Synthesis and Molecular Dynamics Simulation of Amphiphilic Low Molecular Weight Polymer Viscosity Reducer for Heavy Oil Cold Recovery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Molecular Dynamics Simulation Test
2.2.1. Establishment of Heavy Oil Structure Model
2.2.2. Kinetic Simulation of Viscosity Reducer Synthesis Process
2.2.3. Synthesis of Viscosity Reducer Synthesis Method of Viscosity Reducer
2.2.4. Characterization and Viscosity Reduction Effect
3. Results
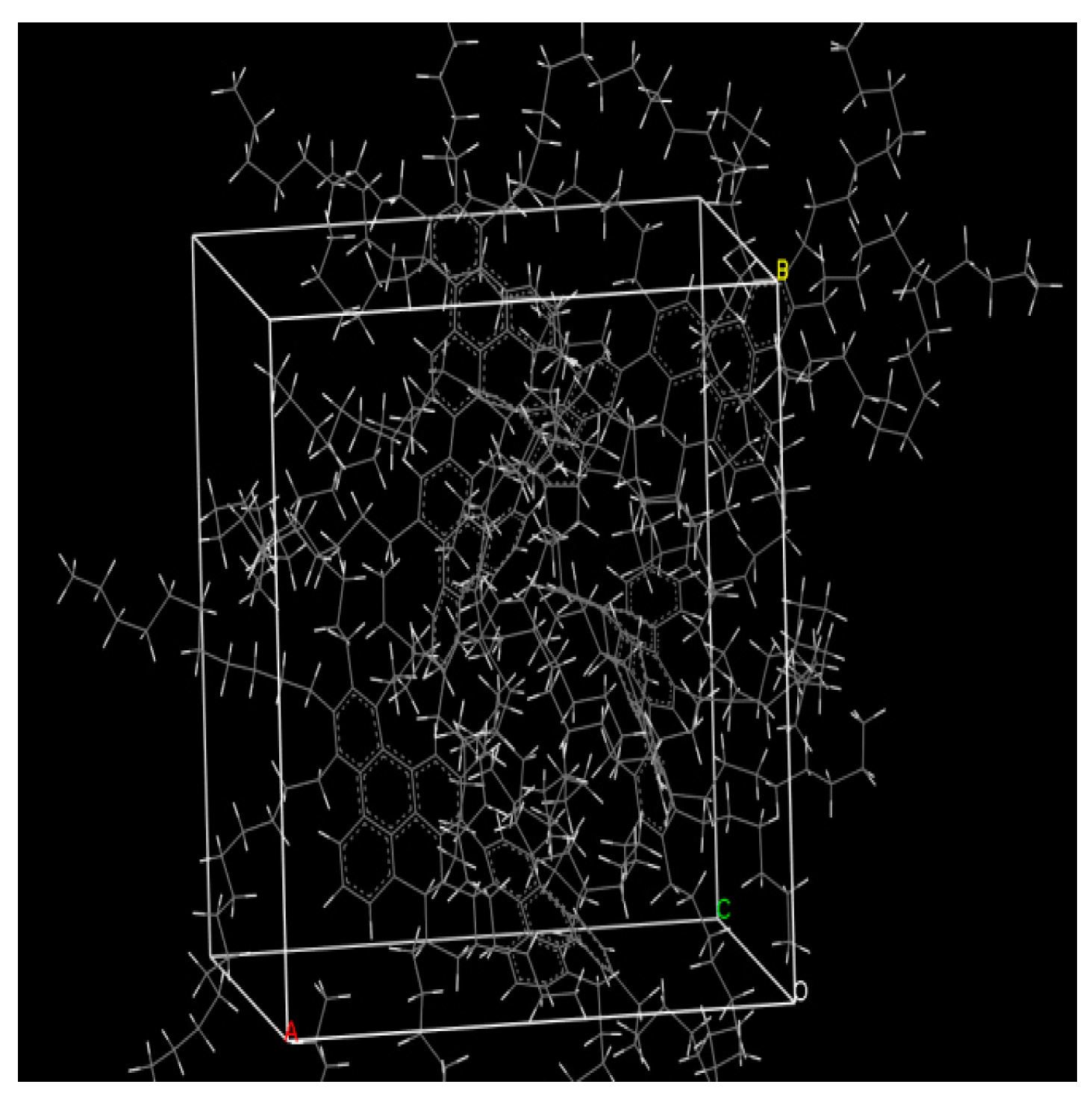
3.1. Establishment of Heavy Oil Structure Model
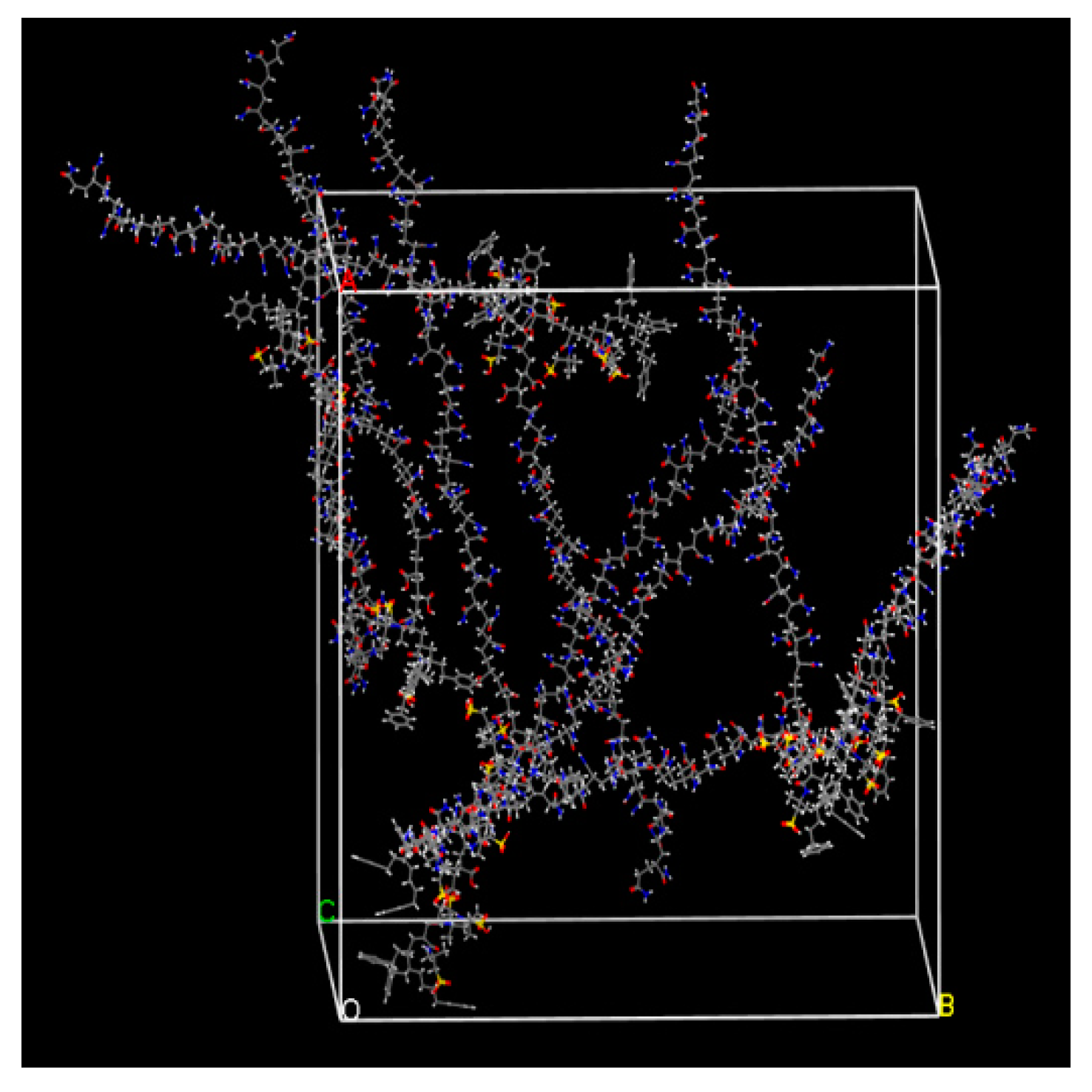
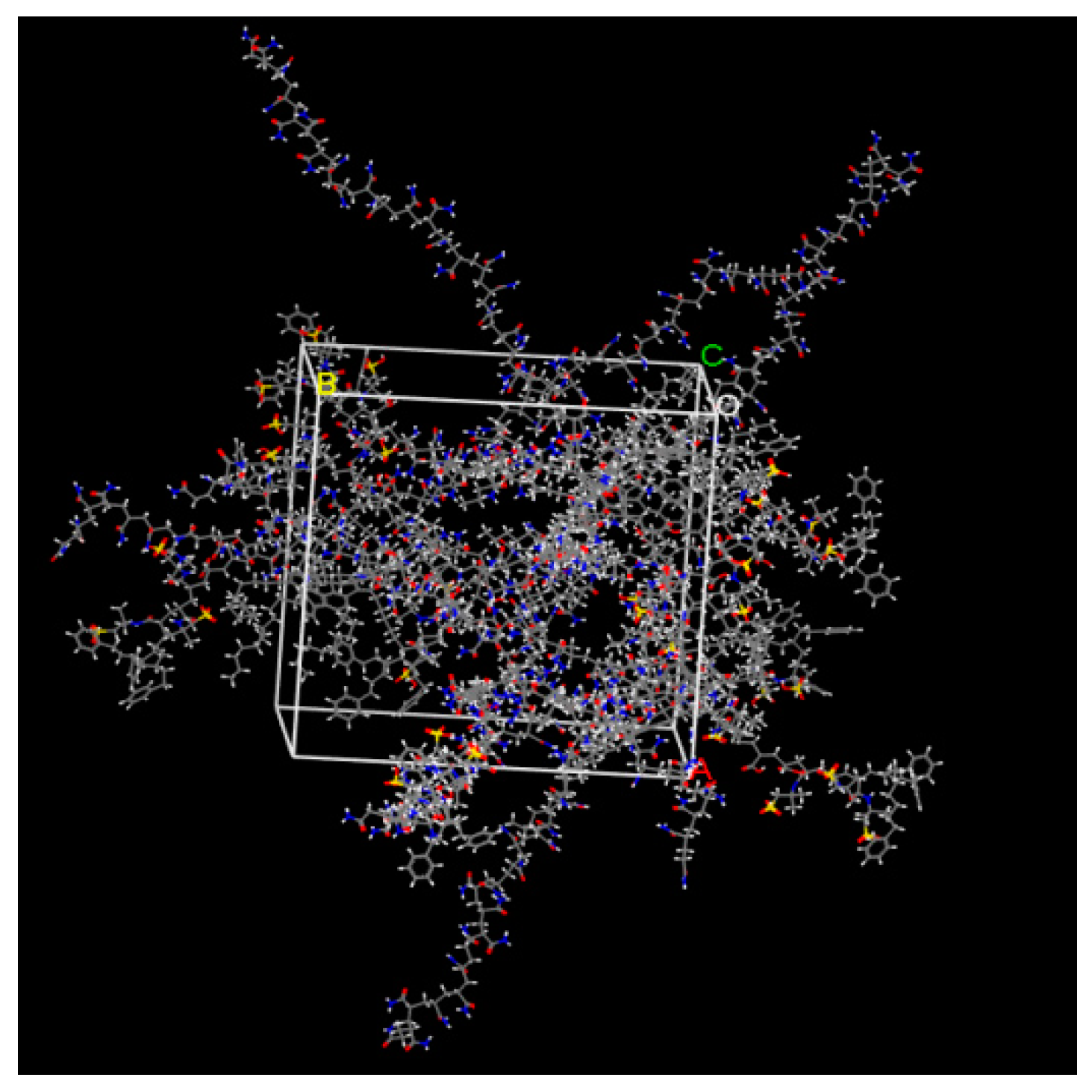
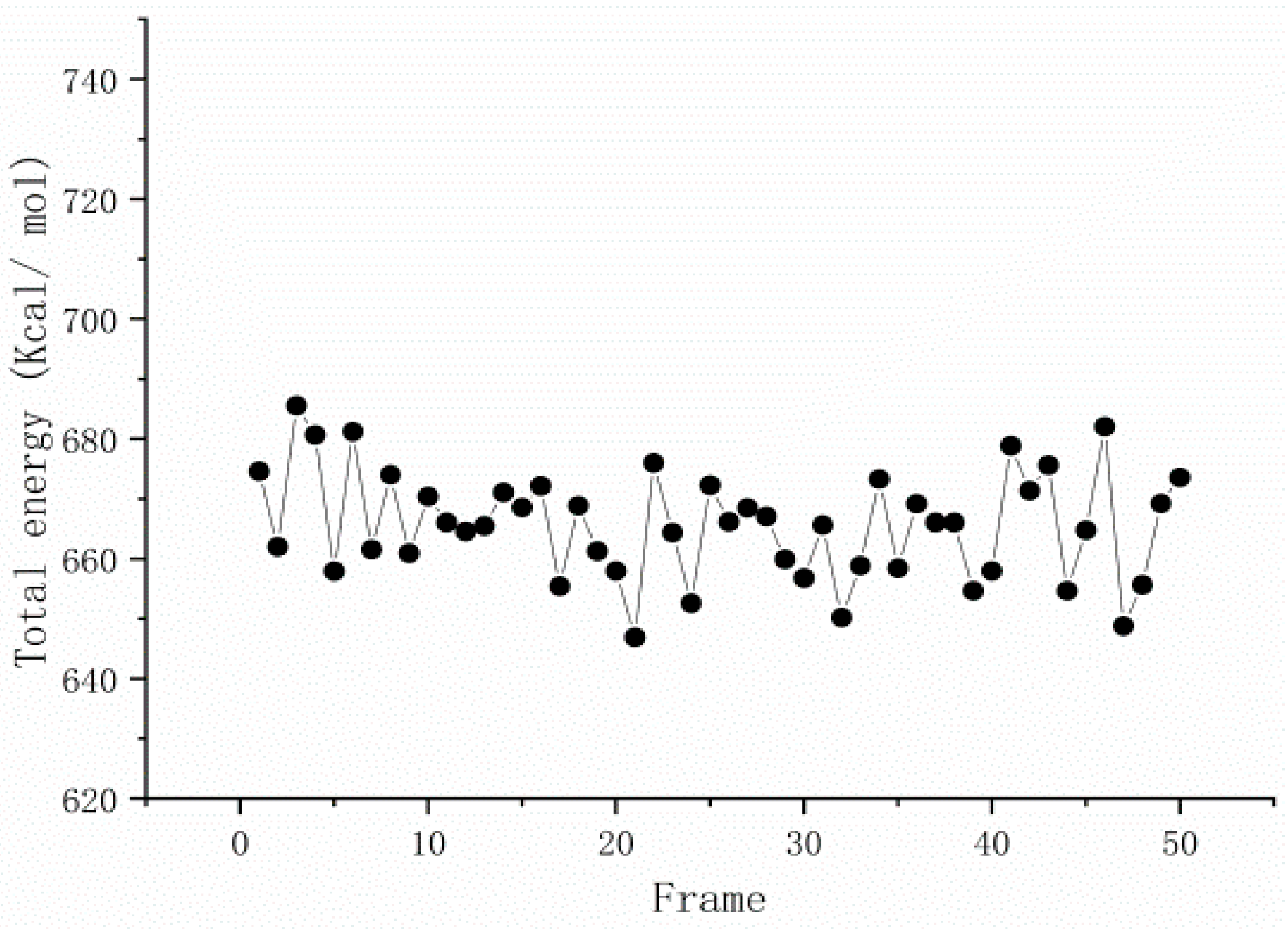
3.2. Dynamic Simulation of Viscosity Reducer Synthesis Process
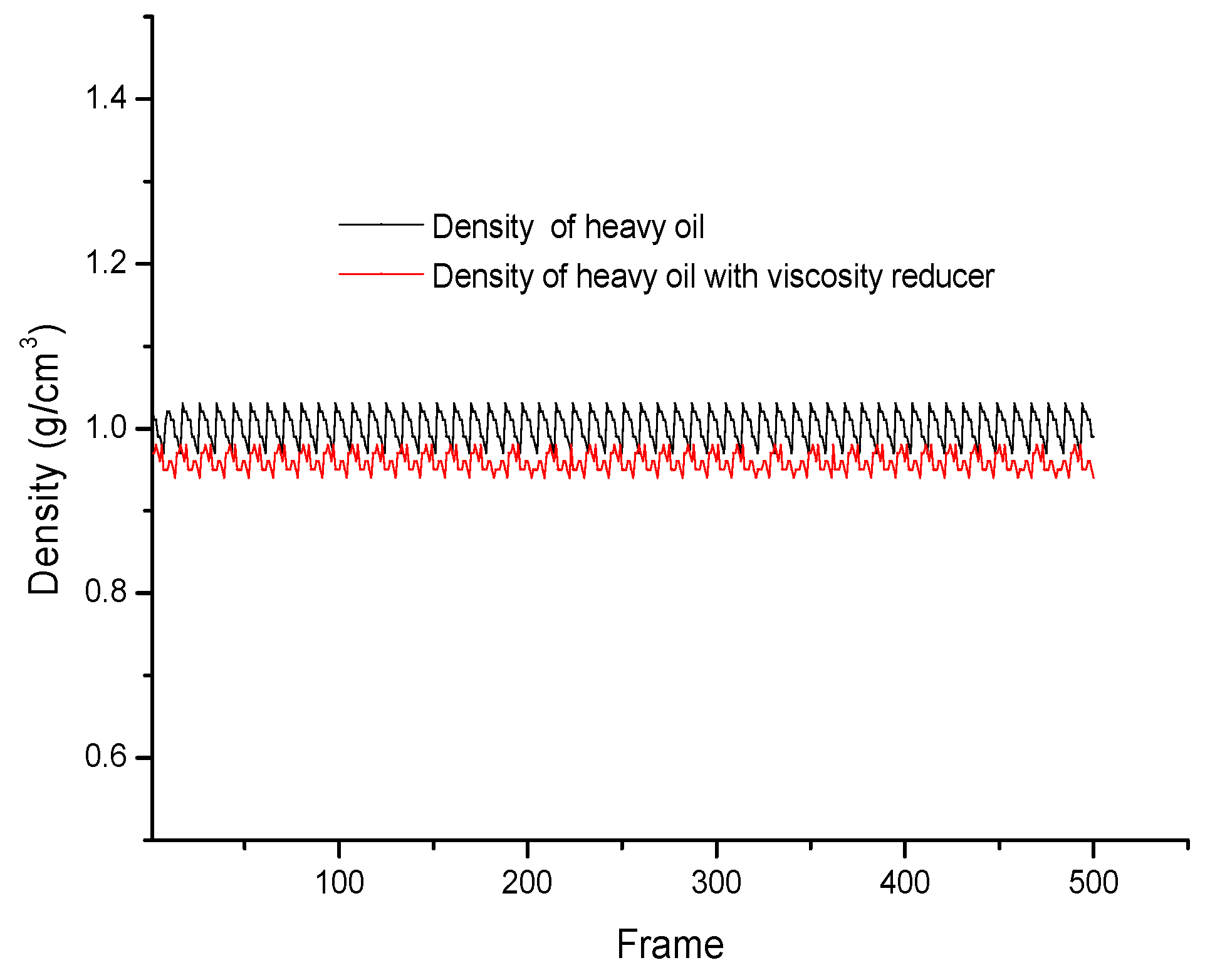
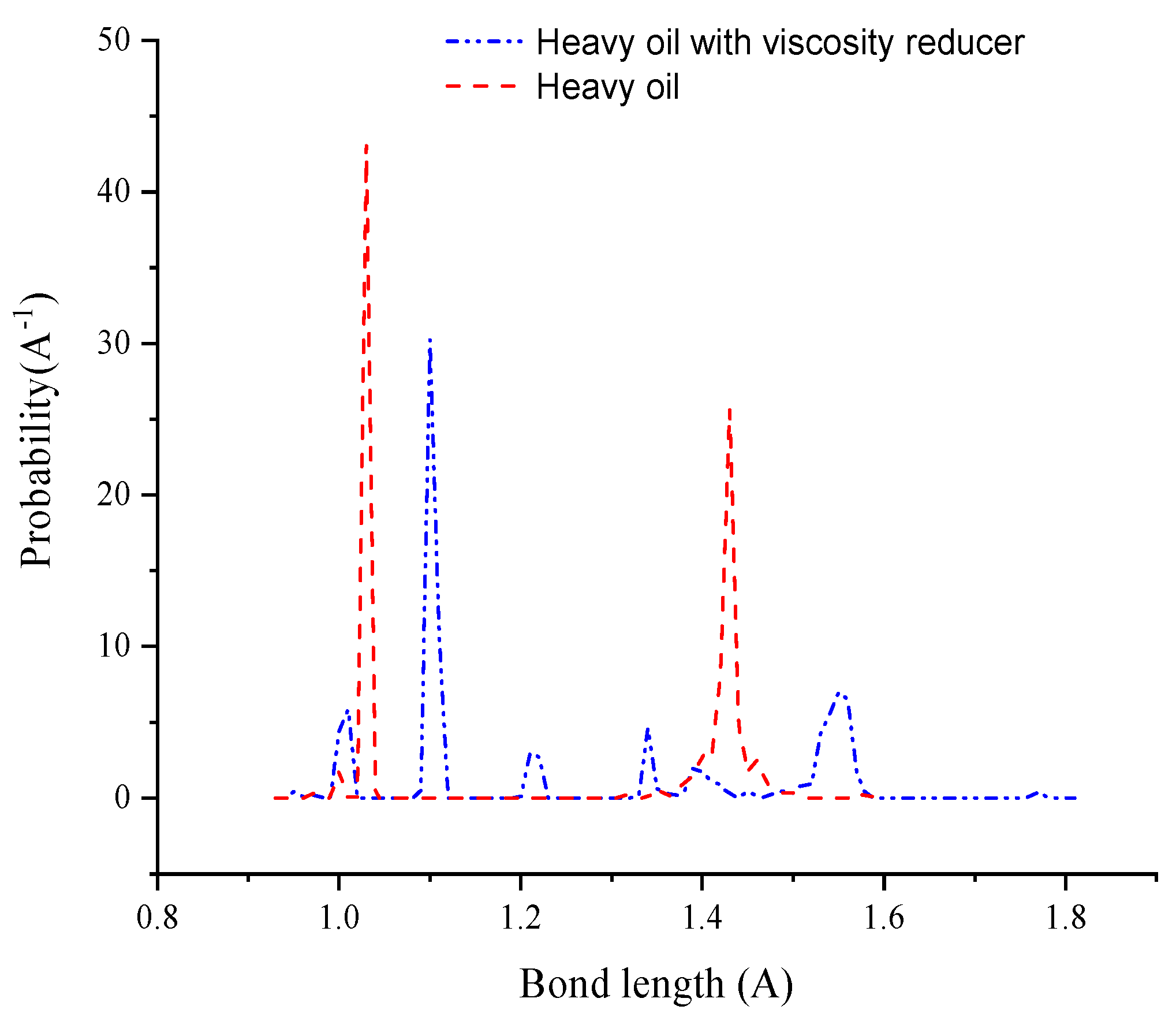
3.3. Dynamic Simulation of Viscosity Reduction Mechanism
3.4. Structure and Performance Characterization of Viscosity Reducer
3.4.1. IR Characterization of Viscosity Reducer
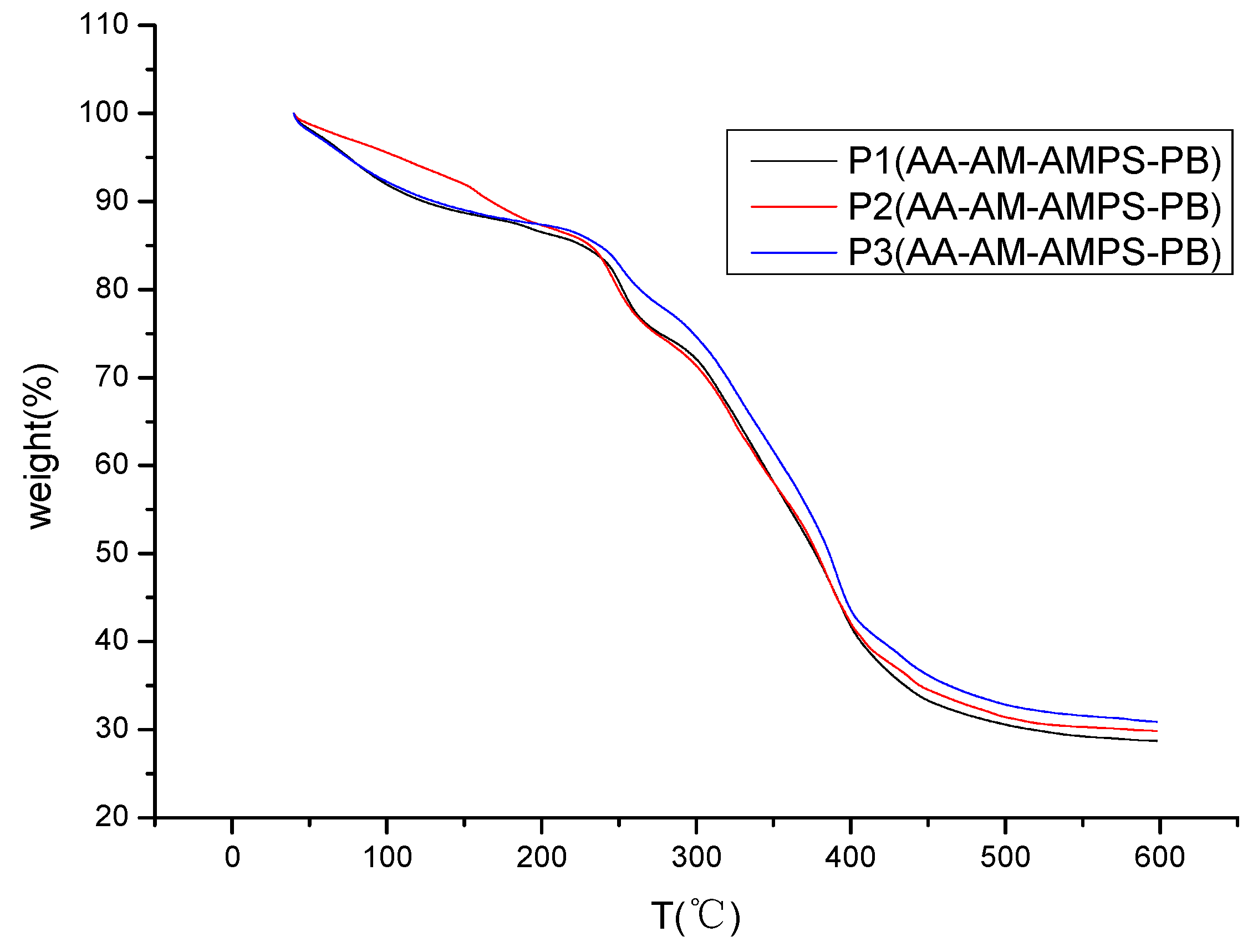
3.4.2. TGA Analysis of Viscosity Reducer
3.4.3. Molecular Weight Test of Viscosity Reducer
3.4.4. Effect of Viscosity Reducer on Heavy Oil Viscosity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xu, G.R.; Li, X.; Xie, K. Mechanism and oil dis-placement of heavy oil emulsification and viscosity reducer: A case study on heavy oil reservoir and fluid conditions of Bohai oilfield. J. Petrochem. Univ. 2016, 29, 57–62. [Google Scholar]
