Experimental Measurements of Wax Precipitation Using a Modified Method of Simultaneous Centrifugation and High-Temperature Gas Chromatography
Abstract
1. Introduction
2. Experimental Study
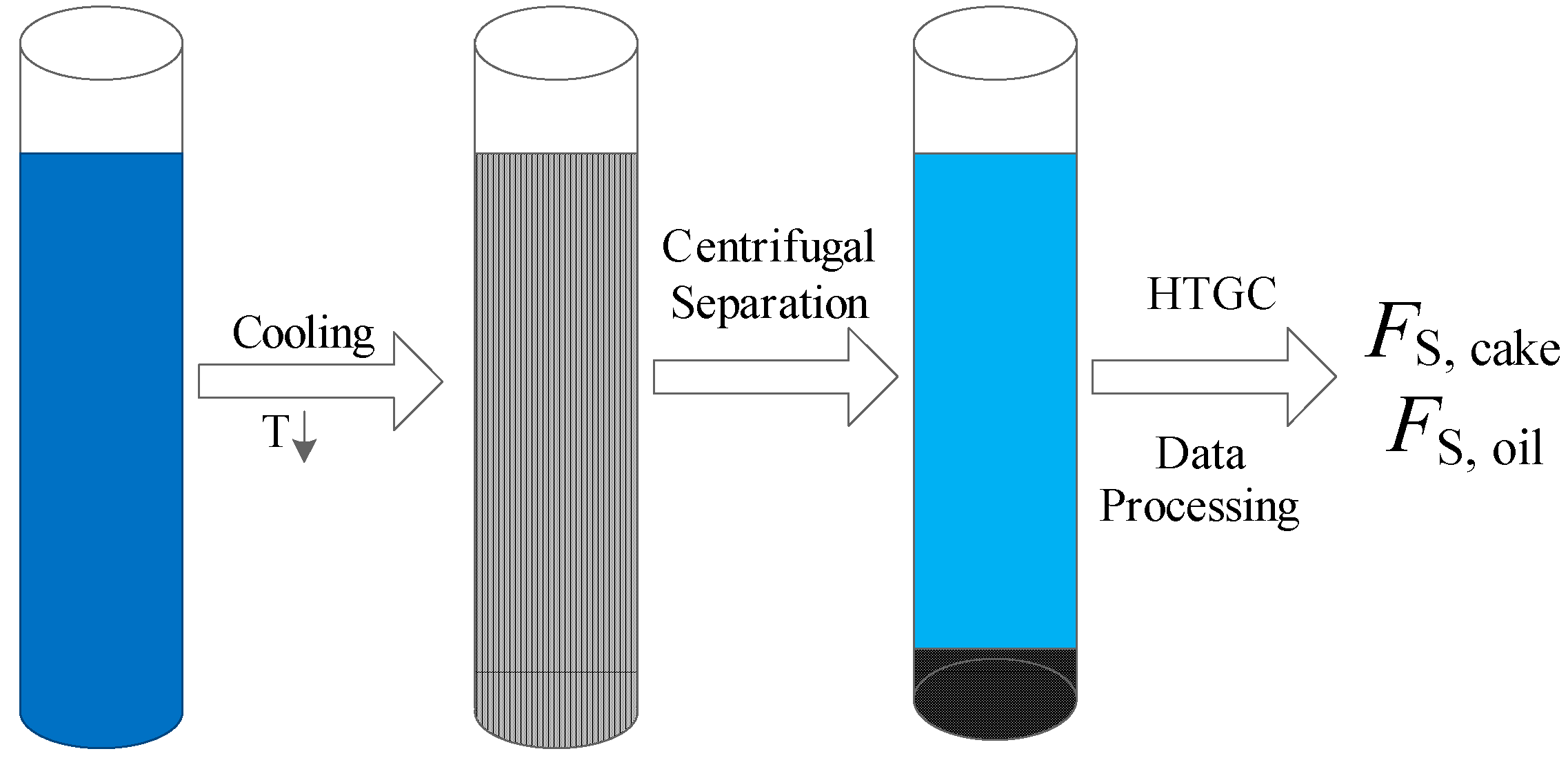
2.1. Conventional C-HTGC Method
2.2. Modified C-HTGC Method
2.3. Experimental Facility
3. Result and Discussion
3.1. Artificial Il
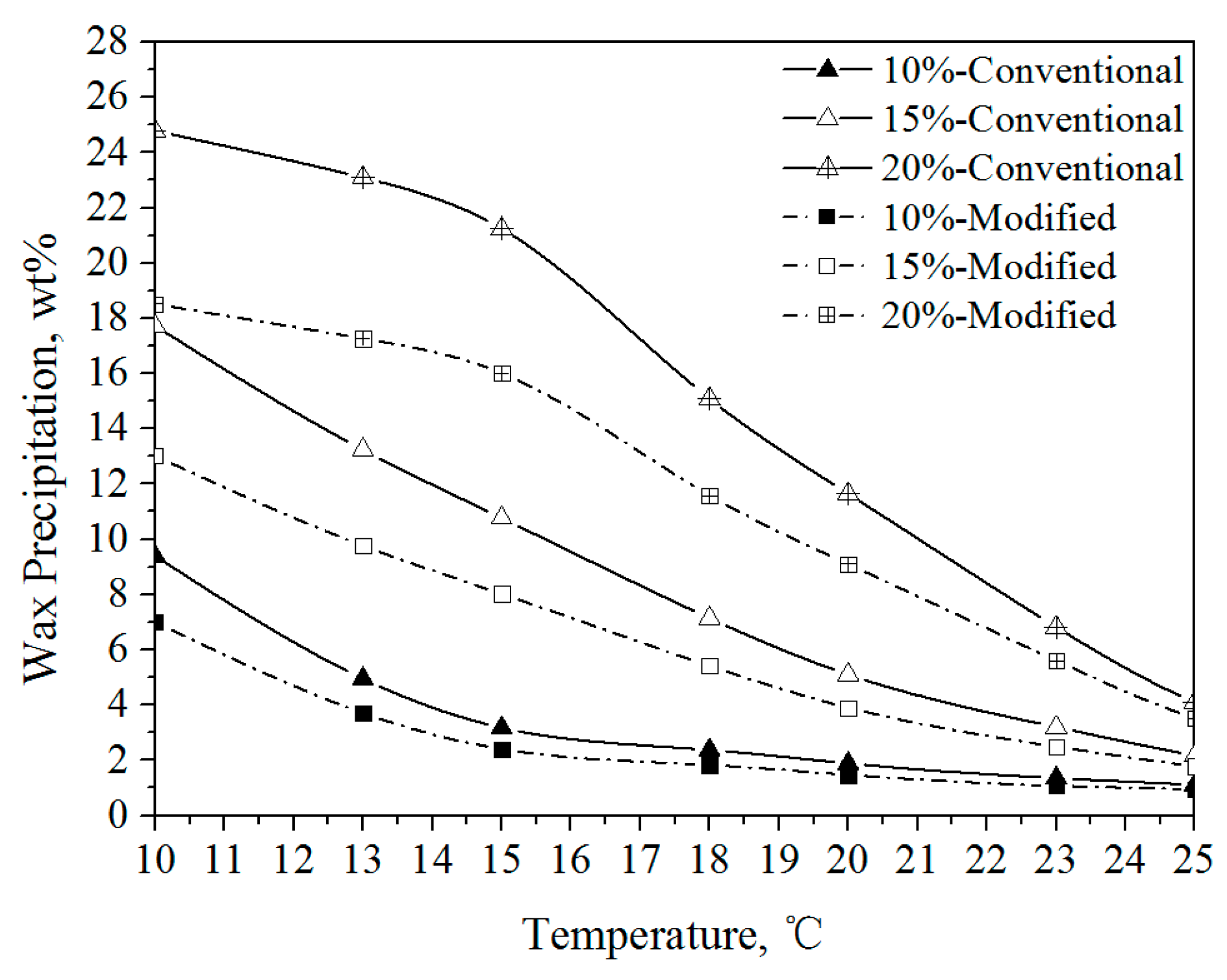
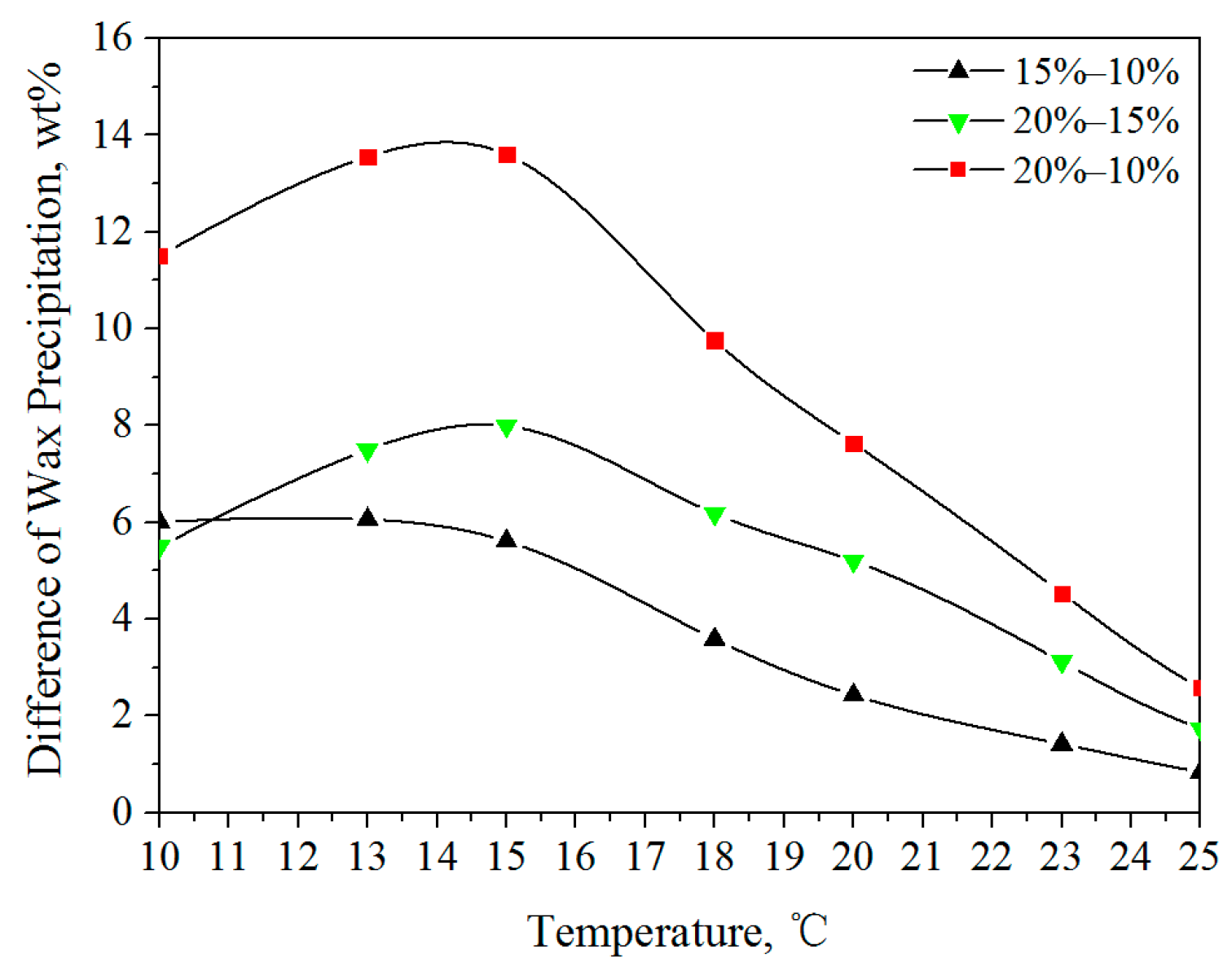
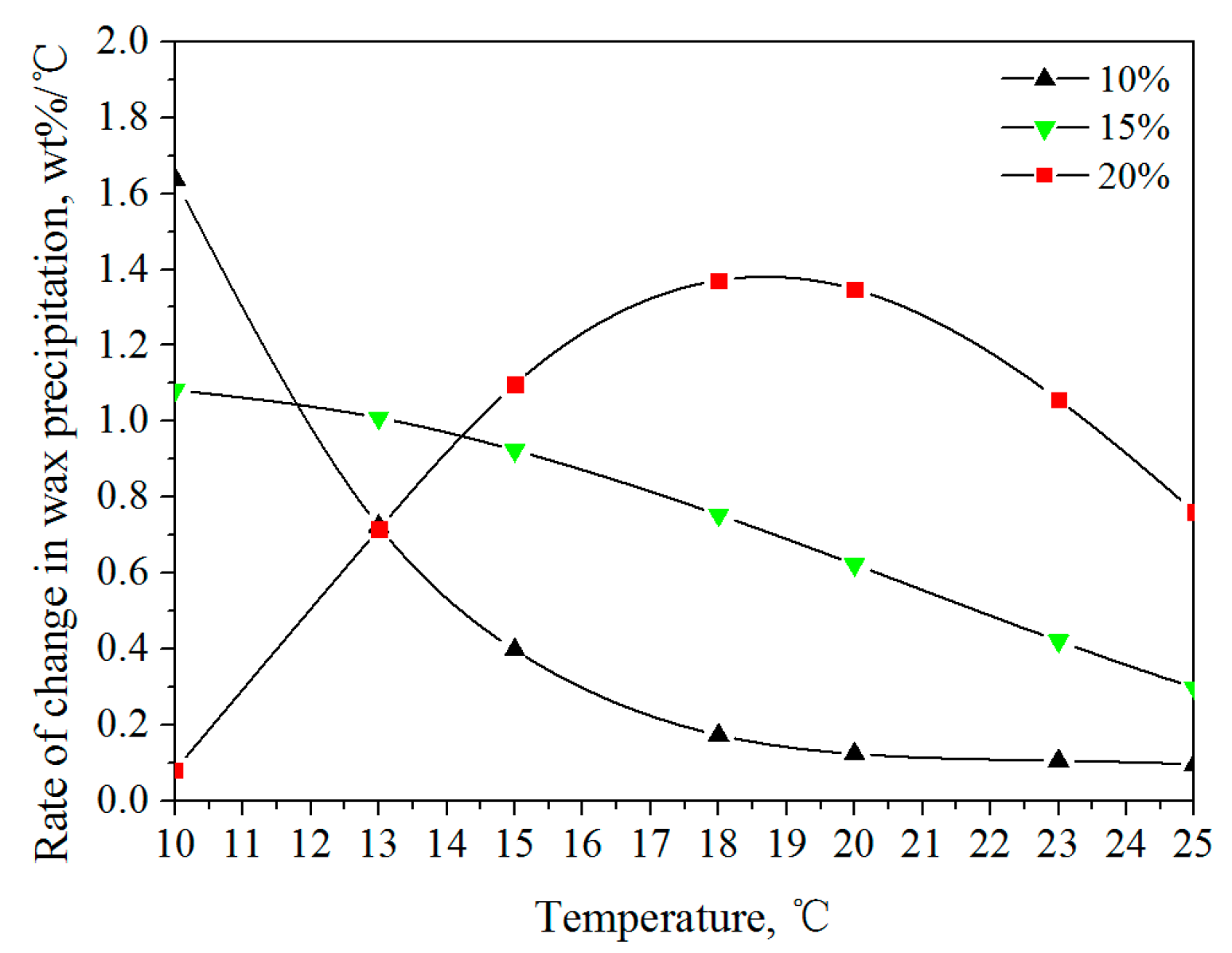
3.1.1. Wax Precipitation Amount
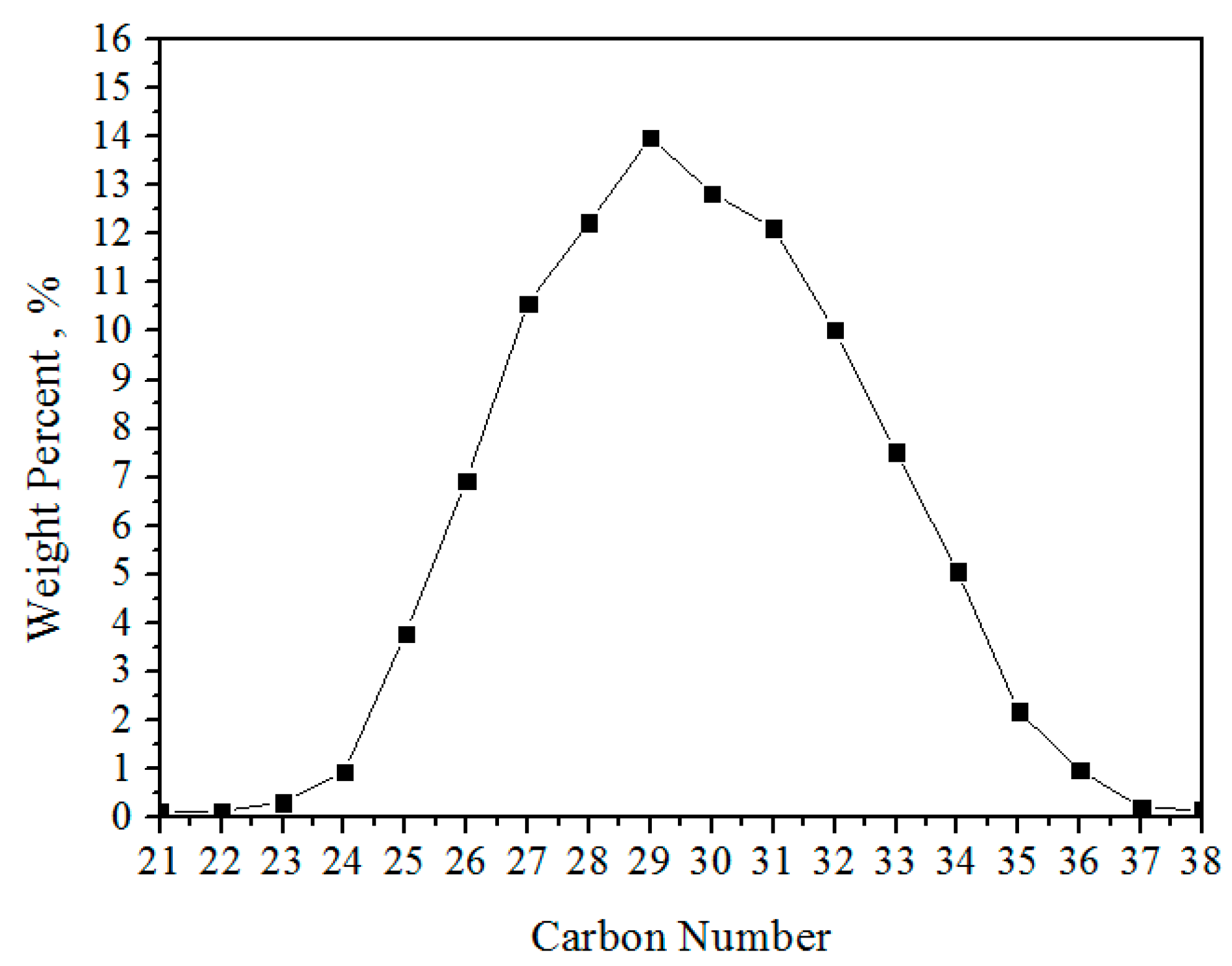
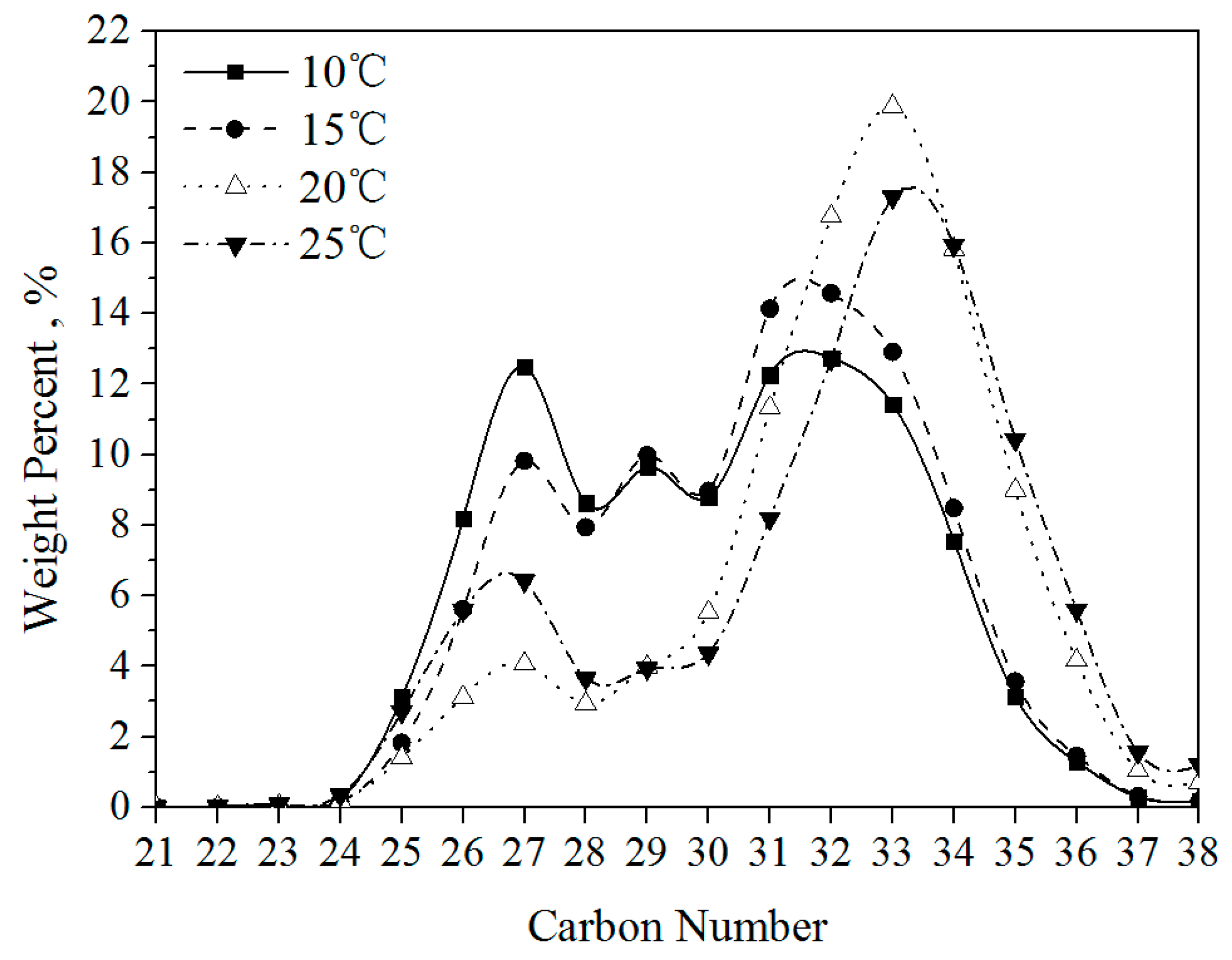
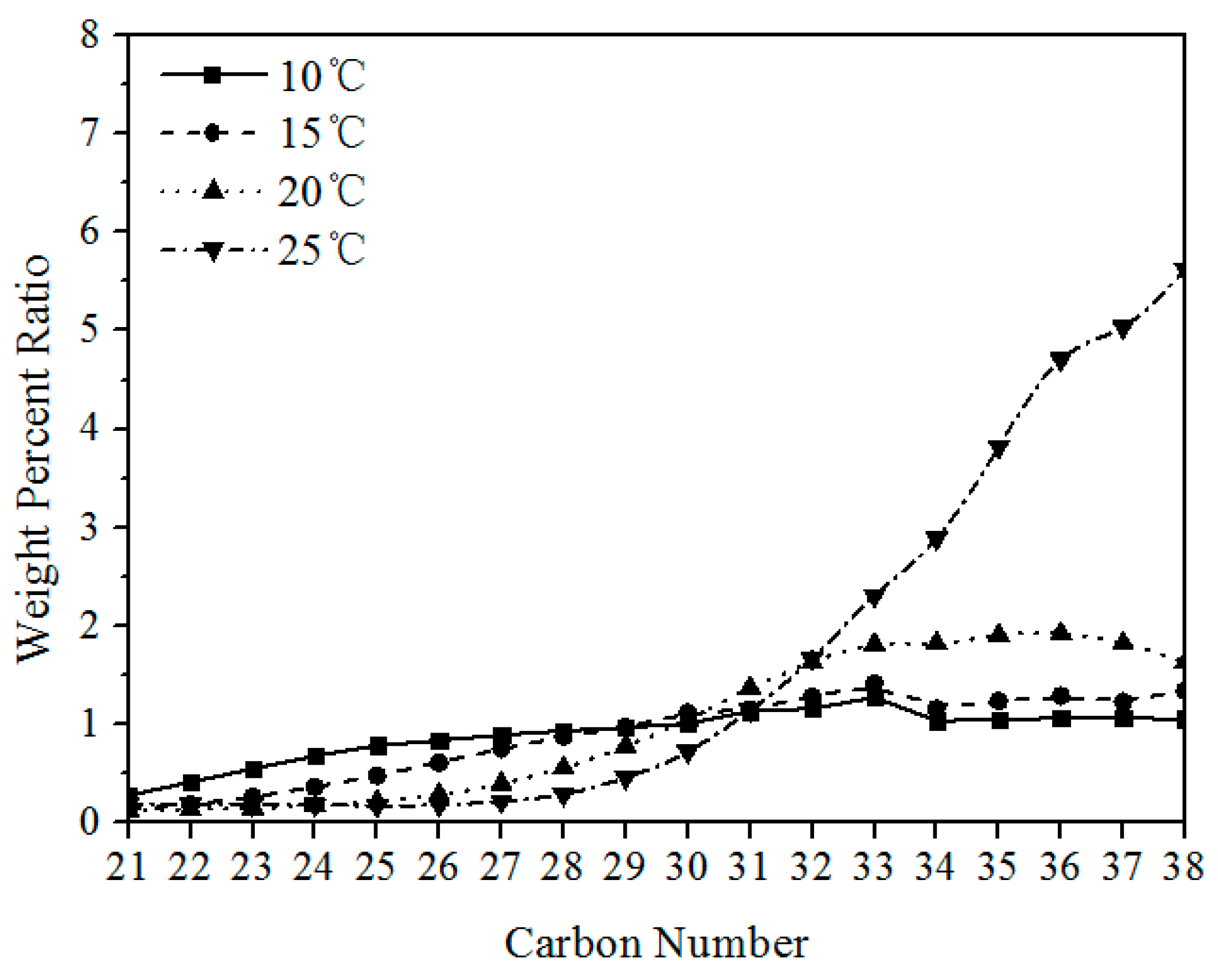
3.1.2. Composition Distribution of Solid Wax
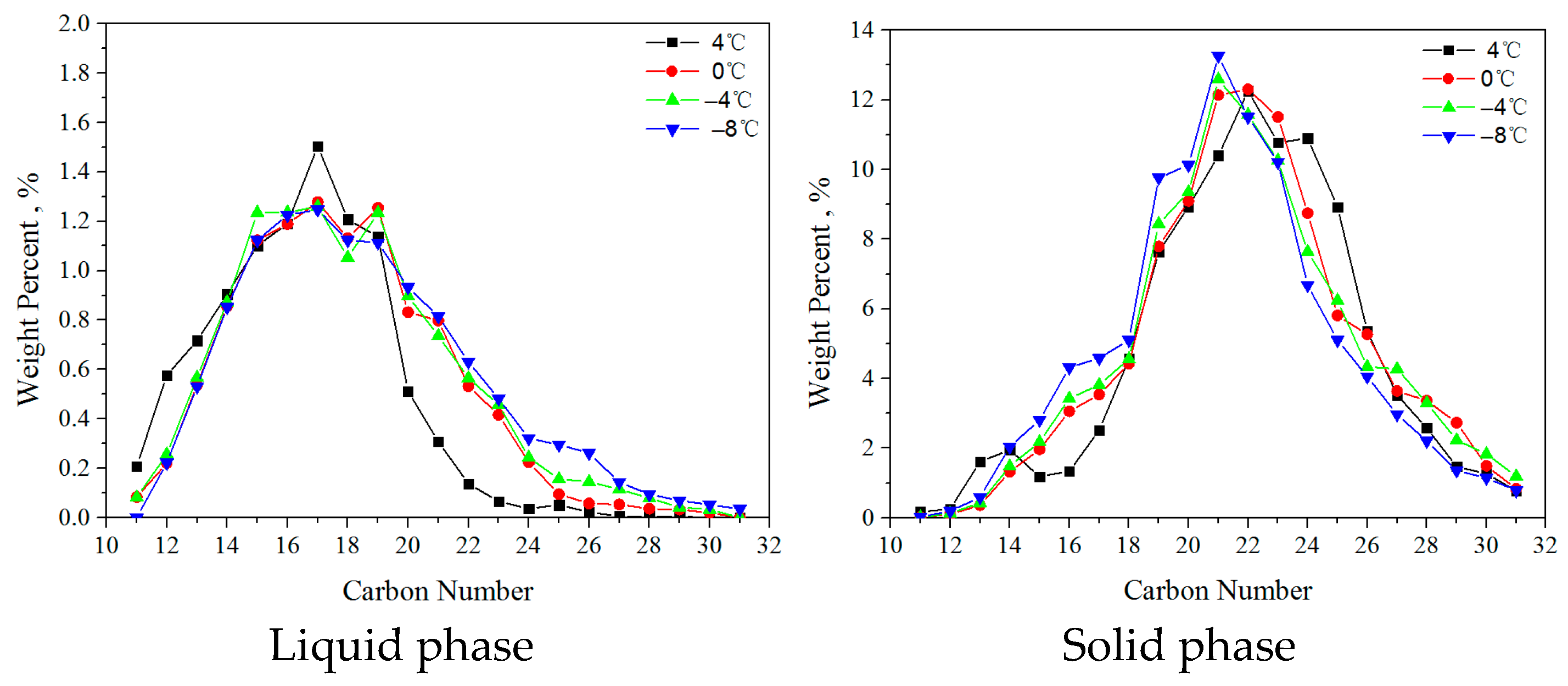
3.2. 0#. Diesel
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Yang, J.; Wang, W.; Huang, H.; Wang, J.; Duan, J.; Wang, D.; Shi, G.; Gong, J.; Shi, B. A further discussion on solid-liquid equilibrium in complex synthetic paraffinic systems with the effects of solid-solid transition. Fluid Phase Equilib. 2017, 440, 9–18. [Google Scholar] [CrossRef]
- Sun, W.; Wang, W.; Gu, Y.; Xu, X.; Gong, J. Study on the wax/asphaltene aggregation with diffusion limited aggregation model. Fuel 2017, 191, 106–113. [Google Scholar] [CrossRef]
- Meighani, H.M.; Ghotbi, C.; Behbahani, T.J. A modified thermodynamic modeling of wax precipitation in crude oil based on PC-SAFT model. Fluid Ph. Equilib. 2016, 429, 313–324. [Google Scholar] [CrossRef]
