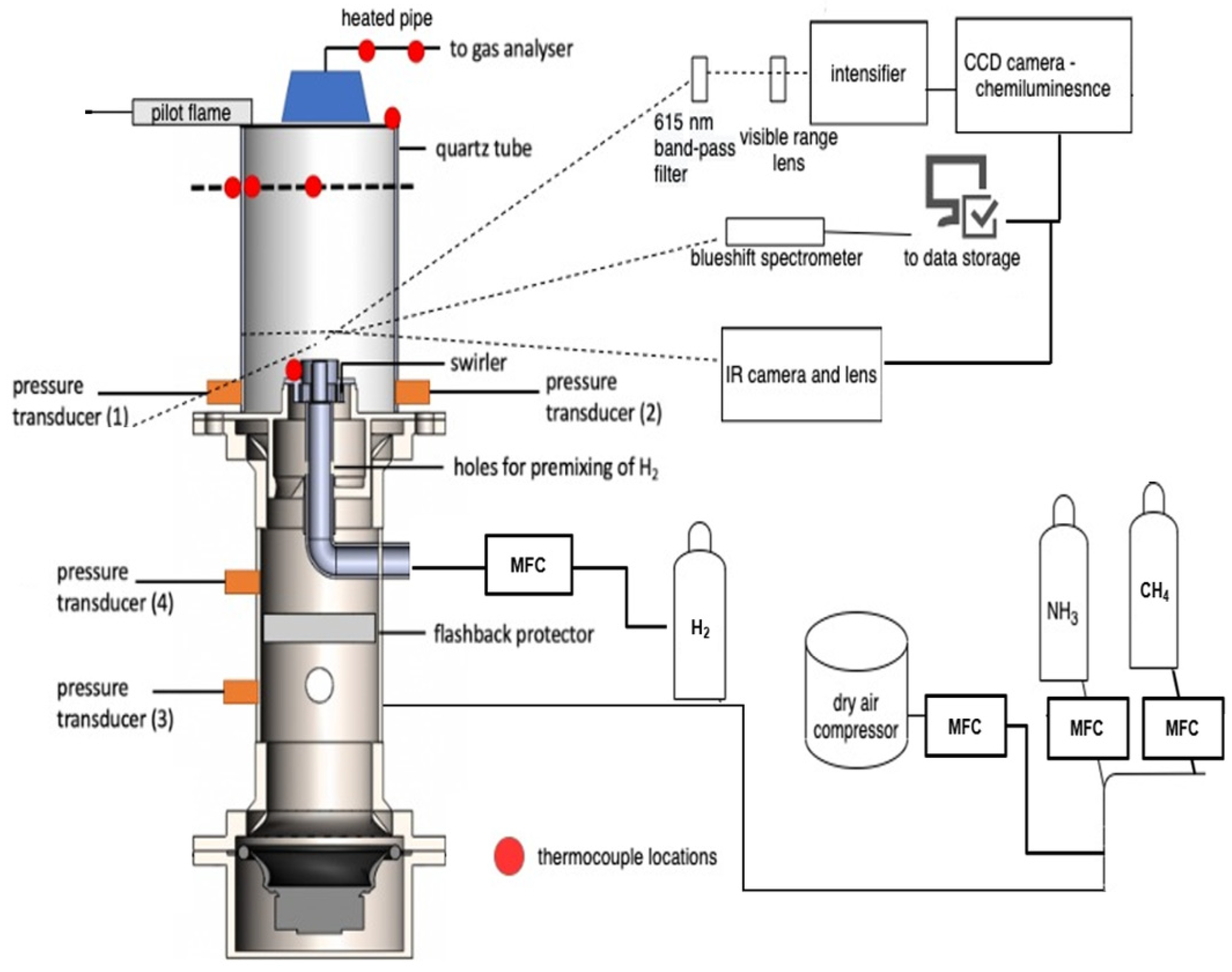
2. Methods
3. Results
4. Conclusions
- Chemiluminescence imaging of OH*, CH*, NH*, and NH2* radicals in these flames denotes complex evolution of species that can be better understood using centre of gravity (CoG) assessments;
- The presence of NH2* follows different trends to CH*, NH*, and OH*. NH2* tends to concentrate at the core of the flame under rich conditions, whilst lean conditions show a wider distribution towards the end of the flame zone;
- A blend of 40/60 (vol%) methane/ammonia shows the best potential for use in combustion applications, with the formation of large pools of radicals that can be employed for second-stage combustion. Additionally, the temperatures are sufficiently high for these blends to be employed as a substitute for fossil fuels;
- Heat release rates (HRR) need to be addressed in methane/ammonia blends using not only OH and CH signatures but also NH2, as the combination of these three molecules shows to be more in line with the high HRR produced by ammonia blends.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elishav, O.; Lis, B.M.; Miller, E.M.; Arent, D.J.; Valera-Medina, A.; Dana, A.G.; Shter, G.E.; Grader, G.S. Progress and Prospective of Nitrogen-Based Alternative Fuels. Chem. Rev. 2020, 120, 5352–5436. [Google Scholar] [CrossRef] [PubMed]
- Valera-Medina, A.; Amer-Hatem, F.; Azad, A.K.; Dedoussi, I.C.; de Joannon, M.; Fernandes, R.X.; Glarborg, P.; Hashemi, H.; He, X.; Mashruk, S.; et al. Review on Ammonia as a Potential Fuel: From Synthesis to Economics. Energy Fuels 2021, 35, 6964–7029. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Roldan, A. Ammonia from Steelworks. In Sustainable Ammonia Production; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Salmon, N.; Bañares-Alcántara, R. Green ammonia as a spatial energy vector: A review. Sustain. Energy Fuels 2021. [Google Scholar] [CrossRef]