- Xiang, Y.C.; Ning, W.; Shu, Q.X. Research progress and development trend of heavy oil emulsifying viscosity reducer: A review. Pet. Sci. Technol. 2021, 39, 550–563. [Google Scholar] [CrossRef]
- Martínez-Palou, R.; Mosqueira, M.D.L.; Zapata-Rendón, B.; Mar-Juárez, E.; Bernal-Huicochea, C.; Clavel-López, J.D.L.C.; Aburto, J. Transportation of heavy and extra-heavy crude oil by pipeline: A review. J. Pet. Sci. Eng. 2011, 75, 274–282. [Google Scholar] [CrossRef]
- Alimohammadi, S.; Zendehboudi, S.; James, L. A comprehensive review of asphaltene deposition in petroleum reservoirs: Theory, challenges, and tips. Fuel 2019, 252, 753–791. [Google Scholar] [CrossRef]
- Fakher, S.; Ahdaya, M.; Elturki, M.; Imqam, A. Critical review of asphaltene properties and factors impacting its stability in crude oil. J. Pet. Explor. Prod. Technol. 2020, 10, 1183–1200. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.H.; Zhang, F.S.; Wu, Y.W.; Liu, G.L.; Li, X.N.; Su, H.M.; Zhu, Z.Y. Overview of emulsified viscosity reducer for enhancing heavy oil recovery. IOP Conf. Ser. Mater. Sci. Eng. 2019, 479, 012009. [Google Scholar] [CrossRef]