- Morozov, E.V.; Falaleev, O.V.; Martyanov, O.N. New Insight into the Wax Precipitation Process: In Situ NMR Imaging Study in a Cold Finger Cell. Energy Fuels 2016, 30, 9003–9013. [Google Scholar] [CrossRef]
- Huang, Z.; Lee, H.S.; Senra, M.; Fogler, H.S. A fundamental model of wax deposition in subsea oil pipelines. AIChE J. 2011, 57, 2955–2964. [Google Scholar] [CrossRef]
- Quan, Q.; Wang, W.; Wang, P.; Duan, J.; Yang, J.; Yao, H.; Gong, J. Wax Deposition From Emulsion-water in Stratified Flow. Pet. Sci. Technol. 2015, 33, 749–755. [Google Scholar] [CrossRef]
- Japper-Jaafar, A.; Bhaskoro, P.T.; Mior, Z.S. A new perspective on the measurements of wax appearance temperature: Comparison between DSC, thermomicroscopy and rheometry and the cooling rate effects. J. Pet. Sci. Eng. 2016, 147, 672–681. [Google Scholar] [CrossRef]
- Duan, J.; Liu, H.; Jiang, J.; Xue, S.; Wu, J.; Gong, J. Numerical prediction of wax deposition in oil–gas stratified pipe flow. Int. J. Heat Mass Transf. 2017, 105, 279–289. [Google Scholar] [CrossRef]
- Han, S.; Huang, Z.; Senra, M.; Hoffmann, R.; Fogler, H.S. Method to Determine the Wax Solubility Curve in Crude Oil from Centrifugation and High Temperature Gas Chromatography Measurements. Energy Fuels 2010, 1753–1761. [Google Scholar] [CrossRef]
