Effect of Localized Temperature Difference on Hydrogen Fermentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inoculum and Substrate
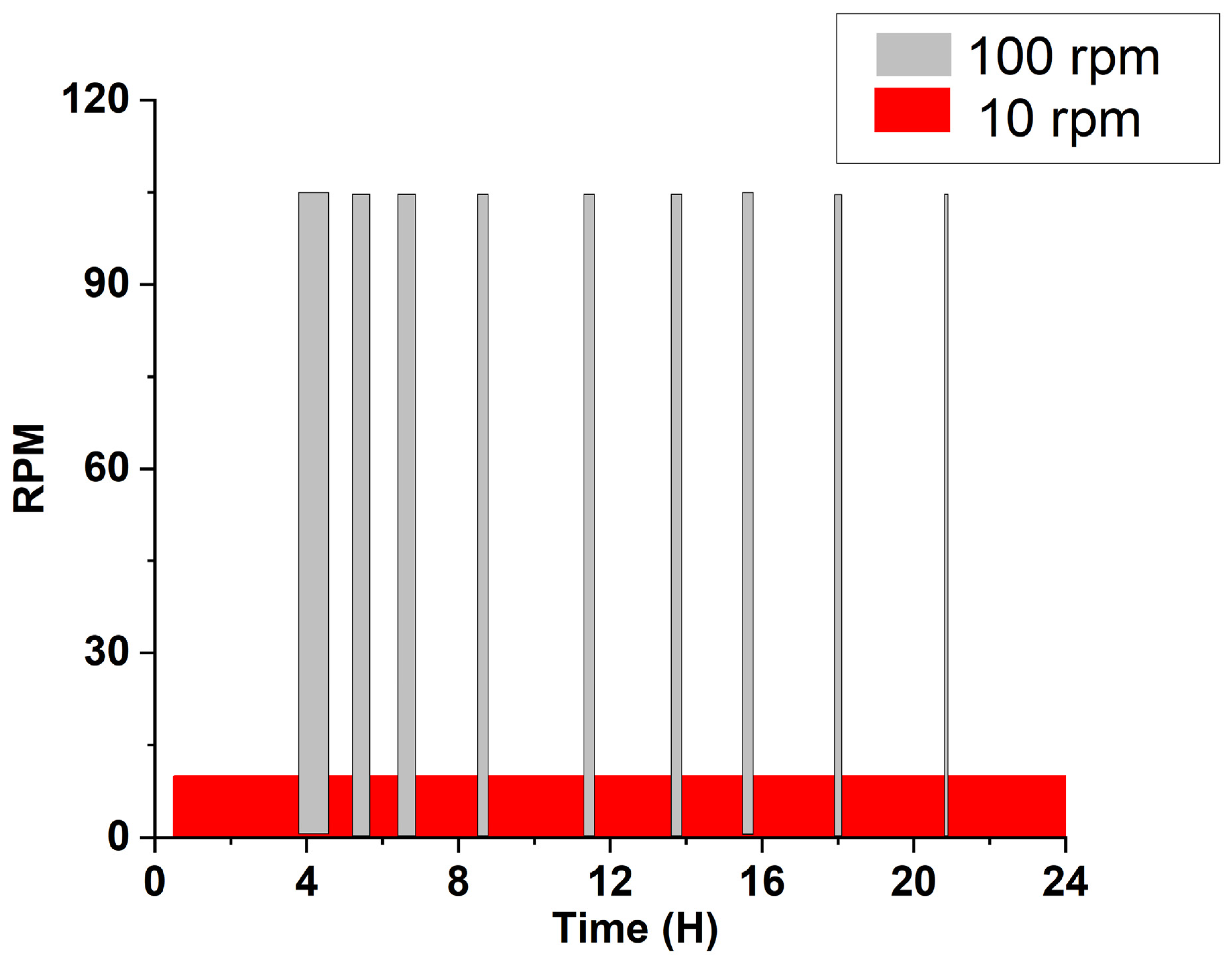
2.2. Experiment
2.3. Analysis
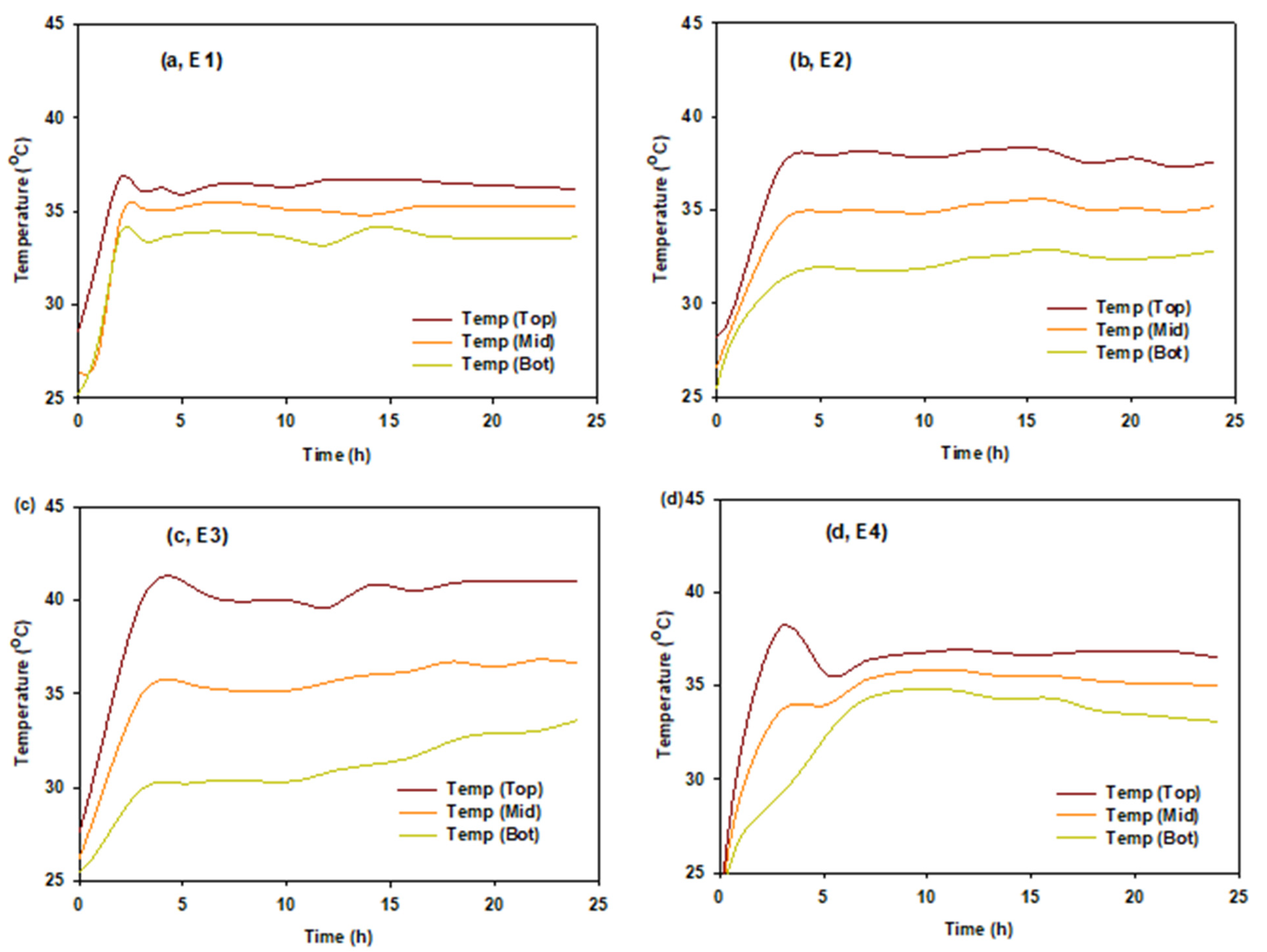
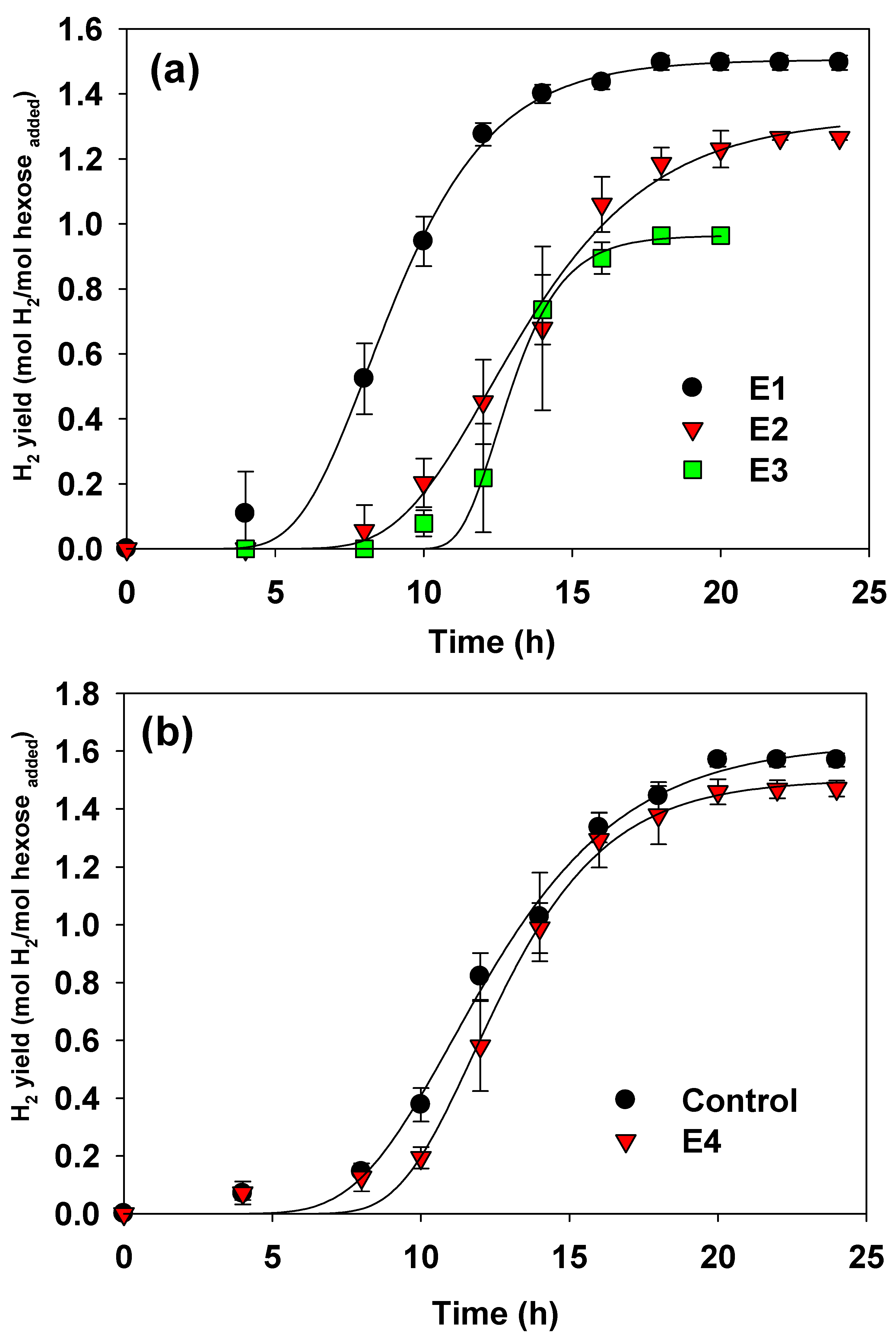
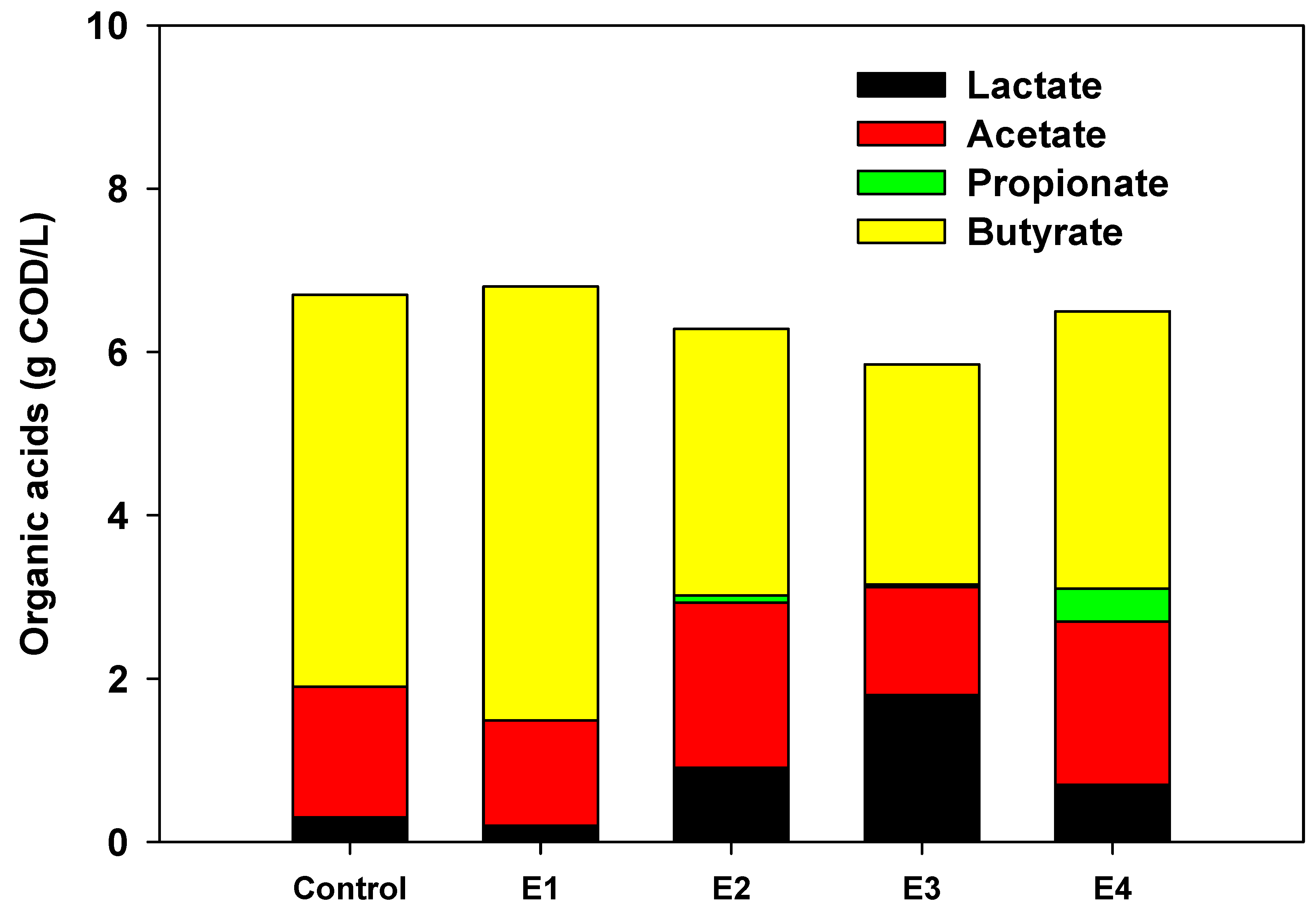
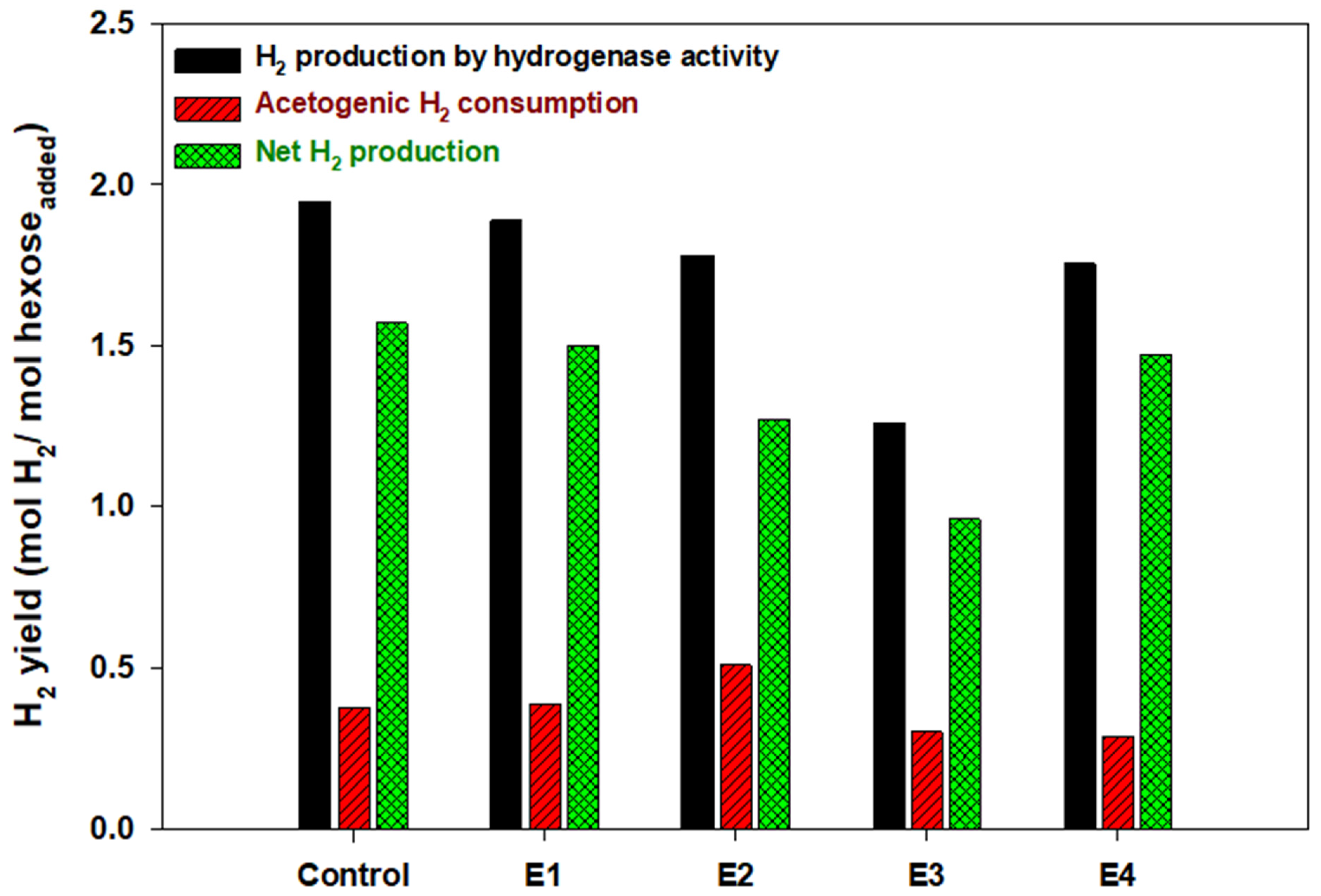
3. Results and Discussion
3.1. Fermentation Performance
3.2. Energy Reduction by Agitation Speed Control
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cieciura-Włoch, W.; Binczarski, M.; Tomaszewska, J.; Borowski, S.; Domański, J.; Dziugan, P.; Witońska, I. The use of acidic hydrolysates after furfural production from sugar waste biomass as a fermentation medium in the biotechnological production of hydrogen. Energies 2019, 12, 3222. [Google Scholar] [CrossRef] [Green Version]
- Prapinagsorn, W.; Sittijunda, S.; Reungsang, A. Co-digestion of napier grass and its silage with cow dung for methane production. Energies 2017, 10, 1654. [Google Scholar] [CrossRef] [Green Version]
- Kieckhäfer, K.; Quante, G.; Müller, C.; Spengler, T.S.; Lossau, M.; Jonas, W. Simulation-based analysis of the potential of alternative fuels towards reducing CO2 emissions from aviation. Energies 2018, 11, 186. [Google Scholar] [CrossRef] [Green Version]
