Development of Thermochemical Heat Storage Based on CaO/CaCO3 Cycles: A Review
Abstract
:1. Introduction
2. CaO/CaCO3 TCHS
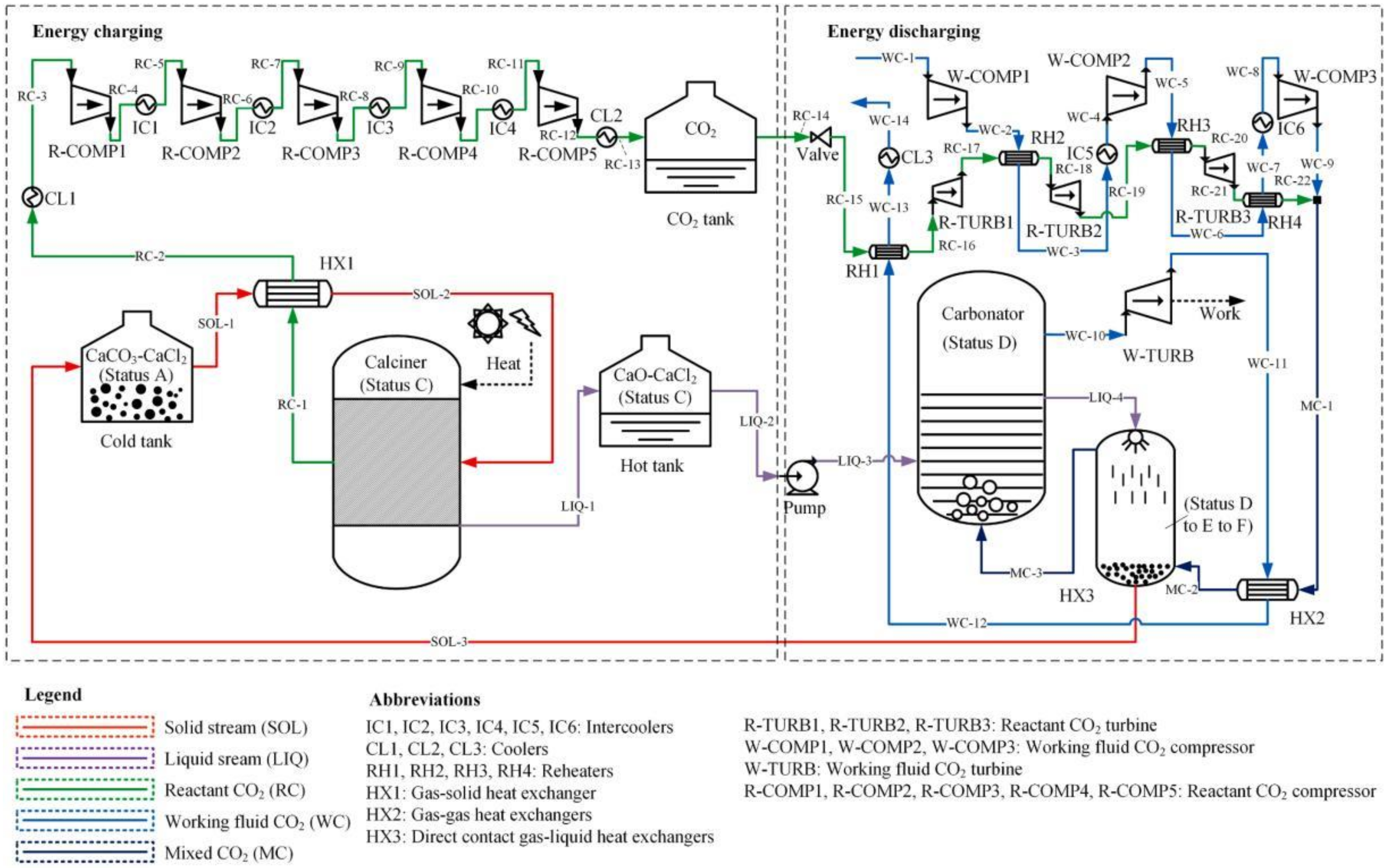
2.1. CSP-CaL Schemes
2.2. CSP-CaL Equipment
2.3. CSP-CaL Techno-Economics
3. Effect of Reaction Conditions on Performance of CaO-Based Materials in CaO/CaCO3 TCHS
3.1. Effect of Calcination Conditions
3.2. Effect of Carbonation Conditions
3.3. Effect of Particle Size
4. Performance of CaO-Based Materials in CaO/CaCO3 TCHS
4.1. Natural CaO-Based Materials
4.2. Waste CaO-Based Materials
5. Improvement on Cyclic Thermal Storage Stability of CaO-Based Materials in CaO/CaCO3 TCHS
5.1. CaO/SiO2 Composites
5.2. CaO/Al2O3 Composites
5.3. Other CaO-Based Composites
5.4. Organic Acid-Treated CaO-Based Composites
6. Improvement on Optical and Thermal Properties of CaO-Based Materials in CaO/CaCO3 TCHS
7. Conclusions
- (1)
- System integration is currently limited to simulation research, which faces technological challenges, such as the fluctuation of solar radiation, the separation of gas-solid two phases, and so on, to realize industrial applications. The integrated system of CaO/CaCO3 heat storage and CSP plant should be further designed and optimized to improve the sensible heat recovery network of the system and reduce energy loss. The design and production of equipment that is more compatible with CaO/CaCO3 heat storage, especially particle receivers, need to meet the main requirements of great scalability and high thermal efficiency.