- Kok, M.V.; Létoffé, J.M.; Claudy, P.; Martin, D.; Garcin, M.; Volle, J.L. Comparison of wax appearance temperatures of crude oils by differential scanning calorimetry, thermomicroscopy and viscometry. Fuel 1996, 75, 787–790. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, J.; Li, H. Determining the wax content of crude oils by using differential scanning calorimetry. Thermochim. Acta 2004, 410, 23–26. [Google Scholar] [CrossRef]
- Wang, W.; Huang, Q.; Wang, C.; Li, S.; Qu, W.; Zhao, J.; He, M. Effect of operating conditions on wax deposition in a laboratory flow loop characterized with DSC technique. J. Therm. Anal. Calorim. 2015, 119, 471–485. [Google Scholar] [CrossRef]
- Roehner, R.M.; Hanson, F.V. Determination of Wax Precipitation Temperature and Amount of Precipitated Solid Wax versus Temperature for Crude Oils Using FT-IR Spectroscopy. Energy Fuels 2001, 15, 756–763. [Google Scholar] [CrossRef]
- Alghanduri, L.M.; Elgarni, M.M.; Daridon, J.L.; Coutinho, J.A. Characterization of Libyan Waxy Crude Oils. Energy Fuels 2010, 24, 3101–3107. [Google Scholar] [CrossRef]
- Espada, J.J.; Coutinho, J.A.P.; Peña, J.L. Evaluation of Methods for the Extraction and Characterization of Waxes from Crude Oils. Energy Fuels 2010, 24, 1837–1843. [Google Scholar] [CrossRef]
- Coto, B.; Martos, C.; Peña, J.L.; Espada, J.J.; Robustillo, M.D. A new method for the determination of wax precipitation from non-diluted crude oils by fractional precipitation. Fuel 2008, 87, 2090–2094. [Google Scholar] [CrossRef]