- López Ortiz, A.; Meléndez Zaragoza, M.J.; Collins-Martínez, V. Hydrogen production research in Mexico: A review. Int. J. Hydrog. Energy 2016, 41, 23363–23379. [Google Scholar] [CrossRef]
- Da Silva Veras, T.; Mozer, T.S.; da Costa Rubim Messeder dos Santos, D.; da Silva César, A. Hydrogen: Trends, production and characterization of the main process worldwide. Int. J. Hydrog. Energy 2017, 42, 2018–2033. [Google Scholar] [CrossRef]
- Islam, M.S.; Guo, C.; Liu, C.Z. Enhanced hydrogen and volatile fatty acid production from sweet sorghum stalks by two-steps dark fermentation with dilute acid treatment in between. Int. J. Hydrog. Energy 2018, 43, 659–666. [Google Scholar] [CrossRef]
- Salem, A.H.; Brunstermann, R.; Mietzel, T.; Widmann, R. Effect of pre-treatment and hydraulic retention time on biohydrogen production from organic wastes. Int. J. Hydrog. Energy 2018, 43, 4856–4865. [Google Scholar] [CrossRef]
- Boodhun, B.S.F.; Mudhoo, A.; Kumar, G.; Kim, S.H.; Lin, C.Y. Research perspectives on constraints, prospects and opportunities in biohydrogen production. Int. J. Hydrog. Energy 2017, 42, 27471–27481. [Google Scholar] [CrossRef]
- Wong, Y.M.; Wu, T.Y.; Juan, J.C. A review of sustainable hydrogen production using seed sludge via dark fermentation. Renew. Sustain. Energy Rev. 2014, 34, 471–482. [Google Scholar] [CrossRef]
