Numerical Simulation of Combustion of Natural Gas Mixed with Hydrogen in Gas Boilers
Abstract
:1. Introduction
2. Numerical Simulation
2.1. Establishment of Model
2.2. Numerical Solution Method Settings
2.3. Model Rationality Verification
2.4. Fuel Interchangeability Analysis
2.5. Boundary Conditions and Calculation Conditions
3. Simulation Results and Analysis
3.1. Analysis of Combustion Temperature Field
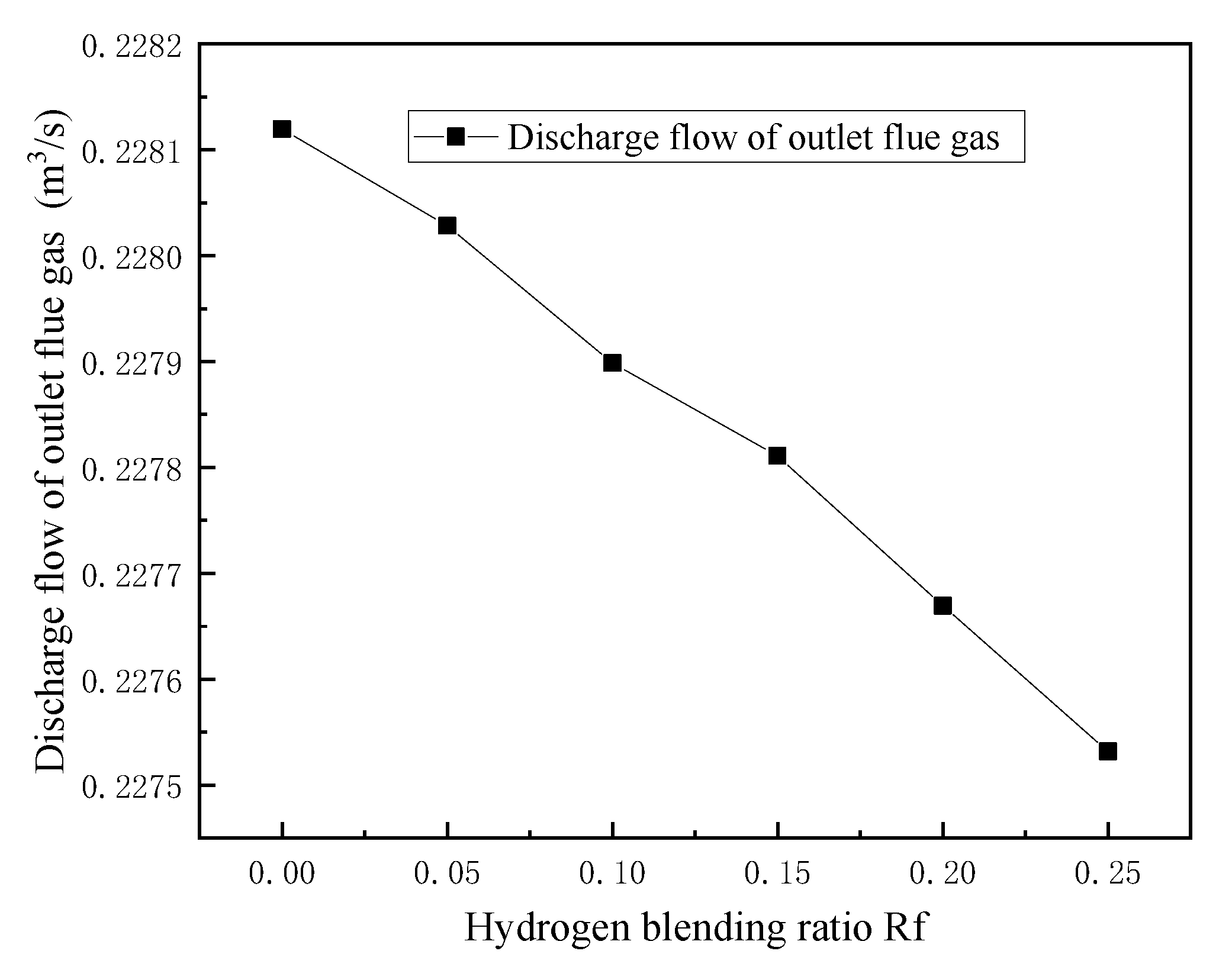
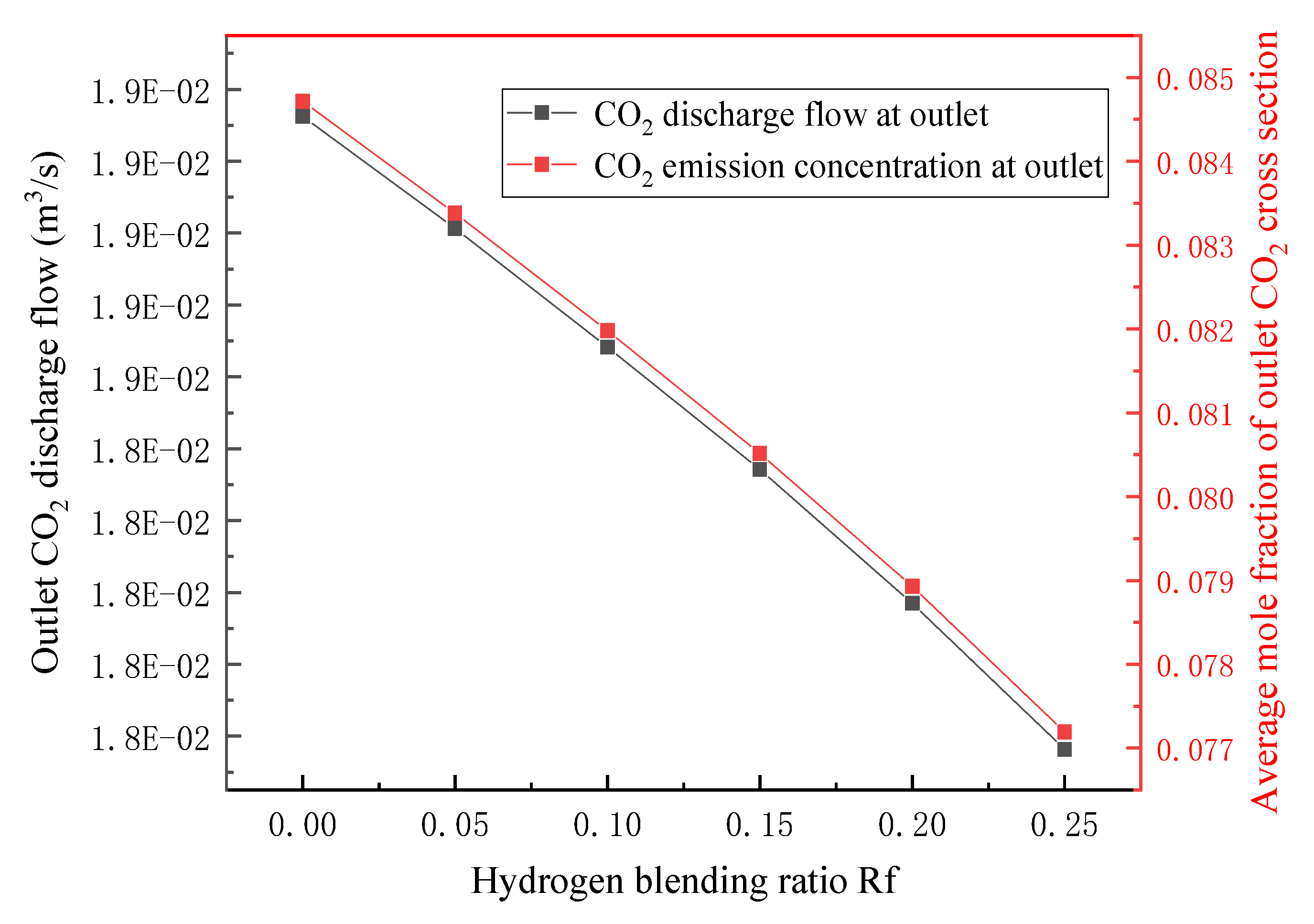
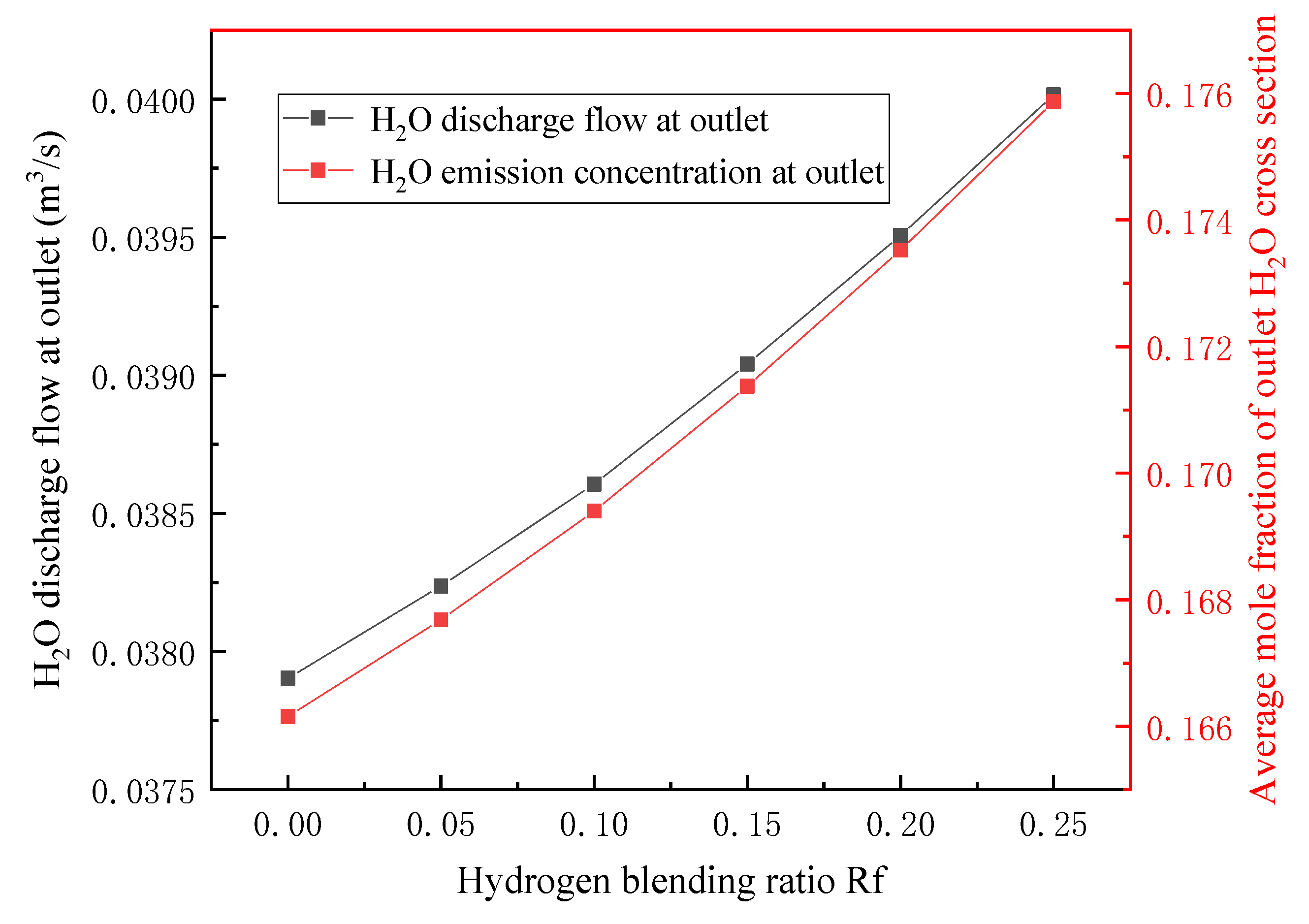
3.2. Flue Gas Analysis
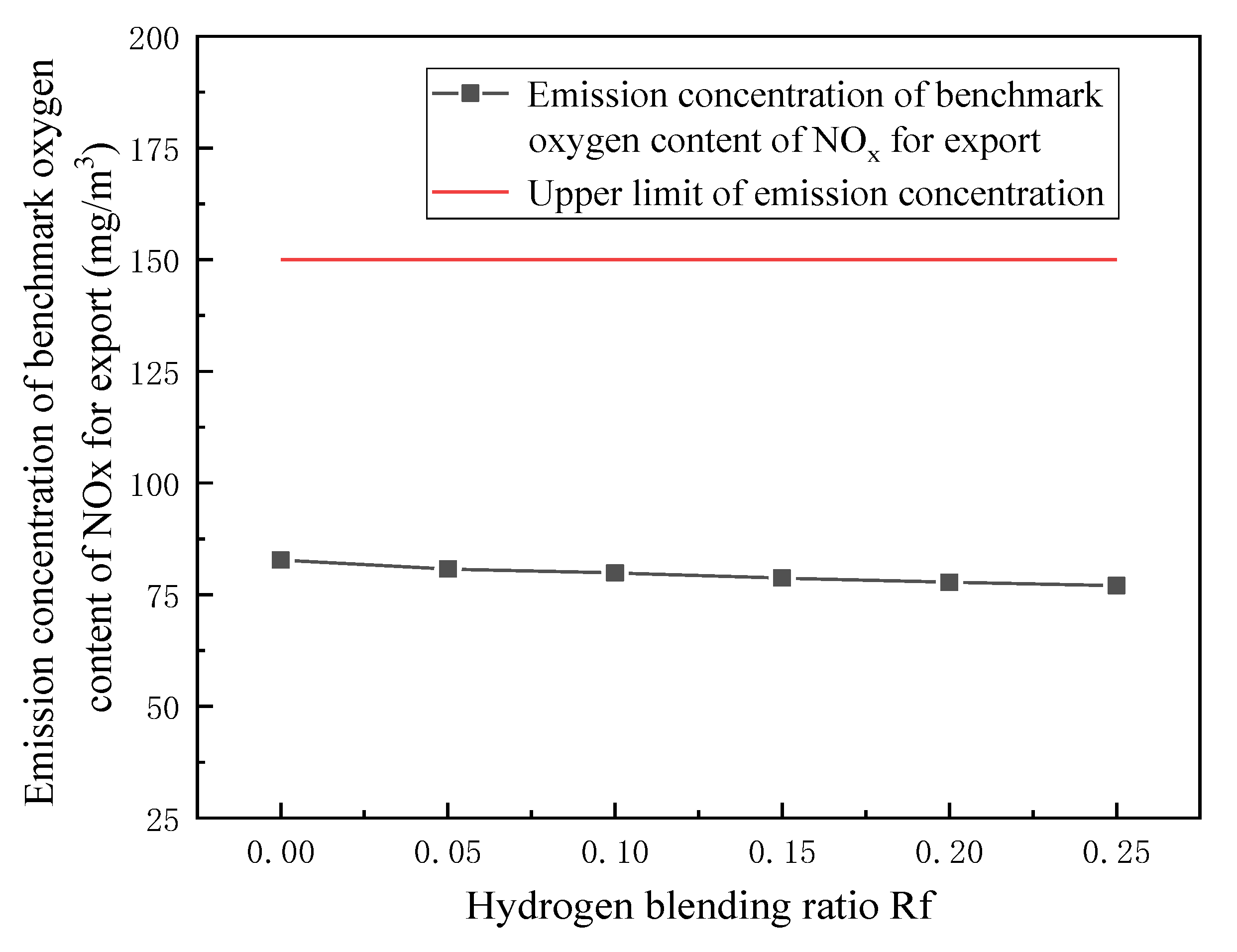
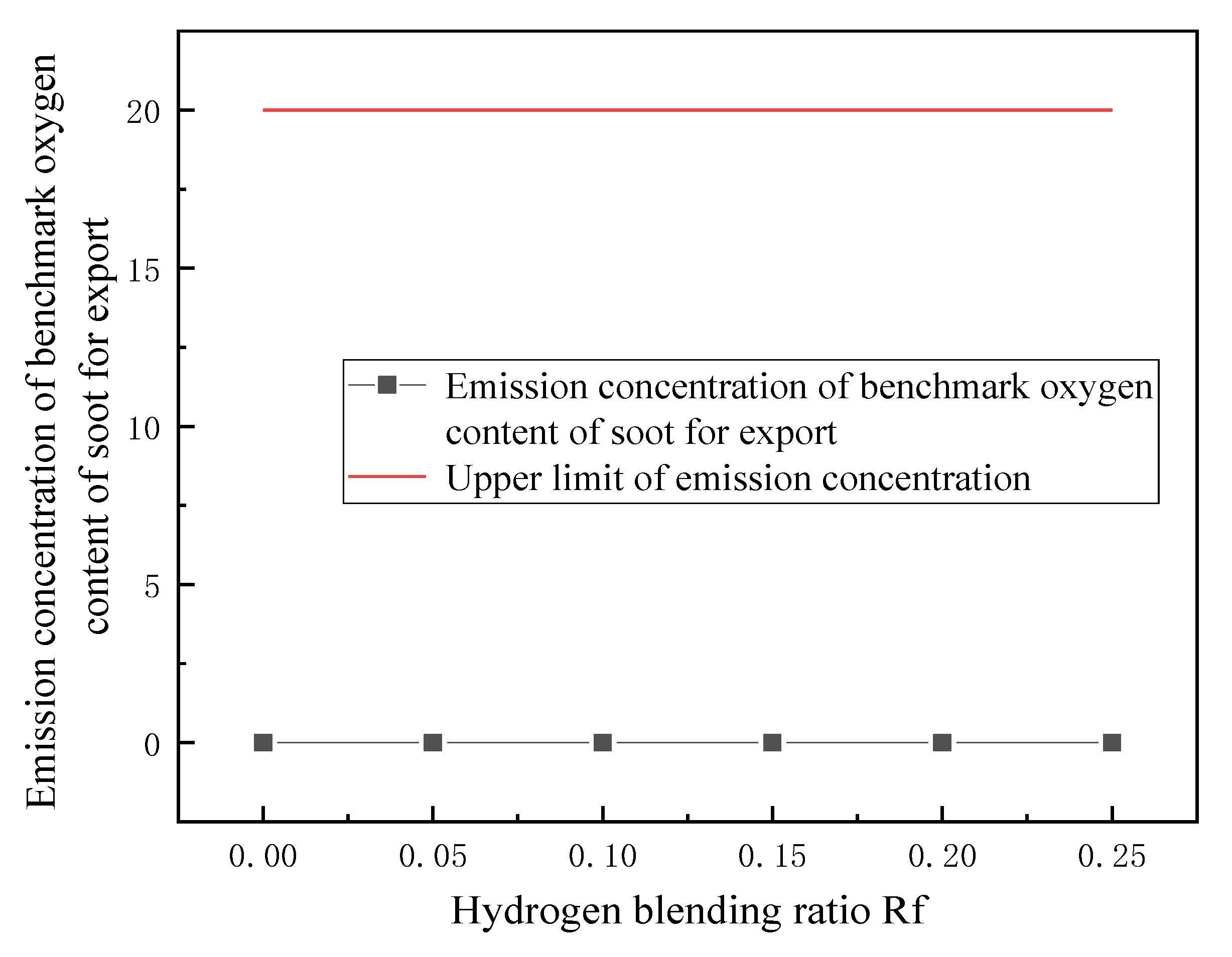
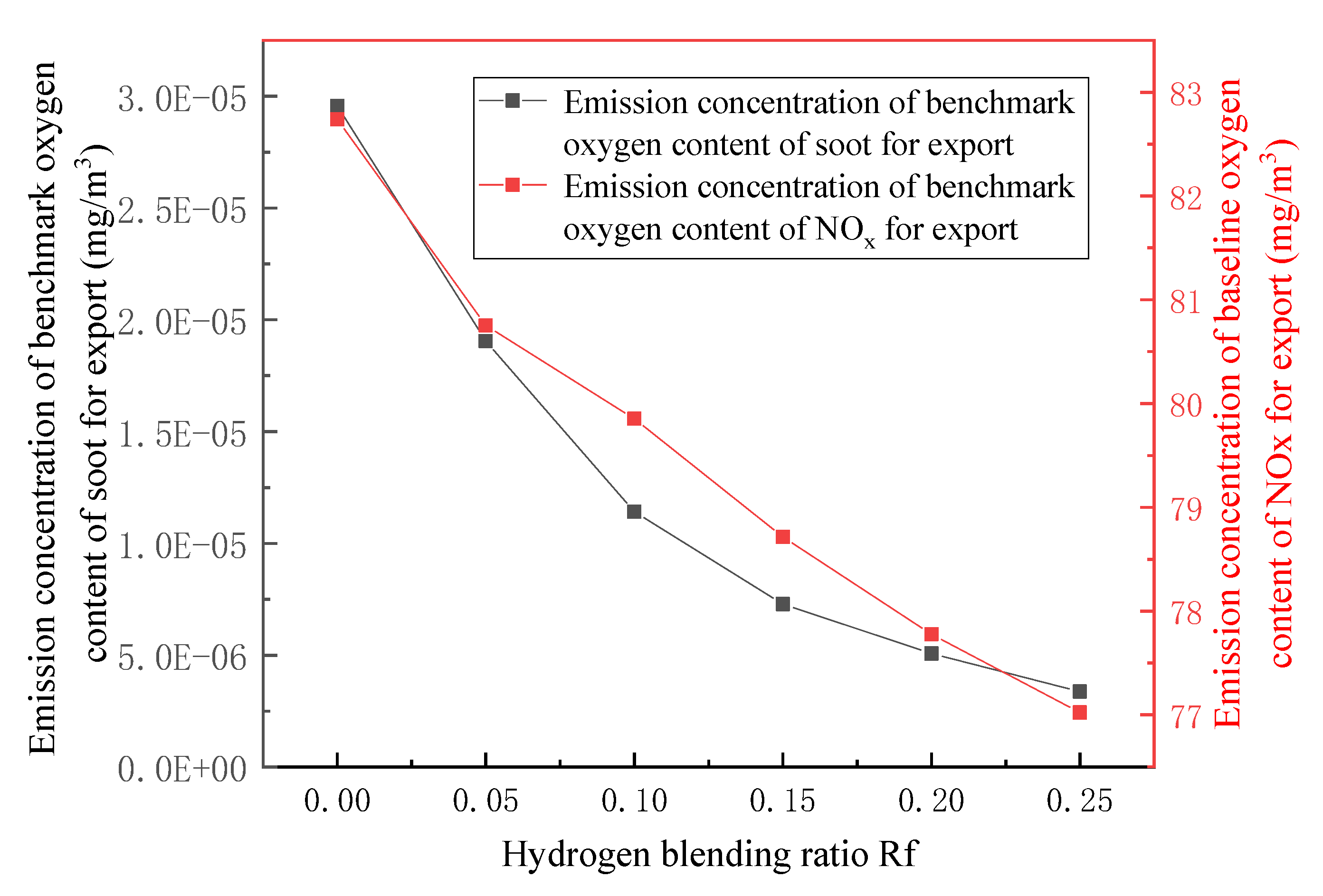
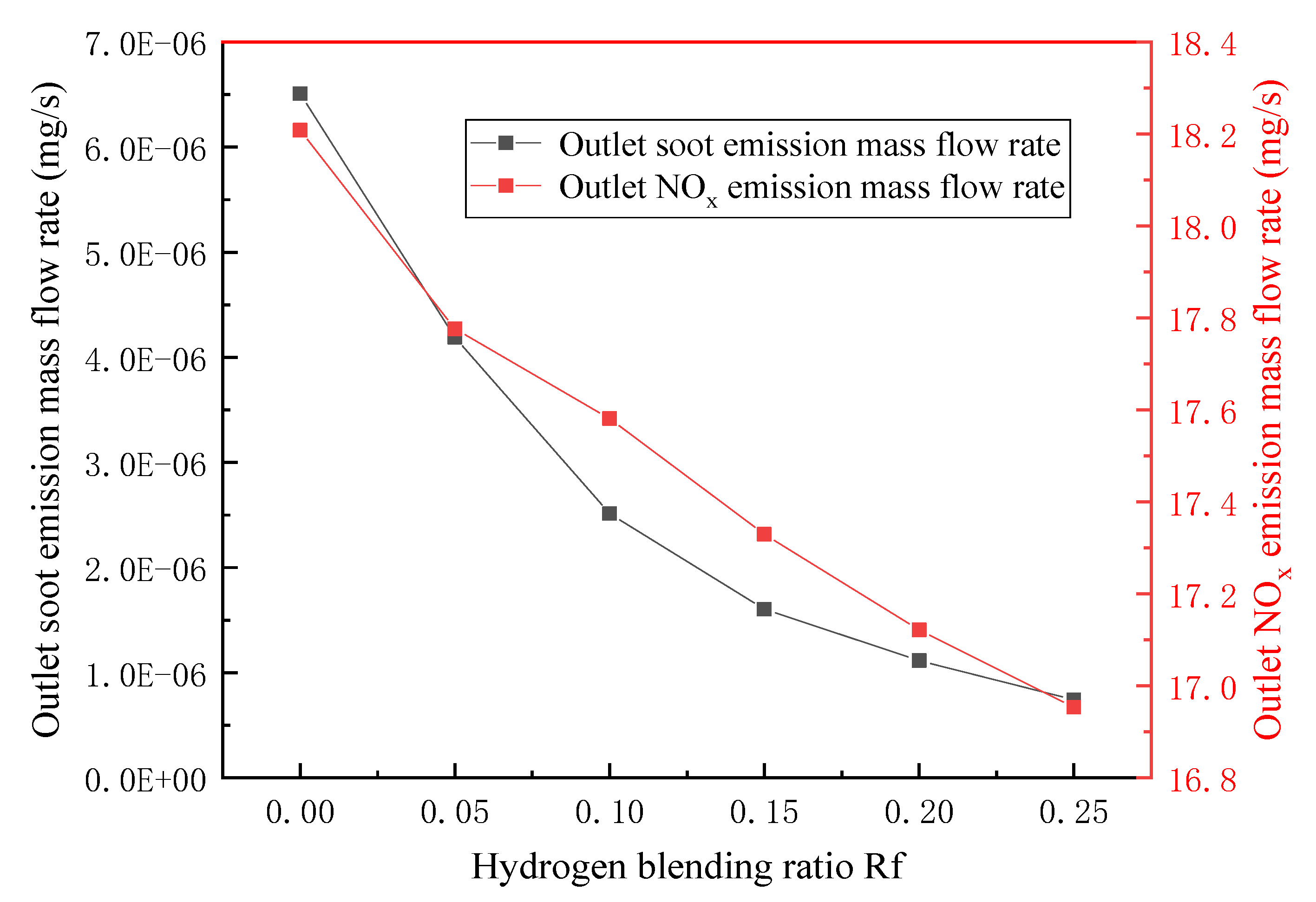
3.3. Emission Detection of Combustion Pollutants
4. Conclusions
- (1)
