Review on Carbon Capture in ICE Driven Transport
Abstract
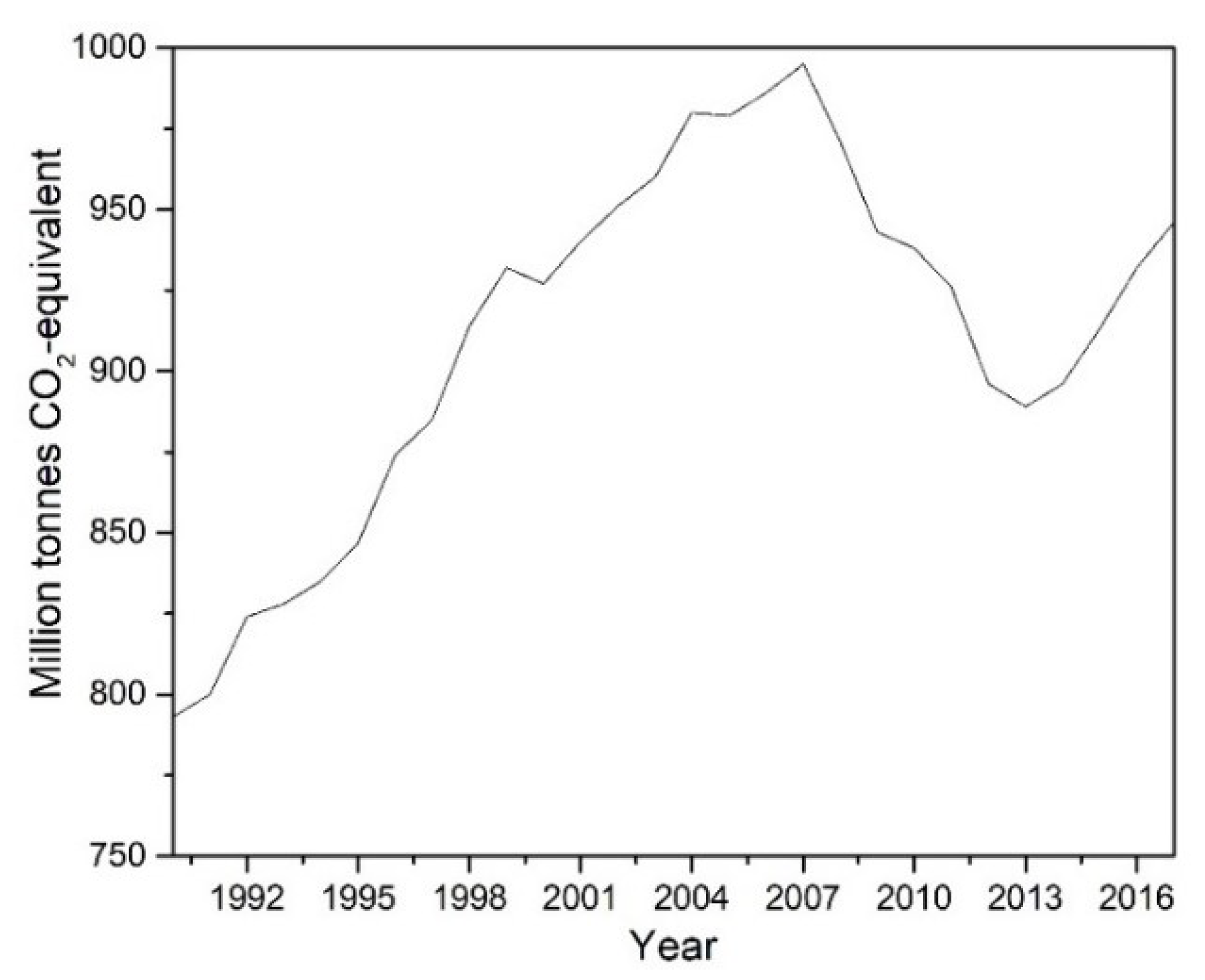
:1. Introduction
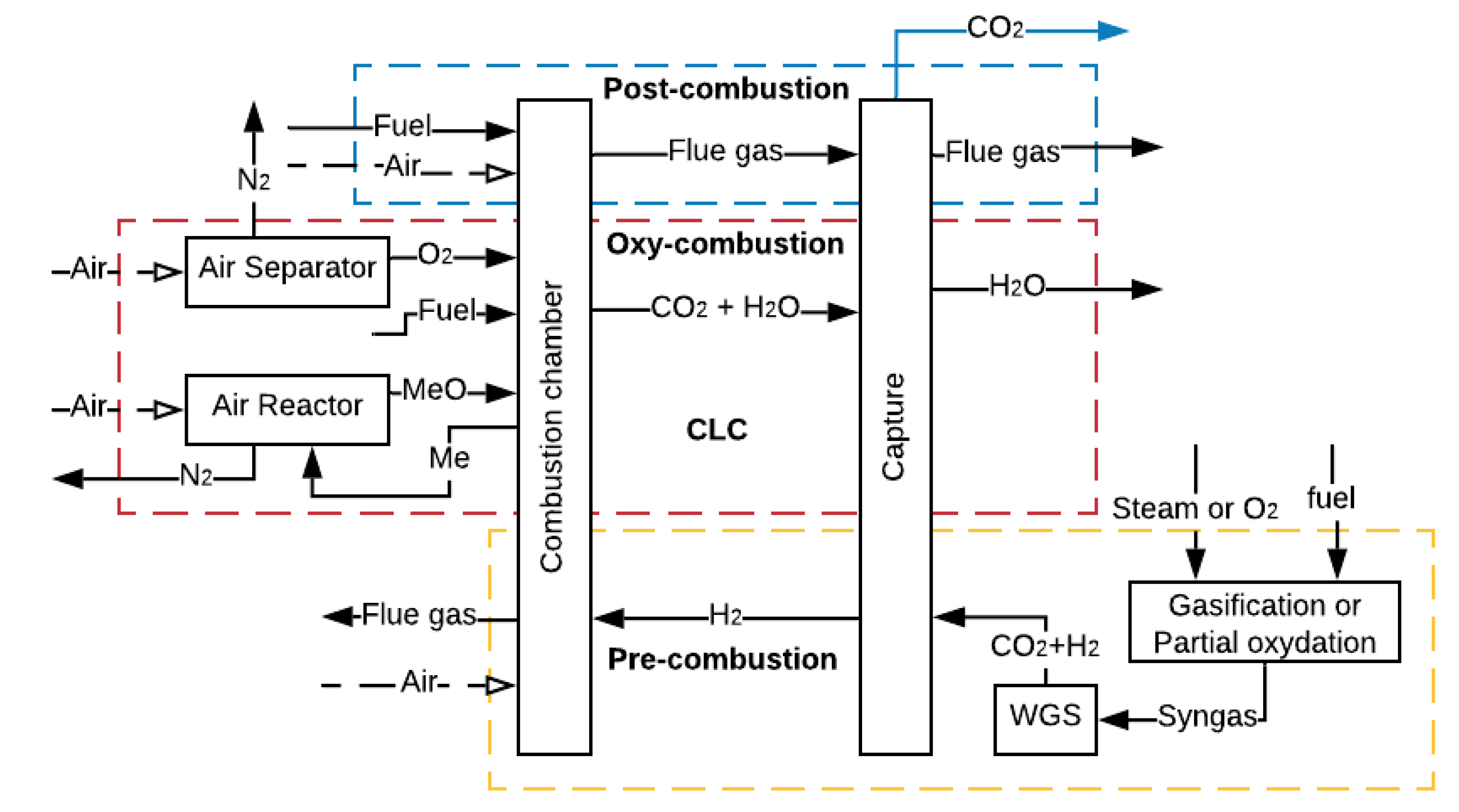
2. Carbon Capture and Storage
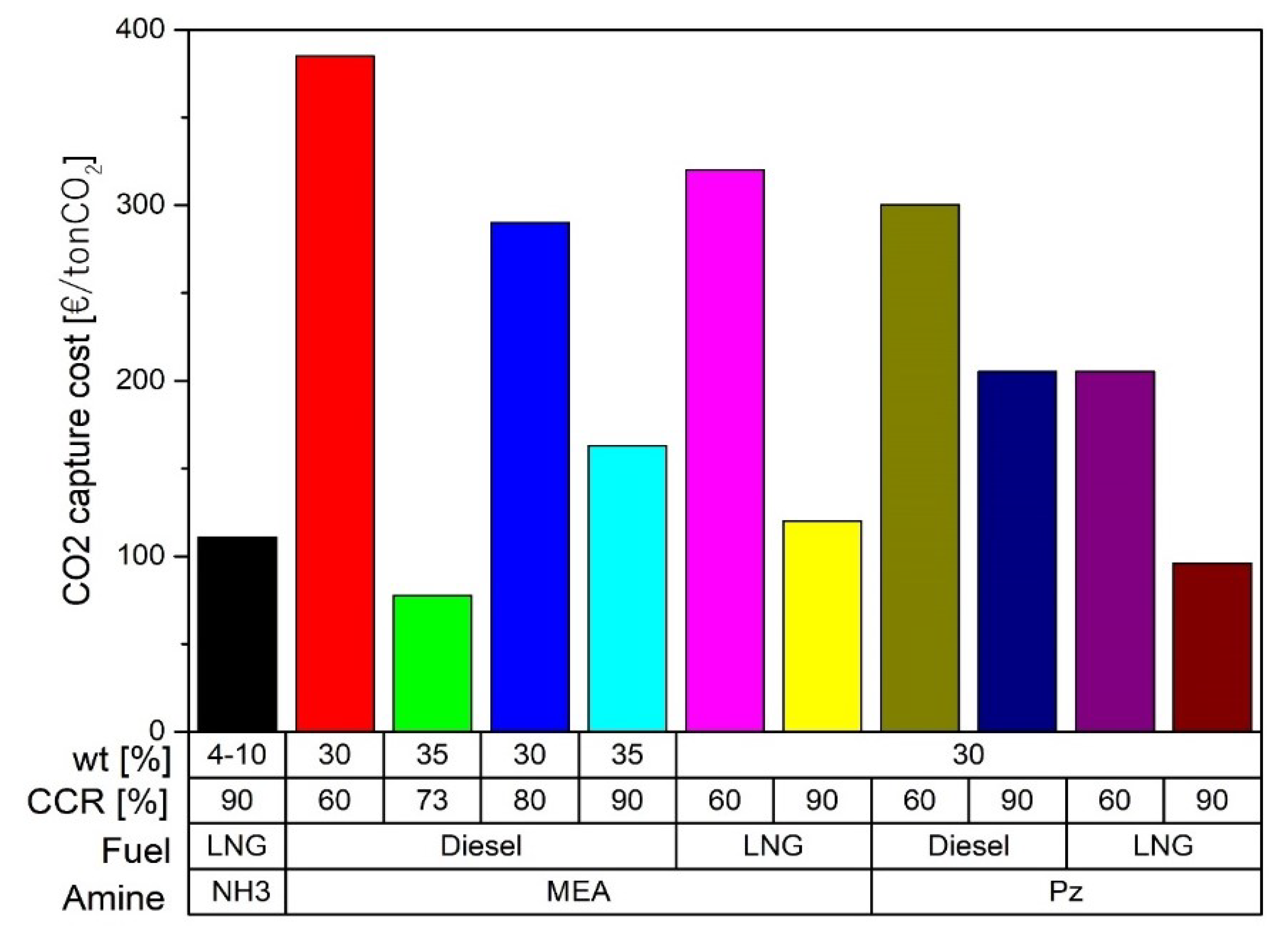
2.1. Amine-Absorption
2.2. Adsorption
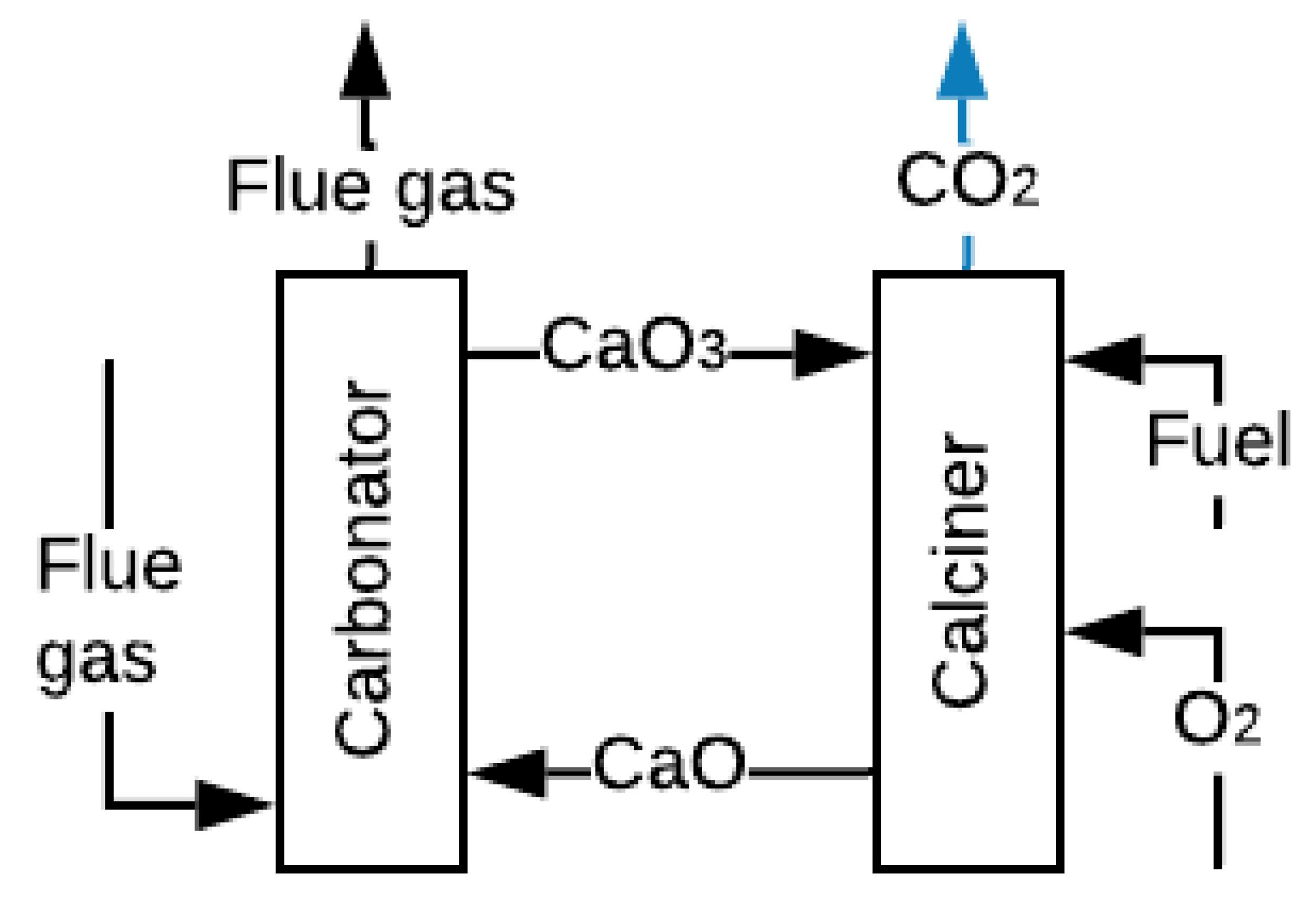
2.3. Carbonate Looping
2.4. Membranes
2.5. Other Forms for CCS
3. CCS in the Transport Sector
3.1. Maritime Sector
3.2. Internal Combustion Engines Vehicles (ICEv)
4. Case Study
4.1. Engine Selection
4.2. Sorbent Selection
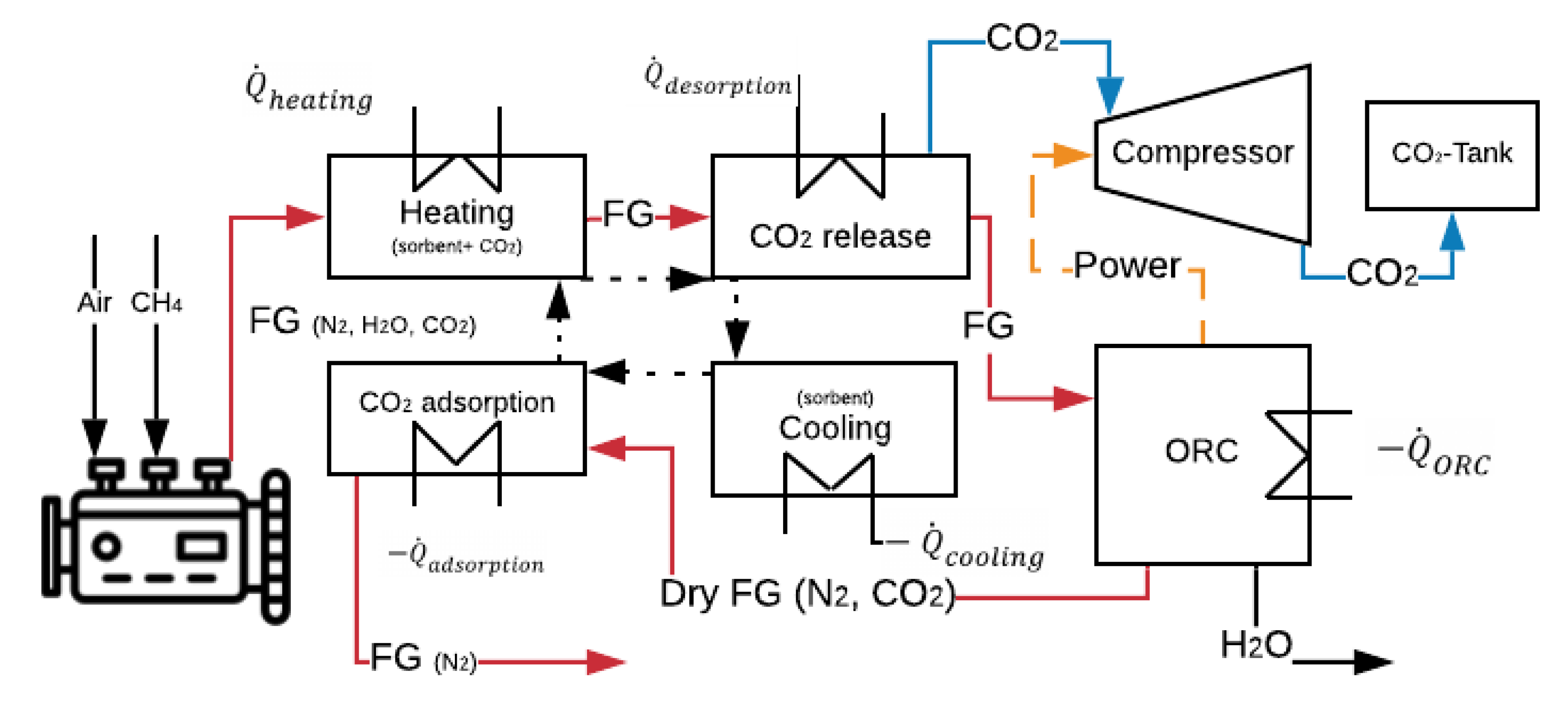
4.3. CCS System Description
4.4. Energy Balance
4.5. Space Requirement
4.6. Results
4.6.1. Energy Results
4.6.2. Space Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Desorption Heat | |
| Sorbent Regeneration Heat | |
| Remaining Heat in the FG | |
| Sensible Heat | |
| CO2 Compressor Power | |
| ORC Power Output | |
| Absorption Heat | |
| Adsorption Heat | |
| Specific Heat | |
| Difference Temperature | |
| AC | Activate Carbon |
| ASU | Air Separator Units |
| CCS | CO2 Capture and Storage |
| CL | Carbonate Looping |