- Ghimire, A.; Frunzo, L.; Pirozzi, F.; Trably, E.; Escudie, R.; Lens, P.N.L.; Esposito, G. A review on dark fermentative biohydrogen production from organic biomass: Process parameters and use of by-products. Appl. Energy 2015, 144, 73–95. [Google Scholar] [CrossRef]
- Urbaniec, K.; Bakker, R.R. Biomass residues as raw material for dark hydrogen fermentation—A review. Int. J. Hydrog. Energy 2015, 40, 3648–3658. [Google Scholar] [CrossRef]
- Sivagurunathan, P.; Kumar, G.; Bakonyi, P.; Kim, S.H.; Kobayashi, T.; Xu, K.Q.; Lakner, G.; Tóth, G.; Nemestóthy, N.; Bélafi-Bakó, K. A critical review on issues and overcoming strategies for the enhancement of dark fermentative hydrogen production in continuous systems. Int. J. Hydrog. Energy 2016, 41, 3820–3836. [Google Scholar] [CrossRef]
- Kumar, G.; Sivagurunathan, P.; Pugazhendhi, A.; Thi, N.B.D.; Zhen, G.; Chandrasekhar, K.; Kadier, A. A comprehensive overview on light independent fermentative hydrogen production from wastewater feedstock and possible integrative options. Energy Convers. Manag. 2017, 141, 390–402. [Google Scholar] [CrossRef]
- Dareioti, M.A.; Vavouraki, A.I.; Tsigkou, K.; Zafiri, C.; Kornaros, M. Dark fermentation of sweet sorghum stalks, cheese whey and cow manure mixture: Effect of PH, pretreatment and organic load. Processes 2021, 9, 1017. [Google Scholar] [CrossRef]