- (2)
- The preparation of CaO-based heat storage materials should be carried out in the direction of high cyclic activity and cyclic stability, and the preparation process of the materials should be optimized. The ideal CaO-based heat storage materials possess high heat storage density, great heat storage stability, and great absorption of sunlight, while economical preparation processes are also required. In addition, the specific application of the material needs to be combined with the reactor design.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oyekale, J.; Petrollese, M.; Tola, V.; Cau, G. Impacts of Renewable Energy Resources on Effectiveness of Grid-Integrated Systems: Succinct Review of Current Challenges and Potential Solution Strategies. Energies 2020, 13, 4856. [Google Scholar] [CrossRef]
- Masson-Delmotte, V.; Zhai, P.; Pörtner, H.-O.; Roberts, D.; Skea, J.; Shukla, P.R.; Pirani, A.; Moufouma-Okia, W.; Péan, C.; Pidcock, R. Global Warming of 1.5 ℃. An IPCC Special Report on the Impacts of Global Warming; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2018; Volume 1, pp. 1–9. [Google Scholar]
- Dudley, B. BP Energy Outlook. Report–BP Energy Economics; B.P.: London, UK, 2019; Volume 9. [Google Scholar]
- Li, J.; Liu, J.; Yan, P.; Li, X.; Zhou, G.; Yu, D. Operation Optimization of Integrated Energy System under a Renewable Energy Dominated Future Scene Considering Both Independence and Benefit: A Review. Energies 2021, 14, 1103. [Google Scholar] [CrossRef]
- Bravo, R.; Ortiz, C.; Chacartegui, R.; Friedrich, D. Hybrid solar power plant with thermochemical energy storage: A multi-objective operational optimisation. Energy Convers. Manag. 2020, 205, 112421. [Google Scholar] [CrossRef]
- del Río, P.; Peñasco, C.; Mir-Artigues, P. An overview of drivers and barriers to concentrated solar power in the European Union. Renew. Sustain. Energy Rev. 2018, 81, 1019–1029. [Google Scholar] [CrossRef]
- Peng, X.; Root, T.W.; Maravelias, C.T. Storing solar energy with chemistry: The role of thermochemical storage in concentrating solar power. Green Chem. 2017, 19, 2427–2438. [Google Scholar] [CrossRef]
- Ellingwood, K.; Mohammadi, K.; Powell, K. A novel means to flexibly operate a hybrid concentrated solar power plant and improve operation during non-ideal direct normal irradiation conditions. Energy Convers. Manag. 2020, 203, 112275. [Google Scholar] [CrossRef]
- Ortiz, C.; Binotti, M.; Romano, M.C.; Valverde, J.M.; Chacartegui, R. Off-design model of concentrating solar power plant with thermochemical energy storage based on calcium-looping. AIP Conf. Proc. 2019, 2126, 210006. [Google Scholar]
- Heuberger, C.F.; Mac Dowell, N. Real-World Challenges with a Rapid Transition to 100% Renewable Power Systems. Joule 2018, 2, 367–370. [Google Scholar] [CrossRef] [Green Version]
- Chang, C. Tracking solar collection technologies for solar heating and cooling systems. In Advances in Solar Heating and Cooling; Elsevier: Amsterdam, The Netherlands, 2016; pp. 81–93. [Google Scholar]
- Kuravi, S.; Trahan, J.; Goswami, D.Y.; Rahman, M.M.; Stefanakos, E.K. Thermal energy storage technologies and systems for concentrating solar power plants. Prog. Energy Combust. Sci. 2013, 39, 285–319. [Google Scholar] [CrossRef]
- Pelay, U.; Luo, L.; Fan, Y.; Stitou, D.; Rood, M. Thermal energy storage systems for concentrated solar power plants. Renew. Sustain. Energy Rev. 2017, 79, 82–100. [Google Scholar] [CrossRef]
- Fernandez, A.I.; Martínez, M.; Segarra, M.; Martorell, I.; Cabeza, L.F. Selection of materials with potential in sensible thermal energy storage. Sol. Energy Mater. Sol. Cells 2010, 94, 1723–1729. [Google Scholar] [CrossRef]
- Riffat, S.; Mempouo, B.; Fang, W. Phase change material developments: A review. Int. J. Ambient Energy 2015, 36, 102–115. [Google Scholar] [CrossRef]
- Regin, A.F.; Solanki, S.C.; Saini, J.S. Heat transfer characteristics of thermal energy storage system using PCM capsules: A review. Renew. Sustain. Energy Rev. 2008, 12, 2438–2458. [Google Scholar] [CrossRef]
- Abedin, A.H.; Rosen, M.A. Closed and open thermochemical energy storage: Energy-and exergy-based comparisons. Energy 2012, 41, 83–92. [Google Scholar] [CrossRef]
- Wei, L.; Wei, C.; Dandan, W. Research and development of thermochemical energy storage based on hydrated salt. Refrig. Air-Cond. 2017, 17, 14–21. [Google Scholar]
- Yan, T.; Wang, R.; Li, T.; Wang, L.; Fred, I.T. A review of promising candidate reactions for chemical heat storage. Renew. Sustain. Energy Rev. 2015, 43, 13–31. [Google Scholar] [CrossRef]
- Pardo, P.; Deydier, A.; Anxionnaz-Minvielle, Z.; Rougé, S.; Cabassud, M.; Cognet, P. A review on high temperature thermochemical heat energy storage. Renew. Sustain. Energy Rev. 2014, 32, 591–610. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Wu, S.; Li, Y. Research progress of CO2 capture with the assist CaO-based energy storage materials at coal-fired power station. Clean Coal Technol. 2019, 25, 1–8. [Google Scholar]
- Mahon, D.; Claudio, G.; Eames, P. An Experimental Study of the Decomposition and Carbonation of Magnesium Carbonate for Medium Temperature Thermochemical Energy Storage. Energies 2021, 14, 1316. [Google Scholar] [CrossRef]
- Rhodes, N.R.; Barde, A.; Randhir, K.; Li, L.; Hahn, D.W.; Mei, R.; Klausner, J.F.; AuYeung, N.J. Solar Thermochemical Energy Storage Through Carbonation Cycles of SrCO3/SrO Supported on SrZrO3. ChemSusChem 2015, 8, 3793–3798. [Google Scholar] [CrossRef]