| CLC | Chemical Looping Combustion |
| DEA | Diethanolamine |
| EU | European Union |
| EVs | Electric Vehicles |
| FG | Flue gas |
| ICE | Internal Combustion Engines |
| ICEv | Internal Combustion Engine Vehicles |
| ICRC | Internal Combustion Rankine Cycle |
| IMO | International Maritime Organization |
| LNG | Liquified Natural Gas |
| LPG | Liquefied Petroleum Gas |
| MDEA | Methyl Diethanolamine |
| MEA | Ethanolamine |
| MOFs | Metal-Organic Frameworks |
| NG | Natural Gas |
| ORC | Organic Rankine Cycle |
| PPNs | Porous Polymer Networks |
| PTG | Power to Gas |
| Pz | Piperazine |
| RWGS | Reverse Water-Gas Shift |
| TEG | Thermo-Electric Generators |
| TSA | Temperature Swing Adsorption |
| US-EPA | United States Environmental Protection Agency |
| CO2 Loading Capacity | |
| Mass Fraction | |
| Density |
References
- IEA Total Final Consumption (TFC) by Sector, World 1990–2017. Available online: https://www.iea.org/data-and-statistics?country=WORLD&fuel=Energy%20consumption&indicator=Total%20final%20consumption%20(TFC)%20by%20sector (accessed on 27 November 2019).