- Mohamed, N.H.; Zaky, M.T. Separation of Microcrystalline Waxes from Local Crude Petrolatums using Solventâ “Antisolvent Mixtures. Pet. Sci. Technol. 2004, 22, 1553–1569. [Google Scholar] [CrossRef]
- Roenningsen, H.P.; Bjoerndal, B.; Hansen, A.B.; Pedersen, W.B. Wax precipitation from North Sea crude oils: 1. Crystallization and dissolution temperatures, and Newtonian and non-Newtonian flow properties. Energy Fuels 1991, 5, 895–908. [Google Scholar] [CrossRef]
- Roehner, R.M.; Fletcher, J.V.; Hanson, F.V.; Dahdah, N.F. Comparative Compositional Study of Crude Oil Solids from the Trans Alaska Pipeline System Using High-Temperature Gas Chromatography. Energy Fuels 2002, 16, 211–217. [Google Scholar] [CrossRef]
- Martos, C.; Coto, B.; Espada, J.J.; Robustillo, M.D.; Gómez, S.; Peña, J.L. Experimental Determination and Characterization of Wax Fractions Precipitated as a Function of Temperature. Energy Fuels 2008, 22, 708–714. [Google Scholar] [CrossRef]
- Jimiao, D.; Wei, W.; Huishu, L.; Jing, G. Modeling the characterization of the plus fractions by using continuous distribution function. Fluid Ph. Equilib. 2013, 345, 1–10. [Google Scholar]
- Martos, C.; Coto, B.; Espada, J.J.; Robustillo, M.D.; Peña, J.L.; Merino-Garcia, D. Characterization of Brazilian Crude Oil Samples To Improve the Prediction of Wax Precipitation in Flow Assurance Problems. Energy Fuels 2010, 24, 2221–2226. [Google Scholar] [CrossRef]