- Jiang, F.; Peng, Z.; Li, H.; Li, J.; Wang, S. Effect of hydraulic retention time on anaerobic baffled reactor operation: Enhanced biohydrogen production and enrichment of hydrogen-producing acetogens. Process 2020, 8, 339. [Google Scholar] [CrossRef] [Green Version]
- Ohnishi, A.; Hasegawa, Y.; Fujimoto, N.; Suzuki, M. Biohydrogen production by mixed culture of megasphaera elsdenii with lactic acid bacteria as lactate-driven dark fermentation. Bioresour. Technol. 2021, 343, 126076. [Google Scholar] [CrossRef]
- Lee, K.S.; Lin, P.J.; Chang, J.S. Temperature effects on biohydrogen production in a granular sludge bed induced by activated carbon carriers. Int. J. Hydrog. Energy 2006, 31, 465–472. [Google Scholar] [CrossRef]
- Wang, J.; Wan, W. Effect of temperature on fermentative hydrogen production by mixed cultures. Int. J. Hydrog. Energy 2008, 33, 5392–5397. [Google Scholar] [CrossRef]
- Özgür, E.; Uyar, B.; Öztürk, Y.; Yücel, M.; Gündüz, U.; Eroǧlu, I. Biohydrogen production by Rhodobacter capsulatus on acetate at fluctuating temperatures. Resour. Conserv. Recycl. 2010, 54, 310–314. [Google Scholar] [CrossRef]
- Kress, P.; Nägele, H.J.; Oechsner, H.; Ruile, S. Effect of agitation time on nutrient distribution in full-scale CSTR biogas digesters. Bioresour. Technol. 2018, 247, 1–6. [Google Scholar] [CrossRef]
