Influence of Operation Conditions on Temperature Hazard of Lithium-Iron-Phosphate (LiFePO4) Cells
Abstract
:1. Introduction
2. Considered Application to Mining Machine
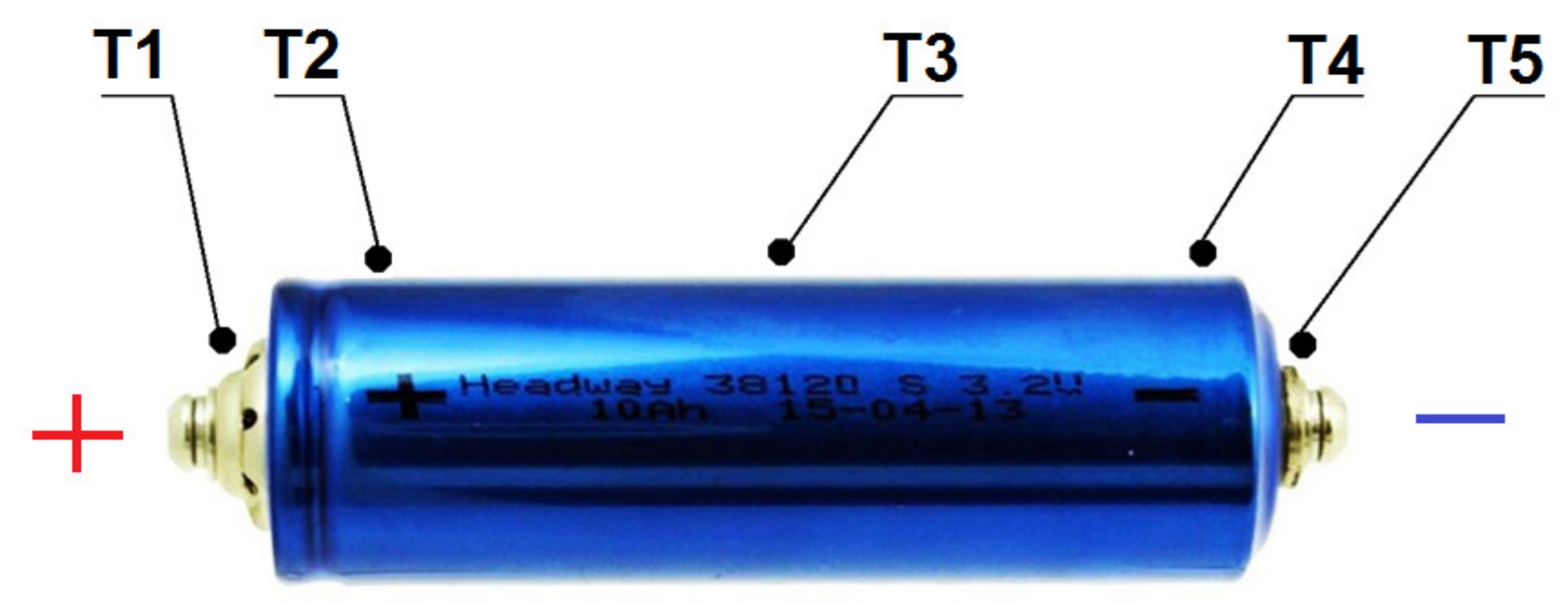
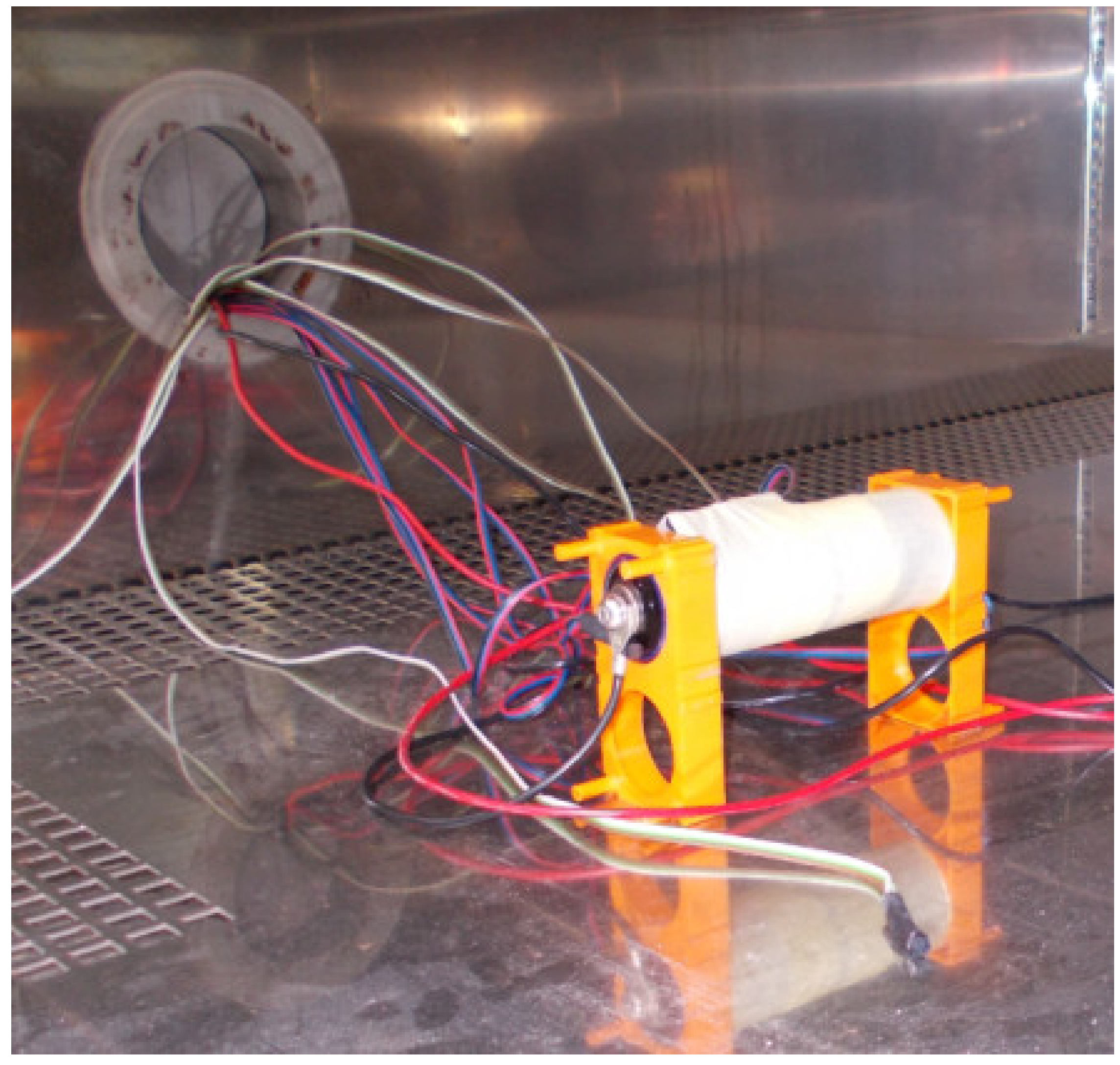
3. The Scope and Method of Conducted Research
- ▪ Voltage: 3.2 V,
- ▪ Capacity: 10 Ah,
- ▪ Internal resistance: <6 mOhm,
- ▪ Charging voltage: 3.65 ± 0.05 V,
- ▪ Energy density: 105 Wh/kg,
- ▪ Technology: lithium-iron-phosphate (LiFePO4),
- ▪ Maximum discharge voltage: 2.5–2.0 V,
- ▪ Range of operational temperatures:
- ▪ Charging: 0 ÷ 45 °C,
- ▪ Discharging: −20 ÷ 65 °C,
- ▪ Life: over 2000 cycles (80% of capacity when loading with 10 A current).