- Duan, J.; Deng, S.; Xu, S.; Liu, H.; Chen, M.; Gong, J. The effect of gas flow rate on the wax deposition in oil-gas stratified pipe flow. J. Pet. Sci. Eng. 2018, 162, 539–547. [Google Scholar] [CrossRef]
- Alcazar-Vara, L.A.; Buenrostro-Gonzalez, E. Experimental study of the influence of solvent and asphaltenes on liquid–solid phase behavior of paraffinic model systems by using DSC and FT-IR techniques. J. Therm. Anal. Calorim. 2012, 107, 1321–1329. [Google Scholar] [CrossRef]
- Do Carmo, R.P.; da Silva, V.M.; Fleming, F.P.; Daridon, J.L.; Pauly, J.; Tavares, F.W. Paraffin solubility curves of diesel fuels from thermodynamic model adjusted through experimental DSC thermograms. Fuel 2018, 230, 266–273. [Google Scholar] [CrossRef]
- Parsa, S.; Javanmardi, J.; Aftab, S.; Nasrifar, K. Experimental measurements and thermodynamic modeling of wax disappearance temperature for the binary systems n-C14H30 +n-C16H34, n-C16H34+n-C18H38 and n-C11H24+n-C18H38. Fluid Ph. Equilib. 2015, 388, 93–99. [Google Scholar] [CrossRef]
- Coto, B.J.A.P.; Coutinho, J.A.P.; Martos, C.; Robustillo, M.D.; Espada, J.J.; Pena, J.L. Assessment and Improvement of n-Paraffin Distribution Obtained by HTGC To Predict Accurately Crude Oil Cold Properties. Energy Fuels 2011, 25, 1153–1160. [Google Scholar] [CrossRef]