- Bicer, Y.; Dincer, I. Life cycle assessment of ammonia utilization in city transportation and power generation. J. Clean. Prod. 2018, 170, 1594–1601. [Google Scholar] [CrossRef]
- Siddiqui, O.; Dincer, I. Experimental investigation and assessment of direct ammonia fuel cells utilizing alkaline molten and solid electrolytes. Energy 2019, 169, 914–923. [Google Scholar] [CrossRef]
- Razon, L.F.; Valera-Medina, A. A Comparative Environmental Life Cycle Assessment of the Combustion of Ammonia/Methane Fuels in a Tangential Swirl Burner. Front. Chem. Eng. 2021, 3. [Google Scholar] [CrossRef]
- Duijm, N.J.; Markert, F.; Paulsen, J.L. Safety Assessment of Ammonia as a Transport Fuel; Risø DTU National Laboratory for Sustainable Energy: Roskilde, Denmark, 2005. [Google Scholar]
- Quest Consultants Inc. Comparative Quantitative Risk Analysis of Motor Gasoline, LPG and Anhydrous Ammonia as an Automotive Fuel; Quest: Norman, OK, USA, 2009. [Google Scholar]
- Crolius, S.; Pugh, D.; Morris, S.; Valera-Medina, A. Safety Aspects. In Techno-Economic Challenges Ammon as an Energy Vector; Elsevier: Amsterdam, The Netherlands, 2021; pp. 221–257. [Google Scholar]
- Kondo, S.; Takahashi, A.; Tokuhashi, K.; Sekiya, A. RF number as a new index for assessing combustion hazard of flammable gases. J. Hazard. Mater. 2002, 93, 259–267. [Google Scholar] [CrossRef]
- NCRFP. Report 18, Marine Highway Transport of Toxic Inhalation Hazard Materials. 2012. Available online: https://www.nap.edu/catalog/22737/marine-highway-transport-of-toxic-inhalation-hazard-materials (accessed on 12 June 2021).
- Crolius, S. An Open Letter to the International Energy Agency. Ammon Energy 2019. Available online: https://www.ammoniaenergy.org/an-open-letter-to-the-international-energy-agency/ (accessed on 12 July 2019).