- Criado, Y.; Alonso, M.; Abanades, J.; Anxionnaz-Minvielle, Z. Conceptual process design of a CaO/Ca(OH)2 thermochemical energy storage system using fluidized bed reactors. Appl. Therm. Eng. 2014, 73, 1087–1094. [Google Scholar] [CrossRef] [Green Version]
- Seitz, G. Modeling fixed-bed reactors for thermochemical heat storage with the reaction system CaO/Ca(OH)2. Appl. Sci. 2021, 11, 682. [Google Scholar] [CrossRef]
- Nyallang Nyamsi, S.; Tolj, I.; Lototskyy, M. Metal hydride beds-phase change materials: Dual mode thermal energy storage for medium-high temperature industrial waste heat recovery. Energies 2019, 12, 3949. [Google Scholar] [CrossRef] [Green Version]
- Preisner, N.C.; Linder, M. A moving bed reactor for thermochemical energy storage based on metal oxides. Energies 2020, 13, 1232. [Google Scholar] [CrossRef] [Green Version]
- Müller, D.; Knoll, C.; Gravogl, G.; Jordan, C.; Eitenberger, E.; Friedbacher, G.; Artner, W.; Welch, J.M.; Werner, A.; Harasek, M. Medium-temperature thermochemical energy storage with transition metal ammoniates—A systematic material comparison. Appl. Energy 2021, 285, 116470. [Google Scholar] [CrossRef]
- Li, W.; Hao, Y. Efficient solar power generation combining photovoltaics and mid-/low-temperature methanol thermochemistry. Appl. Energy 2017, 202, 377–385. [Google Scholar] [CrossRef]
- Wong, B.; Brown, L.; Buckingham, R.; Sweet, W.; Russ, B.; Gorensek, M. Sulfur dioxide disproportionation for sulfur based thermochemical energy storage. Sol. Energy 2015, 118, 134–144. [Google Scholar] [CrossRef] [Green Version]
- Sunku Prasad, J.; Muthukumar, P.; Desai, F.; Basu, D.N.; Rahman, M.M. A critical review of high-temperature reversible thermochemical energy storage systems. Appl. Energy 2019, 254, 113733. [Google Scholar] [CrossRef]
- Ortiz, C.; Valverde, J.M.; Chacartegui, R.; Perez-Maqueda, L.A.; Giménez, P. The Calcium-Looping (CaCO3/CaO) process for thermochemical energy storage in Concentrating Solar Power plants. Renew. Sustain. Energy Rev. 2019, 113, 109252. [Google Scholar] [CrossRef]
- Abedin, A.H.; Rosen, M.A. A critical review of thermochemical energy storage systems. Open Renew. Energy J. 2011, 4, 42–46. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Gu, F.; Clough, P.T.; Zhao, P.; Anthony, E.J. Porous MgO-stabilized CaO-based powders/pellets via a citric acid-based carbon template for thermochemical energy storage in concentrated solar power plants. Chem. Eng. J. 2020, 390, 124163. [Google Scholar] [CrossRef]
- Barin, I.; Platzki, G. Thermochemical Data of Pure Substances; Wiley Online Library: Hoboken, NJ, USA, 1989; Volume 304. [Google Scholar]
- Ortiz, C.; Chacartegui, R.; Valverde, J.; Alovisio, A.; Becerra, J. Power cycles integration in concentrated solar power plants with energy storage based on calcium looping. Energy Convers. Manag. 2017, 149, 815–829. [Google Scholar] [CrossRef]
- Kyaw, K.; Kubota, M.; Watanabe, F.; Matsuda, H.; Hasatani, M. Study of carbonation of CaO for high temperature thermal energy storage. J. Chem. Eng. Jpn. 1998, 31, 281–284. [Google Scholar] [CrossRef]
- Cormos, C.-C. Economic evaluations of coal-based combustion and gasification power plants with post-combustion CO2 capture using calcium looping cycle. Energy 2014, 78, 665–673. [Google Scholar] [CrossRef]
- Zsembinszki, G.; Solé, A.; Barreneche, C.; Prieto, C.; Fernández, A.I.; Cabeza, L.F. Review of reactors with potential use in thermochemical energy storage in concentrated solar power plants. Energies 2018, 11, 2358. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Li, Y.; Zhao, J. Development on Thermochemical Energy Storage Based on CaO-Based Materials: A Review. Sustainability 2018, 10, 2660. [Google Scholar] [CrossRef] [Green Version]
- Felderhoff, M.; Urbanczyk, R.; Peil, S. Thermochemical heat storage for high temperature applications—A review. Green 2013, 3, 113–123. [Google Scholar] [CrossRef]
- Wentworth, W.; Chen, E. Simple thermal decomposition reactions for storage of solar thermal energy. Sol. Energy 1976, 18, 205–214. [Google Scholar] [CrossRef]
- Chen, J.; Duan, L.; Sun, Z. Review on the Development of Sorbents for Calcium Looping. Energy Fuels 2020, 34, 7806–7836. [Google Scholar] [CrossRef]
- Fedunik-Hofman, L.; Bayon, A.; Donne, S.W. Comparative kinetic analysis of CaCO3/CaO reaction system for energy storage and carbon capture. Appl. Sci. 2019, 9, 4601. [Google Scholar] [CrossRef] [Green Version]
- Valverde, J.M. The Ca-looping process for CO2 capture and energy storage: Role of nanoparticle technology. J. Nanoparticle Res. 2018, 20, 1–16. [Google Scholar] [CrossRef]
- Chen, J.; Duan, L.; Sun, Z. Accurate control of cage-like CaO hollow microspheres for enhanced CO2 capture in calcium looping via a template-assisted synthesis approach. Environ. Sci. Technol. 2019, 53, 2249–2259. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Ou, Z.; Qin, C.; Deng, T.; Yin, J.; Pu, G. Understanding the catalytic acceleration effect of steam on CaCO3 decomposition by density function theory. Chem. Eng. J. 2020, 379, 122348. [Google Scholar] [CrossRef]
- Martínez, I.; Grasa, G.; Murillo, R.; Arias, B.; Abanades, J. Modelling the continuous calcination of CaCO3 in a Ca-looping system. Chem. Eng. J. 2013, 215, 174–181. [Google Scholar] [CrossRef]
- Ortiz, C.; Valverde, J.M.; Chacartegui, R.; Perez-Maqueda, L.A. Carbonation of Limestone Derived CaO for Thermochemical Energy Storage: From Kinetics to Process Integration in Concentrating Solar Plants. ACS Sustain. Chem. Eng. 2018, 6, 6404–6417. [Google Scholar] [CrossRef] [Green Version]
- Borgwardt, R.H. Sintering of nascent calcium oxide. Chem. Eng. Sci. 1989, 44, 53–60. [Google Scholar] [CrossRef]