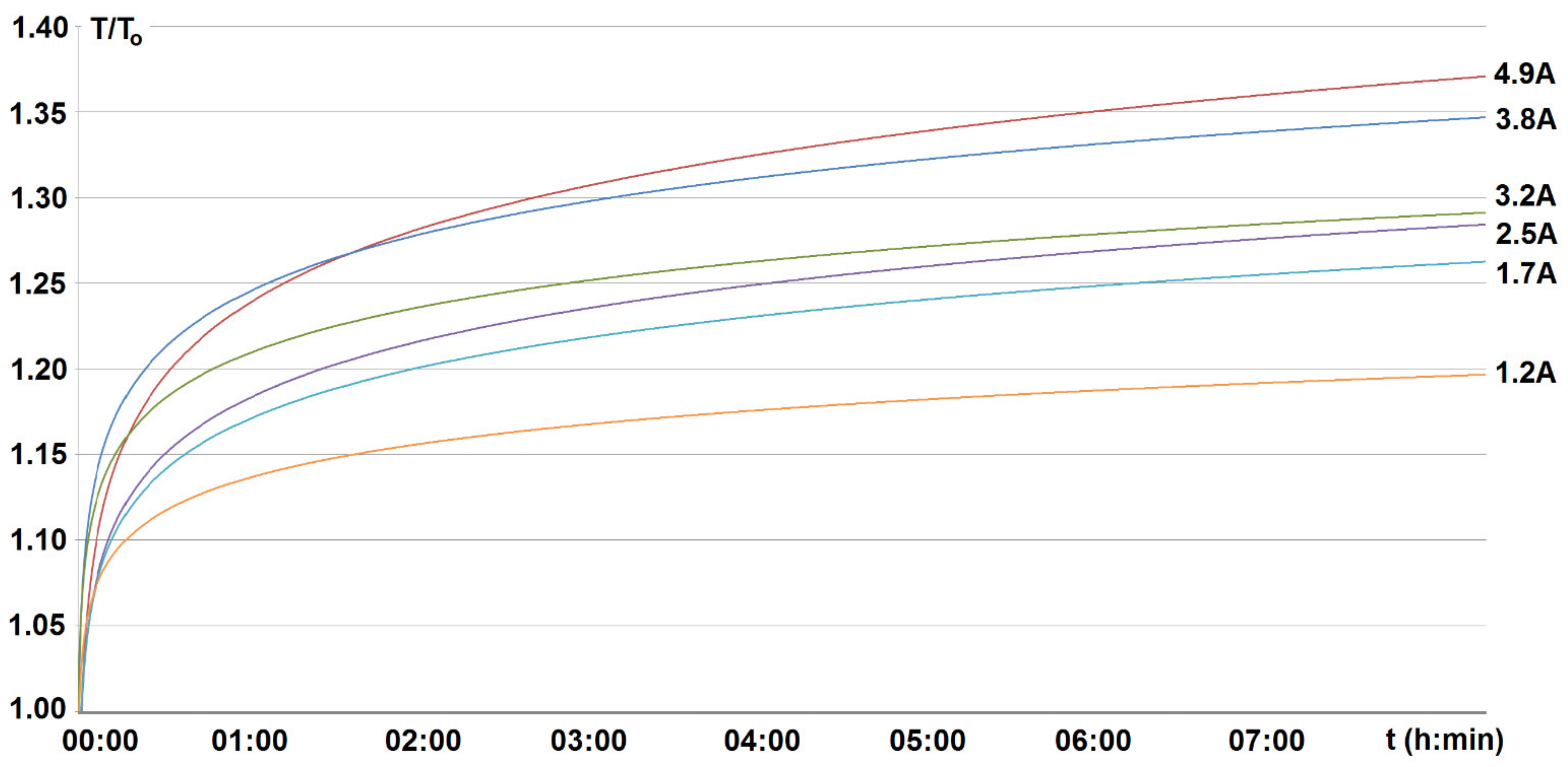
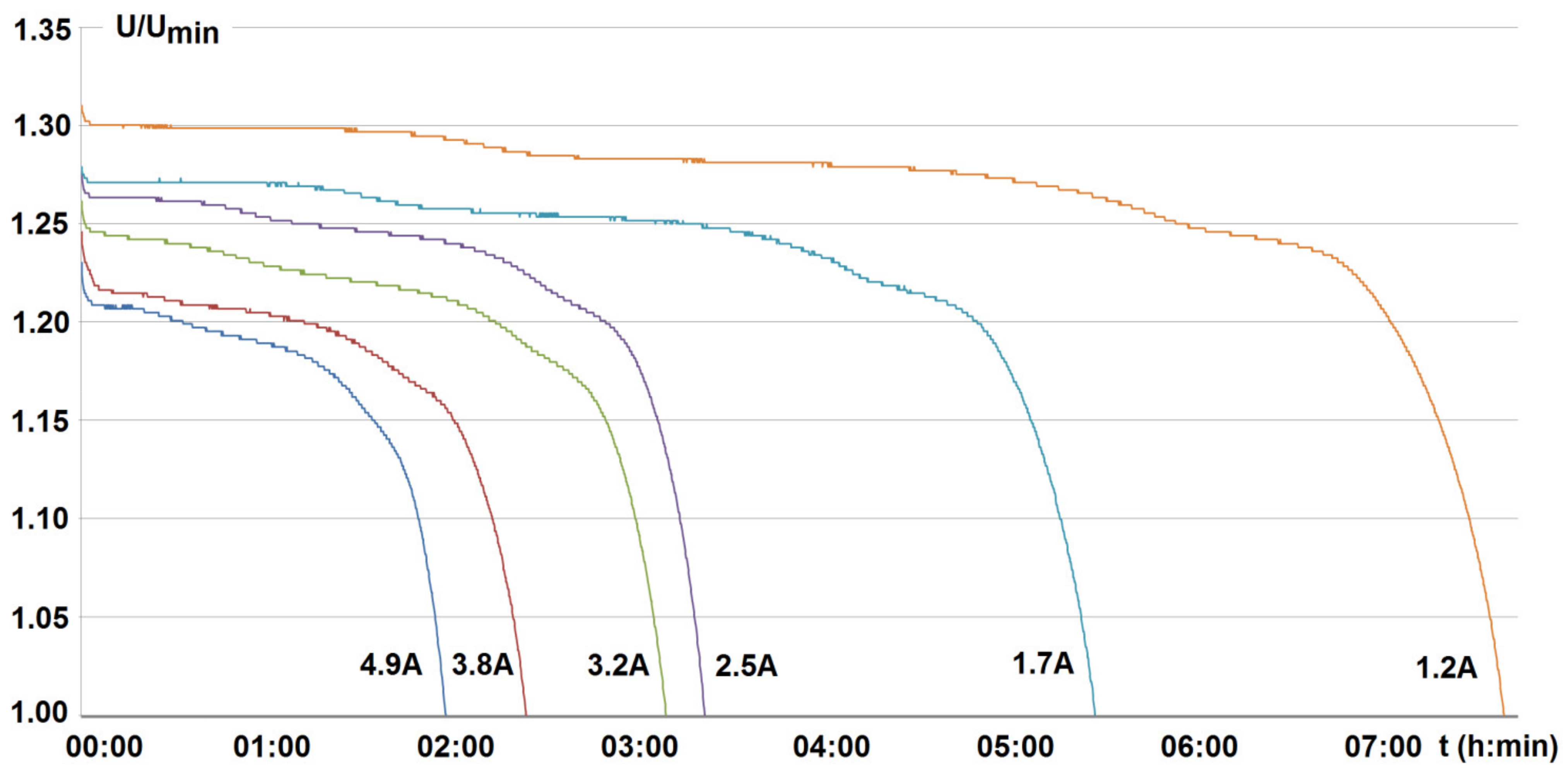
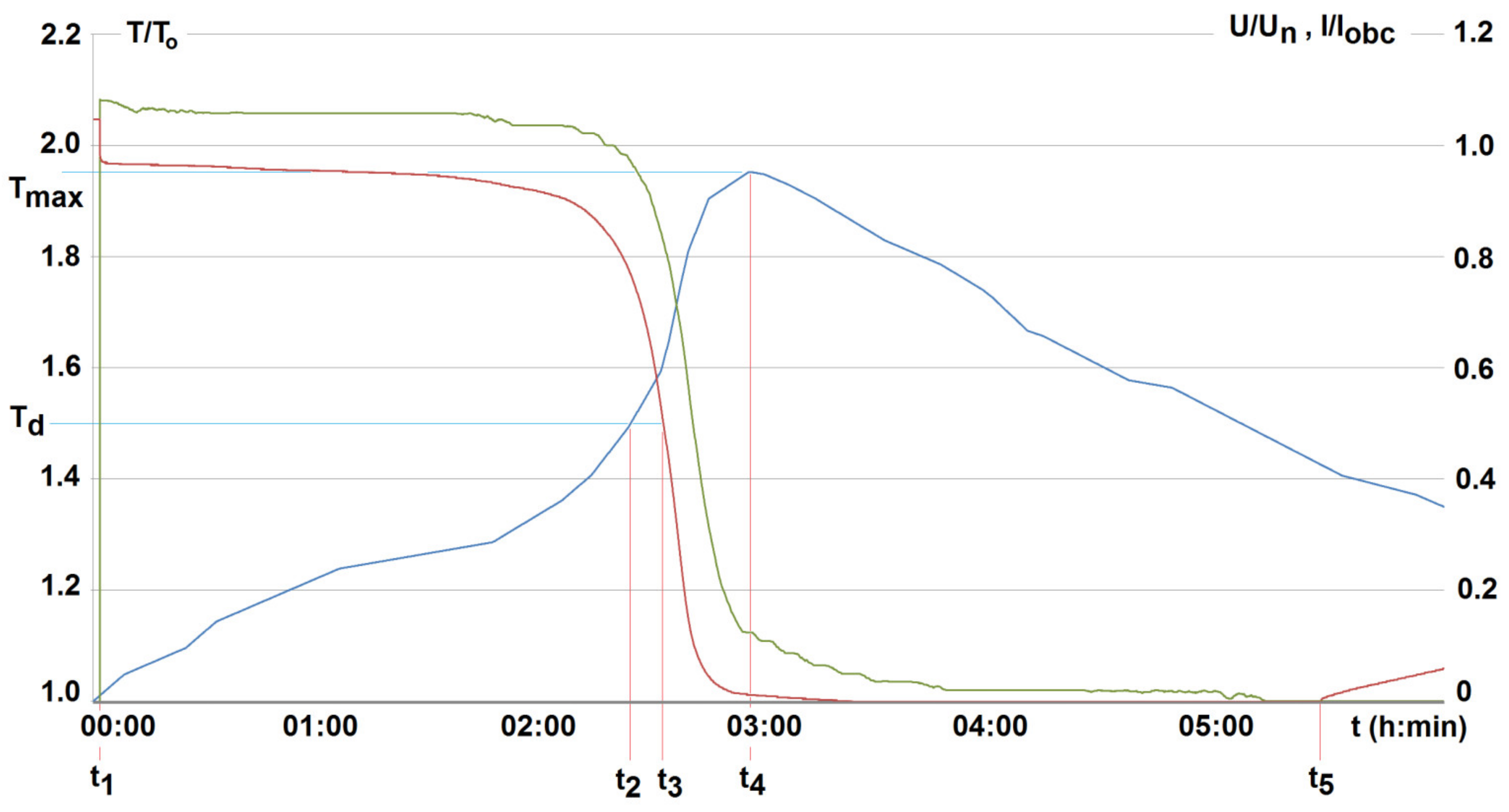
4. Investigated Results and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kurpiel, W.; Polnik, B.; Miedziński, B. Właściwości eksploatacyjne ogniw litowych. Elektro Info 2018, 10, 44–48. [Google Scholar]
- Polnik, B. Tests of the Hk-1 Module of the Mobile Mining Machine. ECS Trans. 2019, 95, 389–396. [Google Scholar] [CrossRef]
- Omar, N.; Monem, M.A.; Firouz, Y.; Salminen, J.; Smekens, J.; Hegazy, O.; Gaulous, H.; Mulder, G.; Van den Bossche, P.; Coosemans, T.; et al. Lithium iron phosphate based battery—Assesment of the aging parameters and development of cycle life model. Appl. Energy 2014, 113, 1575–1585. [Google Scholar] [CrossRef]
- Vetter, J.; Novak, P.; Wagner, M.R.; Veit, C.; Möller, K.C.; Besenhard, J.O.; Winter, M.; Wohlfahrt-Mehrens, M.; Vogler, C.; Hammouche, A. Ageing mechanisms in lithium-ion batteries. J. Power Sources 2005, 147, 269–281. [Google Scholar] [CrossRef]
- Santhanagopalan, S.; Kim, G.H.; Keyers, M.; Pesaran, A.; Smith, K.; Neubauer, J. Design and Analysis of Large Lithium-Ion Battery Systems; Artech House: London, UK, 2015; pp. 81–93. [Google Scholar]
