Micro CT and Experimental Study of Carbonate Precipitation from CO2 and Produced Water Co-Injection into Sandstone
Abstract
:1. Introduction
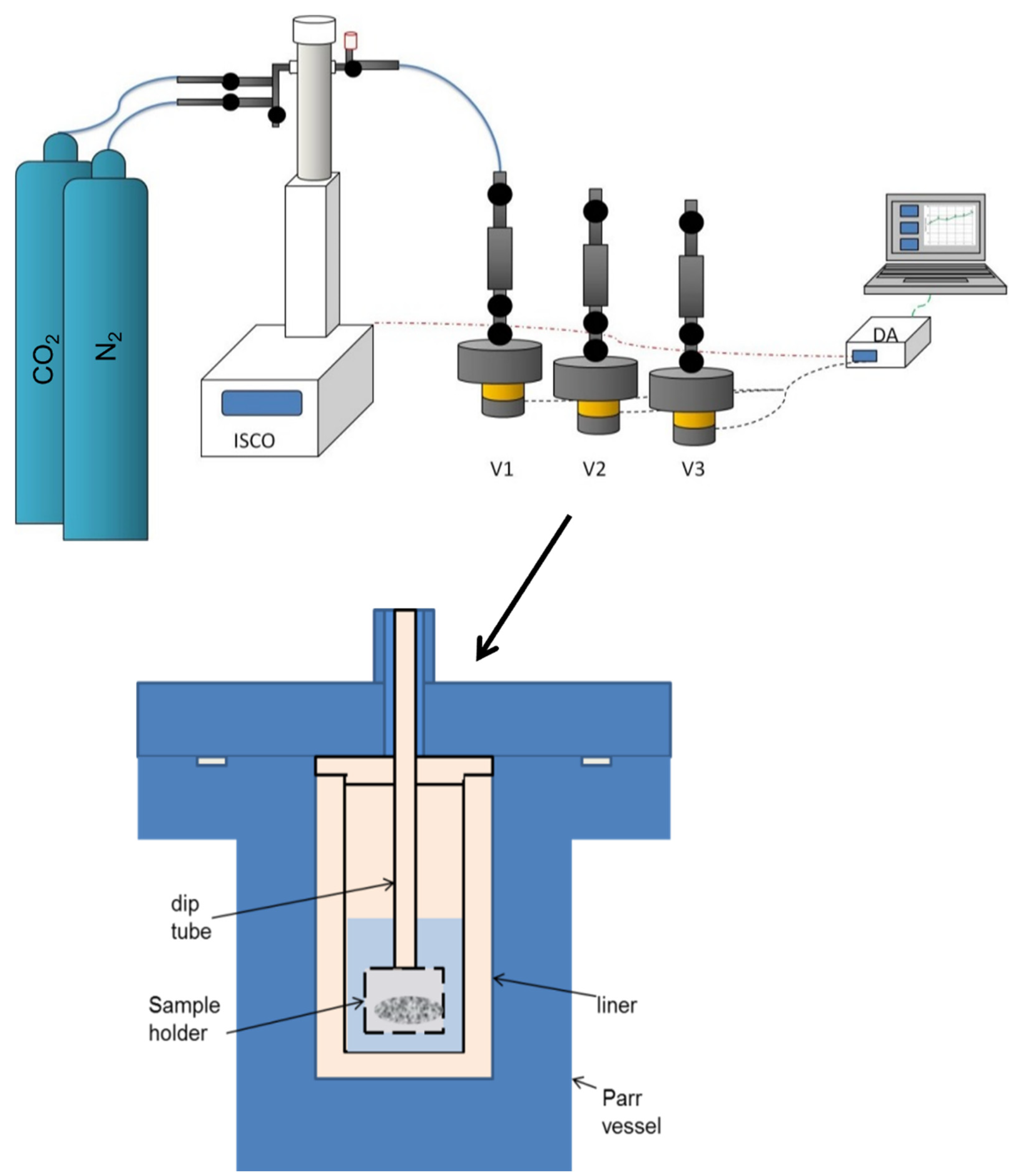
2. Materials and Methods
3. Results
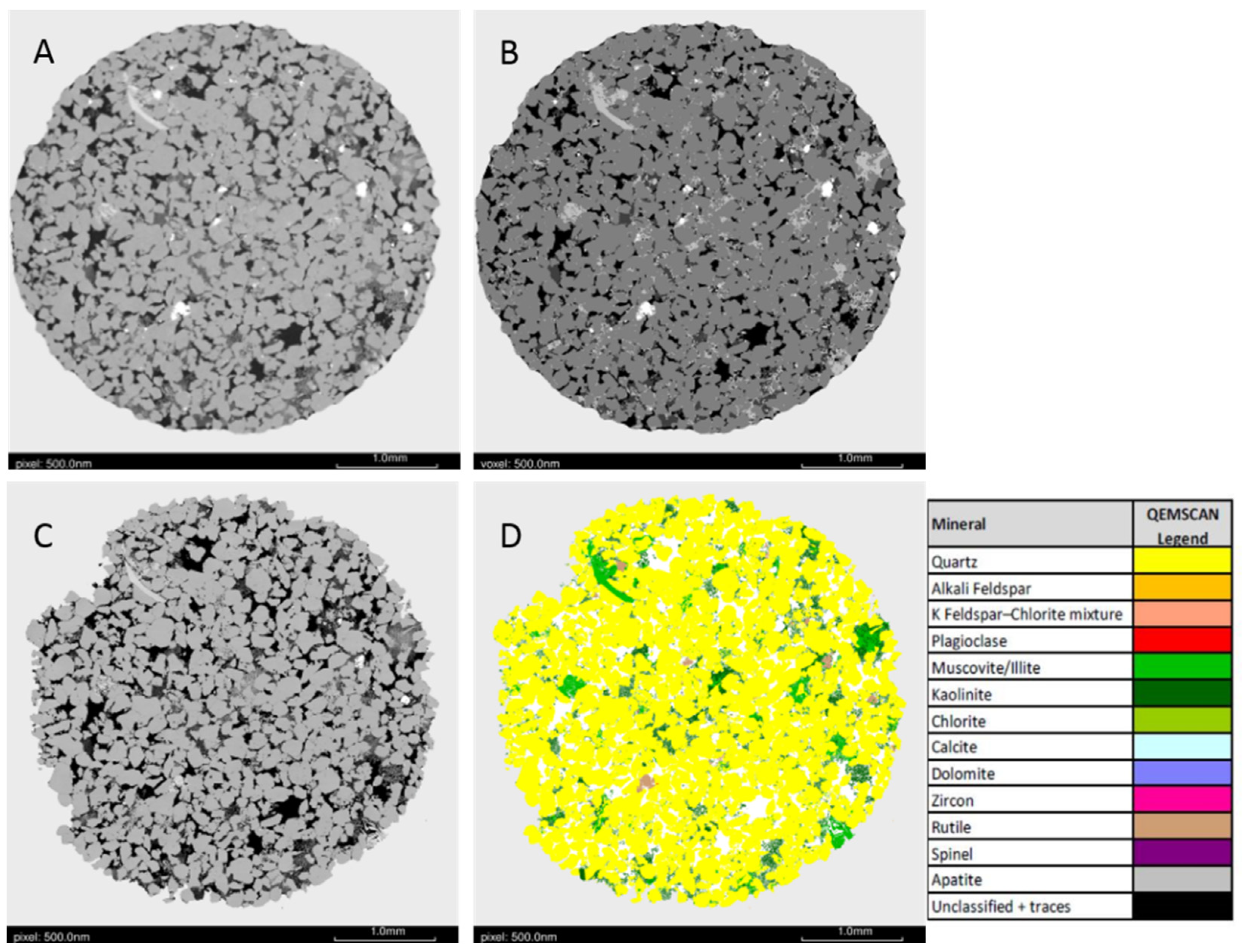
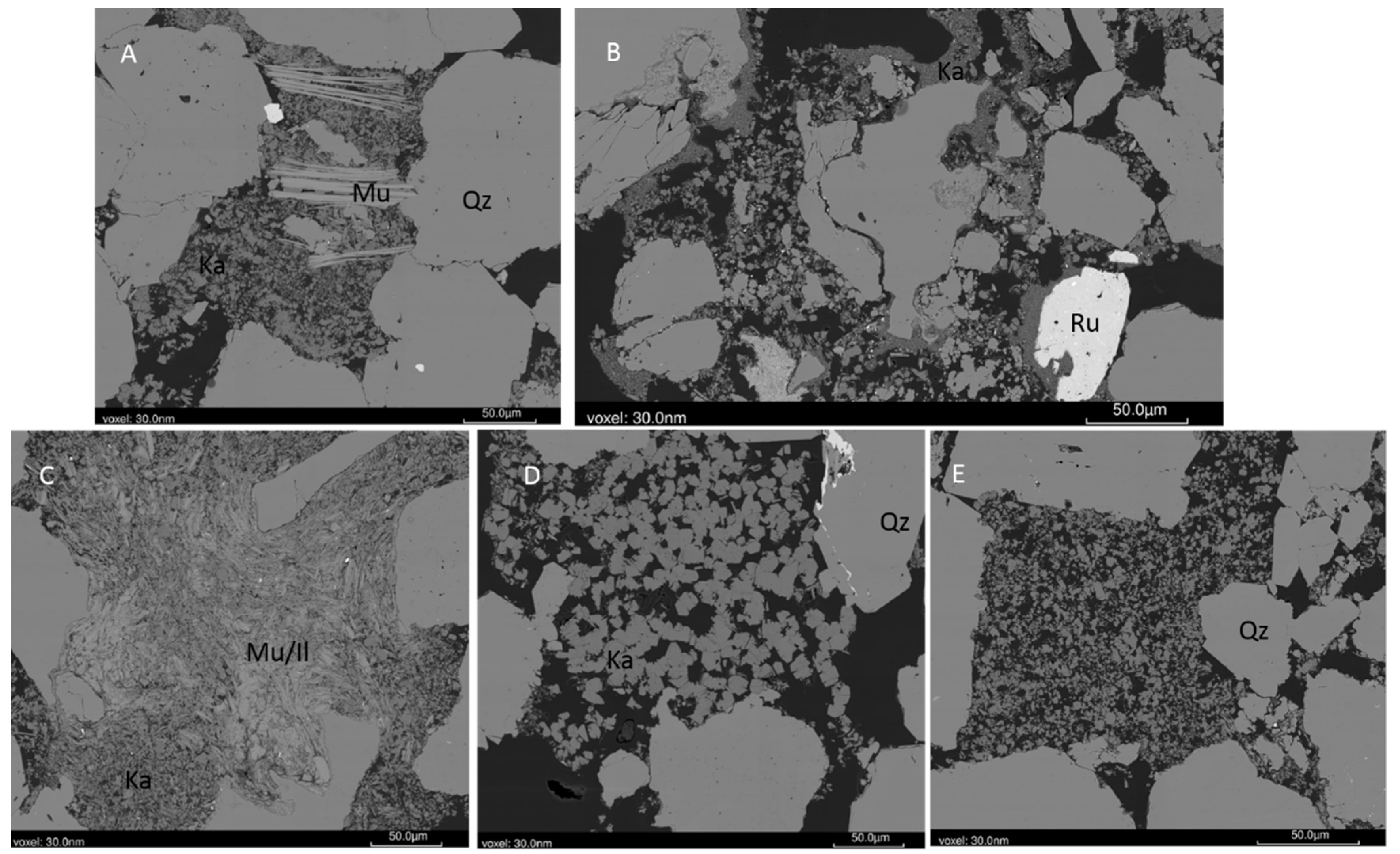
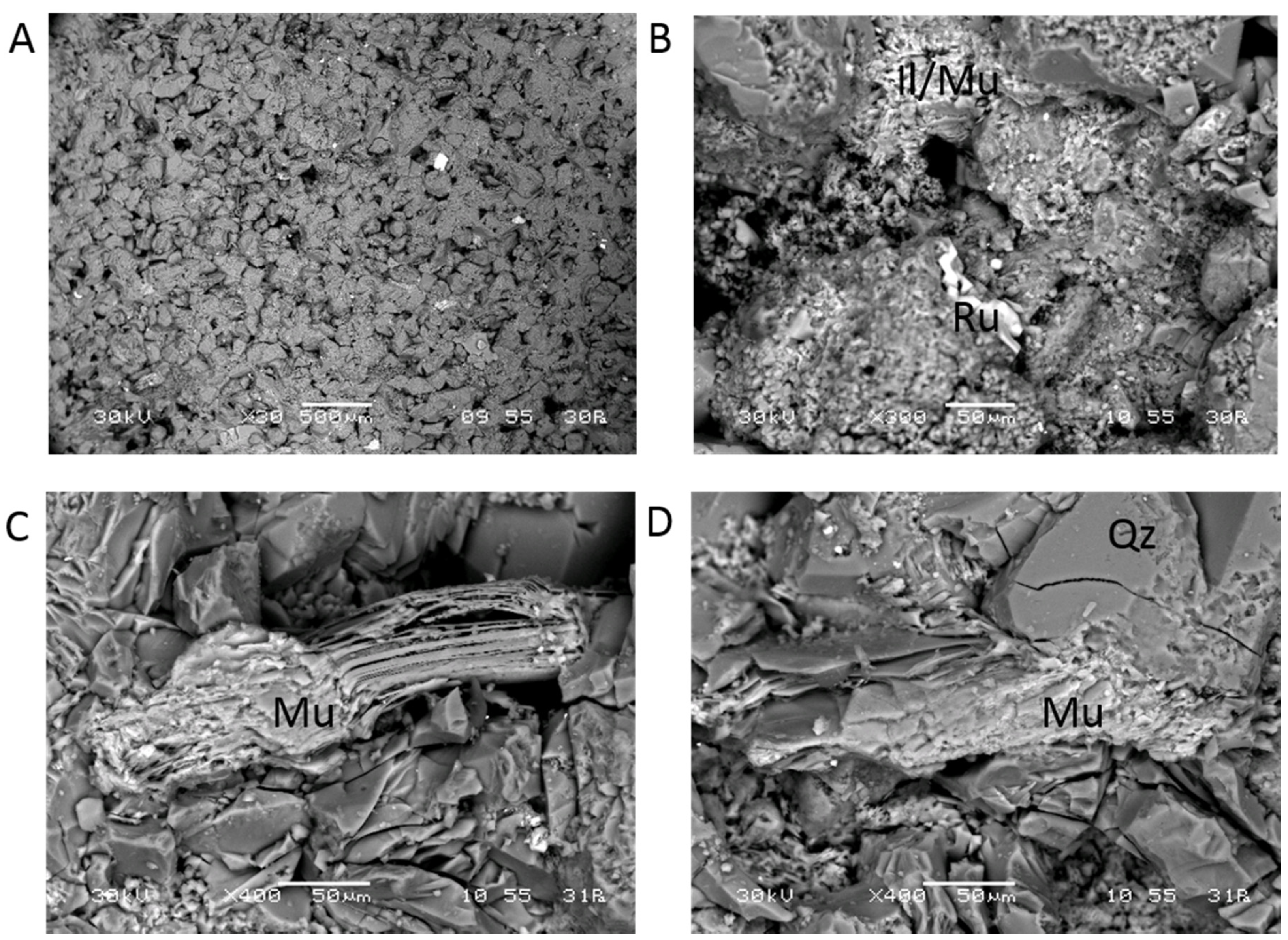
3.1. Pre-Reaction Characterization
3.2. Post-Reaction Characterization
4. Discussion
5. Conclusions
- A quartz rich sandstone was reacted at carbon storage conditions with supercritical CO2 and a synthetic produced water.
- Calcite and dolomite carbonate mineral precipitation was observed with QEMSCAN and SEM-EDS imaging.
- Micro CT image registration before and after the reaction showed no significant change in porosity. However, kaolinite and fine grain movement occurred in the pores. No significant change in porosity is favorable to avoid plugging or injectivity loss near the wellbore. However, clay and fines movement have the potential to alter permeability. Additionally, other rock types may show different responses in porosity, permeability, and mechanical properties and should be further studied.
- Mineral trapping of CO2 as precipitated carbonate minerals was observed.