- Bazaikin, Y.V.; Malkovich, E.; Derevschikov, V.; Lysikov, A.; Okunev, A. Evolution of sorptive and textural properties of CaO-based sorbents during repetitive sorption/regeneration cycles. Chem. Eng. Sci. 2016, 152, 709–716. [Google Scholar] [CrossRef]
- Sarrion, B.; Valverde, J.M.; Perejon, A.; Perez-Maqueda, L.; Sanchez-Jimenez, P.E. On the Multicycle Activity of Natural Limestone/Dolomite for Thermochemical Energy Storage of Concentrated Solar Power. Energy Technol. 2016, 4, 1013–1019. [Google Scholar] [CrossRef]
- Avila-Marin, A.L. Volumetric receivers in solar thermal power plants with central receiver system technology: A review. Sol. Energy 2011, 85, 891–910. [Google Scholar] [CrossRef]
- Reich, L.; Melmoth, L.; Yue, L.; Bader, R.; Gresham, R.; Simon, T.; Lipiński, W. A solar reactor design for research on calcium oxide-based carbon dioxide capture. J. Sol. Energy Eng. 2017, 139, 054501. [Google Scholar] [CrossRef]
- Valverde, J.M.; Medina, S. Crystallographic transformation of limestone during calcination under CO2. Phys. Chem. Chem. Phys. 2015, 17, 21912–21926. [Google Scholar] [CrossRef] [PubMed]
- Charitos, A.; Rodríguez, N.; Hawthorne, C.; Alonso, M.; Zieba, M.; Arias, B.; Kopanakis, G.; Scheffknecht, G.n.; Abanades, J.C. Experimental validation of the calcium looping CO2 capture process with two circulating fluidized bed carbonator reactors. Ind. Eng. Chem. Res. 2011, 50, 9685–9695. [Google Scholar] [CrossRef] [Green Version]
- Arcenegui-Troya, J.; Sánchez-Jiménez, P.E.; Perejón, A.; Moreno, V.; Valverde, J.M.; Pérez-Maqueda, L.A. Kinetics and cyclability of limestone (CaCO3) in presence of steam during calcination in the CaL scheme for thermochemical energy storage. Chem. Eng. J. 2021, 417, 129194. [Google Scholar] [CrossRef]
- Khosa, A.A.; Xu, T.; Xia, B.Q.; Yan, J.; Zhao, C.Y. Technological challenges and industrial applications of CaCO3/CaO based thermal energy storage system—A review. Sol. Energy 2019, 193, 618–636. [Google Scholar] [CrossRef]
- Lanchi, M.; Turchetti, L.; Sau, S.; Liberatore, R.; Cerbelli, S.; Murmura, M.A.; Annesini, M.C. A Discussion of Possible Approaches to the Integration of Thermochemical Storage Systems in Concentrating Solar Power Plants. Energies 2020, 13, 4940. [Google Scholar] [CrossRef]
- Chang, M.-H.; Chen, W.-C.; Huang, C.-M.; Liu, W.-H.; Chou, Y.-C.; Chang, W.-C.; Chen, W.; Cheng, J.-Y.; Huang, K.-E.; Hsu, H.-W. Design and experimental testing of a 1.9 MWth calcium looping pilot plant. Energy Procedia 2014, 63, 2100–2108. [Google Scholar] [CrossRef] [Green Version]
- Valverde, J.M.; Barea-López, M.; Perejón, A.; Sánchez-Jiménez, P.E.; Pérez-Maqueda, L.A. Effect of Thermal Pretreatment and Nanosilica Addition on Limestone Performance at Calcium-Looping Conditions for Thermochemical Energy Storage of Concentrated Solar Power. Energy Fuels 2017, 31, 4226–4236. [Google Scholar] [CrossRef]
- Edwards, S.E.B.; Materić, V. Calcium looping in solar power generation plants. Sol. Energy 2012, 86, 2494–2503. [Google Scholar] [CrossRef]
- Chacartegui, R.; Alovisio, A.; Ortiz, C.; Valverde, J.M.; Verda, V.; Becerra, J.A. Thermochemical energy storage of concentrated solar power by integration of the calcium looping process and a CO2 power cycle. Appl. Energy 2016, 173, 589–605. [Google Scholar] [CrossRef]
- Alovisio, A.; Chacartegui, R.; Ortiz, C.; Valverde, J.M.; Verda, V. Optimizing the CSP-Calcium Looping integration for Thermochemical Energy Storage. Energy Convers. Manag. 2017, 136, 85–98. [Google Scholar] [CrossRef]
- Karasavvas, E.; Panopoulos, K.D.; Papadopoulou, S.; Voutetakis, S. Design of an integrated CSP-calcium looping for uninterrupted power production through energy storage. Chem. Eng. Trans. 2018, 70, 2131–2136. [Google Scholar]
- Tesio, U.; Guelpa, E.; Verda, V. Optimal Indirect Integration of Steam Rankine Cycles in Concentrated Solar Power Coupled with Thermochemical Storage. E3S Web Conf. 2020, 197, 01005. [Google Scholar] [CrossRef]
- Ortiz, C.; Romano, M.C.; Valverde, J.M.; Binotti, M.; Chacartegui, R. Process integration of Calcium-Looping thermochemical energy storage system in concentrating solar power plants. Energy 2018, 155, 535–551. [Google Scholar] [CrossRef]
- Karasavvas, E.; Panopoulos, K.D.; Papadopoulou, S.; Voutetakis, S. Energy and exergy analysis of the integration of concentrated solar power with calcium looping for power production and thermochemical energy storage. Renew. Energy 2020, 154, 743–753. [Google Scholar] [CrossRef]
- Cannone, S.F.; Stendardo, S.; Lanzini, A. Solar-Powered Rankine Cycle Assisted by an Innovative Calcium Looping Process as an Energy Storage System. Ind. Eng. Chem. Res. 2020, 59, 6977–6993. [Google Scholar] [CrossRef]
- Wu, S.; Zhou, C.; Tremain, P.; Doroodchi, E.; Moghtaderi, B. A phase change calcium looping thermochemical energy storage system based on CaCO3/CaO-CaCl2. Energy Convers. Manag. 2021, 227, 113503. [Google Scholar] [CrossRef]
- Habibi, R.; Mehrpooya, M.; Pourmoghadam, P. Integrated Mg-Cl hydrogen production process and CaO/CaCO3-CaCl2 thermochemical energy storage phase change system using solar tower system. Energy Convers. Manag. 2021, 245, 114555. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, D.; Wang, Y.; Ling, X.; Jin, X. The role of sensible heat in a concentrated solar power plant with thermochemical energy storage. Energy Convers. Manag. 2019, 190, 42–53. [Google Scholar] [CrossRef]