- Lemmer, A.; Naegele, H.J.; Sondermann, J. How efficient are agitators in biogas digesters? Determination of the efficiency of submersible motor mixers and incline agitators by measuring nutrient distribution in full-scale agricultural biogas digesters. Energies 2013, 6, 6255–6273. [Google Scholar] [CrossRef] [Green Version]
- Kaparaju, P.; Buendia, I.; Ellegaard, L.; Angelidaki, I. Effects of mixing on methane production during thermophilic anaerobic digestion of manure: Lab-scale and pilot-scale studies. Bioresour. Technol. 2008, 99, 4919–4928. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.J.; Chang, J.S.; Yang, L.H.; Lin, C.Y.; Wu, S.Y.; Lee, K.S. Enhancing the performance of pilot-scale fermentative hydrogen production by proper combinations of HRT and substrate concentration. Int. J. Hydrog. Energy 2011, 36, 14289–14294. [Google Scholar] [CrossRef]
- Jung, K.W.; Kim, D.H.; Shin, H.S. A simple method to reduce the start-up period in a H2-producing UASB reactor. Int. J. Hydrog. Energy 2017, 36, 1466–1473. [Google Scholar] [CrossRef]
- Angelidaki, I.; Sanders, W. Assessment of the anaerobic biodegradability of macropollutants. Rev. Environ. Sci. Biotechnol. 2004, 3, 117–129. [Google Scholar] [CrossRef]