- Bellocchi, S.; De Falco, M.; Gambini, M.; Manno, M.; Stilo, T.; Vellini, M. Opportunities for power-to-Gas and Power-to-liquid in CO2-reduced energy scenarios: The Italian case. Energy 2019, 175, 847–861. [Google Scholar] [CrossRef]
- Hof, A.F.; den Elzen, M.G.J.; Admiraal, A.; Roelfsema, M.; Gernaat, D.E.H.J.; van Vuuren, D.P. Global and regional abatement costs of Nationally Determined Contributions (NDCs) and of enhanced action to levels well below 2 °C and 1.5 °C. Environ. Sci. Policy 2017, 71, 30–40. [Google Scholar] [CrossRef]
- European Commission. A Clean Planet for all A European Long-Term Strategic Vision for a Prosperous, Modern, Competitive and Climate Neutral Economy; European Commission: Luxembourg, 2018; Volume 773, p. 114. [Google Scholar]
- Tamborra, M. European Commission, DG Research. 2006. Available online: https://www.iccr.org/ (accessed on 27 November 2019).
- Akram, M.; Ali, U.; Best, T.; Blakey, S.; Finney, K.N.; Pourkashanian, M. Performance evaluation of PACT Pilot-plant for CO2 capture from gas turbines with Exhaust Gas Recycle. Int. J. Greenh. Gas Control. 2016, 47, 137–150. [Google Scholar] [CrossRef]
- Leach, F.; Kalghatgi, G.; Stone, R.; Miles, P. The scope for improving the efficiency and environmental impact of internal combustion engines. Transp. Eng. 2020, 1, 100005. [Google Scholar] [CrossRef]
- Huang, Y.; Ng, E.C.Y.; Zhou, J.L.; Surawski, N.C.; Chan, E.F.C.; Hong, G. Eco-driving technology for sustainable road transport: A review. Renew. Sustain. Energy Rev. 2018, 93, 596–609. [Google Scholar] [CrossRef]
- Fafoutellis, P.; Mantouka, E.G.; Vlahogianni, E.I. Eco-driving and its impacts on fuel efficiency: An overview of technologies and data-driven methods. Sustainability 2021, 13, 226. [Google Scholar] [CrossRef]
- Agudelo, A.F.; García-Contreras, R.; Agudelo, J.R.; Armas, O. Potential for exhaust gas energy recovery in a diesel passenger car under European driving cycle. Appl. Energy 2016, 174, 201–212. [Google Scholar] [CrossRef]
- Merkisz, J.; Fuc, P.; Lijewski, P.; Ziolkowski, A.; Wojciechowski, K.T. The Analysis of Exhaust Gas Thermal Energy Recovery Through a TEG Generator in City Traffic Conditions Reproduced on a Dynamic Engine Test Bed. J. Electron. Mater. 2015, 44, 1704–1715. [Google Scholar] [CrossRef] [Green Version]
- Arsie, I.; Cricchio, A.; Pianese, C.; Ricciardi, V.; De Cesare, M. Modeling analysis of waste heat recovery via thermo-electric generator and electric turbo-compound for CO2 reduction in automotive SI engines. Energy Procedia 2015, 82, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Sprouse, C.; Depcik, C. Review of organic Rankine cycles for internal combustion engine exhaust waste heat recovery. Appl. Therm. Eng. 2013, 51, 711–722. [Google Scholar] [CrossRef]
- Kühlwein, J. Driving Resistances of Light-Duty Vehicles in Europe: Present Situation, Trends and Scenarios for 2025. Communications 2016, 49, 847129-102. [Google Scholar]
- European Commission. Roadmap to a Single European Transport Area—Towards a Competitive and Resource Efficient Transport System; European Commission: Brussels, Belgium, 2011. [Google Scholar]
- EV-Volumes—The Electric Vehicle World Sales Database. Available online: https://www.ev-volumes.com/news/global-bev-phev-sales-for-2019/%0Ahttp://www.ev-volumes.com/country/total-euefta-plug-in-vehicle-volumes-2/ (accessed on 29 November 2019).
- Amarakoon, S.; Smith, J.; Segal, B. Lithium-ion Batteries and Nanotechnology for Electric Vehicles; EPA 744-R-12-001; US Environmental Protection Agency: Wasinghton, DC, USA, 2012.
- European Environmental Agency. Greenhouse Gas Emissions from Transport in Europe—European Environment Agency; European Environmental Agency: København, Denmark, 2018; pp. 1–7. [Google Scholar]
- Wang, D.; Li, S.; Liu, F.; Gao, L.; Sui, J. Post combustion CO2 capture in power plant using low temperature steam upgraded by double absorption heat transformer. Appl. Energy 2018, 227, 603–612. [Google Scholar] [CrossRef]
- New Wave of CCS Activity: Ten Large-Scale Projects Announced—Global CCS Institute. Available online: https://www.globalccsinstitute.com/news-media/press-room/media-releases/new-wave-of-ccs-activity-ten-large-scale-projects-announced/ (accessed on 17 February 2020).
- International Energy Agency. 20 Years Carbon Capture Storage; OECD: Paris, France, 2016. [Google Scholar] [CrossRef]
- Eveloy, V.; Gebreegziabher, T. A Review of Projected Power-to-Gas Deployment Scenarios. Energies 2018, 11, 1824. [Google Scholar] [CrossRef] [Green Version]
- Llera, E.; Romeo, L.M.; Bailera, M.; Osorio, J.L. Exploring the integration of the power to gas technologies and the sustainable transport. Int. J. Energy Prod. Manag. 2018, 3, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Streibel, M.; Nakaten, N.; Kempka, T.; Kühn, M. Analysis of an integrated carbon cycle for storage of renewables. Energy Procedia 2013, 40, 202–211. [Google Scholar] [CrossRef] [Green Version]
- Kühn, M.; Nakaten, N.; Streibel, M.; Kempka, T. CO2 geological storage and utilization for a carbon neutral “power-to-gas-to-power” cycle to even out fluctuations of renewable energy provision. Energy Procedia 2014, 63, 8044–8049. [Google Scholar] [CrossRef] [Green Version]
- Götz, M.; Lefebvre, J.; Mörs, F.; McDaniel Koch, A.; Graf, F.; Bajohr, S.; Reimert, R.; Kolb, T. Renewable Power-to-Gas: A technological and economic review. Renew. Energy 2016, 85, 1371–1390. [Google Scholar] [CrossRef] [Green Version]
- Rönsch, S.; Schneider, J.; Matthischke, S.; Schlüter, M.; Götz, M.; Lefebvre, J.; Prabhakaran, P.; Bajohr, S. Review on methanation—From fundamentals to current projects. Fuel 2016, 166, 276–296. [Google Scholar] [CrossRef]
- Hagos, D.A.; Ahlgren, E. A State-of-the Art Review on the Development of CNG/LNG Infrastructure and Natural Gas Vehicles (NGVs); Chalmers University of Technology: Göteborg, Sweden, 2017. [Google Scholar]
- Osorio-Tejada, J.L.; Llera-Sastresa, E.; Scarpellini, S. A multi-criteria sustainability assessment for biodiesel and liquefied natural gas as alternative fuels in transport systems. J. Nat. Gas Sci. Eng. 2017, 42, 169–186. [Google Scholar] [CrossRef] [Green Version]
- Osorio-Tejada, J.L.; Llera-Sastresa, E.; Scarpellini, S. Liquefied natural gas: Could it be a reliable option for road freight transport in the EU? Renew. Sustain. Energy Rev. 2017, 71, 785–795. [Google Scholar] [CrossRef] [Green Version]
- Kalghatgi, G.; Levinsky, H.; Colket, M. Future transportation fuels. Prog. Energy Combust. Sci. 2018, 69, 103–105. [Google Scholar] [CrossRef]
- Horvath, S.; Fasihi, M.; Breyer, C. Techno-economic analysis of a decarbonized shipping sector: Technology suggestions for a fleet in 2030 and 2040. Energy Convers. Manag. 2018, 164, 230–241. [Google Scholar] [CrossRef]
- Mikulčić, H.; Ridjan Skov, I.; Dominković, D.F.; Wan Alwi, S.R.; Manan, Z.A.; Tan, R.; Duić, N.; Hidayah Mohamad, S.N.; Wang, X. Flexible Carbon Capture and Utilization technologies in future energy systems and the utilization pathways of captured CO2. Renew. Sustain. Energy Rev. 2019, 114, 109338. [Google Scholar] [CrossRef]
- Boot-Handford, M.E.; Abanades, J.C.; Anthony, E.J.; Blunt, M.J.; Brandani, S.; Mac Dowell, N.; Fernández, J.R.; Ferrari, M.C.; Gross, R.; Hallett, J.P.; et al. Carbon capture and storage update. Energy Environ. Sci. 2014, 7, 130–189. [Google Scholar] [CrossRef]
- Belaissaoui, B.; Le Moullec, Y.; Willson, D.; Favre, E. Hybrid membrane cryogenic process for post-combustion CO2 capture. J. Memb. Sci. 2012, 415–416, 424–434. [Google Scholar] [CrossRef]
- Voldsund, M.; Gardarsdottir, S.O.; De Lena, E.; Pérez-Calvo, J.F.; Jamali, A.; Berstad, D.; Fu, C.; Romano, M.; Roussanaly, S.; Anantharaman, R.; et al. Comparison of technologies for CO2 capture from cement production—Part 1: Technical evaluation. Energies 2019, 12, 559. [Google Scholar] [CrossRef] [Green Version]
- MacDowell, N.; Florin, N.; Buchard, A.; Hallett, J.; Galindo, A.; Jackson, G.; Adjiman, C.S.; Williams, C.K.; Shah, N.; Fennell, P. An overview of CO2 capture technologies. Energy Environ. Sci. 2010, 3, 1645–1669. [Google Scholar] [CrossRef] [Green Version]