- Pascual, S.; Lisbona, P.; Bailera, M.; Romeo, L.M. Design and operational performance maps of calcium looping thermochemical energy storage for concentrating solar power plants. Energy 2021, 220, 119715. [Google Scholar] [CrossRef]
- Peng, X.; Yao, M.; Root, T.W.; Maravelias, C.T. Design and analysis of concentrating solar power plants with fixed-bed reactors for thermochemical energy storage. Appl. Energy 2020, 262, 114543. [Google Scholar] [CrossRef]
- Obermeier, J.; Müller, K.; Karagiannakis, G.; Stubos, A.; Arlt, W. A novel thermochemical energy storage and transportation concept based on concentrated solar irradiation-aided CaO-looping. AIP Conf. Proc. 2016, 1734, 050033. [Google Scholar]
- Flamant, G.; Benoit, H.; Jenke, M.; Santos, A.F.; Tescari, S.; Moumin, G.; Rodriguez, A.; Azapagic, A.; Stamford, L.; Baeyens, J. Solar processing of reactive particles up to 900 °C, the SOLPART project. AIP Conf. Proc. 2018, 2033, 20004. [Google Scholar]
- Sun, J.; Sun, Y.; Yang, Y.; Tong, X.; Liu, W. Plastic/rubber waste-templated carbide slag pellets for regenerable CO2 capture at elevated temperature. Appl. Energy 2019, 242, 919–930. [Google Scholar] [CrossRef]
- Flamant, G.; Hernandez, D.; Bonet, C.; Traverse, J.-P. Experimental aspects of the thermochemical conversion of solar energy; Decarbonation of CaCO3. Sol. Energy 1980, 24, 385–395. [Google Scholar] [CrossRef]
- Da, Y.; Xuan, Y.; Teng, L.; Zhang, K.; Liu, X.; Ding, Y. Calcium-based composites for direct solar-thermal conversion and thermochemical energy storage. Chem. Eng. J. 2020, 382, 122815. [Google Scholar] [CrossRef]
- Cuevas, A.; Martínez, L.; Romero, R.; Dalchiele, E.A.; Marotti, R.; Leinen, D.; Ramos-Barrado, J.R.; Martin, F. Electrochemically grown cobalt-alumina composite layer for solar thermal selective absorbers. Sol. Energy Mater. Sol. Cells 2014, 130, 380–386. [Google Scholar] [CrossRef]
- Zeng, J.; Xuan, Y.; Duan, H. Tin-silica-silver composite nanoparticles for medium-to-high temperature volumetric absorption solar collectors. Sol. Energy Mater. Sol. Cells 2016, 157, 930–936. [Google Scholar] [CrossRef]
- Teng, L.; Xuan, Y.; Da, Y.; Liu, X.; Ding, Y. Modified Ca-Looping materials for directly capturing solar energy and high-temperature storage. Energy Storage Mater. 2020, 25, 836–845. [Google Scholar] [CrossRef]
- Lisbona, P.; Bailera, M.; Hills, T.; Sceats, M.; Díez, L.I.; Romeo, L.M. Energy consumption minimization for a solar lime calciner operating in a concentrated solar power plant for thermal energy storage. Renew. Energy 2020, 156, 1019–1027. [Google Scholar] [CrossRef]
- Neises, M.; Tescari, S.; de Oliveira, L.; Roeb, M.; Sattler, C.; Wong, B. Solar-heated rotary kiln for thermochemical energy storage. Sol. Energy 2012, 86, 3040–3048. [Google Scholar] [CrossRef]
- Bertocchi, R.; Karni, J.; Kribus, A. Experimental evaluation of a non-isothermal high temperature solar particle receiver. Energy 2004, 29, 687–700. [Google Scholar] [CrossRef]
- Röger, M.; Amsbeck, L.; Gobereit, B.; Buck, R. Face-down solid particle receiver using recirculation. J. Sol. Energy Eng. 2011, 133. [Google Scholar] [CrossRef]
- Tregambi, C.; Padula, S.; Galbusieri, M.; Coppola, G.; Montagnaro, F.; Salatino, P.; Troiano, M.; Solimene, R. Directly irradiated fluidized bed reactor for thermochemical energy storage and solar fuels production. Powder Technol. 2020, 366, 460–469. [Google Scholar] [CrossRef]
- Yao, Y.; Wei, X.; Chen, L.; Li, S.; Tan, H. Research and application progress of the key technologies for high efficiency and low emission of cement kilns. Clean Coal Technol. 2020, 26, 1–10. [Google Scholar]
- Moumin, G.; Tescari, S.; Sundarraj, P.; de Oliveira, L.; Roeb, M.; Sattler, C. Solar treatment of cohesive particles in a directly irradiated rotary kiln. Sol. Energy 2019, 182, 480–490. [Google Scholar] [CrossRef]
- Alonso, E.; Romero, M. Review of experimental investigation on directly irradiated particles solar reactors. Renew. Sustain. Energy Rev. 2015, 41, 53–67. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, K.; Clough, P.T.; Anthony, E.J. Developments in calcium/chemical looping and metal oxide redox cycles for high-temperature thermochemical energy storage: A review. Fuel Process. Technol. 2020, 199, 106280. [Google Scholar] [CrossRef]
- Hanak, D.P.; Manovic, V. Economic feasibility of calcium looping under uncertainty. Appl. Energy 2017, 208, 691–702. [Google Scholar] [CrossRef] [Green Version]
- Michalski, S.; Hanak, D.P.; Manovic, V. Techno-economic feasibility assessment of calcium looping combustion using commercial technology appraisal tools. J. Clean. Prod. 2019, 219, 540–551. [Google Scholar] [CrossRef]
- Bayon, A.; Bader, R.; Jafarian, M.; Fedunik-Hofman, L.; Sun, Y.; Hinkley, J.; Miller, S.; Lipiński, W. Techno-economic assessment of solid–gas thermochemical energy storage systems for solar thermal power applications. Energy 2018, 149, 473–484. [Google Scholar] [CrossRef]
- Tesio, U.; Guelpa, E.; Verda, V. Integration of thermochemical energy storage in concentrated solar power. Part 1: Energy and economic analysis/optimization. Energy Convers. Manag. X 2020, 6, 100039. [Google Scholar] [CrossRef]
- Muto, A.; McCabe, K.; Real, D. High-temperature calcium-based thermochemical energy storage system for use with CSP facilities. AIP Conf. Proc. 2018, 2033, 100006. [Google Scholar]
- Benitez-Guerrero, M.; Sarrion, B.; Perejon, A.; Sanchez-Jimenez, P.E.; Perez-Maqueda, L.A.; Manuel Valverde, J. Large-scale high-temperature solar energy storage using natural minerals. Sol. Energy Mater. Sol. Cells 2017, 168, 14–21. [Google Scholar] [CrossRef]