- APHA. Standard Methods For the Examination of Water and Wastewater, 21st ed.; Amer Public Health Assn: Washington, DC, USA, 2005. [Google Scholar]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Chaganti, S.R.; Kim, D.-H.; Lalman, J.A. Flux balance analysis of mixed anaerobic microbial communities: Effects of linoleic acid (LA) and pH on biohydrogen production. Int. J. Hydrog. Energy 2011, 36, 14141–14152. [Google Scholar] [CrossRef]
- Lalman, J.A.; Chaganti, S.R.; Moon, C.; Kim, D.-H. Elucidating acetogenic H2 consumption in dark fermentation using flux balance analysis. Bioresour. Technol. 2013, 146, 775–778. [Google Scholar] [CrossRef]
- Mostafa, A.; El-Dissouky, A.; Fawzy, A.; Farghaly, A.; Peu, P.; Dabert, P.; Le Roux, S.; Tawfik, A. Magnetite/graphene oxide nano-composite for enhancement of hydrogen production from gelatinaceous wastewater. Bioresour. Technol. 2016, 216, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, A.; Im, S.; Song, Y.-C.; Park, J.-H.; Kim, S.-H.; Lim, K.-H.; Kim, D.-H. Unravelling the enhancement of biohydrogen production via adding magnetite nanoparticles and applying electrical energy input. Int. J. Hydrog. Energy 2021. [Google Scholar] [CrossRef]
- Kumar, G.; Park, J.; Kim, M.; Kim, D.-H.; Kim, S. Hydrogen fermentation of different galactose compositions during various hydraulic retention times (HRTs). Int. J. Hydrog. Energy 2014, 39, 20625–20631. [Google Scholar] [CrossRef]