- Injection of CO2 dissolved in produced water or other co-injection schemes may be favorable for inducing or accelerating mineral trapping in low reactivity sandstone, where the water can provide cations such as Ca, Mg or Fe.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
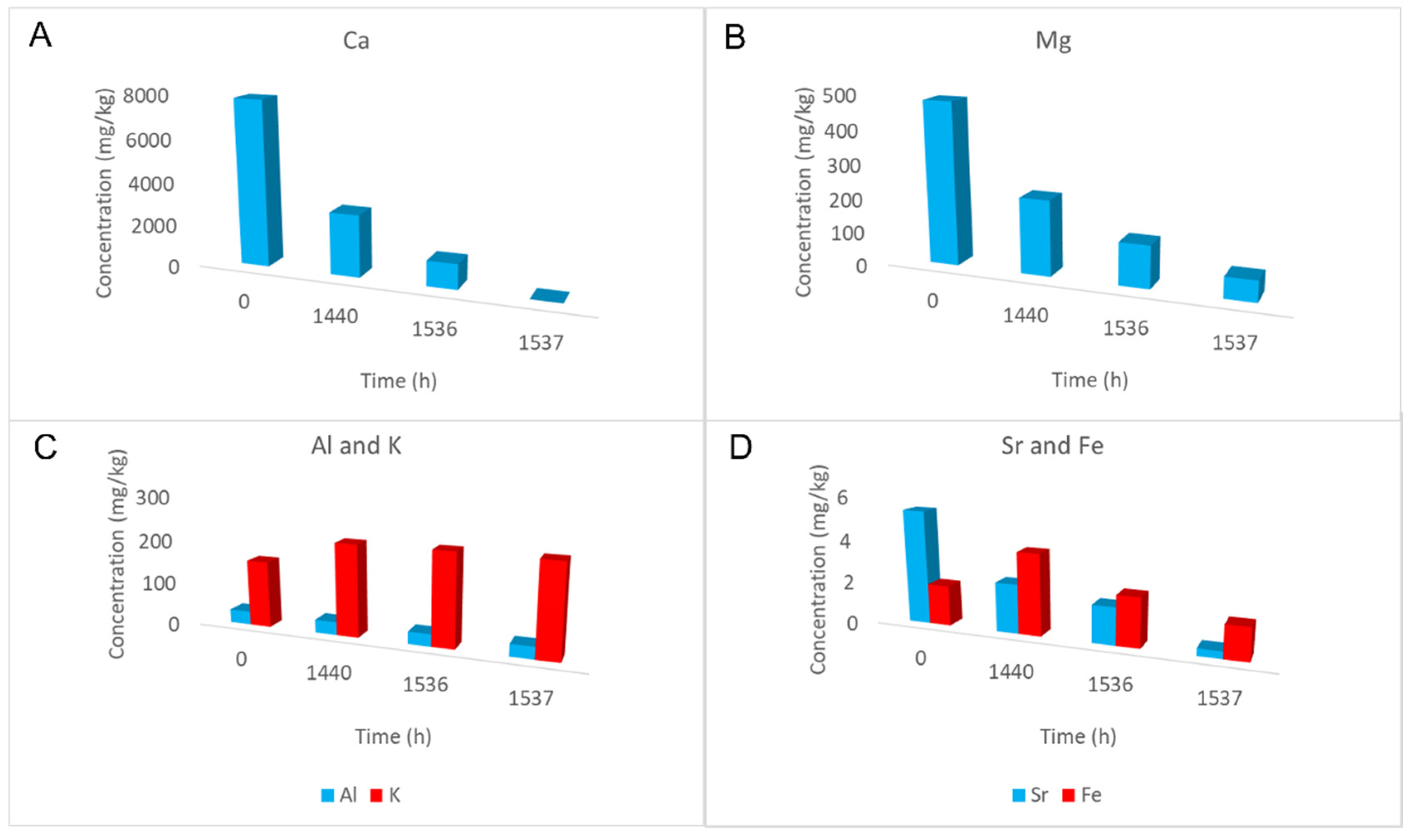
| Time (h) | 0 | 1440 | 1536 | 1537 |
|---|---|---|---|---|
| pH | 7.2 | 7.12 | 7.10 | |
| Ca | 7797.5 | 2903.4 | 1176.3 | <0.01 |
| Mg | 480.0 | 221.8 | 124.3 | 61.7 |
| Al | 30.9 | 30.0 | 28.1 | 28.0 |
| K | 152.8 | 210.2 | 212.3 | 210.4 |
| Sr | 5.3 | 2.3 | 1.7 | 0.3 |
| Fe | 1.9 | 3.8 | 2.3 | 1.5 |
| Si | <0.1 | <0.1 | <0.1 | <0.1 |
| Cr | 0.6 | 0.7 | 0.4 | 0.2 |
| Cu | 1.8 | 2.2 | 1.3 | 1.5 |
| Ni | 3.3 | 4.2 | 4.1 | 6.8 |
| Ti | 0.2 | <0.001 | <0.001 | <0.001 |
References
- Bachu, S.; Gunter, W.; Perkins, E. Aquifer disposal of CO2: Hydrodynamic and mineral trapping. Energy Convers. Manag. 1994, 35, 269–279. [Google Scholar] [CrossRef]
- Pearce, J.K.; Dawson, G.K.W.; Golab, A.; Knuefing, L.; Sommacal, S.; Rudolph, V.; Golding, S.D. A combined geochemical and μCT study on the CO2 reactivity of Surat Basin reservoir and cap-rock cores: Porosity changes, mineral dissolution and fines migration. Int. J. Greenh. Gas Control 2019, 80, 10–24. [Google Scholar] [CrossRef] [Green Version]
- Wilke, F.D.; Vásquez, M.; Wiersberg, T.; Naumann, R.; Erzinger, J. On the interaction of pure and impure supercritical CO2 with rock forming minerals in saline aquifers: An experimental geochemical approach. Appl. Geochem. 2012, 27, 1615–1622. [Google Scholar] [CrossRef]
- Dawson, G.; Pearce, J.; Biddle, D.; Golding, S.D. Experimental mineral dissolution in Berea Sandstone reacted with CO2 or SO2–CO2 in NaCl brine under CO2 sequestration conditions. Chem. Geol. 2015, 399, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Farquhar, S.M.; Pearce, J.K.; Dawson, G.K.W.; Golab, A.; Kirste, D.; Biddle, D.; Golding, S.D. A fresh approach to investigating CO2 storage: Experimental CO2-water-rock interactions in a freshwater reservoir system. Chem. Geol. 2015, 399, 98–122. [Google Scholar] [CrossRef]
- Pearce, J.K.; Law, A.C.; Dawson, G.K.; Golding, S.D. SO2–CO2 and pure CO2 reactivity of ferroan carbonates at carbon storage conditions. Chem. Geol. 2015, 411, 112–124. [Google Scholar] [CrossRef]
- Smith, M.M.; Sholokhova, Y.; Hao, Y.; Carroll, S.A. CO2-induced dissolution of low permeability carbonates. Part I: Characterization and experiments. Adv. Water Resour. 2013, 62, 370–387. [Google Scholar] [CrossRef]
- Wunsch, A.; Navarre-Sitchler, A.K.; Moore, J.; McCray, J. Metal release from limestones at high partial-pressures of CO2. Chem. Geol. 2014, 363, 40–55. [Google Scholar] [CrossRef]
- Pearce, J.; La Croix, A.; Underschultz, J.; Golding, S. Long term reactivity of CO2 in a low salinity reservoir-seal complex. Appl. Geochem. 2020, 114, 104529. [Google Scholar] [CrossRef]
- Pearce, J.K.; La Croix, A.D.; Brink, F.J.; Hayes, P.J.; Underschultz, J.R. CO2 mineral trapping comparison in different regions: Predicted geochemical reactivity of the Precipice Sandstone reservoir and overlying Evergreen Formation. Pet. Geosci. 2021, 27. [Google Scholar] [CrossRef]