- In terms of combustion characteristics, with the increase in hydrogen ratio, the combustion temperature in the furnace increases, the peak temperature position is advanced, and the combustion rate of the gas is accelerated.
- (2)
- In terms of flue gas detection, with the increase in hydrogen mixing ratio, the total flue gas emission decreases together with the emission concentration and emission of CO2 and N2 in the main flue gas components, while the emission concentration and emission of H2O increase. Additionally, the concentration and emission of pollutants NOx and soot in the flue gas are also reduced. Hydrogen-doped combustion can effectively inhibit the generation of NOx and soot by increasing the specific heat capacity of flue gas and the generation of water vapor.
- (3)
- Based on the requirement of fuel interchangeability, the optimal hydrogen mixing volume fraction of the gas-fired boiler is 24.7%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- BP. Statistical Review of World Energy 2020; BP: London, UK, 2020. [Google Scholar]
- Pan, J.P.; Yang, L.L.; Wang, L.X.; Lou, Y.; Wang, S.Y. Strategy on the development of natural gas resources in China under the new situation. Int. Pet. Econ. 2017, 25, 12–18. [Google Scholar]
- Xin, Y.; Zhang, Y.; Xue, P.; Wang, K.; Adu, E.; Tontiwachwuthikul, P. The Optimization and Thermodynamic and Economic Estimation Analysis for CO2 Compression-liquefaction Process of CCUS System Using LNG Cold Energy. Energy 2021, 236, 121–376. [Google Scholar] [CrossRef]
- Melaina, M.W.; Antonia, O.; Penev, M. Blending Hydrogen into Natural Gas Pipeline Networks: A Review of Key Issues; Technical Report; National Renewable Energy Laboratory: Golden, CO, USA, 2013. [Google Scholar]