- Sipöcz, N.; Hernandez-Nogales, A.; Gonzalez-Salazar, M.A.; Shisler, R.; Lissianski, V. Low temperature CO2 capture for near-term applications. Energy Procedia 2013, 37, 1228–1238. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Yan, J. Characterization of flue gas in oxy-coal combustion processes for CO2 capture. Appl. Energy 2012, 90, 113–121. [Google Scholar] [CrossRef]
- Olajire, A.A. CO2 capture and separation technologies for end-of-pipe applications—A review. Energy 2010, 35, 2610–2628. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, P.; Wang, Z. Reviews on current carbon emission reduction technologies and projects and their feasibilities on ships. J. Mar. Sci. Appl. 2017, 16, 129–136. [Google Scholar] [CrossRef]
- Sullivan, J.M.; Sivak, M. Carbon Capture in Vehicles: A Review of General Support, Available Mechanisms, and Consumer Acceptance Issues; University of Michigan, Ann Arbor, Transportation Research Institute: Ann Arbor, MI, USA, 2012. [Google Scholar]
- Echevarria Huaman, R.N. A Review on: CO2 Capture Technology on Fossil Fuel Power Plant. J. Fundam. Renew. Energy Appl. 2015, 5. [Google Scholar] [CrossRef] [Green Version]
- Joel, A.S.; Wang, M.; Ramshaw, C. Modelling and simulation of intensified absorber for post-combustion CO2 capture using different mass transfer correlations. Appl. Therm. Eng. 2015, 74, 47–53. [Google Scholar] [CrossRef]
- Salazar, J.; Diwekar, U.; Joback, K.; Berger, A.H.; Bhown, A.S. Solvent selection for post-combustion CO2 capture. Energy Procedia 2013, 37, 257–264. [Google Scholar] [CrossRef] [Green Version]
- Mondal, M.K.; Balsora, H.K.; Varshney, P. Progress and trends in CO2 capture/separation technologies: A review. Energy 2012, 46, 431–441. [Google Scholar] [CrossRef]
- Jonshagen, K.; Sipöcz, N.; Genrup, M. A Novel Approach of Retrofitting a Combined Cycle with Post Combustion CO2 Capture. J. Eng. Gas Turbines Power 2011, 133, 1–7. [Google Scholar] [CrossRef]
- Xue, B.; Yu, Y.; Chen, J.; Luo, X.; Wang, M. A comparative study of MEA and DEA for post-combustion CO2 capture with different process configurations. Int. J. Coal Sci. Technol. 2017, 4, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Zhai, R.; Liu, H.; Wu, H.; Yu, H.; Yang, Y. Analysis of integration of MEA-based CO2 capture and solar energy system for coal-based power plants based on thermo-economic structural theory. Energies 2018, 11, 1284. [Google Scholar] [CrossRef] [Green Version]
- Goto, K.; Yogo, K.; Higashii, T. A review of efficiency penalty in a coal-fired power plant with post-combustion CO2 capture. Appl. Energy 2013, 111, 710–720. [Google Scholar] [CrossRef]
- Eldardiry, H.; Habib, E. Carbon capture and sequestration in power generation: Review of impacts and opportunities for water sustainability. Energy. Sustain. Soc. 2018, 8, 6. [Google Scholar] [CrossRef] [Green Version]
- Hanak, D.P.; Biliyok, C.; Manovic, V. Efficiency improvements for the coal-fired power plant retrofit with CO2 capture plant using chilled ammonia process. Appl. Energy 2015, 151, 258–272. [Google Scholar] [CrossRef]
- Borhani, T.N.; Wang, M. Role of solvents in CO2 capture processes: The review of selection and design methods. Renew. Sustain. Energy Rev. 2019, 114, 109299. [Google Scholar] [CrossRef]
- Sada, E.; Kumazawa, H.; Butt, M.A. Gas absorption with consecutive chemical reaction: Absorption of carbon dioxide into aqueous amine solutions. Can. J. Chem. Eng. 1976, 54, 421–424. [Google Scholar] [CrossRef]
- Kim, Y.E.; Lim, J.A.; Jeong, S.K.; Yoon, Y.I.; Bae, S.T.; Nam, S.C. Comparison of carbon dioxide absorption in aqueous MEA, DEA, TEA, and AMP solutions. Bull. Korean Chem. Soc. 2013, 34, 783–787. [Google Scholar] [CrossRef] [Green Version]
- El Hadri, N.; Quang, D.V.; Goetheer, E.L.V.; Abu Zahra, M.R.M. Aqueous amine solution characterization for post-combustion CO2 capture process. Appl. Energy 2017, 185, 1433–1449. [Google Scholar] [CrossRef]
- Derks, P.W.J.; Versteeg, G.F. Kinetics of absorption of carbon dioxide in aqueous ammonia solutions. Energy Procedia 2009, 1, 1139–1146. [Google Scholar] [CrossRef] [Green Version]
- Qin, F.; Wang, S.; Kim, I.; Svendsen, H.F.; Chen, C. Heat of absorption of CO2 in aqueous ammonia and ammonium carbonate/carbamate solutions. Int. J. Greenh. Gas Control. 2011, 5, 405–412. [Google Scholar] [CrossRef]
- Qi, G.; Wang, S.; Yu, H.; Wardhaugh, L.; Feron, P.; Chen, C. Development of a rate-based model for CO2 absorption using aqueous NH3 in a packed column. Int. J. Greenh. Gas Control. 2013, 17, 450–461. [Google Scholar] [CrossRef]
- Yang, N.; Yu, H.; Li, L.; Xu, D.; Han, W.; Feron, P. Aqueous Ammonia (NH3) Based Post Combustion CO2 Capture: A Review. Oil Gas Sci. Technol. 2014, 69, 931–945. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Morgan, S.; Allport, A.; Cottrell, A.; Do, T.; Wardhaugh, J.M.G.L.; Feron, P. Results from trialling aqueous NH3 based post combustion capture in a pilot plant at Munmorah power station: Desorption. Chem. Eng. Res. Des. 2012, 89, 753–758. [Google Scholar] [CrossRef]
- Bishnoi, S.; Rochelle, G.T. Absorption of carbon dioxide into aqueous piperazine: Reaction kinetics, mass transfer and solubility. Chem. Eng. Sci. 2000, 55, 5531–5543. [Google Scholar] [CrossRef]
- Chen, X.; Rochelle, G.T. Aqueous piperazine derivatives for CO2 capture: Accurate screening by a wetted wall column. Chem. Eng. Res. Des. 2011, 89, 1693–1710. [Google Scholar] [CrossRef]
- Singto, S.; Supap, T.; Idem, R.; Tontiwachwuthikul, P.; Tantayanon, S. The Effect of Chemical Structure of Newly Synthesized Tertiary Amines Used for the Post Combustion Capture Process on Carbon dioxide (CO2): Kinetics of CO2 Absorption Using the Stopped-Flow Apparatus and Regeneration, and Heat Input of CO2 Regeneration. Energy Procedia 2017, 114, 852–859. [Google Scholar] [CrossRef]
- Ali, B.S.; Aroua, M.K. Effect of piperazine on CO2 loading in aqueous solutions of MDEA at low pressure. Int. J. Thermophys. 2004, 25, 1863–1870. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, L.; Otto, A.; Robinius, M.; Stolten, D. A Review of Post-combustion CO2 Capture Technologies from Coal-fired Power Plants. Energy Procedia 2017, 114, 650–665. [Google Scholar] [CrossRef]
- Conway, W.; Yang, Q.; James, S.; Wei, C.C.; Bown, M.; Feron, P.; Puxty, G. Designer amines for post combustion CO2 capture processes. Energy Procedia 2014, 63, 1827–1834. [Google Scholar] [CrossRef] [Green Version]
- Thompson, J.; Matin, N.; Liu, K. Solubility and Thermodynamic Modeling of Carcinogenic Nitrosamines in Aqueous Amine Solvents for CO2 Capture. Energy Procedia 2017, 114, 1038–1044. [Google Scholar] [CrossRef]
- Kimura, H.; Kubo, T.; Shimada, M.; Kitamura, H.; Fujita, K.; Suzuki, K.; Yamamoto, K.; Akai, M. Environmental Risk Assessment of MEA and its Degradation Products from Post-combustion CO2 Capture Pilot Plant: Drafting Technical Guidelines. Energy Procedia 2017, 114, 6490–6500. [Google Scholar] [CrossRef]
- Kumar, S.; Srivastava, R.; Koh, J. Utilization of zeolites as CO2 capturing agents: Advances and future perspectives. J. CO2 Util. 2020, 41, 101251. [Google Scholar] [CrossRef]
- Lu, C.; Bai, H.; Wu, B.; Su, F.; Hwang, J.F. Comparative study of CO2 capture by carbon nanotubes, activated carbons, and zeolites. Energy Fuels 2008, 22, 3050–3056. [Google Scholar] [CrossRef]
- Hinkov, I.; Lamari, F.D.; Langlois, P.; Dicko, M.; Chilev, C.; Pentchev, I. Carbon dioxide capture by adsorption (review). J. Chem. Technol. Metall. 2016, 51, 609–626. [Google Scholar]
- Verdegaal, W.M.; Wang, K.; Sculley, J.P.; Wriedt, M.; Zhou, H.C. Evaluation of Metal-Organic Frameworks and Porous Polymer Networks for CO2-Capture Applications. ChemSusChem 2016, 9, 636–643. [Google Scholar] [CrossRef]
- Yu, P.; Luo, Z.; Wang, Q.; Fang, M.; Zhou, J.; Wang, W.; Liang, X.; Cai, W. Activated carbon-based CO2 uptake evaluation at different temperatures: The correlation analysis and coupling effects of the preparation conditions. J. CO2 Util. 2020, 40, 101214. [Google Scholar] [CrossRef]
- Veneman, R.; Kamphuis, H.; Brilman, D.W.F. Post-combustion CO2 capture using supported amine sorbents: A process integration study. Energy Procedia 2013, 37, 2100–2108. [Google Scholar] [CrossRef] [Green Version]