- Snæbjörnsdóttir, S.; Gislason, S.R.; Galeczka, I.M.; Oelkers, E.H. Reaction path modelling of in-situ mineralisation of CO2 at the CarbFix site at Hellisheidi, SW-Iceland. Geochim. Cosmochim. Acta 2018, 220, 348–366. [Google Scholar] [CrossRef] [Green Version]
- Watson, M.; Zwingmann, N.; Lemon, N. The Ladbroke Grove–Katnook carbon dioxide natural laboratory: A recent CO2 accumulation in a lithic sandstone reservoir. Energy 2004, 29, 1457–1466. [Google Scholar] [CrossRef]
- Higgs, K.E.; Funnell, R.H.; Reyes, A.G. Changes in reservoir heterogeneity and quality as a response to high partial pressures of CO2 in a gas reservoir, New Zealand. Mar. Pet. Geol. 2013, 48, 293–322. [Google Scholar] [CrossRef]
- Uysal, I.T.; Golding, S.D.; Bolhar, R.; Zhao, J.-X.; Feng, Y.-X.; Baublys, K.A.; Greig, A. CO2 degassing and trapping during hydrothermal cycles related to Gondwana rifting in eastern Australia. Geochim. Cosmochim. Acta 2011, 75, 5444–5466. [Google Scholar] [CrossRef]
- Black, J.R.; Haese, R.R. Chlorite dissolution rates under CO2 saturated conditions from 50 to 120 °C and 120 to 200 bar CO2. Geochim. Cosmochim. Acta 2014, 125, 225–240. [Google Scholar] [CrossRef]
- Higgs, K.E.; Haese, R.R.; Golding, S.D.; Schacht, U.; Watson, M. The Pretty Hill Formation as a natural analogue for CO2 storage; an investigation of mineralogical and isotopic changes associated with sandstones exposed to low, intermediate and high CO2 concentrations over geological time. Chem. Geol. 2015, 399, 36–64. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.M.; Wolery, T.J.; Carroll, S.A. Kinetics of chlorite dissolution at elevated temperatures and CO2 conditions. Chem. Geol. 2013, 347, 1–8. [Google Scholar] [CrossRef]
- Palandri, J.L.; Rosenbauer, R.J.; Kharaka, Y.K. Ferric iron in sediments as a novel CO2 mineral trap: CO2–SO2 reaction with hematite. Appl. Geochem. 2005, 20, 2038–2048. [Google Scholar] [CrossRef]
- Matter, J.M.; Kelemen, P. Permanent storage of carbon dioxide in geological reservoirs by mineral carbonation. Nat. Geosci. 2009, 2, 837–841. [Google Scholar] [CrossRef]
- Xu, T.; Apps, J.A.; Pruess, K. Mineral sequestration of carbon dioxide in a sandstone–shale system. Chem. Geol. 2005, 217, 295–318. [Google Scholar] [CrossRef]
- Baublys, K.; Hamilton, S.; Golding, S.; Vink, S.; Esterle, J. Microbial controls on the origin and evolution of coal seam gases and production waters of the Walloon Subgroup; Surat Basin, Australia. Int. J. Coal Geol. 2015, 147, 85–104. [Google Scholar] [CrossRef]
- Underschultz, J.; Vink, S.; Garnett, A. Coal seam gas associated water production in Queensland: Actual vs predicted. J. Nat. Gas Sci. Eng. 2018, 52, 410–422. [Google Scholar] [CrossRef] [Green Version]
- Gregory, K.B.; Vidic, R.D.; Dzombak, D.A. Water Management Challenges Associated with the Production of Shale Gas by Hydraulic Fracturing. Elements 2011, 7, 181–186. [Google Scholar] [CrossRef]