- Mai, A.; Bryan, J.; Goodarzi, N.; Kantzas, A. Insights Into Non-Thermal Recovery of Heavy Oil. J. Can. Pet. Technol. 2009, 48, 27–35. [Google Scholar] [CrossRef]
- Babadagli, T.; Er, V.; Naderi, K.; Burkus, Z.; Ozum, B. Use of Biodiesel as an Additive in Thermal Recovery of Heavy Oil and Bitumen. J. Can. Pet. Technol. 2010, 49, 43–48. [Google Scholar] [CrossRef]
- Alvarado, V.; Manrique, E. Enhanced Oil Recovery: An Update Review. Energies 2010, 3, 1529–1575. [Google Scholar] [CrossRef]
- Su, B.; Fujimitsu, Y. Research on Heavy Oil Thermal Recovery by CO2 Steam Flooding with Help of Combination of Borehole-Surface Electric Potential and Cross-Borehole Electric Potential. Energy Explor. Exploit. 2011, 29, 797–815. [Google Scholar] [CrossRef]
- Babadagli, T.; Ozum, B. BioDiesel as Additive in High Pressure and Temperature Steam Recovery of Heavy Oil and Bitumen. Oil Gas Sci. Technol.-Rev. d’IFP Energ. Nouv. 2012, 67, 413–421. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.B.; Wang, N.; Xia, N.; Huan, S.X.; Yan, D. Evaluation and mechanism analysis of emulsion viscosity reduction performance of enhanced dispersion system. J. Petrochem. Univ. 2016, 29, 18–22. [Google Scholar]