- Sun, H.; Li, Y.; Bian, Z.; Yan, X.; Wang, Z.; Liu, W. Thermochemical energy storage performances of Ca-based natural and waste materials under high pressure during CaO/CaCO3 cycles. Energy Convers. Manag. 2019, 197, 111885. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, N.; Ling, X.; Wang, Y. Parameter analysis of discharging process for CaCO3/CaO thermochemical energy storage. Energy Procedia 2019, 158, 4617–4622. [Google Scholar] [CrossRef]
- Fedunik-Hofman, L.; Bayon, A.; Hinkley, J.; Lipiński, W.; Donne, S.W. Friedman method kinetic analysis of CaO-based sorbent for high-temperature thermochemical energy storage. Chem. Eng. Sci. 2019, 200, 236–247. [Google Scholar] [CrossRef]
- Barker, R. The reactivity of calcium oxide towards carbon dioxide and its use for energy storage. J. Appl. Chem. Biotechnol. 1974, 24, 221–227. [Google Scholar] [CrossRef]
- Sarrión, B.; Perejón, A.; Sánchez-Jiménez, P.E.; Pérez-Maqueda, L.A.; Valverde, J.M. Role of calcium looping conditions on the performance of natural and synthetic Ca-based materials for energy storage. J. CO2 Util. 2018, 28, 374–384. [Google Scholar] [CrossRef]
- Tammann, G.; Westerhold, F.; Garre, B.; Kordes, E.; Kalsing, H. Chemische Reaktionen in pulverförmigen Gemengen zweier Kristallarten. Z. Anorg. Allg. Chem. 1925, 149, 21–98. [Google Scholar] [CrossRef]
- Valverde, J.M.; Medina, S. Limestone calcination under calcium-looping conditions for CO2 capture and thermochemical energy storage in the presence of H2O: An in situ XRD analysis. Phys. Chem. Chem. Phys. 2017, 19, 7587–7596. [Google Scholar] [CrossRef] [PubMed]
- Sarrión, B.; Perejón, A.; Sánchez-Jiménez, P.E.; Amghar, N.; Chacartegui, R.; Valverde, J.M.; Pérez-Maqueda, L.A. Calcination under low CO2 pressure enhances the calcium Looping performance of limestone for thermochemical energy storage. Chem. Eng. J. 2021, 417, 127922. [Google Scholar] [CrossRef]
- Champagne, S.; Lu, D.Y.; Macchi, A.; Symonds, R.T.; Anthony, E.J. Influence of steam injection during calcination on the reactivity of CaO-based sorbent for carbon capture. Ind. Eng. Chem. Res. 2013, 52, 2241–2246. [Google Scholar] [CrossRef]
- Ma, Z.; Li, Y.; Zhang, W.; Wang, Y.; Zhao, J.; Wang, Z. Energy storage and attrition performance of limestone under fluidization during CaO/CaCO3 cycles. Energy 2020, 207, 118291. [Google Scholar] [CrossRef]
- Obermeier, J.; Sakellariou, K.G.; Tsongidis, N.I.; Baciu, D.; Charalambopoulou, G.; Steriotis, T.; Müller, K.; Karagiannakis, G.; Konstandopoulos, A.G.; Stubos, A.; et al. Material development and assessment of an energy storage concept based on the CaO-looping process. Sol. Energy 2017, 150, 298–309. [Google Scholar] [CrossRef]
- Sarrion, B.; Sanchez-Jimenez, P.E.; Perejon, A.; Perez-Maqueda, L.A.; Valverde, J.M. Pressure Effect on the Multicycle Activity of Natural Carbonates and a Ca/Zr Composite for Energy Storage of Concentrated Solar Power. ACS Sustain. Chem. Eng. 2018, 6, 7849–7858. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Li, Y.; Sun, H.; Wang, Y.; Wang, Z. Thermochemical Heat Storage Performance of CaO Pellets Fabricated by Extrusion–Spheronization under Harsh Calcination Conditions. Energy Fuels 2020, 34, 6462–6473. [Google Scholar] [CrossRef]
- Durán-Martín, J.D.; Jimenez, P.E.S.; Valverde, J.M.; Perejón, A.; Arcenegui-Troya, J.; Triñanes, P.G.; Maqueda, L.A.P. Role of particle size on the multicycle calcium looping activity of limestone for thermochemical energy storage. J. Adv. Res. 2020, 22, 67–76. [Google Scholar] [CrossRef]
- Prieto, C.; Cooper, P.; Fernández, A.I.; Cabeza, L.F. Review of technology: Thermochemical energy storage for concentrated solar power plants. Renew. Sustain. Energy Rev. 2016, 60, 909–929. [Google Scholar] [CrossRef] [Green Version]
- Abanades, J.C. The maximum capture efficiency of CO2 using a carbonation/calcination cycle of CaO/CaCO3. Chem. Eng. J. 2002, 90, 303–306. [Google Scholar] [CrossRef]
- Jiang, L. Comprehensiveutnization situation of fly ash in coal-fired power plants and its development suggestions. Clean Coal Technol. 2020, 26, 31–39. [Google Scholar]
- Benitez-Guerrero, M.; Valverde, J.M.; Sanchez-Jimenez, P.E.; Perejon, A.; Perez-Maqueda, L.A. Multicycle activity of natural CaCO3 minerals for thermochemical energy storage in Concentrated Solar Power plants. Sol. Energy 2017, 153, 188–199. [Google Scholar] [CrossRef]
- Setoodeh Jahromy, S.; Azam, M.; Jordan, C.; Harasek, M.; Winter, F. The Potential Use of Fly Ash from the Pulp and Paper Industry as Thermochemical Energy and CO2 Storage Material. Energies 2021, 14, 3348. [Google Scholar] [CrossRef]
- Perejón, A.; Valverde, J.M.; Miranda-Pizarro, J.; Sánchez-Jiménez, P.E.; Pérez-Maqueda, L.A. Large-Scale Storage of Concentrated Solar Power from Industrial Waste. ACS Sustain. Chem. Eng. 2017, 5, 2265–2272. [Google Scholar] [CrossRef]
- Valverde, J.M.; Miranda-Pizarro, J.; Perejón, A.; Sánchez-Jiménez, P.E.; Pérez-Maqueda, L.A. Calcium-Looping performance of steel and blast furnace slags for thermochemical energy storage in concentrated solar power plants. J. CO2 Util. 2017, 22, 143–154. [Google Scholar] [CrossRef]
- Bai, S.; Zhou, Y.; Chen, Y.; Wang, Z.; Sun, J.; Zhao, C. Thermochemical Energy Storage Performances of Steel Slag-Derived CaO-Based Composites. Chem. Eng. Technol. 2020, 43, 2190–2197. [Google Scholar] [CrossRef]
- Setoodeh Jahromy, S.; Jordan, C.; Azam, M.; Werner, A.; Harasek, M.; Winter, F. Fly Ash from Municipal Solid Waste Incineration as a Potential Thermochemical Energy Storage Material. Energy Fuels 2019, 33, 5810–5819. [Google Scholar] [CrossRef]