- Haines, M.R.; Polman, E.A.; Laat, J. Reduction of CO2 Emissions by Adding Hydrogen to Natural Gas; International Energy Agency: Apeldoorn, The Netherlands,, 2005. [Google Scholar]
- Hu, Z.Q.; Zhang, X. Study on laminar combustion characteristic of low calorific value gas blended with hydrogen in a constant volume combustion bomb. Int. J. Hydrogen Energy 2019, 44, 487–493. [Google Scholar] [CrossRef]
- Leng, Y.X.; Ge, Q.Q.; He, Z.X.; He, D.Z.; Long, W.Q. Numerical study on the combustion and emission characteristics of a pre-chamber engine fueled with hydrogen enriched compressed natural gas. Trans. Csice 2021, 39, 26–33. [Google Scholar]
- Zhao, R.; Xu, L.P.; Feng, S.Q.; Li, C.X.; Yao, L.; Hu, S.P.; Wang, Z.C. Effects of hydrogen addition in fuel of marine LNG fueled engines on combustion and emissions. J. Propuls. Technol. 2020, 41, 2549–2557. [Google Scholar]
- Wang, J.H.; Huang, Z.H.; Mao, H.Y. Kinetic simulation of CH4-H2-O2-Ar laminar premixed flame chemical reaction. In Proceedings of the 2008 Combustion Academic Conference of Chinese Society of Engineering Thermophysics, Xi’an, China, 25 October 2008. [Google Scholar]
- Wei, J. Study on the Combustion Mechanism and Characteristics of Light Hydrocarbon Mixed Gas [D]; Xi’an Shiyou University: Xi’an, China, 2019. [Google Scholar]
- Standardization Administration of the People’s Republic of China. Classification and Basic Characteristics of Urban Gas: GB-T13611-2018; China Quality and Standards Press: Beijing, China, 2018; p. 3. [Google Scholar]
- Ren, J.H. The Study of Numerical Simulation of Combustion and Heat Transfer in the WNS Gas-Fired Boiler; Beijing Institute of Petrochemical Technology: Beijing, China, 2015; p. 17. [Google Scholar]