- Coutinho, J.A.P.; Dauphin, C.; Daridon, J.L. Measurements and modelling of wax formation in diesel fuels. Fuel 2000, 79, 607–616. [Google Scholar] [CrossRef][Green Version]
- Kazmierczak, P.R.; Paredes, M.L.L.; Lima, E.R.A. Enhancing the objective function used to predict the composition of n -paraffin mixtures from calorimetric curves. Thermochim. Acta 2017, 650, 56–65. [Google Scholar] [CrossRef]
- Coutinho, J.A.P. A Thermodynamic Model for Predicting Wax Formation in Jet and Diesel Fuels. Energy Fuels 2000, 14, 625–631. [Google Scholar] [CrossRef]








| Alkane Component | Weight Fraction % | Alkane Component | Weight Fraction % | Alkane Component | Weight Fraction % |
|---|---|---|---|---|---|
| C10 | 0.092 | C18 | 1.038 | C25 | 0.196 |
| C11 | 0.332 | C19 | 0.954 | C26 | 0.129 |
| C12 | 0.512 | C20 | 0.821 | C27 | 0.085 |
| C13 | 0.956 | C21 | 0.637 | C28 | 0.062 |
| C14 | 1.212 | C22 | 0.359 | C29 | 0.028 |
| C15 | 1.235 | C23 | 0.332 | C30 | 0.020 |
| C16 | 1.252 | C24 | 0.235 | C31 | 0.092 |
| C17 | 1.137 |
| Temperature, °C | 4 | 2 | 0 | −2 | −4 | −6 | −8 |
|---|---|---|---|---|---|---|---|
| Wax precipitate, wt% | 0.182 | 1.034 | 1.764 | 3.188 | 5.187 | 7.963 | 11.210 |
| Alkane Component | 4 °C | 0 °C | −4 °C | −8 °C | ||||
|---|---|---|---|---|---|---|---|---|
| Liquid Phase xiL | Solid Phase xiS | Liquid Phase xiL | Solid Phase xiS | Liquid Phase xiL | Solid Phase xiS | Liquid Phase xiL | Solid Phase xiS | |