- Kim, D.-H.; Kim, S.; Kim, K.; Shin, H. Experience of a pilot-scale hydrogen-producing anaerobic sequencing batch reactor (ASBR) treating food waste. Int. J. Hydrog. Energy 2010, 35, 1590–1594. [Google Scholar] [CrossRef]
- Jung, K.W.; Kim, D.-H.; Kim, S.H.; Shin, H.S. Bioreactor design for continuous dark fermentative hydrogen production. Bioresour. Technol. 2011, 102, 8612–8620. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Han, S.K.; Kim, S.H.; Shin, H.S. Effect of gas sparging on continuous fermentative hydrogen production. Int. J. Hydrog. Energy 2006, 31, 2158–2169. [Google Scholar] [CrossRef]
- Bundhoo, M.A.Z.; Mohee, R. Inhibition of dark fermentative bio-hydrogen production: A review. Int. J. Hydrog. Energy 2016, 41, 6713–6733. [Google Scholar] [CrossRef]
- Gomes, S.D.; Fuess, L.T.; Mañunga, T.; De Lima Gomes, P.C.F.; Zaiat, M. Bacteriocins of lactic acid bacteria as a hindering factor for biohydrogen production from cassava flour wastewater in a continuous multiple tube reactor. Int. J. Hydrog. Energy 2016, 41, 8120–8131. [Google Scholar] [CrossRef] [Green Version]
- Manish, S.; Venkatesh, K.V.; Banerjee, R. Metabolic flux analysis of biological hydrogen production by Escherichia coli. Int. J. Hydrog. Energy 2007, 32, 3820–3830. [Google Scholar] [CrossRef]