- Zhang, L.; Pan, Z.; Zhang, Z.; Yan, S.L.; Wen, J.; Chen, S. Thermodynamic and Economic Analysis Between Organic Rankine Cycle and Kalina Cycle for Waste Heat Recovery From Steam-Assisted Gravity Drainage Process in Oilfield. J. Energy Resour. Technol. 2018, 140, 122005. [Google Scholar] [CrossRef]
- Maity, S.K.; Ancheyta, J.; Marroquín, G. Catalytic Aquathermolysis Used for Viscosity Reduction of Heavy Crude Oils: A Review. Energy Fuels 2010, 24, 2809–2816. [Google Scholar] [CrossRef]
- Roscioli, J.R.; Herndon, S.C.; Yacovitch, T.I.; Knighton, W.B.; Zavala-Araiza, D.; Johnson, M.R.; Tyner, D.R. Characterization of methane emissions from five cold heavy oil production with sands (CHOPS) facilities. J. Air Waste Manag. Assoc. 2018, 68, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.T.; Lu, X.G.; Cao, W.J.; Zhang, Y.B.; Wang, N.; Xia, H. Viscosity reduction effect and mechanism of heavy oil surfactant emulsification-Bohai BZ25-1s reservoir as an example. J. Pet. Chem. Univ. 2017, 30, 26–31. [Google Scholar]
- Kang, W.L.; Liu, Y.L.; Meng, L.W. Screening of spontaneous emulsified viscosity reducer for heavy oil in Yongping Oilfield and evaluation of oil displacement effect. Pet. Geol. Recovery Effic. 2012, 1, 59–61. [Google Scholar]
- Zhang, X. Synthesis and Solution Properties of Microblock Amphiphilic Polymers; Southwest Petroleum University: Chengdu, China, 2015. [Google Scholar]