| C11 | 0.208 | 0.175 | 0.083 | 0.018 | 0.082 | 0.018 | 0.040 | 0.027 |
| C12 | 0.576 | 0.251 | 0.221 | 0.104 | 0.256 | 0.129 | 0.222 | 0.193 |
| C13 | 0.717 | 1.614 | 0.538 | 0.367 | 0.567 | 0.433 | 0.531 | 0.595 |
| C14 | 0.903 | 1.948 | 0.856 | 1.320 | 0.873 | 1.480 | 0.852 | 2.039 |
| C15 | 1.102 | 1.186 | 1.123 | 1.964 | 1.235 | 2.178 | 1.123 | 2.810 |
| C16 | 1.190 | 1.342 | 1.189 | 3.060 | 1.236 | 3.429 | 1.225 | 4.315 |
| C17 | 1.505 | 2.518 | 1.278 | 3.537 | 1.259 | 3.816 | 1.247 | 4.584 |
| C18 | 1.208 | 4.559 | 1.131 | 4.423 | 1.053 | 4.560 | 1.123 | 5.104 |
| C19 | 1.137 | 7.644 | 1.254 | 7.791 | 1.234 | 8.443 | 1.113 | 9.762 |
| C20 | 0.512 | 8.928 | 0.832 | 9.094 | 0.897 | 9.350 | 0.933 | 10.126 |
| C21 | 0.308 | 10.403 | 0.798 | 12.138 | 0.736 | 12.584 | 0.815 | 13.263 |
| C22 | 0.137 | 12.255 | 0.532 | 12.302 | 0.565 | 11.570 | 0.631 | 11.508 |
| C23 | 0.066 | 10.767 | 0.416 | 11.508 | 0.457 | 10.255 | 0.482 | 10.211 |
| C24 | 0.036 | 10.900 | 0.224 | 8.748 | 0.243 | 7.640 | 0.321 | 6.679 |
| C25 | 0.051 | 8.923 | 0.095 | 5.810 | 0.156 | 6.242 | 0.295 | 5.104 |
| C26 | 0.023 | 5.356 | 0.057 | 5.267 | 0.145 | 4.335 | 0.262 | 4.055 |
| C27 | 0.005 | 3.528 | 0.053 | 3.643 | 0.115 | 4.276 | 0.143 | 2.967 |
| C28 | 0.006 | 2.579 | 0.036 | 3.367 | 0.079 | 3.294 | 0.095 | 2.205 |
| C29 | 0.003 | 1.470 | 0.033 | 2.734 | 0.042 | 2.232 | 0.069 | 1.357 |