- General Administration of Quality Supervision. Inspection and Quarantine. Emission Standard of Air Pollutants for Coal-Burning Oil-Burning Gas-Fired Boiler: GB 13271-2014; China Environmental Science Press: Beijing, China, 2014; Volume 3. [Google Scholar]
- Liu, G.Q.; Ma, L.X.; Liu, J. Chemical and Chemical Engineering Physical Property Data Manual, Inorganic Volume; Chemical Industry Press: Beijing, China, 2002; pp. 112–119. [Google Scholar]
- Zhu, Q.H.; Niu, Y.; Chen, X.F. Impact of water vapor on the soot formation in the laminar methane/air diffusion flame. J. Saf. Environ. 2017, 17, 174–177. [Google Scholar]
- Le Cong, T.; Dagaut, P. Experimental and detailed modeling study of the effect of water vapor on the kinetics of combustion of hydrogen and natural gas, impact on NOx. Energy Fuel 2009, 23, 725–734. [Google Scholar] [CrossRef]
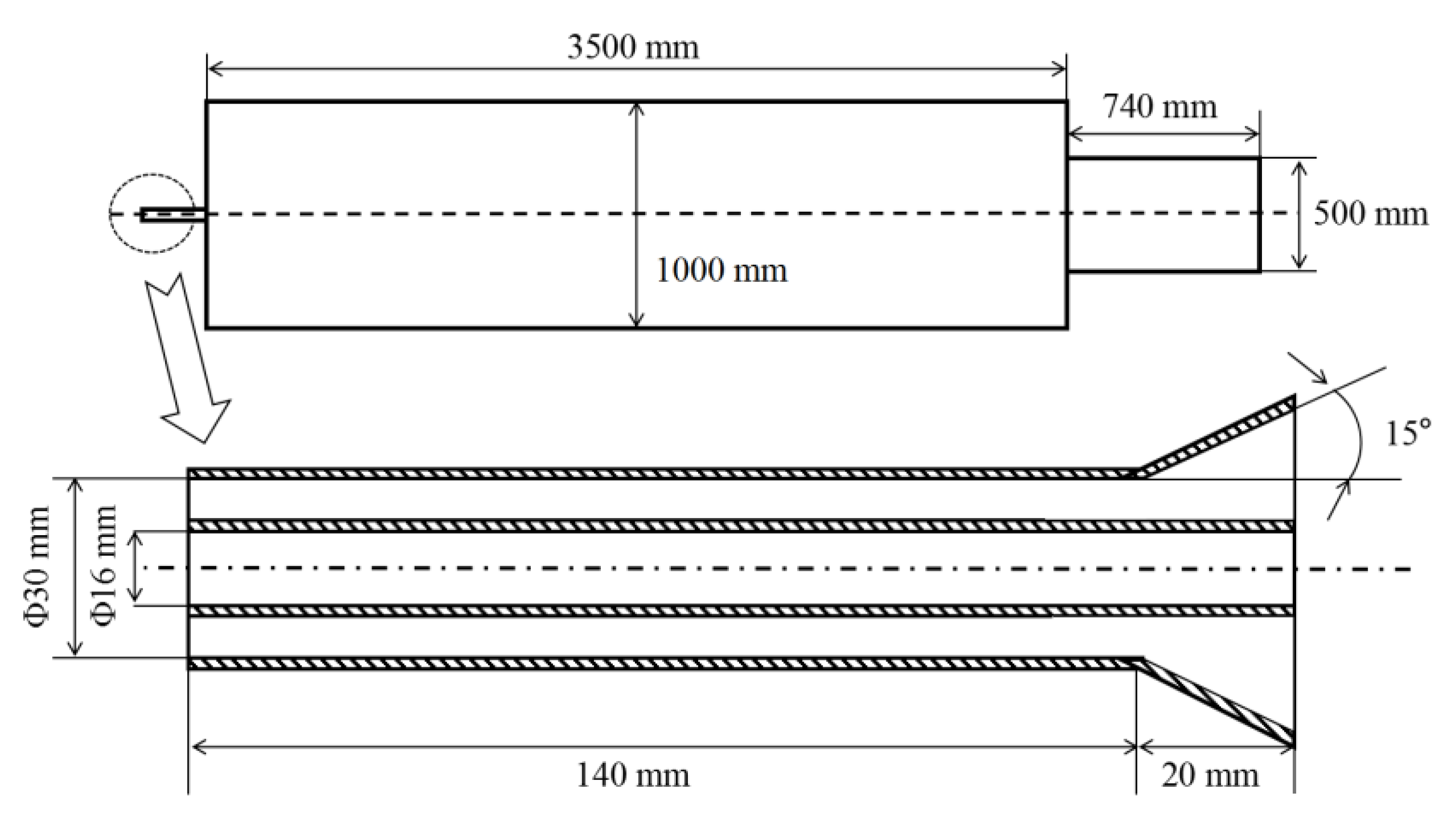

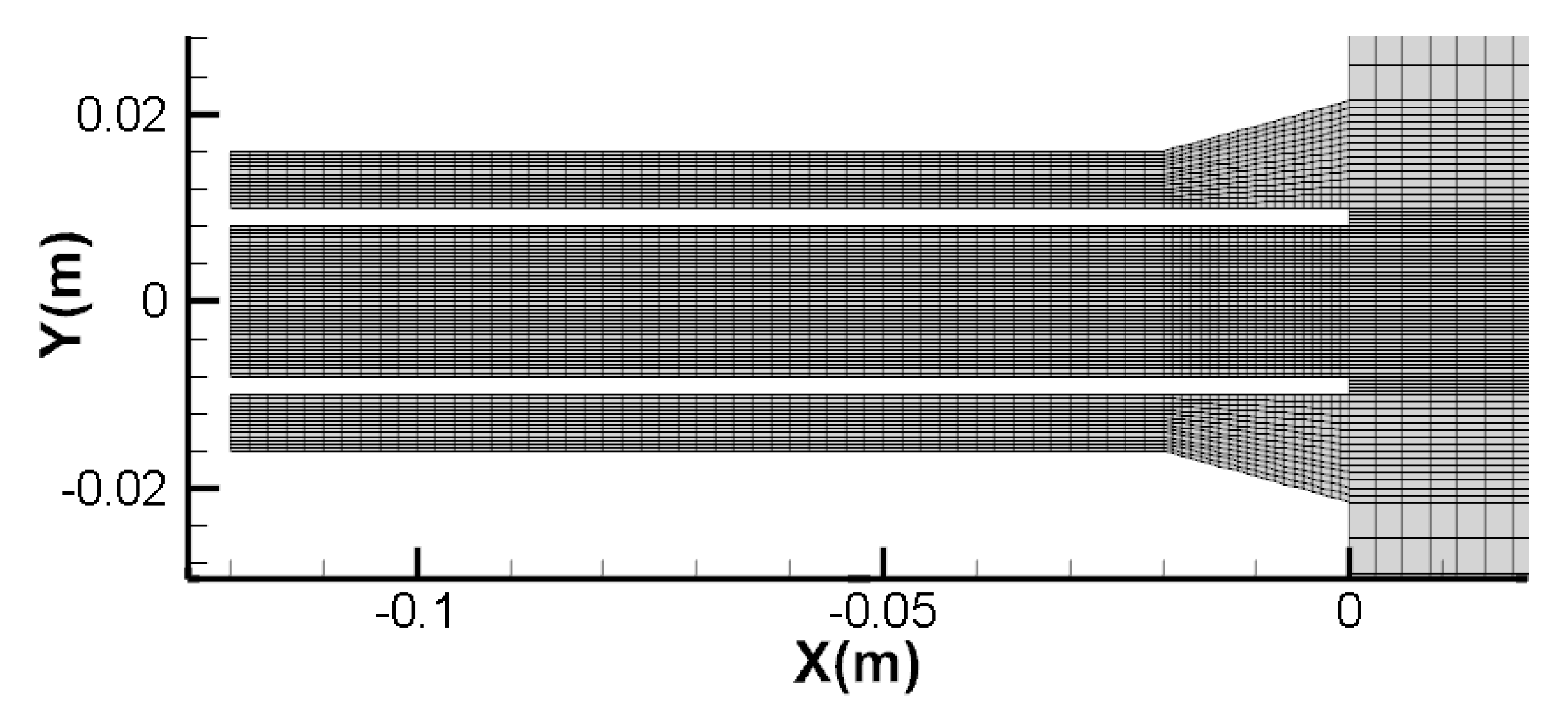





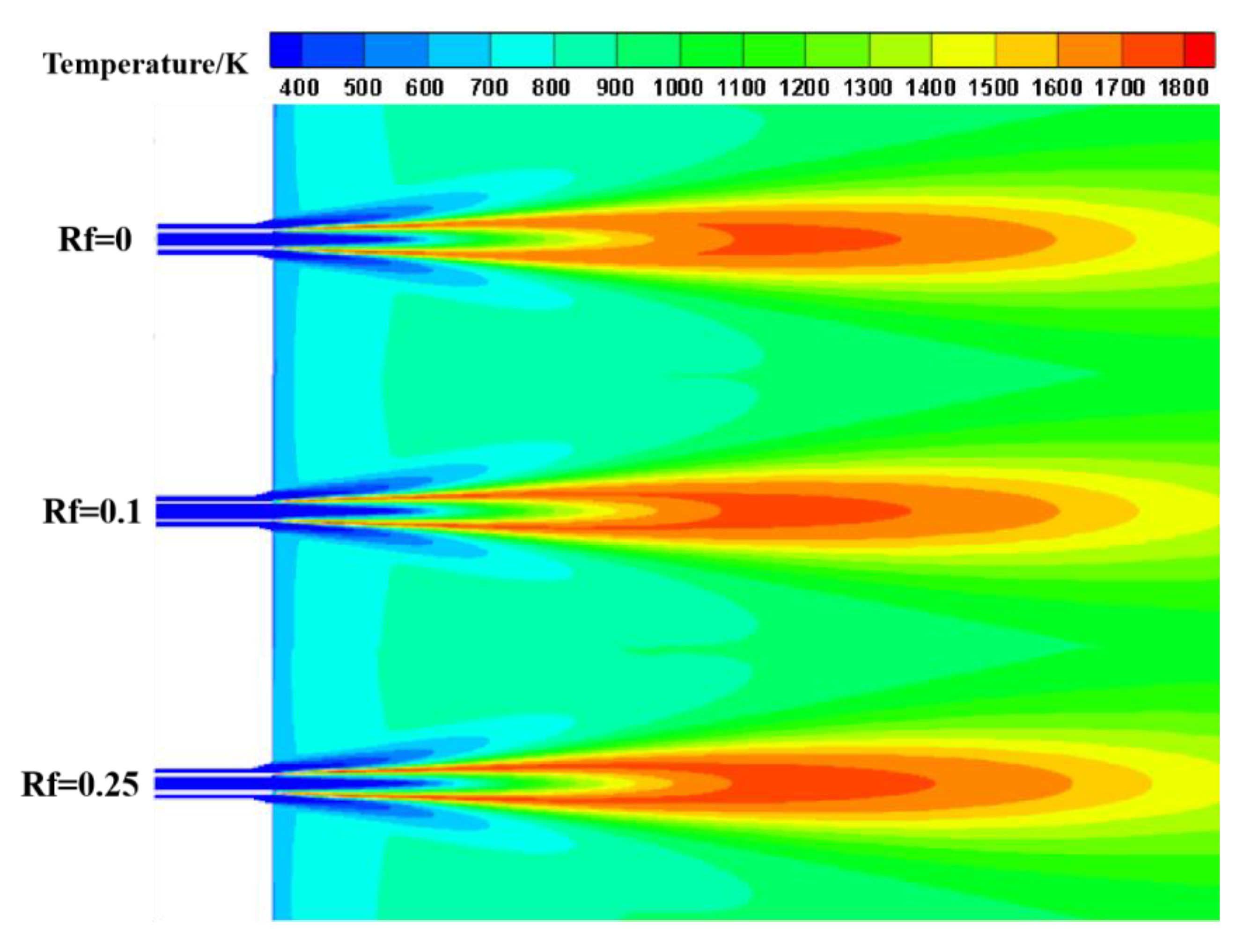
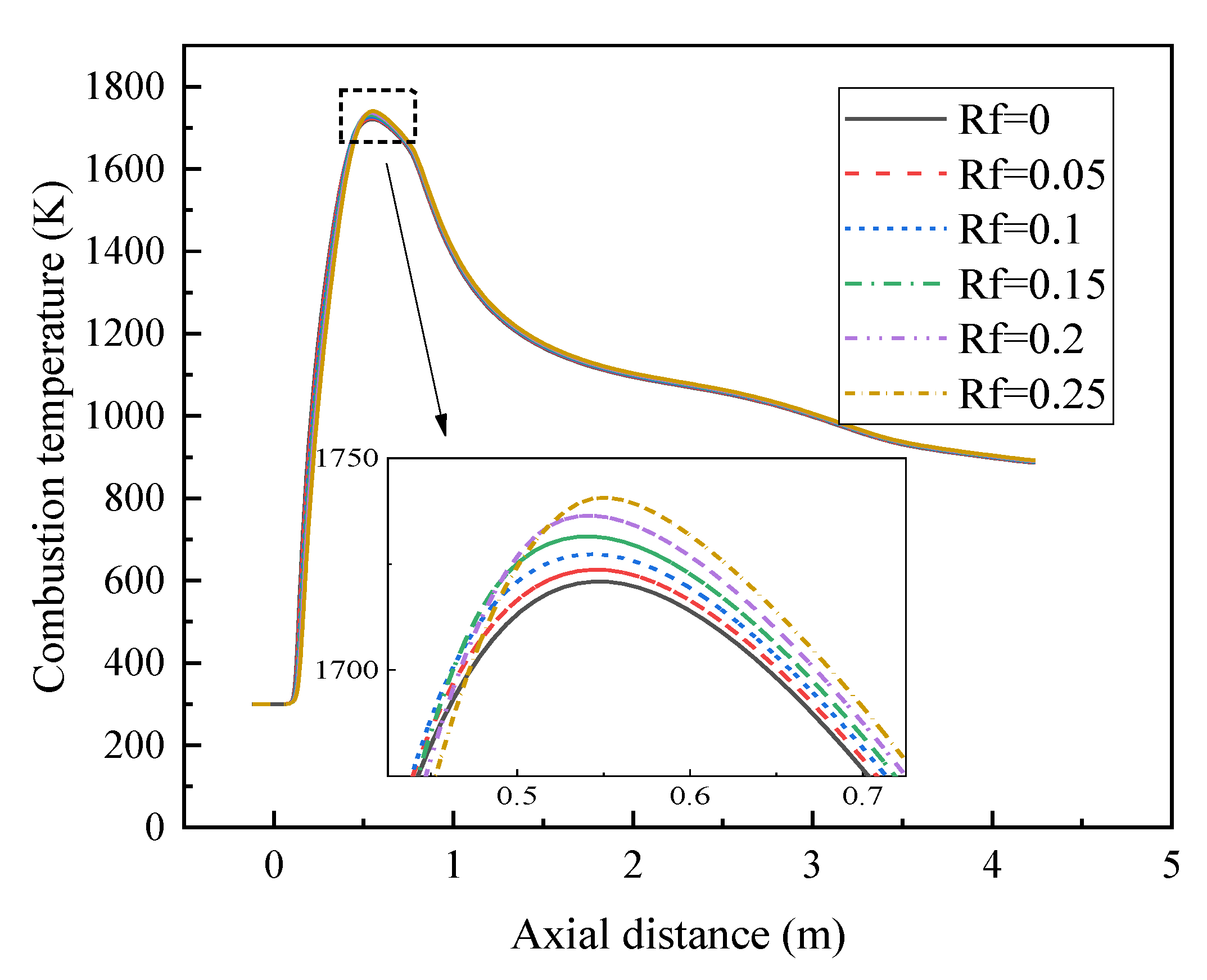


| Name | Type | Temperature/K | Turbulent Intensity/% | Hydraulic Diameter/m |
|---|---|---|---|---|
| Fuel inlet | Velocity inlet | 300 | 10 | 0.016 |
| Air inlet | Velocity inlet | 300 | 10 | 0.012 |
| Outlet | Pressure outlet | 300 | 5 | 0.5 |
| Wall | Wall | 300 | – | – |
| Hydrogen Blending Ratio (Rf) | Fuel | Oxidizer | |||
|---|---|---|---|---|---|
| Component | Flow Rate (m/s) | Component | Flow Rate (m/s) | ||
| 0 | 100% natural gas | 0% H2 | 31.87 | Air atmosphere | 151.07 |
| 0.05 | 95% natural gas | 5% H2 | 33.04 | 150.69 | |
| 0.1 | 90% natural gas | 10% H2 | 34.29 | 150.28 | |
| 0.15 | 85% natural gas | 15% H2 | 35.65 | 149.83 | |
| 0.2 | 80% natural gas | 20% H2 | 37.11 | 149.35 | |
| 0.25 | 75% natural gas | 25% H2 | 38.70 | 148.83 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xin, Y.; Wang, K.; Zhang, Y.; Zeng, F.; He, X.; Takyi, S.A.; Tontiwachwuthikul, P. Numerical Simulation of Combustion of Natural Gas Mixed with Hydrogen in Gas Boilers. Energies 2021, 14, 6883. https://doi.org/10.3390/en14216883
Xin Y, Wang K, Zhang Y, Zeng F, He X, Takyi SA, Tontiwachwuthikul P. Numerical Simulation of Combustion of Natural Gas Mixed with Hydrogen in Gas Boilers. Energies. 2021; 14(21):6883. https://doi.org/10.3390/en14216883
Chicago/Turabian StyleXin, Yue, Ke Wang, Yindi Zhang, Fanjin Zeng, Xiang He, Shadrack Adjei Takyi, and Paitoon Tontiwachwuthikul. 2021. "Numerical Simulation of Combustion of Natural Gas Mixed with Hydrogen in Gas Boilers" Energies 14, no. 21: 6883. https://doi.org/10.3390/en14216883