- Srirugsa, T.; Prasertsan, S.; Theppaya, T.; Leevijit, T.; Prasertsan, P. Appropriate mixing speeds of Rushton turbine for biohydrogen production from palm oil mill effluent in a continuous stirred tank reactor. Energy 2019, 179, 823–830. [Google Scholar] [CrossRef]
- Lindmark, J.; Eriksson, P.; Thorin, E. The effects of different mixing intensities during anaerobic digestion of the organic fraction of municipal solid waste. Waste Manag. 2014, 34, 1391–1397. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, Z.; Zhang, H.; Jing, Y.; Li, Y.; Zhang, Q. Rheological properties of corn stover hydrolysate and photo-fermentation bio-hydrogen producing capacity under intermittent stirring. Int. J. Hydrog. Energy 2020, 45, 3721–3728. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Im, S.; Lee, M.-K.; Mostafa, A.; Prakash, O.; Lim, K.-H.; Kim, D.-H. Effect of Localized Temperature Difference on Hydrogen Fermentation. Energies 2021, 14, 6885. https://doi.org/10.3390/en14216885
Im S, Lee M-K, Mostafa A, Prakash O, Lim K-H, Kim D-H. Effect of Localized Temperature Difference on Hydrogen Fermentation. Energies. 2021; 14(21):6885. https://doi.org/10.3390/en14216885
Chicago/Turabian StyleIm, Seongwon, Mo-Kwon Lee, Alsayed Mostafa, Om Prakash, Kyeong-Ho Lim, and Dong-Hoon Kim. 2021. "Effect of Localized Temperature Difference on Hydrogen Fermentation" Energies 14, no. 21: 6885. https://doi.org/10.3390/en14216885