- De Vries, N.; Okafor, E.; Gutesa-Bozo, M.; Xiao, H.; Valera-Medina, A. Use of Ammonia for Heat, Power and Propulsion. In Techno-Economic Challenges Green Ammon as an Energy Vector; Elsevier: Amsterdam, The Netherlands, 2020; pp. 105–154. [Google Scholar] [CrossRef]
- Hayakawa, A.; Goto, T.; Mimoto, R.; Kudo, T.; Kobayashi, H. NO formation/reduction mechanisms of ammonia/air premixed flames at various equivalence ratios and pressures. Mech. Eng. J. 2015, 2, 14–00402. [Google Scholar] [CrossRef] [Green Version]
- Okafor, E.C.; Somarathne, K.K.A.; Hayakawa, A.; Kudo, T.; Kurata, O.; Iki, N.; Kobayashi, H. Towards the development of an efficient low-NOx ammonia combustor for a micro gas turbine. Proc. Combust. Inst. 2019, 37, 4597–4606. [Google Scholar] [CrossRef]
- Okafor, E.C.; Somarathne, K.K.A.; Ratthanan, R.; Hayakawa, A.; Kudo, T.; Kurata, O.; Iki, N.; Tsujimura, T.; Furutani, H.; Kobayashi, H. Control of NOx and other emissions in micro gas turbine combustors fuelled with mixtures of methane and ammonia. Combust. Flame 2020, 211, 406–416. [Google Scholar] [CrossRef]
- Pugh, D.; Medina, A.V.; Bowen, P.; Giles, A.; Goktepe, B.; Runyon, J.P.; Morris, S.; Hewlett, S.; Marsh, R. Emissions Performance of Staged Premixed and Diffusion Combustor Concepts for an NH3/Air Flame With and Without Reactant Humidification. J. Eng. Gas Turbines Power 2021, 143, 1–10. [Google Scholar] [CrossRef]
- Pugh, D.; Bowen, P.; Valera-Medina, A.; Giles, A.; Runyon, J.; Marsh, R. Influence of steam addition and elevated ambient conditions on NOx reduction in a staged premixed swirling NH3/H2 flame. Proc. Combust. Inst. 2018, 37, 5401–5409. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, X.; Qin, X.; Huang, Z. Effect of hydrogen blending on the high temperature auto-ignition of ammonia at elevated pressure. Fuel 2020, 287, 119563. [Google Scholar] [CrossRef]
- Thomas, D.; Northrop, W. Ammonia-Hydrogen Combustion in Diffusion Flames. In Proceedings of the 38th International Symposium on Combustion, Adelaide, Australia, 24–29 January 2021. [Google Scholar]
- Iki, N.; Kurata, O.; Matsunuma, T.; Inoue, T.; Suzuki, M.; Tsujimura, T.; Furutani, H. Micro Gas Turbine Firing Kerosene and Ammonia. In Proceedings of the Turbo Expo: Power for Land, Sea, and Air, Montreal, QC, Canada, 15–19 June 2015; Volume 8. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Marsh, R.; Runyon, J.; Pugh, D.; Beasley, P.; Hughes, T.; Bowen, P. Ammonia–methane combustion in tangential swirl burners for gas turbine power generation. Appl. Energy 2017, 185, 1362–1371. [Google Scholar] [CrossRef] [Green Version]
- Xiao, H.; Howard, M.; Valera-Medina, A.; Dooley, S.; Bowen, P.J. Study on Reduced Chemical Mechanisms of Ammonia/Methane Combustion under Gas Turbine Conditions. Energy Fuels 2016, 30, 8701–8710. [Google Scholar] [CrossRef]
- Khateeb, A.A.; Guiberti, T.F.; Zhu, X.; Younes, M.; Jamal, A.; Roberts, W.L. Stability limits and NO emissions of technically-premixed ammonia-hydrogen-nitrogen-air swirl flames. Int. J. Hydrogen Energy 2020, 45, 22008–22018. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Morris, S.; Runyon, J.; Pugh, D.; Marsh, R.; Beasley, P.; Hughes, T. Ammonia, Methane and Hydrogen for Gas Turbines. Energy Procedia 2015, 75, 118–123. [Google Scholar] [CrossRef] [Green Version]