- Hasan, M.M.F.; Baliban, R.C.; Elia, J.A.; Floudas, C.A. Modeling, simulation, and optimization of postcombustion CO2 capture for variable feed concentration and flow rate. 2. Pressure swing adsorption and vacuum swing adsorption processes. Ind. Eng. Chem. Res. 2012, 51, 15665–15682. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.; Sun, Y.; Cakstins, J.; Sun, C.; Snape, C.E. Parametric study on the regeneration heat requirement of an amine-based solid adsorbent process for post-combustion carbon capture. Appl. Energy 2016, 168, 394–405. [Google Scholar] [CrossRef]
- Huck, J.M.; Lin, L.C.; Berger, A.H.; Shahrak, M.N.; Martin, R.L.; Bhown, A.S.; Haranczyk, M.; Reuter, K.; Smit, B. Supporting Information: Evaluating different classes of porous materials for carbon capture. Energy Environ. Sci. 2014, 7, 4132–4146. [Google Scholar] [CrossRef]
- Quang, D.V.; El Hadri, N.; Abu-zahra, M.R.M. Reduction in the regeneration energy of CO2 capture process by impregnating amine solvent onto precipitated silica. Eur. Sci. J. 2013, 9, 82–102. [Google Scholar]
- Won, W.; Lee, S.; Lee, K.S. Modeling and parameter estimation for a fixed-bed adsorption process for CO2 capture using zeolite 13X. Sep. Purif. Technol. 2012, 85, 120–129. [Google Scholar] [CrossRef]
- Al Mousa, A.; Abouelnasr, D.; Loughlin, K.F. Saturation loadings on 13X (faujasite) zeolite above and below the critical conditions. Part II: Unsaturated and cyclic hydrocarbons data evaluation and modeling. Adsorption 2015, 21, 321–332. [Google Scholar] [CrossRef]
- Hedin, N.; Andersson, L.; Bergström, L.; Yan, J. Adsorbents for the post-combustion capture of CO2 using rapid temperature swing or vacuum swing adsorption. Appl. Energy 2013, 104, 418–433. [Google Scholar] [CrossRef]
- Creamer, A.E.; Gao, B. Carbon-based adsorbents for postcombustion CO2 capture: A critical review. Environ. Sci. Technol. 2016, 50, 7276–7289. [Google Scholar] [CrossRef] [PubMed]
- Raganati, F.; Chirone, R.; Ammendola, P. CO2 Capture by Temperature Swing Adsorption: Working Capacity as Affected by Temperature and CO2 Partial Pressure. Ind. Eng. Chem. Res. 2020, 59, 3593–3605. [Google Scholar] [CrossRef]
- Song, C.; Kansha, Y.; Fu, Q.; Ishizuka, M.; Tsutsumi, A. Reducing energy consumption of advanced PTSA CO2 capture process-Experimental and numerical study. J. Taiwan Inst. Chem. Eng. 2016, 64, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Zanco, S.E.; Joss, L.; Hefti, M.; Gazzani, M.; Mazzotti, M. Addressing the Criticalities for the Deployment of Adsorption-based CO2 Capture Processes. Energy Procedia 2017, 114, 2497–2505. [Google Scholar] [CrossRef]
- Marx, D.; Joss, L.; Hefti, M.; Mazzotti, M. Temperature Swing Adsorption for Postcombustion CO2 Capture: Single- and Multicolumn Experiments and Simulations. Ind. Eng. Chem. Res. 2016, 55, 1401–1412. [Google Scholar] [CrossRef]
- Dhoke, C.; Zaabout, A.; Cloete, S.; Amini, S. Review on Reactor Configurations for Adsorption-Based CO2 Capture. Ind. Eng. Chem. Res. 2021, 60, 3779–3798. [Google Scholar] [CrossRef]
- Ströhle, J.; Junk, M.; Kremer, J.; Galloy, A.; Epple, B. Carbonate looping experiments in a 1 MWth pilot plant and model validation. Fuel 2014, 127, 13–22. [Google Scholar] [CrossRef]
- Perejón, A.; Romeo, L.M.; Lara, Y.; Lisbona, P.; Martínez, A.; Valverde, J.M. The Calcium-Looping technology for CO2 capture: On the important roles of energy integration and sorbent behavior. Appl. Energy 2016, 162, 787–807. [Google Scholar] [CrossRef] [Green Version]
- Alonso, M.; Rodríguez, N.; González, B.; Grasa, G.; Murillo, R.; Abanades, J.C. Carbon dioxide capture from combustion flue gases with a calcium oxide chemical loop. Experimental results and process development. Int. J. Greenh. Gas Control. 2010, 4, 167–173. [Google Scholar] [CrossRef]
- Ortiz, C.; Romano, M.C.; Valverde, J.M.; Binotti, M.; Chacartegui, R. Process integration of Calcium-Looping thermochemical energy storage system in concentrating solar power plants. Energy 2018, 155, 535–551. [Google Scholar] [CrossRef]
- Ahmad, J.; Rehman, W.U.; Deshmukh, K.; Basha, S.K.; Ahamed, B.; Chidambaram, K. Recent Advances in Poly (Amide-B-Ethylene) Based Membranes for Carbon Dioxide (CO2) Capture: A Review. Polym. Technol. Mater. 2019, 58, 366–383. [Google Scholar] [CrossRef]
- Turi, D.M.; Ho, M.; Ferrari, M.C.; Chiesa, P.; Wiley, D.E.; Romano, M.C. CO2 capture from natural gas combined cycles by CO2 selective membranes. Int. J. Greenh. Gas Control. 2017, 61, 168–183. [Google Scholar] [CrossRef] [Green Version]
- Sifat, N.S.; Haseli, Y. A critical review of CO2 capture technologies and prospects for clean power generation. Energies 2019, 12, 4143. [Google Scholar] [CrossRef] [Green Version]
- Khaisri, S.; deMontigny, D.; Tontiwachwuthikul, P.; Jiraratananon, R. Comparing membrane resistance and absorption performance of three different membranes in a gas absorption membrane contactor. Sep. Purif. Technol. 2009, 65, 290–297. [Google Scholar] [CrossRef]
- Norahim, N.; Yaisanga, P.; Faungnawakij, K.; Charinpanitkul, T.; Klaysom, C. Recent Membrane Developments for CO2 Separation and Capture. Chem. Eng. Technol. 2018, 41, 211–223. [Google Scholar] [CrossRef]
- Fernández-Barquín, A.; Casado-Coterillo, C.; Etxeberria-Benavides, M.; Zuñiga, J.; Irabien, A. Comparison of Flat and Hollow-Fiber Mixed-Matrix Composite Membranes for CO2 Separation with Temperature. Chem. Eng. Technol. 2017, 40, 997–1007. [Google Scholar] [CrossRef] [Green Version]
- Franz, J.; Schiebahn, S.; Zhao, L.; Riensche, E.; Scherer, V.; Stolten, D. Investigating the influence of sweep gas on CO2/N2 membranes for post-combustion capture. Int. J. Greenh. Gas Control. 2013, 13, 180–190. [Google Scholar] [CrossRef]
- Arias, A.M.; Mussati, M.C.; Mores, P.L.; Scenna, N.J.; Caballero, J.A.; Mussati, S.F. Optimization of multi-stage membrane systems for CO2 capture from flue gas. Int. J. Greenh. Gas Control. 2016, 53, 371–390. [Google Scholar] [CrossRef]
- Favre, E. Carbon dioxide recovery from post-combustion processes: Can gas permeation membranes compete with absorption? J. Memb. Sci. 2007, 294, 50–59. [Google Scholar] [CrossRef]
- Zhao, L.; Riensche, E.; Blum, L.; Stolten, D. Multi-stage gas separation membrane processes used in post-combustion capture: Energetic and economic analyses. J. Memb. Sci. 2010, 359, 160–172. [Google Scholar] [CrossRef]
- Jafari, M.; Ghasemzadeh, K.; Amiri, T.Y.; Basile, A. Comparative Study of Membrane and Absorption Processes Performance and their Economic Evaluation for CO2 Capturing from Flue Gas. Gas Process. J. 2019, 7, 37–52. [Google Scholar]
- Zhao, L.; Weber, M.; Stolten, D. Comparative investigation of polymer membranes for post-combustion capture. Energy Procedia 2013, 37, 1125–1134. [Google Scholar] [CrossRef] [Green Version]
- Yousef, A.M.; El-Maghlany, W.M.; Eldrainy, Y.A.; Attia, A. New approach for biogas purification using cryogenic separation and distillation process for CO2 capture. Energy 2018, 156, 328–351. [Google Scholar] [CrossRef]
- Song, C.; Liu, Q.; Deng, S.; Li, H.; Kitamura, Y. Cryogenic-based CO2 capture technologies: State-of-the-art developments and current challenges. Renew. Sustain. Energy Rev. 2019, 101, 265–278. [Google Scholar] [CrossRef]
- Berger, A.H.; Hoeger, C.; Baxter, L.; Bhown, A.S. Evaluation of Cryogenic Systems for Post Combustion CO2 Capture. In Proceedings of the 14th Greenhouse Gas Control Technologies Conference, Melbourne, Australia, 21–26 October 2018; pp. 1–8. [Google Scholar]
- Razzak, S.A.; Ali, S.A.M.; Hossain, M.M.; deLasa, H. Biological CO2 fixation with production of microalgae in wastewater—A review. Renew. Sustain. Energy Rev. 2017, 76, 379–390. [Google Scholar] [CrossRef]
- Christodoulou, A.; Gonzalez-Aregall, M.; Linde, T.; Vierth, I.; Cullinane, K. Targeting the reduction of shipping emissions to air. Marit. Bus. Rev. 2019, 4, 16–30. [Google Scholar] [CrossRef]
- Roussanaly, S.; Jakobsen, J.P.; Hognes, E.H.; Brunsvold, A.L. Benchmarking of CO2 transport technologies: Part I-Onshore pipeline and shipping between two onshore areas. Int. J. Greenh. Gas Control. 2013, 19, 584–594. [Google Scholar] [CrossRef] [Green Version]
- IMO Energy Efficiency and the Reduction of GHG Emissions from Ships. Available online: http://www.imo.org/en/MediaCentre/HotTopics/GHG/Pages/default.aspx (accessed on 10 June 2020).