- Cadar, D.V.; Petreus, D.M.; Patarau, T.M. An Energy Converter Method for Battery Cell Balancing. In Proceedings of the IEEE 33rd International Spring Seminar on Electronics Technology (ISSE), Warsaw, Poland, 12–16 May 2010; pp. 290–293. [Google Scholar]
- Gabano, J.P. Lithium Batteries; JohnWiley & Sons, Inc.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Ehrlich, G.M. Lithium-Ion Batteries. In Handbook of Batteries, 3rd ed.; Linden, D., Reddy, T., Eds.; McGraw-Hill: New York, NY, USA, 2002; pp. 53–59. [Google Scholar]
- Aurbach, J.D. Review of Selected Electrode-Solution Interactions with Determine the Performance of Li and Li Ion Batteries. Power Sources 2000, 89, 206. [Google Scholar] [CrossRef]
- Schalkwijk, W.V.; Scrosati, B. Advances in Lithium-Ion Batteries; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002. [Google Scholar]
- Zhang, Z.J.; Ramadass, P.; Fang, W. Safety of Lithium-Ion Batteries. In Lithium-Ion Batteries: Advances and Applications; Pistoia, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Levy, S.C. Safety and Reliability Considerations for Lithium Batteries. J. Power Sources 1997, 68, 75–77. [Google Scholar] [CrossRef]
- Zhang, S.S. A Review on Electrolyte Additives for Lithium-Ion Batteries. J. Power Sources 2006, 162, 1379–1394. [Google Scholar] [CrossRef]
