Recent Achievements in Microalgal Photobiological Hydrogen Production
Abstract
:1. Introduction
2. Genetic Modification
3. O2 Removal
4. Co-Cultures
5. Immobilization
6. Hydrogen Production without Nutrient Starvation
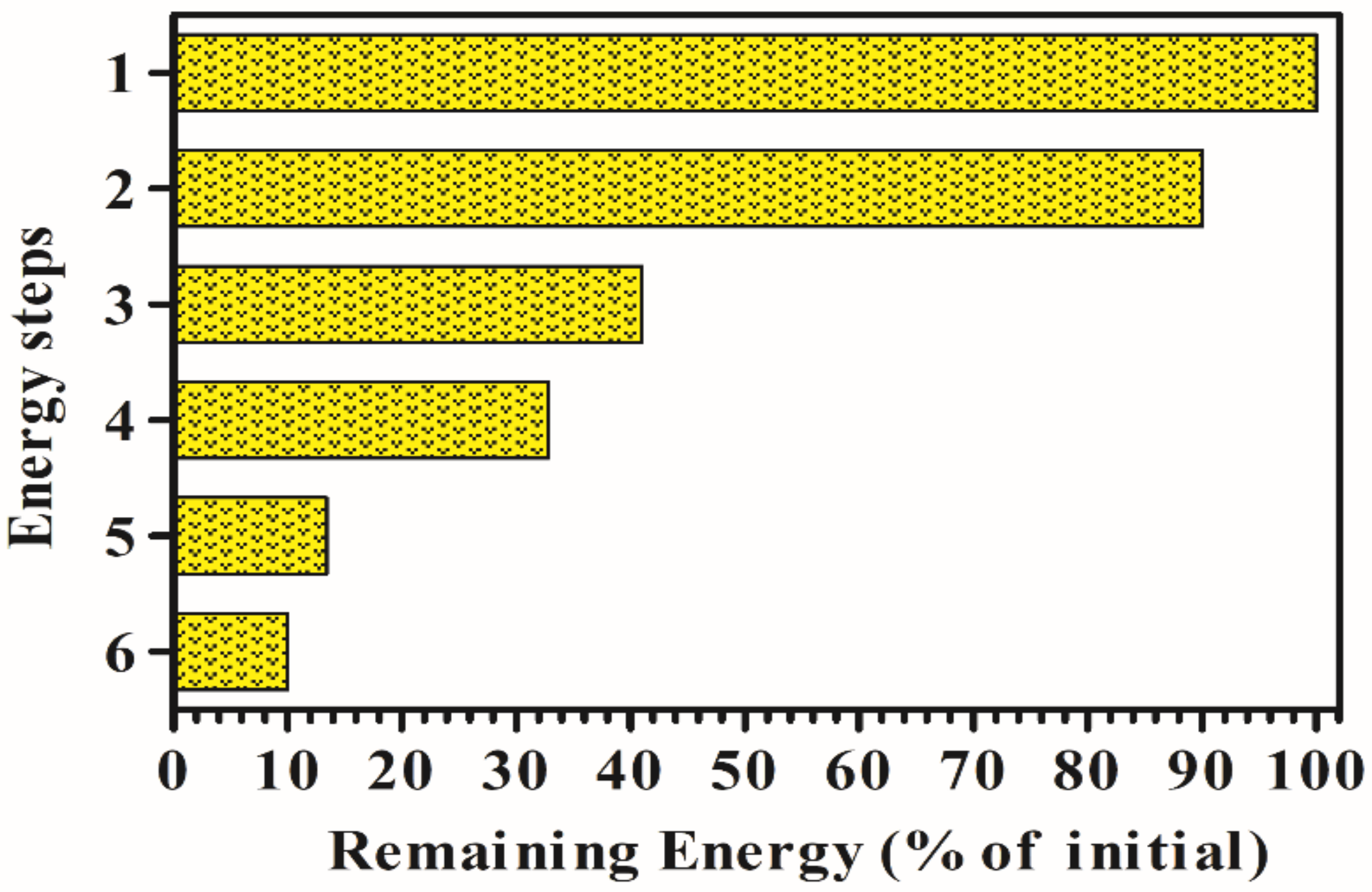
7. Theoretical Limit for Biological Hydrogen Production
- Approximately 10% is lost by reflection and scattering (90% of initial remaining).
- Approximately 55% of radiation is not available to drive photosynthesis since it falls outside of the photosynthetically active radiation (400–700 nm) and thus is not utilized by photosynthetic pigments. As a result, the total amount of available light drops to 41%.
- About 20.4% of the radiation is lost as heat [82].