- Maaten, B.; Konist, A.; Siirde, A. Potential of solid residues from power plants as thermochemical energy storage materials. J. Therm. Anal. Calorim. 2020, 142, 1799–1805. [Google Scholar] [CrossRef]
- Arcenegui-Troya, J.; Sánchez-Jiménez, P.E.; Perejón, A.; Valverde, J.M.; Chacartegui, R.; Pérez-Maqueda, L.A. Calcium-Looping Performance of Biomineralized CaCO3 for CO2 Capture and Thermochemical Energy Storage. Ind. Eng. Chem. Res. 2020, 59, 12924–12933. [Google Scholar] [CrossRef]
- Scaltsoyiannes, A.A.; Lemonidou, A.A. On the factors affecting the deactivation of limestone under calcium looping conditions: A new comprehensive model. Chem. Eng. Sci. 2021, 243, 116797. [Google Scholar] [CrossRef]
- Huang, C.-H.; Chang, K.-P.; Yu, C.-T.; Chiang, P.-C.; Wang, C.-F. Development of high-temperature CO2 sorbents made of CaO-based mesoporous silica. Chem. Eng. J. 2010, 161, 129–135. [Google Scholar] [CrossRef]
- Sanchez-Jimenez, P.E.; Perez-Maqueda, L.A.; Valverde, J. Nanosilica supported CaO: A regenerable and mechanically hard CO2 sorbent at Ca-looping conditions. Appl. Energy 2014, 118, 92–99. [Google Scholar] [CrossRef] [Green Version]
- Benitez-Guerrero, M.; Valverde, J.M.; Perejon, A.; Sanchez-Jimenez, P.E.; Perez-Maqueda, L.A. Low-cost Ca-based composites synthesized by biotemplate method for thermochemical energy storage of concentrated solar power. Appl. Energy 2018, 210, 108–116. [Google Scholar] [CrossRef]
- Chen, X.; Jin, X.; Liu, Z.; Ling, X.; Wang, Y. Experimental investigation on the CaO/CaCO3 thermochemical energy storage with SiO2 doping. Energy 2018, 155, 128–138. [Google Scholar] [CrossRef]
- Bird, J.E.; Humphries, T.D.; Paskevicius, M.; Poupin, L.; Buckley, C.E. Thermal properties of thermochemical heat storage materials. Phys. Chem. Chem. Phys. 2020, 22, 4617–4625. [Google Scholar] [CrossRef] [PubMed]
- Khosa, A.A.; Zhao, C.Y. Heat storage and release performance analysis of CaCO3/CaO thermal energy storage system after doping nano silica. Sol. Energy 2019, 188, 619–630. [Google Scholar] [CrossRef]
- Møller, K.T.; Ibrahim, A.; Buckley, C.E.; Paskevicius, M. Inexpensive thermochemical energy storage utilising additive enhanced limestone. J. Mater. Chem. A 2020, 8, 9646–9653. [Google Scholar] [CrossRef]
- Benitez-Guerrero, M.; Valverde, J.M.; Sanchez-Jimenez, P.E.; Perejon, A.; Perez-Maqueda, L.A. Calcium-Looping performance of mechanically modified Al2O3-CaO composites for energy storage and CO2 capture. Chem. Eng. J. 2018, 334, 2343–2355. [Google Scholar] [CrossRef]
- Fedunik-Hofman, L.A.; Bayon, A.; Lipinski, W.; Donne, S.W. Investigation of novel hydroxyapatite-doped CaO material for calcination-carbonation thermochemical energy storage. AIP Conf. Proc. 2018, 2033, 100004. [Google Scholar]
- Han, R.; Gao, J.; Wei, S.; Su, Y.; Su, C.; Li, J.; Liu, Q.; Qin, Y. High-performance CaO-based composites synthesized using a space-confined chemical vapor deposition strategy for thermochemical energy storage. Sol. Energy Mater. Sol. Cells 2020, 206, 110346. [Google Scholar] [CrossRef]
- Sun, H.; Li, Y.; Yan, X.; Zhao, J.; Wang, Z. Thermochemical energy storage performance of Al2O3/CeO2 co-doped CaO-based material under high carbonation pressure. Appl. Energy 2020, 263, 114650. [Google Scholar] [CrossRef]
- Sun, H.; Li, Y.; Yan, X.; Wang, Z.; Liu, W. CaO/CaCO3 thermochemical heat storage performance of CaO-based micrometre-sized tubular composite. Energy Convers. Manag. 2020, 222, 113222. [Google Scholar] [CrossRef]
- Møller, K.T.; Humphries, T.D.; Berger, A.; Paskevicius, M.; Buckley, C.E. Thermochemical energy storage system development utilising limestone. Chem. Eng. J. Adv. 2021, 8, 100168. [Google Scholar] [CrossRef]
- Lu, S.; Wu, S. Calcination–carbonation durability of nano CaCO3 doped with Li2SO4. Chem. Eng. J. 2016, 294, 22–29. [Google Scholar] [CrossRef]
- Khosa, A.A.; Yan, J.; Zhao, C.Y. Investigating the effects of ZnO dopant on the thermodynamic and kinetic properties of CaCO3/CaO TCES system. Energy 2021, 215, 119132. [Google Scholar] [CrossRef]
- Xu, T.; Tian, X.; Khosa, A.; Yan, J.; Ye, Q.; Zhao, C. Reaction performance of CaCO3/CaO thermochemical energy storage with TiO2 dopant and experimental study in a fixed-bed reactor. Energy 2021, 236, 121451. [Google Scholar] [CrossRef]
- Sánchez Jiménez, P.E.; Perejón, A.; Benítez Guerrero, M.; Valverde, J.M.; Ortiz, C.; Pérez Maqueda, L.A. High-performance and low-cost macroporous calcium oxide based materials for thermochemical energy storage in concentrated solar power plants. Appl. Energy 2019, 235, 543–552. [Google Scholar] [CrossRef]
- Han, R.; Gao, J.; Wei, S.; Sun, F.; Liu, Q.; Qin, Y. Development of dense Ca-based, Al-stabilized composites with high volumetric energy density for thermochemical energy storage of concentrated solar power. Energy Convers. Manag. 2020, 221, 113201. [Google Scholar] [CrossRef]
- Han, R.; Gao, J.; Wei, S.; Su, Y.; Sun, F.; Zhao, G.; Qin, Y. Strongly coupled calcium carbonate/antioxidative graphite nanosheets composites with high cycling stability for thermochemical energy storage. Appl. Energy 2018, 231, 412–422. [Google Scholar] [CrossRef]
- Han, R.; Xing, S.; Wu, X.; Pang, C.; Lu, S.; Su, Y.; Liu, Q.; Song, C.; Gao, J. Compressing Two-Dimensional Graphite-Nanosheet-Supported CaO for Optimizing Porous Structures toward High-Volumetric-Performance Heat Storage. Energy Fuels 2021, 35, 10841–10849. [Google Scholar] [CrossRef]
- Song, C.; Liu, X.; Zheng, H.; Bao, C.; Teng, L.; Da, Y.; Jiang, F.; Li, C.; Li, Y.; Xuan, Y.; et al. Decomposition kinetics of Al- and Fe-doped calcium carbonate particles with improved solar absorbance and cycle stability. Chem. Eng. J. 2021, 406, 126282. [Google Scholar] [CrossRef]