- Jia, H.; Huang, P.; Han, Y.; Wang, Q.; Wei, X.; Huang, W.; Dai, J.; Song, J.; Yan, H.; Liu, D. Synergistic effects of Janus graphene oxide and surfactants on the heavy oil/water interfacial tension and their application to enhance heavy oil recovery. J. Mol. Liq. 2020, 314, 113791. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Q.; Jiang, Y. Discussion on the status quo and application of heavy oil non-heating gathering technology. Nat. Gas Pet. 2010, 1, 6–9. [Google Scholar]
- Duan, H.B.; Liu, Q.; Luo, W.Y. Study on Viscosity Reduction Technology and Mechanism of Tahe Heavy Oil. Chem. Eng. 2013, 12, 26–30. [Google Scholar]
- Fan, J.L.; Wu, B.F.; Li, Y. Study on Emulsification and Viscosity Reduction of Henan Super Heavy Oil. Oilfield Chem. 2014, 3, 451–456. [Google Scholar]
- Li, M.R.; Qi, L.Y.; Wang, W.; Lin, T.S.K. Study on Emulsification and Viscosity Reduction Mechanism of Shengli Super Heavy Oil. J. Fuel Chem. 2013, 6, 679–684. [Google Scholar]
- Yu, L.L. Preparation and Performance Evaluation of Polymer Heavy Oil Viscosity Reducer; Jilin University: Jilin, China, 2012. [Google Scholar]
- Zhang, L.J.; Yue, X.G.; Guo, Z.J. Study on Emulsification Characteristics of ASP System and Dagang and Daqing Crude Oils. Pet. Geol. Recovery Effic. 2010, 3, 74–76. [Google Scholar]
- Cui, G.S. Study on Emulsification and Viscosity Reduction Method and Mechanism of Heavy Oil; China University of Petroleum: Beijing, China, 2009. [Google Scholar]