- Yoshino, A. Development of the Lithium-Ion Battery and Recent Technological Trends. In Lithium-Ion Batteries: Advances and Applications; Pistoia, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Zaghib, K.; Mauger, A.; Julien, C.M. Rechargeable lithium batteries for energy storage in smart grids. In Rechargeable Lithium Batteries; Franco, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Salminen, J.; Kallio, T.; Omar, N.; Van den Bossche, P.; Van Mierlo, J.; Gualous, H. Transport Energy—Lithium Ion Batteries. In Future Energy; Letcher, T., Ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Lisbona, D.; Snee, T. A review of hazards associated with primary lithium and lithium-ion batteries. Process Saf. Environ. Prot. 2011, 89, 434–442. [Google Scholar] [CrossRef]
- Baird, A.R.; Archibald, E.J.; Marr, K.C.; Ezekoye, O.A. Explosion hazards from lithium-ion battery vent gas. J. Power Sources 2020, 446, 227257. [Google Scholar] [CrossRef]
- Essl, C.; Golubkov, A.W.; Gasser, E.; Nachtnebel, M.; Zankel, A.; Ewert, E.; Fuchs, A. Comprehensive hazard analysis of failing automotive Lithium-ion batteries in overtemperature experiments. Batteries 2020, 6, 30. [Google Scholar] [CrossRef]
- Ouyang, D.; Liu, J.; Chen, M.; Wang, J. Investigation into the fire hazards of lithium-ion batteries under overcharging. Appl. Sci. 2017, 7, 1314. [Google Scholar] [CrossRef] [Green Version]