- Li, B.; Li, Y.; Dou, Y.; Wang, Y.; Zhao, J.; Wang, T. SiC/Mn co-doped CaO pellets with enhanced optical and thermal properties for calcium looping thermochemical heat storage. Chem. Eng. J. 2021, 423, 130305. [Google Scholar] [CrossRef]
- Yang, L.; Huang, Z.; Huang, G. Fe- and Mn-Doped Ca-Based Materials for Thermochemical Energy Storage Systems. Energy Fuels 2020, 34, 11479–11488. [Google Scholar] [CrossRef]
- Zheng, H.; Song, C.; Bao, C.; Liu, X.; Xuan, Y.; Li, Y.; Ding, Y. Dark calcium carbonate particles for simultaneous full-spectrum solar thermal conversion and large-capacity thermochemical energy storage. Sol. Energy Mater. Sol. Cells 2020, 207, 110364. [Google Scholar] [CrossRef]









| SHS | LHS | TCHS | |
|---|---|---|---|
| Heat storage density | Low ~0.2 GJ/m3 | Medium ~0.3–0.5 GJ/m3 | High ~0.5–3 GJ/m3 |
| Working temperature | Low | Low or medium | Medium or high |
| Advantages | Mature technology Low price Long service life | Small heat storage volume Simple system | High heat storage density Small thermal losses Long-distance transportation |
| Disadvantages | High thermal losses Low heat storage density | Poor thermal conductivity Material corrosion High thermal losses | Complex technology High cost |
| Advantages | Disadvantages | |
|---|---|---|
| Falling particle | Simple structure design Low energy consumption | Poor heat transfer Uneven solar radiation distribution |
| Fluidized bed | High heat and mass transfer efficiency Mature industrial technology | Difficulty in gas-solid separation at high temperature Serious particle wear High requirements for flow rate control |
| Rotary kiln | Low particle wear Great heat and mass transfer Suitable for materials with large particle size | Difficult to integrate design with the solar system High equipment maintenance cost under high temperature |
| Cao-Based Materials | Particle Size | Calcination Conditions | Carbonation Conditions | Cycles | Effective Conversion | Reference |
|---|---|---|---|---|---|---|
| Limestone | <45 μm | 725 °C/He/5 min | 850 °C/CO2/5 min | 20 | 0.41 | [97] |
| >45 μm | 725 °C/He/5 min | 850 °C/CO2/5 min | 20 | 0.18 | [97] | |
| >160 μm | 725 °C/He/5 min | 850 °C/CO2/5 min | 20 | 0.21 | [102] | |
| >160 μm | 950 °C/CO2/5 min | 850 °C/CO2/5 min | 20 | 0.18 | [102] | |
| 100–400 μm | 1000 °C/CO2 | 850 °C/CO2/1 bar | 11 | 0.13 | [109] | |
| 100–400 μm | 1000 °C/CO2 | 850 °C/CO2/3 bar | 11 | 0.07 | [109] | |
| - | 725 °C/He/5 min | 850 °C/CO2/5 min | 20 | 0.51 | [115] | |
| Dolomite | <45 μm | 725 °C/He/5 min | 850 °C/CO2/5 min | 20 | 0.41 | [97] |
| >45 μm | 725 °C/He/5 min | 850 °C/CO2/5 min | 20 | 0.42 | [97] | |
| 100–400 μm | 1000 °C/CO2 | 850 °C/CO2/1 bar | 11 | 0.20 | [109] | |
| 100–400 μm | 1000 °C/CO2 | 850 °C/CO2/3 bar | 11 | 0.15 | [109] | |
| Marble | - | 725 °C/He/5 min | 850 °C/CO2/5 min | 20 | 0.27 | [115] |
| Chalk | - | 725 °C/He/5 min | 850 °C/CO2/5 min | 20 | 0.38 | [115] |
| Steel Slag | - | 675 °C/He/5 min | 850 °C/CO2/5 min | 20 | 0.63 | [117] |
| Steel Slag | - | 650 °C/He/5 min | 850 °C/CO2/5 min | 20 | 0.50 | [118] |
| Blast Furnace Slag | - | 650 °C/He/5 min | 850 °C/CO2/5 min | 20 | 0.29 | [118] |
| Carbide Slag | <125 μm | 850 °C/N2/10 min | 850 °C/CO2/5 min/13 bar | 30 | 0.51 | [98] |
| Snail Shell | 20 μm | 750 °C/N2/5 min | 850 °C/CO2/5 min | 20 | 0.24 | [122] |
| Eggshell | 20 μm | 750 °C/N2/5 min | 850 °C/CO2/5 min | 20 | 0.19 | [122] |
| Additives | Doping Ratio (wt%) | Carbonation Pressure (bar) | Cycles | Effective Conversion | Reference |
|---|---|---|---|---|---|
| SiO2 | 10% | 1 | 20 | 0.30 | [126] |
| SiO2 | 30% | 1 | 20 | 0.34 | [126] |
| SiO2 | 5% | 1 | 20 | 0.20 | [127] |
| SiO2 | 37.5% | 1 | 45 | 0.20 | [129] |
| SiO2 | 20% | 5 | 50 | 0.29 | [130] |
| Al2O3 | 20% | 5 | 50 | 0.62 | [130] |
| Al2O3 | 5% | 1 | 20 | 0.55 | [131] |
| ZrO2 | 5% | 1 | 10 | 0.22 | [109] |
| ZrO2 | 20% | 5 | 50 | 0.67 | [130] |
| ZrO2 | 40% | 5 | 50 | 0.45 | [130] |
| ZnO | 20% | 5 | 50 | 0.07 | [130] |
| Fe2O3 | 20% | 5 | 50 | 0.08 | [130] |
| Ni | 20% | 5 | 50 | 0.14 | [130] |
| BaCO3 | 9.5% | 5 | 50 | 0.09 | [130] |
| Li2SO4 | 5% | 1 | 11 | 0.48 | [137] |
| Al2O3/CeO2 | 5%/5% | 13 | 30 | 0.79 | [136] |
| Graphite | 20% | 5 | 50 | 0.25 | [130] |
| H3BO3/Graphite | 3% | 1 | 50 | 0.41 | [142] |
| Mn/Fe | - | 1 | 20 | 0.80 | [79] |
| Al/Citric acid | - | 1 | 20 | 0.7 | [133] |
| Acetic acid(Ac) | - | 1 | 30 | 0.56 | [140] |
| Mg/Ac | - | 1 | 30 | 0.70 | [140] |
| NaY | 20% | 5 | 50 | 0.23 | [130] |
| HY | 20% | 5 | 50 | 0.16 | [130] |
| Mor | 20% | 5 | 50 | 0.15 | [130] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Li, Y.; Yan, X.; Zhao, J.; Zhang, C. Development of Thermochemical Heat Storage Based on CaO/CaCO3 Cycles: A Review. Energies 2021, 14, 6847. https://doi.org/10.3390/en14206847
Yang Y, Li Y, Yan X, Zhao J, Zhang C. Development of Thermochemical Heat Storage Based on CaO/CaCO3 Cycles: A Review. Energies. 2021; 14(20):6847. https://doi.org/10.3390/en14206847
Chicago/Turabian StyleYang, Ying, Yingjie Li, Xianyao Yan, Jianli Zhao, and Chunxiao Zhang. 2021. "Development of Thermochemical Heat Storage Based on CaO/CaCO3 Cycles: A Review" Energies 14, no. 20: 6847. https://doi.org/10.3390/en14206847
APA StyleYang, Y., Li, Y., Yan, X., Zhao, J., & Zhang, C. (2021). Development of Thermochemical Heat Storage Based on CaO/CaCO3 Cycles: A Review. Energies, 14(20), 6847. https://doi.org/10.3390/en14206847