- Comer, A.B.; Chen, C.; Rutherford, D. Relating Short-Term Measures to IMO’ s Minimum 2050 Emissions Reduction Target; International Council on Clean Transportation: Washington, DC, USA, 2018. [Google Scholar]
- Van den Akker, J. Carbon Capture Onboard LNG-Fueled Vessels; Delft University of Technology: Delft, The Netherlands, 2017. [Google Scholar]
- Feenstra, M.; Monteiro, J.; van den Akker, J.T.; Abu-Zahra, M.R.M.; Gilling, E.; Goetheer, E. Ship-based carbon capture onboard of diesel or LNG-fuelled ships. Int. J. Greenh. Gas Control. 2019, 85, 1–10. [Google Scholar] [CrossRef]
- Luo, X.; Wang, M. Study of solvent-based carbon capture for cargo ships through process modelling and simulation. Appl. Energy 2017, 195, 402–413. [Google Scholar] [CrossRef] [Green Version]
- Fang, S.; Xu, Y.; Li, Z. Joint generation and demand-side management for shipboard carbon capture and storage system. Conf. Rec. Ind. Commer. Power Syst. Tech. Conf. 2019, 2019, 1–8. [Google Scholar] [CrossRef]
- Fang, S.; Xu, Y.; Li, Z.; Ding, Z.; Liu, L.; Wang, H. Optimal Sizing of Shipboard Carbon Capture System for Maritime Greenhouse Emission Control. IEEE Trans. Ind. Appl. 2019, 55, 1. [Google Scholar] [CrossRef]
- Awoyomi, A.; Patchigolla, K.; Anthony, E.J. CO2/SO2 emission reduction in CO2 shipping infrastructure. Int. J. Greenh. Gas Control. 2019, 88, 57–70. [Google Scholar] [CrossRef]
- Awoyomi, A.; Patchigolla, K.; Anthony, E.J. Process and Economic Evaluation of an Onboard Capture System for LNG-Fueled CO2 Carriers. Ind. Eng. Chem. Res. 2020, 59, 6951–6960. [Google Scholar] [CrossRef]
- Zhou, P.; Wang, H. Carbon capture and storage—Solidification and storage of carbon dioxide captured on ships. Ocean Eng. 2014, 91, 172–180. [Google Scholar] [CrossRef] [Green Version]
- Stec, M.; Tatarczuk, A.; Iluk, T.; Szul, M. Reducing the energy efficiency design index for ships through a post-combustion carbon capture process. Int. J. Greenh. Gas Control. 2021, 108, 103333. [Google Scholar] [CrossRef]
- Lee, S.; Yoo, S.; Park, H.; Ahn, J.; Chang, D. Novel methodology for EEDI calculation considering onboard carbon capture and storage system. Int. J. Greenh. Gas Control. 2021, 105, 103241. [Google Scholar] [CrossRef]
- Trivyza, N.L.; Rentizelas, A.; Theotokatos, G. Impact of carbon pricing on the cruise ship energy systems optimal configuration. Energy 2019, 175, 952–966. [Google Scholar] [CrossRef] [Green Version]
- Malmgren, E.; Brynolf, S.; Fridell, E.; Grahn, M.; Andersson, K. The environmental performance of a fossil-free ship propulsion system with onboard carbon capture—A life cycle assessment of the HyMethShip concept. Sustain. Energy Fuels 2021, 5, 2753–2770. [Google Scholar] [CrossRef]
- Balsamo, M.; Erto, A.; Lancia, A.; Di Natale, F. Carbon dioxide capture from model marine diesel engine exhaust by means of K2CO3-based sorbents. Chem. Eng. Trans. 2016, 52, 415–420. [Google Scholar] [CrossRef]
- Erto, A.; Balsamo, M.; Paduano, L.P.; Lancia, A.; Di Natale, F. Utilization of alumina-supported K2CO3 as CO2-selective sorbent: A promising strategy to mitigate the carbon footprint of the maritime sector. J. CO2 Util. 2018, 24, 139–148. [Google Scholar] [CrossRef]
- Bilger, R.W.; Wu, Z. Carbon capture for automobiles using internal combustion rankine cycle engines. J. Eng. Gas Turbines Power 2009, 131, 1–4. [Google Scholar] [CrossRef]
- Yu, X.; Wu, Z.; Fu, L.; Deng, J.; Hu, Z.; Li, L. Study of combustion characteristics of a quasi internal combustion rankine cycle engine. SAE Tech. Pap. 2013. [Google Scholar] [CrossRef]
- Yu, X.; Wu, Z.; Wang, C.; Deng, J.; Hu, Z.; Li, L. Study of the combustion and emission characteristics of a quasi ICRC engine under different engine loads. SAE Tech. Pap. 2014. [Google Scholar] [CrossRef]
- Van Blarigan, A.C.; Seiser, R.; Chen, J.Y.; Cattolica, R.; Dibble, R.W. Working fluid composition effects on methane oxycombustion in an SI-engine: EGR vs. CO2. Proc. Combust. Inst. 2013, 34, 2951–2958. [Google Scholar] [CrossRef]
- Van Blarigan, A.; Kozarac, D.; Seiser, R.; Cattolica, R.; Chen, J.-Y.; Dibble, R. Experimental Study of Methane Fuel Oxycombustion in a Spark-Ignited Engine. J. Energy Resour. Technol. 2014, 136, 012203. [Google Scholar] [CrossRef]
- Van Blarigan, A.; Kozarac, D.; Seiser, R.; Chen, J.Y.; Cattolica, R.; Dibble, R. Spark-ignited engine NOx emissions in a low-nitrogen oxycombustion environment. Appl. Energy 2014, 118, 22–31. [Google Scholar] [CrossRef]
- Li, X.; Peng, Z.; Ajmal, T.; Aitouche, A.; Mobasheri, R.; Pei, Y.; Gao, B.; Wellers, M. A feasibility study of implementation of oxy-fuel combustion on a practical diesel engine at the economical oxygen-fuel ratios by computer simulation. Adv. Mech. Eng. 2020, 12, 1–13. [Google Scholar] [CrossRef]
- Yu, X.; Wu, Z. Simulation on Effect of EGR on Oxy-fuel IC Engine.pdf. Appl. Mech. Mater. 2012, 130, 790–795. [Google Scholar] [CrossRef]
- Wu, Z.J.; Yu, X.; Fu, L.Z.; Deng, J.; Li, L.G. Experimental study of the effect of water injection on the cycle performance of an internal-combustion Rankine cycle engine. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2014, 228, 580–588. [Google Scholar] [CrossRef]
- Kumar, P.; Rathod, V.; Parwani, A.K. Experimental investigation on performance of absorbents for carbon dioxide capture from diesel engine exhaust. Environ. Prog. Sustain. Energy 2021, 40, e13651. [Google Scholar] [CrossRef]
- Aramco Capturing Carbon on the Move. Available online: https://www.aramco.com/en/creating-value/technology-development/transport-technologies/mobile-carbon-capture# (accessed on 19 February 2021).
- Al-Meshari, A.A.; Muhaish, F.I.; Aleidan, A.A. Carbon Capture: Saudi Aramco’s Carbon Management Program. J. Pet. Technol. 2014, 66, 72–74. [Google Scholar] [CrossRef]
- Subramanian, T.; Sonthalia, A.; Varuvel, E.G. Effect of calcite/activated carbon-based post- combustion CO2 capture system in a biodiesel-fueled CI engine—An experimental study. Energy Sources Part A Recovery Util. Environ. Eff. 2018, 41, 1972–1982. [Google Scholar] [CrossRef]
- Thiyagarajan, S.; Edwin Geo, V.; Martin, L.J.; Nagalingam, B. Carbon dioxide (CO2) capture and sequestration using biofuels and an exhaust catalytic carbon capture system in a single-cylinder CI engine: An experimental study. Biofuels 2018, 9, 659–668. [Google Scholar] [CrossRef]
- Thiyagarajan, S.; Varuvel, E.G.; Martin, L.J.; Beddhannan, N. Mitigation of carbon footprints through a blend of biofuels and oxygenates, combined with post-combustion capture system in a single cylinder CI engine. Renew. Energy 2019, 130, 1067–1081. [Google Scholar] [CrossRef]
- Thiyagarajan, S.; Geo, V.E.; Martin, L.J.; Nagalingam, B. Combined effect of fuel-design and after-treatment system on reduction of local and global emissions from CI engine. Environ. Technol. 2019, 40, 2802–2812. [Google Scholar] [CrossRef]
- Saravanan, S.; Kumar, C.R. Carbon Dioxide Capture using Adsorption Technology in Diesel Engines. Int. J. Renew. Energy Res. 2020, 10, 1614–1620. [Google Scholar]
- Ajith Kumar, P.S.; Varghese, A.; Vaishanth, S.S.; Balaji, G. Development and test of a new carbon capture system using Zeolite with addition of Activated carbon and Monoethanolamine for IC engines. IOP Conf. Ser. Mater. Sci. Eng. 2020, 912. [Google Scholar] [CrossRef]
- Kumar, R. Experimental Investigations on CO2 Recovery from Engine Exhaust Using Adsorption Technology. SAE Tech. Pap. 2019. [Google Scholar] [CrossRef]
- Kaushal, A.; Ahirwar, R.; Vardhan, A. Investigation of CO2 Capturing Capacity of Solid Adsorbents (PEIs) Polyethylenimines from Automotive Vehicle Exhausts System for 4-Stroke SI Engine. Bonfring Int. J. Ind. Eng. Manag. Sci. 2018, 8, 31–35. [Google Scholar] [CrossRef]
- Rajdurai, M.S.; Rao, A.H.S.; Kamalakkannan, K. CO2 Capture Using Activated Alumina in Gasoline Passenger Vehicles. Fuel 2016, 140, 4300RPM. [Google Scholar]
- Sharma, S.; Maréchal, F. Carbon Dioxide Capture from Internal Combustion Engine Exhaust Using Temperature Swing Adsorption. Front. Energy Res. 2019, 7, 143. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Atilhan, M. Evaluating the Performance of a Newly Developed Carbon Capture Device for Mobile Emission Sources. J. Energy Resour. Technol. 2017, 139, 1–8. [Google Scholar] [CrossRef]
- Pera-Titus, M.; Alshebani, A.; Nicolas, C.H.; Roumégoux, J.P.; Miachon, S.; Dalmon, J.A. Nanocomposite MFI-alumina membranes: High-flux hollow fibers for CO2 capture from internal combustion vehicles. Ind. Eng. Chem. Res. 2009, 48, 9215–9223. [Google Scholar] [CrossRef]
- Bradley, T.; Knackstedt, C.; Jambor, E. Reducing Effective Vehicle Emissions through the Integration of a Carbon Capture and Sequestration System in the CSU EcoCAR Vehicle. SAE Tech. Pap. 2016. [Google Scholar] [CrossRef]
- Telles, E.C.; Yang, S.; Vargas, J.V.C.; Ordonez, J.C.; Mariano, A.B.; Chagas, M.B.; Davis, T. Stationary compression ignition internal combustion engines (CI-ICE) CO2 capturing via microalgae culture using a mini-photobioreactor. In Proceedings of the 2015 IEEE Conference on Technologies for Sustainability (SusTech), Ogden, UT, USA, 30 July–1 August 2015; pp. 209–215. [Google Scholar] [CrossRef]
- Hamad, E.Z.; Al-sadat, W.I. Apparatus and Method for Oxy-Combustion of Fuels in Internal Combustion Engines. US 2013/0247886 A1, 8 November 2013. [Google Scholar]
- Hamad, E.Z.; Al-sadat, W.I. Apparatus and Method for Oxy-Combustion of Fuels in Internal Combustion Engines. US 2017/0074213 A1, 7 May 2017. [Google Scholar]
- Hamad, E.Z.; Al-sadat, W.I. Apparatus and Method for Oxy-Combustion of Fuels in Internal Combustion Engines. US 10,280,877 B2, 7 May 2019. [Google Scholar]
- Athayde, D.D.; Souza, D.F.; Silva, A.M.A.; Vasconcelos, D.; Nunes, E.H.M.; Da Costa, J.C.D.; Vasconcelos, W.L. Review of perovskite ceramic synthesis and membrane preparation methods. Ceram. Int. 2016, 42, 6555–6571. [Google Scholar] [CrossRef] [Green Version]
- Myers, B.A.; Ihms, D.W. Vehicle System to Separate and Store Carbon Dioxide from Engine Exhaust. US 8,480,798B1, 9 July 2013. [Google Scholar]
- Myers, B.A.; Ihms, D.W. Heat Exchanger Equipped with Thermal Electric Device for Engine Exhaust Carbon Dioxide Collection System. US 9,267,415B2, 23 February 2016. [Google Scholar]
- Younes, M.V.; Hamad, E.Z. Integrated Method of Driving a CO2, Compressor of a CO2-Capture System Using Waste Heat from an Internal Combustion Engine on Board a Mobile Source. US 9,222,480B2, 29 December 2015. [Google Scholar]
- Hamad, E.Z.; Al-sadat, W.I. Reversible Solid Adsorption Method and System Utilizing Waste Heat for on-Board Recovery and Storage of CO2. WO 2012/100149A1, 20 January 2012. [Google Scholar]
- Hamad, E.Z. Liquid, Slurry and Flowable Powder Adsorption/Absorption Method Utilizing Waste Heat for on-Board Recovery And Storage of CO2 from Motor Vehicle Internal Combustion Engine Exhaust Gases. US 2016/0059180A1, 8 November 2016. [Google Scholar]
- Mercedes-Benz Citaro NGT Technical Information. Available online: https://www.mercedes-benz-bus.com/content/dam/mbo/markets/common/buy/services-online/download-technical-brochures/images/content/regular-service-buses/citaro-ngt/MB-NGT-2-ES-09_17.pdf (accessed on 8 February 2020).