- Hornbach, M.J.; Jones, M.; Scales, M.; DeShon, H.R.; Magnani, M.B.; Frohlich, C.; Stump, B.; Hayward, C.; Layton, M. Ellenburger wastewater injection and seismicity in North Texas. Phys. Earth Planet. Inter. 2016, 261, 54–68. [Google Scholar] [CrossRef] [Green Version]
- Akob, D.M.; Mumford, A.C.; Orem, W.H.; Engle, M.A.; Klinges, J.G.; Kent, D.B.; Cozzarelli, I.M. Wastewater Disposal from Unconventional Oil and Gas Development Degrades Stream Quality at a West Virginia Injection Facility. Environ. Sci. Technol. 2016, 50, 5517–5525. [Google Scholar] [CrossRef]
- Khan, C.; Pearce, J.; Golding, S.; Rudolph, V.; Underschultz, J. Carbon Storage Potential of North American Oil & Gas Produced Water Injection with Surface Dissolution. Geosciences 2021, 11, 123. [Google Scholar] [CrossRef]
- Clark, C.E.; Veil, J.A. Produced Water Volumes and Managment Practices in the United States; ANL/EVS/R-09/1; Argonne National Laboratoy: Lemont, IL, USA, 2009. [Google Scholar]
- Fossil-Water Produced Water Beneficial Re-Us—High TDS Waters; Fossil Water: Calgary, AB, Canada, 2007.
- Prommer, H.; Rathi, B.; Donn, M.; Siade, A.; Wendling, L.; Martens, E.; Patterson, B. Geochemical Response to Reinjection: Final Report; CSIRO: Canberra, Australia, 2016. [Google Scholar]
- Hayes, P.; Nicol, C.; La Croix, A.; Pearce, J.; Gonzalez, S.; Wang, J.; Harfoush, A.; He, J.; Moser, A.; Helm, L.; et al. Enhancing geological and hydrogeological understanding of the Precipice Sandstone aquifer of the Surat Basin, Great Artesian Basin, Australia, through model inversion of managed aquifer recharge datasets. Hydrogeol. J. 2019, 28, 175–192. [Google Scholar] [CrossRef] [Green Version]
- APLNG Australia Pacific LNG Upstream Phase 1. Reedy Creek Aquifer Injection Management Plan—Precipice Sandstone; Report Q-4255-95-MP-004; APLNG: Brisbane, Australia, 2013. [Google Scholar]
- Wu, Z.; Luhmann, A.J.; Rinehart, A.J.; Mozley, P.S.; Dewers, T.A.; Heath, J.E.; Majumdar, B.S. Chemo-mechanical Alterations Induced from CO2 Injection in Carbonate-Cemented Sandstone: An Experimental Study at 71 °C and 29 MPa. J. Geophys. Res. Solid Earth 2020, 125, e2019JB019096. [Google Scholar] [CrossRef]
- Li, D.; Saraji, S.; Jiao, Z.; Zhang, Y. CO2 injection strategies for enhanced oil recovery and geological sequestration in a tight reservoir: An experimental study. Fuel 2020, 284, 119013. [Google Scholar] [CrossRef]
- Hutcheon, I.; Shevalier, M.; Durocher, K.; Bloch, J.; Johnson, G.; Nightingale, M.; Mayer, B. Interactions of CO2 with formation waters, oil and minerals and CO2 storage at the Weyburn IEA EOR site, Saskatchewan, Canada. Int. J. Greenh. Gas Control. 2016, 53, 354–370. [Google Scholar] [CrossRef] [Green Version]
- Sohrabi, M.; Kechut, N.I.; Riazi, M.; Jamiolahmady, M.; Ireland, S.; Robertson, G. Safe storage of CO2 together with improved oil recovery by CO2-enriched water injection. Chem. Eng. Res. Des. 2011, 89, 1865–1872. [Google Scholar] [CrossRef]
