New Experimental Approach for the Determination of the Heat Generation in a Li-Ion Battery Cell
Abstract
:1. Introduction
2. Materials and Methods
Analytical Solution of the ECM Thermal Response
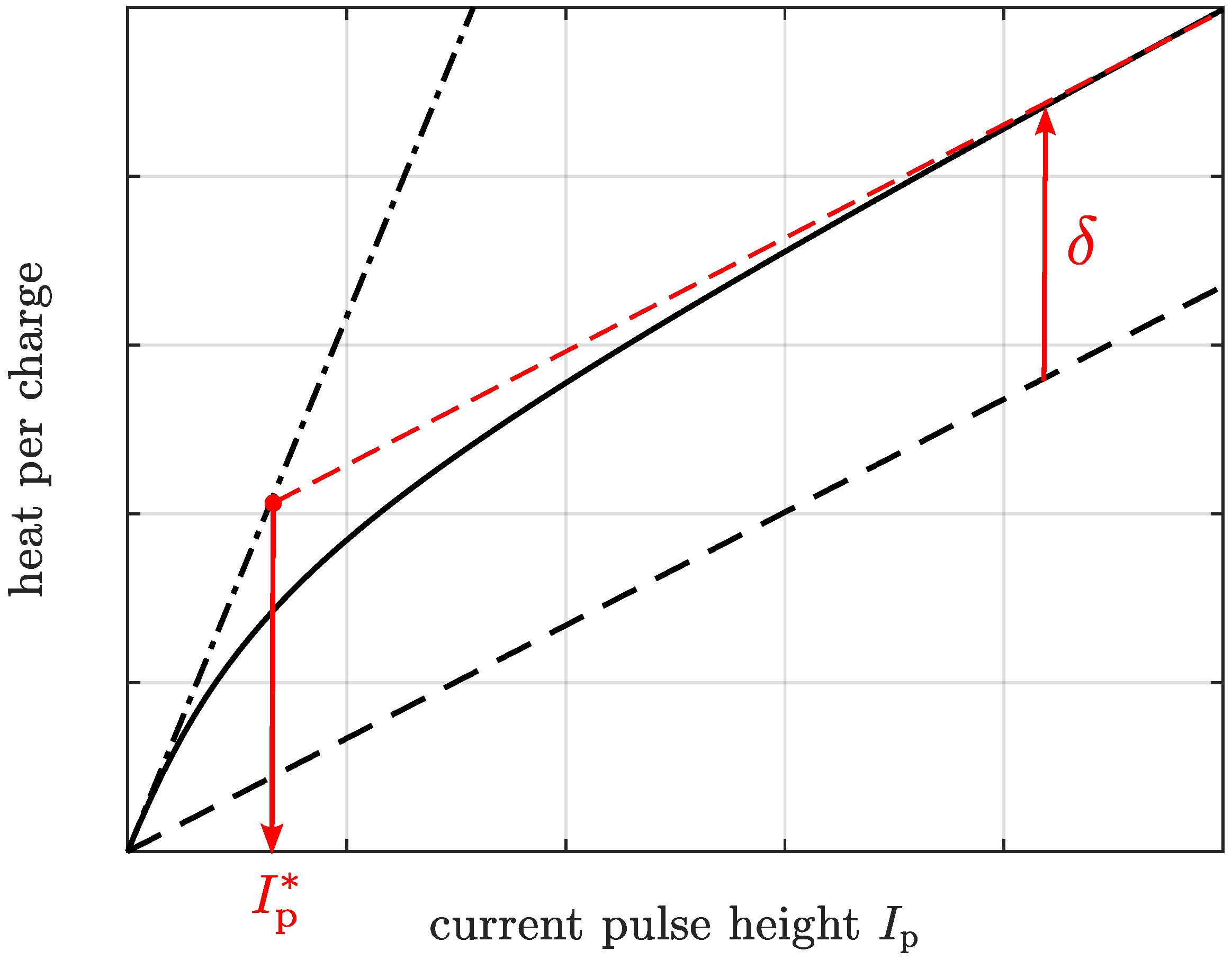
- A certain amount of heat has been generated due to the current pulse.
- Some part of this heat has already been released into the environment due to the existing internal temperature gradient.
- The other part of the generated heat is still stored in the heat capacity of the battery cell.