- Wärtsilä. Wärtsilä 46DF Product Guide; Wärtsilä, Marine Solutions: Vaasa, Finland, 2019. [Google Scholar]
- Revista Viajeros Prueba: Mercedes-Benz Citaro NGT. Available online: https://www.revistaviajeros.com/noticia/9877/prueba-mercedes-benz-citaro-ngt (accessed on 7 May 2021).
- Talebizadehsardari, P.; Ehyaei, M.A.; Ahmadi, A.; Jamali, D.H.; Shirmohammadi, R.; Eyvazian, A.; Ghasemi, A.; Rosen, M.A. Energy, exergy, economic, exergoeconomic, and exergoenvironmental (5E) analyses of a triple cycle with carbon capture. J. CO2 Util. 2020, 41, 101258. [Google Scholar] [CrossRef]
- Einewall, P.; Tunestal, P.; Johansson, B. Lean Burn Natural Gas Operation vs. Stoichiometric Operation with Egr and a Three Way Catalyst. SAE Tech. Pap. 2005, 21, 343–362. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Xu, Z.; Li, M.; Shao, S. Combustion and emissions of a Euro VI heavy-duty natural gas engine using EGR and TWC. J. Nat. Gas Sci. Eng. 2016, 28, 660–671. [Google Scholar] [CrossRef]
- Jiang, N.; Shen, Y.; Liu, B.; Zhang, D.; Tang, Z.; Li, G.; Fu, B. CO2 capture from dry flue gas by means of VPSA, TSA and TVSA. J. CO2 Util. 2020, 35, 153–168. [Google Scholar] [CrossRef]
- Scaccabarozzi, R.; Tavano, M.; Invernizzi, C.M. Comparison of working fluids and cycle optimization for heat recovery ORCs from large internal combustion engines. Energy 2018, 158, 396–416. [Google Scholar] [CrossRef]
- Shu, G.; Li, X.; Tian, H.; Liang, X.; Wei, H.; Wang, X. Alkanes as working fluids for high-temperature exhaust heat recovery of diesel engine using organic Rankine cycle. Appl. Energy 2014, 119, 204–217. [Google Scholar] [CrossRef]
- Corporation, T.M. Toyota Unveils FC Bus Concept—Sora. Available online: https://global.toyota/en/detail/19063778 (accessed on 11 June 2020).
- US Deparment of Energy Gas Mileage Tips. Available online: https://www.fueleconomy.gov/feg/driveHabits.jsp (accessed on 15 February 2021).
- Casadei, A.; Broda, R. Impact of Vehicle Weight Reduction on Fuel Economy for Various Vehicle Architectures; The Aluminum Association, Inc.: Arlington, VA, USA, 2008. [Google Scholar]


| Solvent | Rate Constant Reaction [m3/kmol-s] | Absorption Heat (ΔHabs) [kJ/molCO2] | Loading Capacity (q) | Amine in the Solution [wt%] | |
|---|---|---|---|---|---|
| [molCO2/molamine] | [molCO2/Lamine] | ||||
| Ethanolamine (MEA) | 8400 [54] | −88.91 [55] | 0.59 [56] | 9.77 | 30 |
| Diethanolamine (DEA) | 1340 [54] | −70.44 [55] | 0.61 [56] | 6.32 | 30 |
| Ammonia (NH3) | 7500 [57] | −65.5 [58] | 0.4 [59,60,61] | 16 | 2.5 |
| Piperazine (Pz) | 53,700 [62] | −80.58 [56] | 0.81 [56,63] | 10.34 | 30 |
| Methyl diethanolamine (MDEA) | 11.15 [64] | −52.51 [56] | 0.74 [65] | 6.51 | 30 |
| Sorbent | Adsorption Heat (ΔHads) [kJ/molCO2] | Loading Capacity (q) | Selectivity CO2/N2 | Specific Heat (cp) [kJ/kgK] | * Density (ρ) [kg/m3] | |
|---|---|---|---|---|---|---|
| [kgCO2/kgadsorbent] | ** [molCO2/Ladsorbent] | |||||
| Polyethylenimine/silica (PEI/HMS) [77] | −95.04 | 0.059 | - | 1.81 | - | |
| PPN-6-CH2-TETA [73,78] | −48.22 | 0.06 | 1.21 | >10,000 | 0.985 | 883.8 |
| PPN-6-CH2-DETA [73,78] | −45.32 | 0.112 | 2.04 | >10,000 | 0.985 | 805 |
| PEI-PS-50 [79] | −70.4 | 0.1276 | - | 1.65 | - | |
| Zeolite (13X) [80,81] | −49.72 | 0.176 | 2.6 | 17.46 | 1.07 | 1430 |
| MOF-74-Mg [73,78] | −37.4 | 0.2284 | 4.75 | 209 | 0.896 | 914.9 |
| Ref. | Method | CO2 Technique | Detail | Analysis | Main Results |
|---|---|---|---|---|---|
| [113] | Postcombustion | Amine-absorption | MEA at 30 wt% CCR 90% | Economic | € 73 per ton of captured CO2 |
| [114] | Postcombustion | Amine-absorption | MEA at 30 wt% Pz at 30 wt% CCR 60 and 90% | Technical and economic | Pz is 34% more economical than MEA Desorption pressure with Pz at 5 bar Desorption pressure with MEA at 2 bar CO2 storage at 11 bar |
| [115] | Postcombustion | Amine-absorption | MEA at 35 wt% CCR 73 and 90% | Economic | 77.5 €/ton CO2 at 73% of CCR 163 €/ton CO2 at 90% of CCR |
| [116] | Postcombustion | Amine-absorption | MEA at 35 wt% | Technical and economic | CCR 93% Operational costs decrease when investment is lower than 710,000 USD/MW and the CO2 emission tax per unit mass is higher than 32 USD/ton |
| [117] | Postcombustion | CL | NaOH and CaO | Technical and economic | CCS system requires 24 m2 Operating cost increment of 10.6 and selling the CaCO3 of 6.8% |
| [118] | Postcombustion | Amine-absorption | NH3 at 3.5 and 4.1 wt% CCR of 75% | Energy | Reboiler energy consumption is 6.3 and 4.5 MJ/kg CO2 at 3.5 and 4.2 wt% of NH3 |
| [119] | Postcombustion | Amine-absorption | NH3 between 4 and 10 wt% CCR 90% | Energy | Reboiler energy consumption decrease 28.5% for 4% of NH3 |
| [120] | Postcombustion | CL | NaOH and CaO | Technical | NaOH flow rate required was 12.52 tons/day CCR of 20% |
| [121] | Postcombustion | Amine-absorption | MEA at 35 wt% | Energy | CCR of 56.5% tropical conditions |
| [122] | Postcombustion | Amine-absorption | MDEA at 22% and Pz at 8% | Technical | CCR of 12.2% for 2030 CCR of 34.8% for 2040 CCR of 68.35% for 2050 |
| [123] | Postcombustion | CL | NaOH and CaO | Life cycle | CCR of 37% Life cycle cost is 40% minor than the base configuration |
| [124] | Pre-combustion | Electromethanol | Hymethship | Life cycle | Reduction of 98% of GWP20 and GWP100 |
| [125] | Postcombustion | Adsorption | K2CO3 raw and on an alumina base and support | Technical | CCR of 43% with K2CO3 supported on an alumina base |
| [126] | Postcombustion | Adsorption | K2CO3 supported on porous alumina | Energy | CCR of 30% with K2CO3 supported on an alumina base Carbonation and regeneration temperatures are 60 and 120 °C, respectively |
| Ref. | Method | CO2 Technique | Detail | Analysis | Main Results |
|---|---|---|---|---|---|
| [127] | Oxy-fuel combustion | ICRC | O2 and water injection | Modelling | Demonstration |
| [128] | Oxy-fuel combustion | ICRC | SI-ICE, fuel C3H8, 2000 rpm, 40% of O2 | Performance | Increase in the indicated work of 7.8% |
| [129] | Oxy-fuel combustion | ICRC | SI-ICE, fuel C3H8, 2000 rpm, 45% of O2 | Performance | 2% reduction in the indicated work |
| [130,131,132] | Oxy-fuel combustion | Intake charge EGR and CO2 | SI-ICE, fuel CH4, 35.4% of O2 % | Performance | IMEP: 9.6 bar |
| [133] | Oxy-fuel combustion | Intake charge and CO2 | CI-ICE, Fuel diesel | Performance simulation | 40 kW of brake power with a CO2 fraction 72%, O2 ratio 1.5 compression ratio 22 |