- Matter, J.M.; Stute, M.; Snæbjörnsdottir, S.; Oelkers, E.H.; Gislason, S.R.; Aradottir, E.S.; Sigfusson, B.; Gunnarsson, I.; Sigurdardottir, H.; Gunnlaugsson, E.; et al. Rapid carbon mineralization for permanent disposal of anthropogenic carbon dioxide emissions. Science 2016, 352, 1312–1314. [Google Scholar] [CrossRef] [Green Version]
- Knackstedt, M.; Carnerup, A.; Golab, A.; Sok, R.; Young, B.; Riepe, L. Petrophysical Characterization of Unconventional Reservoir Core at Multiple Scales. Petrophysics 2013, 54, 216–223. [Google Scholar]
- Golab, A.; Knuefing, L.; Goodwin, C.; Sommacal, S.; Carnerup, A.; Dawson, G.; Pearce, J.K.; Golding, S.D. Milestone 5.7: Final Report on Geochemical Reactivity Studies of Core Material Using ScCO2; Report for ANLEC R&D; Lithicon FEI: Hillsboro, OR, USA, 2015. [Google Scholar]
- Pearce, J.; Golab, A.; Dawson, G.K.; Knuefing, L.; Goodwin, C.; Golding, S.D. Mineralogical controls on porosity and water chemistry during O2-SO2-CO2 reaction of CO2 storage reservoir and cap-rock core. Appl. Geochem. 2016, 75, 152–168. [Google Scholar] [CrossRef] [Green Version]
- Golab, A.; Arena, A.; Sommacal, S.; Goodwin, C.; Rajan, P.; Dodd, N.; Khor, J.; Deakin, L.; Zhang, J.; Young, B.; et al. Milestone 2.9: Final Report of Digital Core Analysis Results for Plug Samples from West Wandoan-1 Well; Report for ANLEC R&D; Lithicon FEI: Hillsboro, OR, USA, 2015. [Google Scholar]
- Golab, A.N.; Knackstedt, M.A.; Averdunk, H.; Senden, T.; Butcher, A.; Jaime, P. 3D porosity and mineralogy characterization in tight gas sandstones. Geophysics 2010, 29, 1476–1483. [Google Scholar] [CrossRef]
- Pearce, J.K.; Kirste, D.M.; Dawson, G.K.; Farquhar, S.M.; Biddle, D.; Golding, S.D.; Rudolph, V. SO2 impurity impacts on experimental and simulated CO2–water–reservoir rock reactions at carbon storage conditions. Chem. Geol. 2015, 399, 65–86. [Google Scholar] [CrossRef]
- Dawson, G.K.W.; Biddle, D.; Farquhar, S.M.; Gao, J.; Golding, S.D.; Jiang, X.; Keck, R.; Khan, C.; Law, A.C.K.; Li, Q.; et al. Achieving Risk and Cost Reductions in CO2 Geosequestration through 4D Characterisation of Host Formations; University of Queensland: St. Lucia, Australia, 2015. [Google Scholar]
- Wdowin, M.; Tarkowski, R.; Franus, W. Determination of changes in the reservoir and cap rocks of the Chabowo Anticline caused by CO2–brine–rock interactions. Int. J. Coal Geol. 2014, 130, 79–88. [Google Scholar] [CrossRef]
- Fischer, S.; The Ketzin Team; Liebscher, A.; De Lucia, M.; Hecht, L. Reactivity of sandstone and siltstone samples from the Ketzin pilot CO2 storage site-Laboratory experiments and reactive geochemical modeling. Environ. Earth Sci. 2013, 70, 3687–3708. [Google Scholar] [CrossRef]
- Saeedi, A.; Piane, C.D.; Esteban, L.; Stalker, L. Flood characteristic and fluid rock interactions of a supercritical CO2, brine, rock system: South West Hub, Western Australia. Int. J. Greenh. Gas Control. 2015, 54, 309–321. [Google Scholar] [CrossRef] [Green Version]
- Xu, T.; Apps, J.A.; Pruess, K.; Yamamoto, H. Numerical modeling of injection and mineral trapping of CO2 with H2S and SO2 in a sandstone formation. Chem. Geol. 2007, 242, 319–346. [Google Scholar] [CrossRef] [Green Version]