- Assuming as quantum requirement that 8 photons are required to produce 2 mol of H2, and considering that 1 mol of H2 is 286 KJ, and the mean energy for charge separation at PSII and PSI is 173.5 KJ/mol, it follows that the efficiency of the process will be the following: (286 KJ/mol × 2)/(173.5 KJ/mol × 8) × 100 = 41.2%, with a corresponding loss of energy of 59%. Consequently, the theoretical LCE for H2 production, attainable by direct biophotolysis is about 13.4% of incident solar light [83].
- With a LCE of about 10%, assuming that approximately 20% of the energy can be lost for cell maintenance, it might be possible to produce about 600,000 m3/ha/y of H2 in sunny areas.
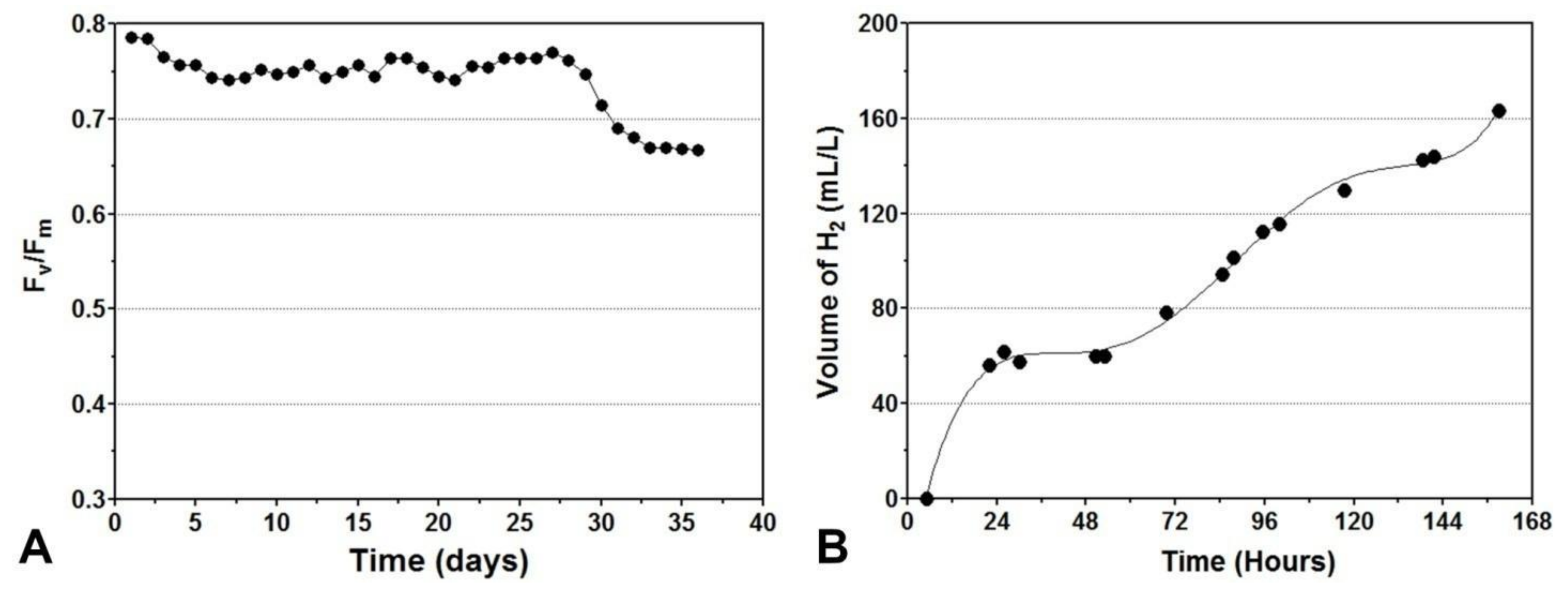
8. Chlorophyll Fluorescence Measurements as a Tool for Monitoring Changes of Photochemical Efficiency during the Hydrogen Production Process
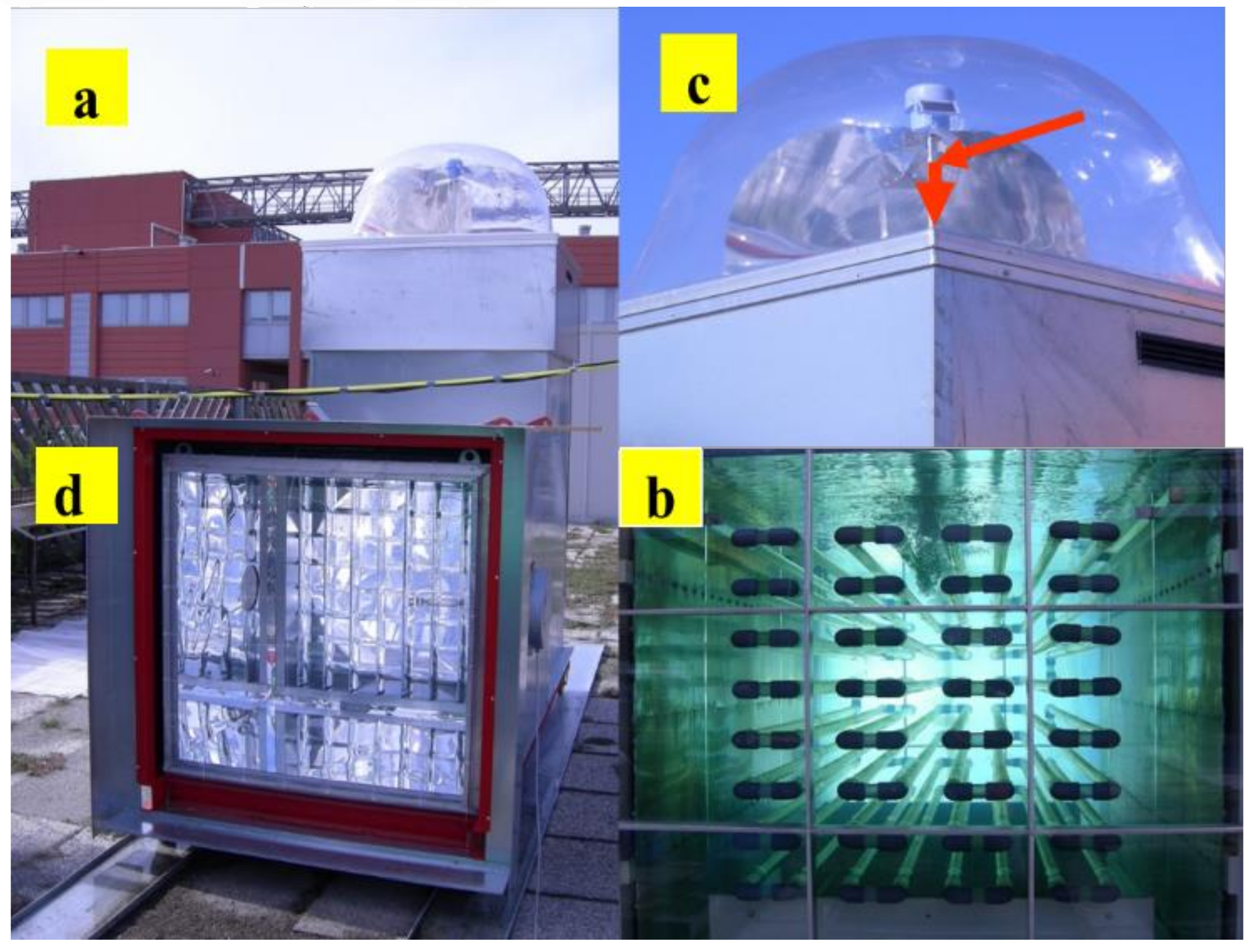
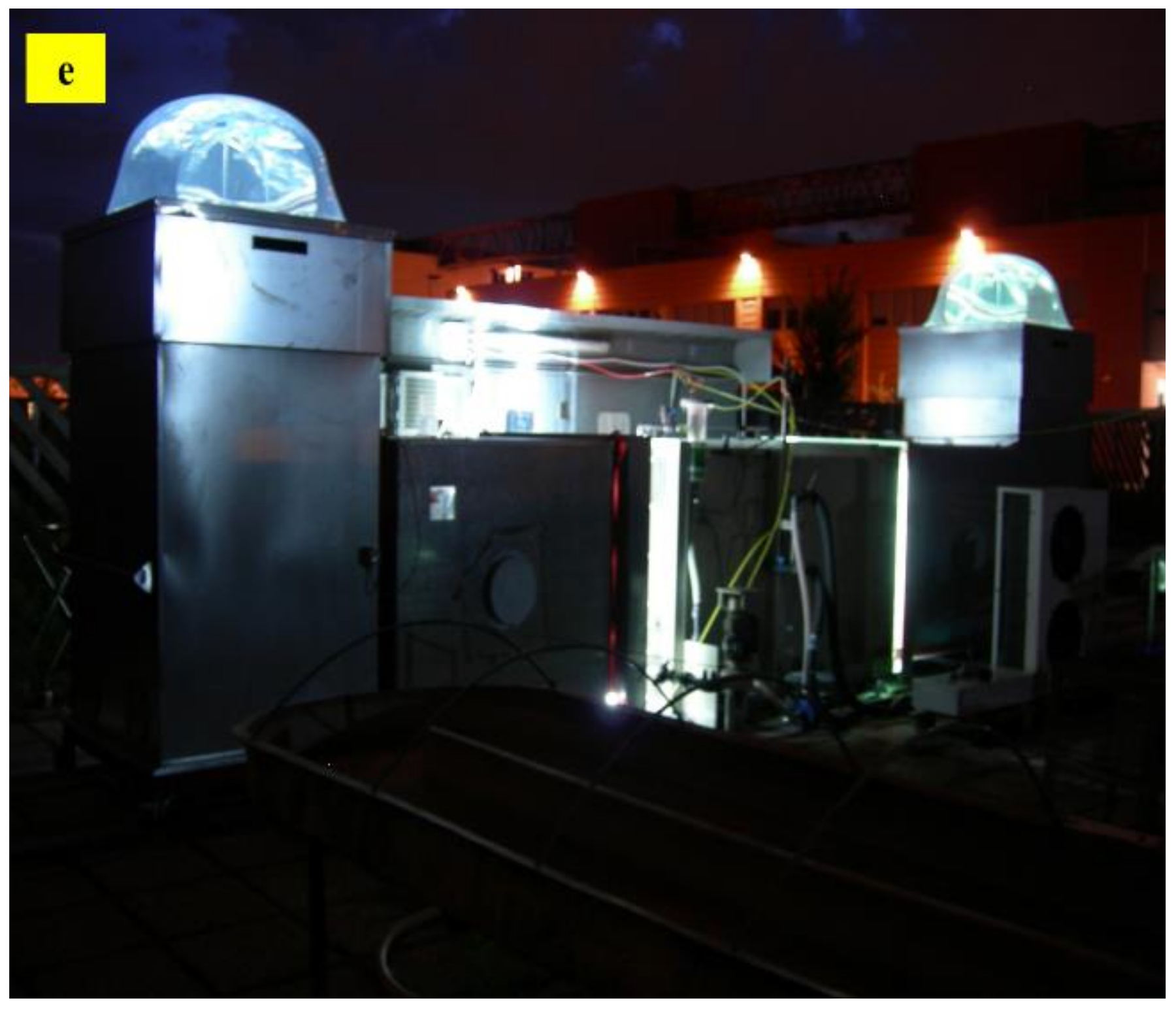
9. Photobiological Hydrogen Production in Outdoor Photobioreactors
10. Optimal Photobioreactor Design for the Hydrogen Production
11. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moshood, T.D.; Nawanir, G.; Mahmud, F. Microalgae biofuels production: A systematic review on socioeconomic prospects of microalgae biofuels and policy implications. Environ. Chall. 2021, 5, 100207. [Google Scholar] [CrossRef]
- Masojídek, J.; Torzillo, G.; Koblízek, M. Photosynthesis in microalgae. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology, 2nd ed.; John Wiley & Sons: Chichester, UK, 2013; pp. 21–36. [Google Scholar]
- Gaffron, H.; Rubin, J. Fermentative and photochemical production of hydrogen in algae. J. Gen. Physiol. 1942, 26, 219–240. [Google Scholar] [CrossRef]
- Martinez-Burgos, W.J.; de Souza Candeo, E.; Pedroni Medeiros, A.B.; Cesar de Carvalho, J.; Oliveira de Andrade Tanobe, V.; Soccol, C.R.; Sydney, E.B. Hydrogen: Current advances and patented technologies of its renewable production. J. Clean. Prod. 2021, 286, 124970. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, H.; Zhang, X.; Han, F.; Tu, W.; Yang, W. Microalgal hydrogen production. Small Methods 2020, 4, 1900514. [Google Scholar] [CrossRef]
- Dębowski, M.; Dudek, M.; Zieliński, M.; Nowicka, A.; Kazimierowicz, J. Microalgal hydrogen production in relation to other biomass-based technologies—A review. Energies 2021, 14, 6025. [Google Scholar] [CrossRef]