3. Results
4. Discussion
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADC | Analog digital converter |
| ARC | Accelerated-rate calorimetry |
| ECM | Equivalent circuit model |
| EHC | Entropic heat coefficient |
| HF | Heat flow |
| OCV | Open circuit voltage |
| RC | Resistor-Capacitor |
| SoC | State of charge |
| THFS | Temperature and heat flow sensor |
References
- Wen, F.; Duan, B.; Zhang, C.; Zhu, R.; Shang, Y.; Zhang, J. High-accuracy parameter identification method for equivalent-circuit models of lithium-ion batteries based on the stochastic theory response reconstruction. Electronics 2019, 8, 834. [Google Scholar] [CrossRef] [Green Version]
- Nemes, R.; Ciornei, S.; Ruba, M.; Hedesiu, H.; Martis, C. Modeling and simulation of first-order Li-Ion battery cell with experimental validation. In Proceedings of the 2019 8th International Conference on Modern Power Systems, MPS 2019, Cluj, Romania, 21–23 May 2019; pp. 8–13. [Google Scholar] [CrossRef]
- Nikolian, A.; Jaguemont, J.; de Hoog, J.; Goutam, S.; Omar, N.; Van Den Bossche, P.; Van Mierlo, J. Complete cell-level lithium-ion electrical ECM model for different chemistries (NMC, LFP, LTO) and temperatures (−5 °C to 45 °C)—Optimized modelling techniques. Int. J. Electr. Power Energy Syst. 2018, 98, 133–146. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, H.; Ning, Z.; Mu, Z.; Sun, C. Comparative research on RC equivalent circuit models for lithium-ion batteries of electric vehicles. Appl. Sci. 2017, 7, 1002. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Li, S.; Peng, H. A comparative study of equivalent circuit models for Li-ion batteries. J. Power Sources 2012, 198, 359–367. [Google Scholar] [CrossRef]
- Zheng, Y.; He, F.; Wang, W. A method to identify lithium battery parameters and estimate SOC based on different temperatures and driving conditions. Electronics 2019, 8, 1391. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Torregrossa, D.; Paolone, M. Surface Temperature Estimation of Li-ion Battery via Thermal Impulse Response Technique. In Proceedings of the 2015 IEEE Applied Power Electronics Conference and Exposition (APEC), Charlotte, NC, USA, 15–19 March 2015; pp. 1089–1095. [Google Scholar]
- Chen, K.; Unsworth, G.; Li, X. Measurements of heat generation in prismatic Li-ion batteries. J. Power Sources 2014, 261, 28–37. [Google Scholar] [CrossRef]
- Karimi, G.; Li, X. Thermal management of lithium-ion batteries for electric vehicles. Int. J. Energy Res. 2013, 37, 13–24. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, J.; Li, Z.; Wu, B.; Nie, Z.; Sun, Y.; An, F.; Wu, N. Comparison and validation of methods for estimating heat generation rate of large-format lithium-ion batteries. J. Therm. Anal. Calorim. 2014, 117, 447–461. [Google Scholar] [CrossRef]
- Bandhauer, T.M.; Garimella, S.; Fuller, T.F. A critical review of thermal issues in lithium-ion batteries. J. Electrochem. Soc. 2011, 158, R1. [Google Scholar] [CrossRef]
- Srinivasan, V.; Wang, C.Y. Analysis of Electrochemical and Thermal Behavior of Li-Ion Cells. J. Electrochem. Soc. 2003, 150, A98–A106. [Google Scholar] [CrossRef] [Green Version]
- Bernardi, D.; Pawlikowski, E.; Newman, J. A General Energy Balance for Battery Systems. J. Electrochem. Soc. 1985, 132, 5–12. [Google Scholar] [CrossRef] [Green Version]
- Zhao, R.; Gu, J.; Liu, J. An investigation on the significance of reversible heat to the thermal behavior of lithium ion battery through simulations. J. Power Sources 2014, 266, 422–432. [Google Scholar] [CrossRef]
- Williford, R.E.; Viswanathan, V.V.; Zhang, J.G. Effects of entropy changes in anodes and cathodes on the thermal behavior of lithium ion batteries. J. Power Sources 2009, 189, 101–107. [Google Scholar] [CrossRef]
- Bazinski, S.J.; Wang, X. The Influence of Cell Temperature on the Entropic Coefficient of a Lithium Iron Phosphate (LFP) Pouch Cell. J. Electrochem. Soc. 2014, 161, A168–A175. [Google Scholar] [CrossRef] [Green Version]
- Madani, S.S.; Schaltz, E.; Kær, S.K.; Knudsen Kær, S. An experimental analysis of entropic coefficient of a lithium titanate oxide battery. Energies 2019, 12, 2685. [Google Scholar] [CrossRef] [Green Version]
- Geifes, F.; Bolsinger, C.; Mielcarek, P.; Birke, K.P. Determination of the entropic heat coefficient in a simple electro-thermal lithium-ion cell model with pulse relaxation measurements and least squares algorithm. J. Power Sources 2019, 419, 148–154. [Google Scholar] [CrossRef]