- Ma, C.; Zhu, Q.; Pan, M.L. Discussion on flocculation of temperature sensitive polymer in heavy oil wastewater. Ind. Water Treat. 2017, 37, 50–53. [Google Scholar]
- Ma, C.; Pan, M.L.; Su, C. The oil displacement effect of a temperature sensitive polymer solution containing benzene ring structure. Appl. Chem. Ind. 2017, 46, 1047–1050. [Google Scholar]
- Ma, C.; Wang, G.; Zhang, X. Synthesis and Solution Properties of Temperature-Sensitive Copolymer OBS/DEAM/AM. Polym. Mater. Sci. Eng. 2016, 32, 28–32. [Google Scholar]
- Test method for separation of asphalt into four fractions. Oil and Gas Industry Standard Assay for Determination of Petroleum Asphaltene Components. (SH /T 0509-2010).
- Wu, C.; Zhang, R.S.; Zhang, Z.G.; Sui, Z.Y.; Wu, Z.B. Molecular simulation and mechanism of viscosity reduction and upgrading of super heavy oil. Pet. J. 2015, 36, 355–360. [Google Scholar]
- Li, Q.; Pu, W.F.; Wang, Y.B.; Geng, X.F. Synthesis and Properties of AM/AMPS Copolymers. Appl. Chem. Ind. 2012, 41, 300–303. [Google Scholar]
- Zhang, S.P. Synthesis of Amphiphilic Polymers Containing Quaternary Quaternary Ammonium Salts and Their Application in Viscosity Reduction of Heavy Oils; Shandong University: Jinan, China, 2017. [Google Scholar]