| [134] | Oxy-fuel combustion | ICRC | SI-ICE | Performance simulation | EGR 5% Thermal efficiency 42% CFR 26.4% |
| [135] | Oxy-fuel combustion | ICRC with EGR 60% | SI-ICE fuel C3H8 | Performance | Indicated work is increased by 7.8%. |
| [136] | Post-combustion | Amine-absorption | CI-ICE, fuel diesel, MEA 30 wt%, DMEA 30 wt%, NH3 30 wt% | Energy | CCR of 90% regeneration energy 2.2 kWh with MEA CCR of 80% regeneration energy 0.7 kWh with NH3 CCR of 90% regeneration energy 1.1 kWh with DMEA |
| [137] | Post-combustion | Adsorption Absorption | Ford F-250 and Toyota Camry’s | Technical | Ford CCR 10%, solid sorbent Toyota CCR 25%, solvent |
| [138] | Post-combustion | Absorption | Volvo heavy-duty truck | Technical | CCR 40% |
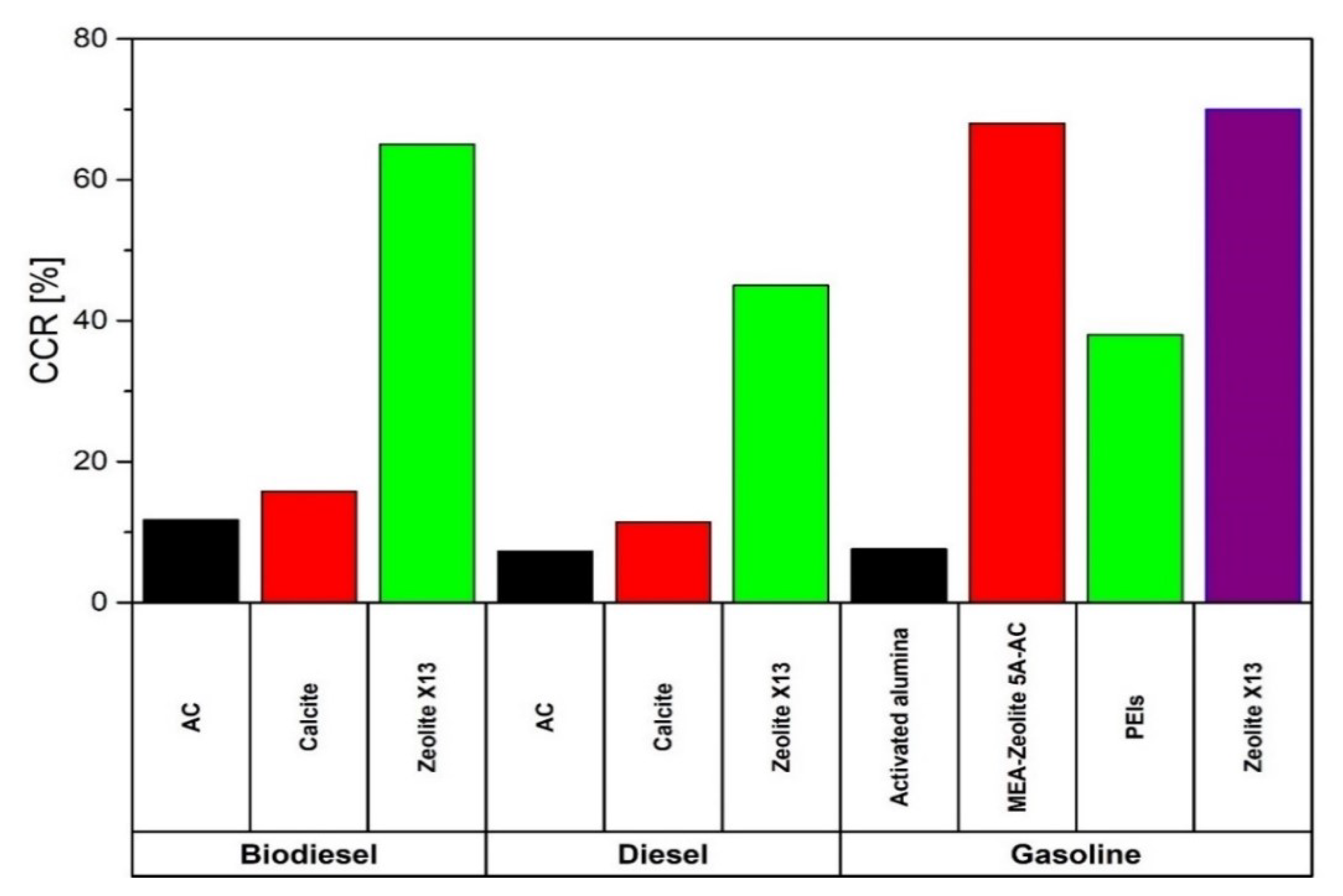
| [139] | Post-combustion | Adsorption | CI-ICE, fuel diesel and Biodiesel, AC and Calcite as sorbents | Technical | Diesel operation: CCR 11.45% with calcite and CCR 7.29% with AC Biodiesel operation: CCR 15.79% with calcite and CCR 11.76% with AC |
| [140,141,142] | Post-combustion | Adsorption | CI-ICE, fuel diesel and blends of KOME, orange oil, acetone, ethanol or butanol or pentanol, sorbent AC and Zeolite X13 | Technical | Maximum CCR 65% with zeolite X13 CI-ICE fuelled with KOME, orange oil and methanol |
| [143] | Post-combustion | Adsorption | CI-ICE, fuel diesel, Sorbent Zeolite X13 | Technical | CCR 45% |
| [144] | Post-combustion | Adsorption | SI-ICE, fuel gasoline, sorbent blend of MEA, zeolite 5A and AC | Technical | CCR 68% |
| [145] | Post-combustion | Adsorption | SI-ICE, fuel gasoline, sorbent zeolite X13 | Technical | CCR 70% |
| [146] | Post-combustion | Adsorption | SI-ICE, fuel gasoline, sorbent PEIs | Technical | CCR 38% |
| [147] | Post-combustion | Adsorption | SI-ICE, fuel gasoline, sorbent activated alumina | Technical | CCR 7.6% |
| [148] | Post-combustion | TSA | CI-ICE, fuel diesel, ORC implementation, Sorbent PPN-6-CH2 TETA, CCR 90% | Simulation | It is possible a CCS system operating in CI-ICE for road freight transport without affecting the engine performance |
| [149] | Post-combustion | absorption Adsorption | Synthetic FG, NaOH and Ca(OH)2 | Technical | With NaO CCR 100% for 70 min |
| [150] | Post-combustion | H2 Injection | Heavy duty vehicle, Membranes | Simulation | CCR 75% |
| [151] | Post-combustion | H2 Injection RWGS | SI-ICE, fuel CH4 operating at 2000 and | Technical | CCR 3.88% |
| [152] | Bio-fixation | Microalgae | CI-ICE, fuel diesel and biodiesel | Technical | No reported some parameter of CO2 reduction |
| Engine | BUS (M936G) [162] | Ship (W9L46DF) [163] |
|---|---|---|
| Architecture | In-line 6-cylinder engine | In-line 9-cylinder engine |
| Intake | Wastegate Turbocharged with Aftercooler | Turbocharged with Aftercooler |
| Injection | Multipoint—Stoichiometric combustion | Multipoint |
| Displacement volume [L] | 7.7 | 867.6 |
| Brake Power [kW] | 222 at 1950 rpm | 10,305 at 600 rpm |
| Fuel Consumption [kg/km] | 0.361 at average velocity of 20.75 km/h [164] | NA |
| Specific Fuel Consumption [g/kWh] | 194 at 1950 rpm and 100% engine load | 165 at 600 rpm and 75% engine load |
| Species | Mass Fraction (x) [%] | cp at 600 K [kJ/kgK] | cp at 700 K [kJ/kgK] | cp at 800 K [kJ/kgK] |
|---|---|---|---|---|
| CO2 | 0.1514 | 1.075 | 1.126 | 1.168 |
| H2O | 0.1239 | 1.954 | 2.08 | 2.147 |
| N2 | 0.7245 | 1.075 | 1.098 | 1.122 |
| Engine | FG Mass Flow [kg/s] | Sorbent | Regeneration Heat for a CCR 90% [kW] | Available Heat [kW] | ||
|---|---|---|---|---|---|---|
| 600 K | 700 K | 800 K | ||||
| M936G | 0.03772 | PPN-6-CH2-DETA | 11.2 | 13.4 | 18.5 | 23.7 |
| MOF-74-Mg | 7.3 | |||||
| Zeolite X13 | 10.1 | |||||
| W9L46DF | 12.3 | PPN-6-CH2-DETA | 2737.5 | NA | 6021.4 | NA |
| MOF-74-Mg | 2240.5 | |||||
| Zeolite X13 | 3291.5 | |||||
| Engine | M936G | W9L46DF | ||||
|---|---|---|---|---|---|---|
| Sorbent | PPN-6-CH2-DETA | MOF-74-Mg | Zeolite X13 | PPN-6-CH2-DETA | MOF-74-Mg | Zeolite X13 |
| at 600 K [kW] | 2.169 | 6.094 | 3.303 | NA | NA | NA |
| at 700 K [kW] | 7.238 | 11.163 | 8.373 | 3283.9 | 3780.9 | 2729.9 |
| at 800 K [kW] | 12.460 | 16.385 | 13.594 | NA | NA | NA |
| Engine | M936G | W9L46DF | ||||
|---|---|---|---|---|---|---|
| Sorbent | PPN-6-CH2-DETA | MOF-74-Mg | Zeolite X13 | PPN-6-CH2-DETA | MOF-74-Mg | Zeolite X13 |
| at 600 K [kW] | 0.434 | 1.219 | 0.661 | NA | NA | NA |
| at 700 K [kW] | 1.448 | 2.233 | 1.675 | 656.8 | 756.2 | 545 |
| at 800 K [kW] | 2.492 | 3.277 | 2.719 | NA | NA | NA |
| Engine | M936G | W9L46DF | ||
|---|---|---|---|---|
| Sorbent | PPN-CH2-DETA | MOF-74-Mg | PPN-CH2-DETA | MOF-74-Mg |
| CO2 Mass (68% of CCR) [kg] | 28.0 | 28.0 | 9116.0 | 9116.0 |
| CO2 loading [kgCO2/kgads] | 0.235 | 0.278 | 0.235 | 0.278 |
| Sorbent mass [kg] | 118.8 | 100.5 | 38726 | 32782 |
| Sorbent density [kg/m3] | 805.00 | 914.88 | 805.00 | 914.88 |
| Sorbent Volume [m3] | 0.148 | 0.110 | 48.106 | 35.832 |
| CO2 Volume stored [m3] | 0.147 | 0.147 | 47.815 | 47.815 |
| Total volume [m3] | 0.295 | 0.257 | 95.922 | 83.647 |
| Engine | M936G | W9L46DF | ||
|---|---|---|---|---|
| Sorbent | PPN-CH2-DETA | MOF-74-Mg | PPN-CH2-DETA | MOF-74-Mg |
| CO2 Mass (68% of CCR) [kg] | 28.0 | 28.0 | 9116.0 | 9116.0 |
| CO2 loading [kgCO2/kgads] | 0.235 | 0.278 | 0.235 | 0.278 |
| Sorbent mass [kg] | 118.8 | 100.5 | 38,726 | 32,782 |
| Sorbent density [kg/m3] | 402.5 | 457.44 | 402.5 | 457.44 |
| Sorbent Volume [m3] | 0.295 | 0.220 | 96.213 | 71.664 |
| CO2 Volume stored [m3] | 0.147 | 0.147 | 47.815 | 47.815 |
| Total volume [m3] | 0.442 | 0.366 | 144.028 | 119.479 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Mariaca, A.; Llera-Sastresa, E. Review on Carbon Capture in ICE Driven Transport. Energies 2021, 14, 6865. https://doi.org/10.3390/en14216865
García-Mariaca A, Llera-Sastresa E. Review on Carbon Capture in ICE Driven Transport. Energies. 2021; 14(21):6865. https://doi.org/10.3390/en14216865
Chicago/Turabian StyleGarcía-Mariaca, Alexander, and Eva Llera-Sastresa. 2021. "Review on Carbon Capture in ICE Driven Transport" Energies 14, no. 21: 6865. https://doi.org/10.3390/en14216865
APA StyleGarcía-Mariaca, A., & Llera-Sastresa, E. (2021). Review on Carbon Capture in ICE Driven Transport. Energies, 14(21), 6865. https://doi.org/10.3390/en14216865