- Seyyedi, M.; Ben Mahmud, H.K.; Verrall, M.; Giwelli, A.; Esteban, L.; Ghasemiziarani, M.; Clennell, M. Pore Structure Changes Occur During CO2 Injection into Carbonate Reservoirs. Sci. Rep. 2020, 10, 3624. [Google Scholar] [CrossRef] [Green Version]
- Lamy-Chappuis, B.; Angus, D.; Fisher, Q.; Grattoni, C.; Yardley, B.W.D. Rapid porosity and permeability changes of calcareous sandstone due to CO2-enriched brine injection. Geophys. Res. Lett. 2013, 41, 399–406. [Google Scholar] [CrossRef] [Green Version]
- Vialle, S.; Vanorio, T. Laboratory measurements of elastic properties of carbonate rocks during injection of reactive CO2-saturated water. Geophys. Res. Lett. 2011, 38. [Google Scholar] [CrossRef]
- Porter, R.T.; Fairweather, M.; Pourkashanian, M.; Woolley, R.M. The range and level of impurities in CO2 streams from different carbon capture sources. Int. J. Greenh. Gas Control 2015, 36, 161–174. [Google Scholar] [CrossRef]

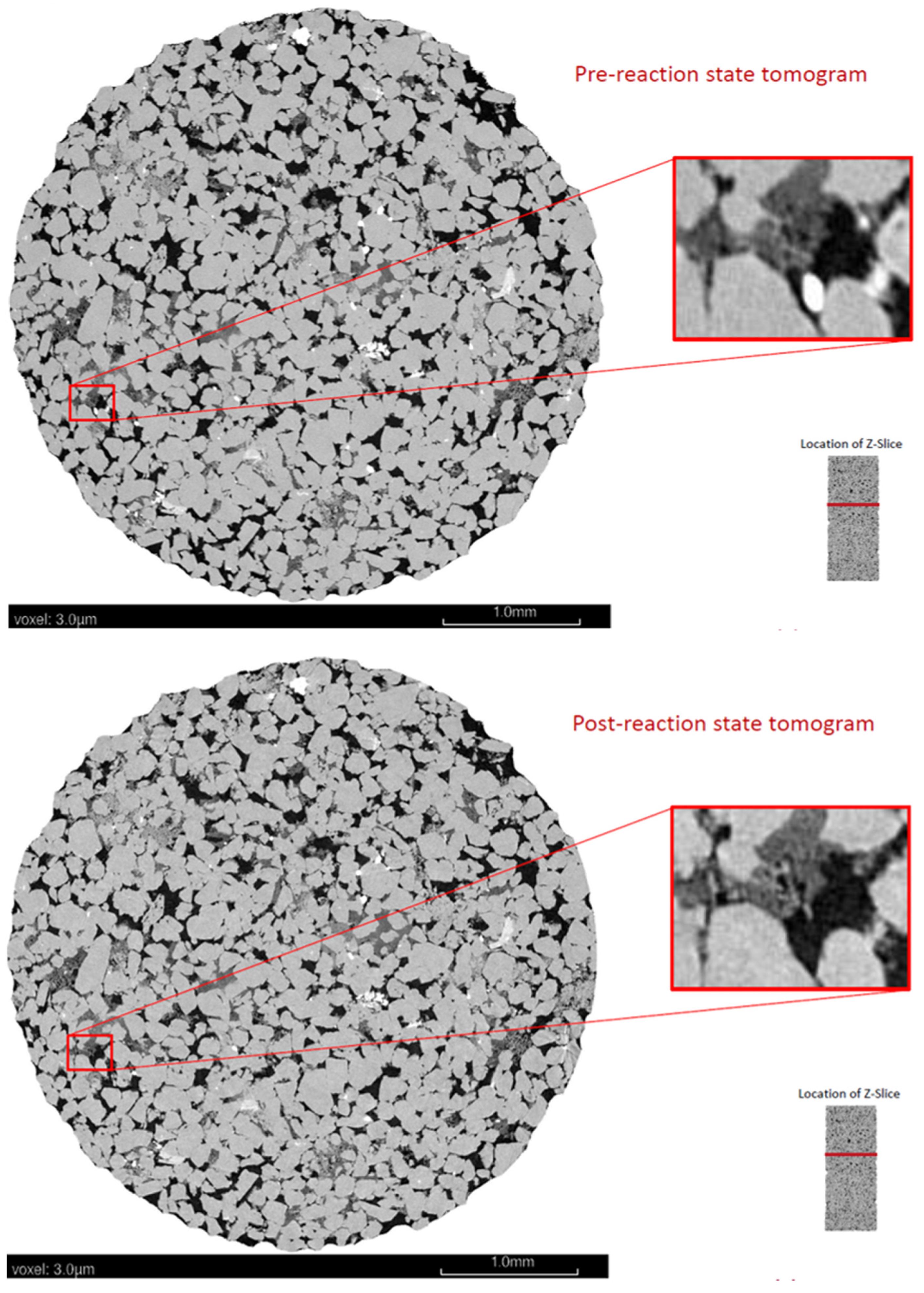




| Formation | Precipice Sandstone |
|---|---|
| Depth (m) | 1211.65 |
| Core offcut size (mm) | 18 × 12 × 12 |
| Voxel size (scoping scan i (µm)) | 15 |
| Sub-plug size (mm) | 4 × 11 |
| Voxel size (sub-plug) (µm) | 3 |
| Property | Grain Type | Main Mineral | Pre | Post |
|---|---|---|---|---|
| Resolved porosity | 11.1 | 11.4 | ||
| Framework-grain | Grain-1 | quartz | 73.3 | 73.4 |
| Framework-grain | Grain-2 | - | - | |
| Lower density Clay | Grain-1 | kaolinite | 8 | 7.7 |
| Higher density Clay | Grain-2 | illite/muscovite | 7.2 | 7.1 |
| High density | Grain-1 | rutile | 0.45 | 0.45 |
| High density | Grain-2 | zircon | - | - |
| Mineral | Pre | Post |
|---|---|---|
| Quartz | 89.2 | 89.9 |
| Alkali Feldspar | - | - |
| Plagioclase | - | - |
| Muscovite/Illite | 3.5 | 2.2 |
| Kaolinite | 6.5 | 6.8 |
| Chlorite | - | <0.1 |
| Calcite | - | 0.3 |
| Dolomite | - | 0.1 |
| Zircon | - | <0.1 |
| Rutile | 0.7 | 0.5 |
| Spinel | - | - |
| Apatite | - | - |
| Unclassified and traces | 0.1 | 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pearce, J.K.; Dawson, G.K.W.; Sommacal, S.; Golding, S.D. Micro CT and Experimental Study of Carbonate Precipitation from CO2 and Produced Water Co-Injection into Sandstone. Energies 2021, 14, 6998. https://doi.org/10.3390/en14216998
Pearce JK, Dawson GKW, Sommacal S, Golding SD. Micro CT and Experimental Study of Carbonate Precipitation from CO2 and Produced Water Co-Injection into Sandstone. Energies. 2021; 14(21):6998. https://doi.org/10.3390/en14216998
Chicago/Turabian StylePearce, Julie K., Grant K. W. Dawson, Silvano Sommacal, and Suzanne D. Golding. 2021. "Micro CT and Experimental Study of Carbonate Precipitation from CO2 and Produced Water Co-Injection into Sandstone" Energies 14, no. 21: 6998. https://doi.org/10.3390/en14216998
APA StylePearce, J. K., Dawson, G. K. W., Sommacal, S., & Golding, S. D. (2021). Micro CT and Experimental Study of Carbonate Precipitation from CO2 and Produced Water Co-Injection into Sandstone. Energies, 14(21), 6998. https://doi.org/10.3390/en14216998