- Spinner, N.S.; Hinnant, K.M.; Tuttle, S.G.; Rose-Pehrsson, S.L. Lithium-Ion Battery Failure: Effects of State of Charge and Packing Configuration; Naval Research Laboratory: Arlington, VA, USA, 2016; p. 21. [Google Scholar]
- Yoshio, M.; Brodd, R.J.; Kozawa, A. Lithium-Ion Batteries: Science and Technologies; Springer: New York, NY, USA, 2009. [Google Scholar]
- Perner, A.; Vetter, J. Post-Lithium-ion batteries for hybrid electric vehicles and battery electric vehicles. In Advances in Battery Technologies for Electric Vehicles; Scrosati, B., Garche, J., Tillmetz, W., Eds.; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Documentation of Battery LiFePO4 3.2 V. Available online: https://www.bto.pl/produkt/31801/headway-lfp38120(s)-10000mah-lifepo4 (accessed on 5 August 2021).
- Documentation of Voltage Output Temperature Sensor with Signal Conditioning. Available online: https://www.analog.com/en/products/ad22100.html# (accessed on 5 August 2021).
- Taylor, R.J. Introduction to Error Analysis, the Study of Uncertainties in Physical Measurements, 2nd ed.; University Science Books: Sausalito, CA, USA, 1997. [Google Scholar]