- Honzawa, T.; Kai, R.; Okada, A.; Valera-Medina, A.; Bowen, P.J.; Kurose, R. Predictions of NO and CO emissions in ammonia/methane/air combustion by LES using a non-adiabatic flamelet generated manifold. Energy 2019, 186, 115771. [Google Scholar] [CrossRef]
- Montgomery, M.J.; Kwon, H.; Dreyer, J.A.; Xuan, Y.; McEnally, C.S.; Pfefferle, L.D. Effect of ammonia addition on suppressing soot formation in methane co-flow diffusion flames. Proc. Combust. Inst. 2020, 38, 2497–2505. [Google Scholar] [CrossRef]
- Stagni, A.; Cavallotti, C.; Arunthanayothin, S.; Song, Y.; Herbinet, O.; Battin-Leclerc, F.; Faravelli, T. An experimental, theoretical and kinetic-modeling study of the gas-phase oxidation of ammonia. React. Chem. Eng. 2020, 5, 696–711. [Google Scholar] [CrossRef] [Green Version]
- Mikulčić, H.; Baleta, J.; Wang, X.; Wang, J.; Qi, F.; Wang, F. Numerical simulation of ammonia/methane/air combustion using reduced chemical kinetics models. Int. J. Hydrog. Energy 2021, 46, 23548–23563. [Google Scholar] [CrossRef]
- Cai, T.; Zhao, D.; Li, X.; Shi, B.; Li, J. Mitigating NO emissions from an ammonia-fueled micro-power system with a perforated plate implemented. J. Hazard. Mater. 2020, 401, 123848. [Google Scholar] [CrossRef]
- Kujiraoka, H.; Izumi, T.; Yoshizuru, Y.; Suemasy, T.; Ueda, M.; Niki, T.; Itou, T.; Nishio, M.; Murai, R.; Akamatsu, F. Evaluation of the Cement Clinker Fired in the Combustion Furnace of Heavy-Oil and NH3. NH3 Assoc 2018. Available online: https://nh3fuelassociation.org/wp-content/uploads/2018/12/1700-549g181031_Kujiraoka_UBE_NoAPP.pdf (accessed on 24 June 2019).
- Mitsubishi Heavy Industries Group. Mitsubishi Power Commences Development of World’s First Ammonia-fired 40MW Class Gas Turbine System 2021. Available online: https://power.mhi.com/news/20210301.html (accessed on 13 August 2021).
- Zhang, F.; Chen, G.; Wu, D.; Li, T.; Zhang, Z.; Wang, N. Characteristics of Ammonia/Hydrogen Premixed Combustion in a Novel Linear Engine Generator. Proceedings 2020, 58, 2. [Google Scholar] [CrossRef]
- Dantec Dynamics. BSA Flow Software for LDA 2017. Available online: https://www.dantecdynamics.com/download-login (accessed on 28 September 2020).
- Van Assen, H.H.; Egmont-Petersen, M.; Reiber, J.J. Accurate object localization in gray level images using the center of gravity measure: Accuracy versus precision. IEEE Trans. Image Process. 2002, 11, 1379–1384. [Google Scholar] [CrossRef] [Green Version]
- Eriksson, P. The Zimont TFC Model Applied to Premixed Bluff Body Stabilized Combustion Using Four Different RANS Turbulence Models. In Proceedings of the Turbo Expo: Power for Land, Sea, and Air, Montreal, QC, Canada, 14–17 May 2007; pp. 353–361. [Google Scholar] [CrossRef]
- Baej, H.; Valera-Medina, A.; Bowen, P.; Syred, N.; O’Doherty, T.; Marsh, R. Impacts on Blowoff by a Variety of CRZs Using Various Gases for Gas Turbines. Energy Procedia 2014, 61, 1606–1609. [Google Scholar] [CrossRef] [Green Version]
- Siemens. Complex Chemistry. Star-CCM+ Doc 2019. Available online: https://documentation.thesteveportal.plm.automation.siemens.coml (accessed on 24 September 2019).