| C30 | 0.002 | 1.267 | 0.020 | 1.494 | 0.033 | 1.833 | 0.052 | 1.150 |
| C31 | 0.000 | 0.776 | 0.000 | 0.832 | 0.000 | 1.194 | 0.035 | 0.786 |
| Non-alkane component | 88.431 | 0.480 | 88.737 | 0.710 | 89.231 | 1.160 | 90.305 | 1.611 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Duan, J.; Li, J.; Yan, H.; Wang, J.; Lin, K.; Guan, L.; Li, C. Experimental Measurements of Wax Precipitation Using a Modified Method of Simultaneous Centrifugation and High-Temperature Gas Chromatography. Energies 2021, 14, 7035. https://doi.org/10.3390/en14217035
Liu H, Duan J, Li J, Yan H, Wang J, Lin K, Guan L, Li C. Experimental Measurements of Wax Precipitation Using a Modified Method of Simultaneous Centrifugation and High-Temperature Gas Chromatography. Energies. 2021; 14(21):7035. https://doi.org/10.3390/en14217035
Chicago/Turabian StyleLiu, Huishu, Jimiao Duan, Jiang Li, Hao Yan, Jian Wang, Keyu Lin, Liang Guan, and Changjun Li. 2021. "Experimental Measurements of Wax Precipitation Using a Modified Method of Simultaneous Centrifugation and High-Temperature Gas Chromatography" Energies 14, no. 21: 7035. https://doi.org/10.3390/en14217035
APA StyleLiu, H., Duan, J., Li, J., Yan, H., Wang, J., Lin, K., Guan, L., & Li, C. (2021). Experimental Measurements of Wax Precipitation Using a Modified Method of Simultaneous Centrifugation and High-Temperature Gas Chromatography. Energies, 14(21), 7035. https://doi.org/10.3390/en14217035