- Li, Y.Y.; Wu, Q.; Xu, P.; Ma, A.Q.; Hou, Y.F. Preparation and performance optimization of a new viscosity-reducing agent for crude oil based on molecular simulation. Chem. Ind. Eng. Progress 2020, 12, 5234–5242. [Google Scholar]
- Zhu, J.; Li, C.X.; Xin, P.G. The effect of structure of viscosity reducer to viscosity reducing effect for heavy oil. J. Petrochem. Univ. 2011, 24, 39–42. [Google Scholar]
- Cui, Q.; Zhang, C.Q.; Xiu, J.X.; Xiu, S.M.; Lu, L.L. Molecular dynamics simulation of viscosity reduction mechanism of heavy oil asphaltene colloid. J. Shandong Univ. (Eng. Sci.) 2017, 47, 123–130. [Google Scholar]
- Khabaz, F.; Khare, R. Effect of chain architecture on the size, shape, and intrinsic viscosity of chains in polymer solutions: A molecular simulation study. J. Chem. Phys. 2014, 141, 214904. [Google Scholar] [CrossRef] [PubMed]

 Hydrophobic group containing benzene ring;
Hydrophobic group containing benzene ring;  Carboxyl group/amide group/sulfonic acid group;
Carboxyl group/amide group/sulfonic acid group;  Water molecule;
Water molecule;  Heavy oil.
Heavy oil.
 Hydrophobic group containing benzene ring;
Hydrophobic group containing benzene ring;  Carboxyl group/amide group/sulfonic acid group;
Carboxyl group/amide group/sulfonic acid group;  Water molecule;
Water molecule;  Heavy oil.