- Zilberman, I.; Rheinfeld, A.; Jossen, A. Uncertainties in entropy due to temperature path dependent voltage hysteresis in Li-ion cells. J. Power Sources 2018, 395, 179–184. [Google Scholar] [CrossRef]
- Schuster, E.; Ziebert, C.; Melcher, A.; Rohde, M.; Seifert, H.J. Thermal behavior and electrochemical heat generation in a commercial 40 Ah lithium ion pouch cell. J. Power Sources 2015, 286, 580–589. [Google Scholar] [CrossRef]
- Drake, S.J.; Martin, M.; Wetz, D.A.; Ostanek, J.K.; Miller, S.P.; Heinzel, J.M.; Jain, A. Heat generation rate measurement in a Li-ion cell at large C-rates through temperature and heat flux measurements. J. Power Sources 2015, 285, 266–273. [Google Scholar] [CrossRef]
- Murashko, K.A.; Mityakov, A.V.; Mityakov, V.Y.; Sapozhnikov, S.Z.; Jokiniemi, J.; Pyrhönen, J. Determination of the entropy change profile of a cylindrical lithium-ion battery by heat flux measurements. J. Power Sources 2016, 330, 61–69. [Google Scholar] [CrossRef]
- Sheng, L.; Zhang, Z.; Su, L.; Zhang, H.; Zhang, H.; Li, K.; Fang, Y.; Ye, W. A calibration calorimetry method to investigate the thermal characteristics of a cylindrical lithium-ion battery. Int. J. Therm. Sci. 2021, 165, 106891. [Google Scholar] [CrossRef]
- Geng, Z.; Groot, J.; Thiringer, T. A Time- and Cost-Effective Method for Entropic Coefficient Determination of a Large Commercial Battery Cell. IEEE Trans. Transp. Electrif. 2020, 6, 257–266. [Google Scholar] [CrossRef]
- Rizzo, G.; Christen, R.; Stöck, M. Calibration methodology for contact heat flux sensors with enhanced accuracy. Meas. Sci. Technol. 2021, 32, 045003. [Google Scholar] [CrossRef]
- Christen, R.; Rizzo, G.; Gadola, A.; Stöck, M. Test Method for Thermal Characterization of Li-Ion Cells and Verification of Cooling Concepts. Batteries 2017, 3, 3. [Google Scholar] [CrossRef] [Green Version]
- Schweiger, H.G.; Obeidi, O.; Komesker, O.; Raschke, A.; Schiemann, M.; Zehner, C.; Gehnen, M.; Keller, M.; Birke, P. Comparison of Several Methods for Determining the Internal Resistance of Lithium Ion Cells. Sensors 2010, 10, 5604–5625. [Google Scholar] [CrossRef] [Green Version]
- Barai, A.; Uddin, K.; Widanage, W.D.; McGordon, A.; Jennings, P. A study of the influence of measurement timescale on internal resistance characterisation methodologies for lithium-ion cells. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]





| SoC before | 0.25C | 0.5C | 1C | 1.5C | 3C | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pulse | ||||||||||
| 90.0% | 12 | 6 | 3 | 2 | 1 | |||||
| 85.0% | 12 | 6 | 3 | 2 | 1 | |||||
| 80.0% | 24 | 12 | 6 | 4 | 2 | |||||
| 69.9% | 24 | 12 | 6 | 4 | 2 | |||||
| 59.9% | 24 | 12 | 6 | 4 | 2 | |||||
| 49.8% | 24 | 12 | 6 | 4 | 2 | |||||
| 39.7% | 24 | 12 | 6 | 4 | 2 | |||||
| 29.7% | 24 | 12 | 6 | 4 | 2 | |||||
| 19.6% | 12 | 6 | 3 | 2 | 1 | |||||
| 14.6% | 12 | 6 | 3 | 2 | 1 | |||||
| 9.5% | 12 | 6 | 3 | 2 | 1 | |||||
| 14.6% | 12 | 6 | 3 | 2 | 1 | |||||
| 19.6% | 24 | 12 | 6 | 4 | 2 | |||||
| 29.6% | 24 | 12 | 6 | 4 | 2 | |||||
| 39.7% | 24 | 12 | 6 | 4 | 2 | |||||
| 49.7% | 24 | 12 | 6 | 4 | 2 | |||||
| 59.8% | 24 | 12 | 6 | 4 | 2 | |||||
| 69.8% | 24 | 12 | 6 | 4 | 2 | |||||
| 79.8% | 12 | 6 | 3 | 2 | 1 | |||||
| 83.9% | 12 | 6 | 3 | 2 | 1 | |||||
| Current Direction | Total Amount of Heat | Heat Released during Pulse | Percentage of Heat Released after |
|---|---|---|---|
| discharge | 81.2% | ||
| charge | 66.6% |
| SoC Range | |||||
|---|---|---|---|---|---|
| 90% to 85% | |||||
| 85% to 80% | |||||
| 80% to 70% | |||||
| 70% to 60% | |||||
| 60% to 50% | |||||
| 50% to 40% | |||||
| 40% to 30% | |||||
| 30% to 20% | |||||
| 20% to 15% | |||||
| 15% to 10% |
| T | 20 | 25 | 30 | 35 |
| OCV |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christen, R.; Martin, B.; Rizzo, G. New Experimental Approach for the Determination of the Heat Generation in a Li-Ion Battery Cell. Energies 2021, 14, 6972. https://doi.org/10.3390/en14216972
Christen R, Martin B, Rizzo G. New Experimental Approach for the Determination of the Heat Generation in a Li-Ion Battery Cell. Energies. 2021; 14(21):6972. https://doi.org/10.3390/en14216972
Chicago/Turabian StyleChristen, Rouven, Björn Martin, and Gerhard Rizzo. 2021. "New Experimental Approach for the Determination of the Heat Generation in a Li-Ion Battery Cell" Energies 14, no. 21: 6972. https://doi.org/10.3390/en14216972