- Montanino, M.; Passerini, S.; Appetecchi, G.B. Electrolytes for rechargeable lithium batteries. In Rechargeable Lithium Batteries; Franco, A.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurpiel, W.; Polnik, B.; Orzech, Ł.; Lesiak, K.; Miedziński, B.; Habrych, M.; Debita, G.; Zamłyńska, M.; Falkowski-Gilski, P. Influence of Operation Conditions on Temperature Hazard of Lithium-Iron-Phosphate (LiFePO4) Cells. Energies 2021, 14, 6728. https://doi.org/10.3390/en14206728
Kurpiel W, Polnik B, Orzech Ł, Lesiak K, Miedziński B, Habrych M, Debita G, Zamłyńska M, Falkowski-Gilski P. Influence of Operation Conditions on Temperature Hazard of Lithium-Iron-Phosphate (LiFePO4) Cells. Energies. 2021; 14(20):6728. https://doi.org/10.3390/en14206728
Chicago/Turabian StyleKurpiel, Wojciech, Bartosz Polnik, Łukasz Orzech, Krzysztof Lesiak, Bogdan Miedziński, Marcin Habrych, Grzegorz Debita, Monika Zamłyńska, and Przemysław Falkowski-Gilski. 2021. "Influence of Operation Conditions on Temperature Hazard of Lithium-Iron-Phosphate (LiFePO4) Cells" Energies 14, no. 20: 6728. https://doi.org/10.3390/en14206728
APA StyleKurpiel, W., Polnik, B., Orzech, Ł., Lesiak, K., Miedziński, B., Habrych, M., Debita, G., Zamłyńska, M., & Falkowski-Gilski, P. (2021). Influence of Operation Conditions on Temperature Hazard of Lithium-Iron-Phosphate (LiFePO4) Cells. Energies, 14(20), 6728. https://doi.org/10.3390/en14206728