Heavy oil.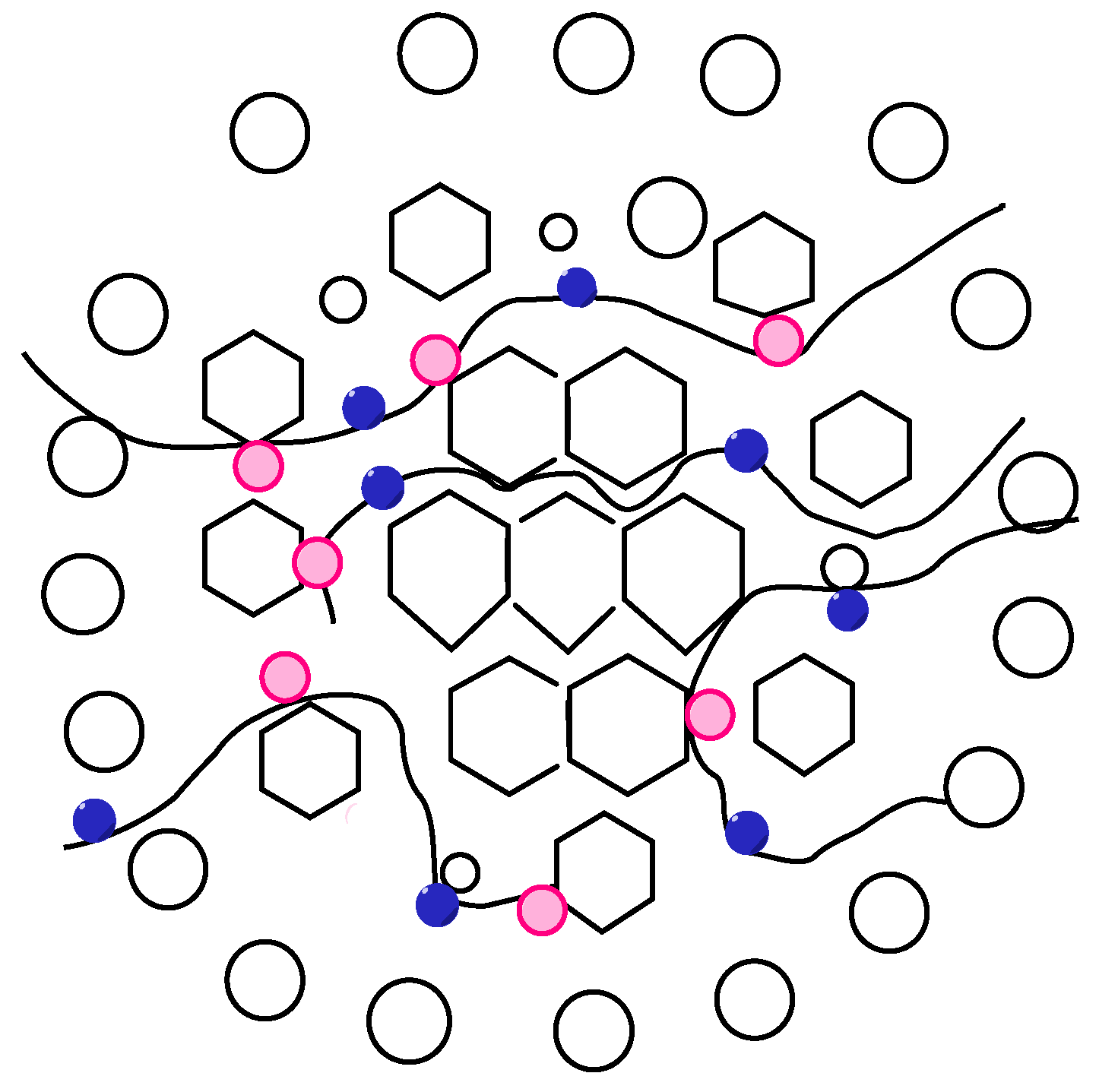




| Component | Unit | Content |
|---|---|---|
| Saturated hydrocarbon content | % | 52.15 |
| Aromatics content | % | 27.10 |
| Resin | % | 31.70 |
| Asphalt | % | 7.68 |
| Viscosity of heavy oil(50 °C) | mPa.s | 1134.5 |
| Pour point of crude oil | °C | 44.5 |
| Component | Representative Component Number | Number of Molecules |
|---|---|---|
| Asphaltene | 1 | 2 |
| 2 | 2 | |
| 3 | 3 | |
| Colloid | 4 | 4 |
| 5 | 8 | |
| 6 | 8 | |
| 7 | 4 | |
| 8 | 2 | |
| 9 | 2 | |
| 10 | 3 | |
| Aromatics hydrocarbon | 11 | 6 |
| 12 | 7 | |
| 13 | 9 | |
| 14 | 5 | |
| Saturated hydrocarbon | 15 | 30 |
| 16 | 22 |
| Sample | Symbol | Meaning | Value |
|---|---|---|---|
| P1 (AA-AM-AMPS-PB) | Mw | The weight average molecular weight | 83,567 |
| Mn | The number average molecular weight | 59,423 | |
| Mw/Mn | The ratio of weight average molecular weight and number average molecular weight | 1.420 | |
| P2 (AA-AM-AMPS-PB) | Mw | The weight average molecular weight | 94,563 |
| Mn | The number average molecular weight | 48,765 | |
| Mw/Mn | The ratio of weight average molecular weight and number average molecular weight | 1.930 | |
| P3 (AA-AM-AMPS-PB) | Mw | The weight average molecular weight | 84,789 |
| Mn | The number average molecular weight | 56,328 | |
| Mw/Mn | The ratio of weight average molecular weight and number average molecular weight | 1.510 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, C.; Liu, X.; Xie, L.; Chen, Y.; Ren, W.; Gu, W.; Zhang, M.; Zhou, H. Synthesis and Molecular Dynamics Simulation of Amphiphilic Low Molecular Weight Polymer Viscosity Reducer for Heavy Oil Cold Recovery. Energies 2021, 14, 6856. https://doi.org/10.3390/en14216856
Ma C, Liu X, Xie L, Chen Y, Ren W, Gu W, Zhang M, Zhou H. Synthesis and Molecular Dynamics Simulation of Amphiphilic Low Molecular Weight Polymer Viscosity Reducer for Heavy Oil Cold Recovery. Energies. 2021; 14(21):6856. https://doi.org/10.3390/en14216856
Chicago/Turabian StyleMa, Chao, Xingyu Liu, Longlong Xie, Yan Chen, Wendong Ren, Wen Gu, Minghua Zhang, and Huili Zhou. 2021. "Synthesis and Molecular Dynamics Simulation of Amphiphilic Low Molecular Weight Polymer Viscosity Reducer for Heavy Oil Cold Recovery" Energies 14, no. 21: 6856. https://doi.org/10.3390/en14216856