- Vigueras-Zuniga, M.-O.; Tejeda-Del-Cueto, M.-E.; Vasquez-Santacruz, J.-A.; Herrera-May, A.-L.; Valera-Medina, A. Numerical Predictions of a Swirl Combustor Using Complex Chemistry Fueled with Ammonia/Hydrogen Blends. Energies 2020, 13, 288. [Google Scholar] [CrossRef] [Green Version]
- Pugh, D.; Runyon, J.; Bowen, P.; Giles, A.; Valera-Medina, A.; Marsh, R.; Goktepe, B.; Hewlett, S. An investigation of ammonia primary flame combustor concepts for emissions reduction with OH*, NH2* and NH* chemiluminescence at elevated conditions. Proc. Combust. Inst. 2020, 38, 6451–6459. [Google Scholar] [CrossRef]
- Glarborg, P.; Dam-Johansen, K.; Miller, J.A.; Kee, R.J.; Coltrin, M.E. Modeling the thermal DENOx process in flow reactors. Surface effects and Nitrous Oxide formation. Int. J. Chem. Kinet. 1994, 26, 421–436. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Mashruk, S.; Xiao, H.; Chiong, M.-C.; Tung Chong, C. Ammonia/Hydrogen Blends for Zero-Carbon Energy. In Proceedings of the 8th International Conference on Fluid Mechanics, Virtual, 25–28 September 2018. [Google Scholar]
- Stow, S.R.; Dowling, A.P. Thermoacoustic Oscillations in an Annular Combustor. In Proceedings of the Turbo Expo: Power for Land, Sea, and Air, New Orleans, LA, USA, 4–7 June 2001. [Google Scholar] [CrossRef]











| Parameter | Value | Parameter | Value |
|---|---|---|---|
| Blends CH4/NH3 (vol%) | 20/80, 40/60, 60/40 | Quartz Temperature | 1185, 1177, 1190 K |
| Mixing | Fully pre-mixed | Burner section | Symmetry (120°) |
| Equivalence Ratios (Φ) | 0.8, 1.0 *, 1.2 | Swirler walls | Adiabatic |
| Power | 8 kW | Ignition Temperature | 3000 K |
| Inlet Temperature | 300 K | Turbulence | 10% |
| Inlet Pressure | 0.11 MPa | Walls | No-slip |
| Outlet Pressure | 0.10 MPa | Method | Segregated Flow |
| Swirl | 1.05 | Mechanisms | Okafor |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vigueras-Zúñiga, M.O.; Tejeda-del-Cueto, M.E.; Mashruk, S.; Kovaleva, M.; Ordóñez-Romero, C.L.; Valera-Medina, A. Methane/Ammonia Radical Formation during High Temperature Reactions in Swirl Burners. Energies 2021, 14, 6624. https://doi.org/10.3390/en14206624
Vigueras-Zúñiga MO, Tejeda-del-Cueto ME, Mashruk S, Kovaleva M, Ordóñez-Romero CL, Valera-Medina A. Methane/Ammonia Radical Formation during High Temperature Reactions in Swirl Burners. Energies. 2021; 14(20):6624. https://doi.org/10.3390/en14206624
Chicago/Turabian StyleVigueras-Zúñiga, Marco Osvaldo, Maria Elena Tejeda-del-Cueto, Syed Mashruk, Marina Kovaleva, Cesar Leonardo Ordóñez-Romero, and Agustin Valera-Medina. 2021. "Methane/Ammonia Radical Formation during High Temperature Reactions in Swirl Burners" Energies 14, no. 20: 6624. https://doi.org/10.3390/en14206624
APA StyleVigueras-Zúñiga, M. O., Tejeda-del-Cueto, M. E., Mashruk, S., Kovaleva, M., Ordóñez-Romero, C. L., & Valera-Medina, A. (2021). Methane/Ammonia Radical Formation during High Temperature Reactions in Swirl Burners. Energies, 14(20), 6624. https://doi.org/10.3390/en14206624