- Kosourov, S.; Nagy, V.; Shevela, D.; Jokel, M.; Messinger, J.; Allahverdiyeva, Y. Water oxidation by photosystem II is the primary source of electron for sustained H2 photoproduction in nutrient-replete green algae. Proc. Natl. Acad. Sci. USA 2020, 117, 29629–29636. [Google Scholar] [CrossRef] [PubMed]
- Touloupakis, E.; Faraloni, C.; Silva Benavides, A.M.; Masojídek, J.; Torzillo, G. Sustained photobiological hydrogen production by Chlorella vulgaris without nutrient starvation. Int. J. Hydrog. Energy 2021, 46, 3684–3694. [Google Scholar] [CrossRef]
- Melis, A. Photosynthetic H2 metabolism in Chlamydomonas reinhardtii (unicellular green algae). Planta 2007, 226, 1075–1086. [Google Scholar] [CrossRef]
- Liu, J.Z.; Ge, Y.M.; Sun, J.Y.; Chen, P.; Addy, M.; Huo, S.H.; Li, K.; Cheng, P.F.; Ruan, R. Exogenic glucose as an electron donor for algal hydrogenases to promote hydrogen photoproduction by Chlorella pyrenoidosa. Bioresour. Technol. 2019, 289, 121762. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Marin, A.; Canedo-López, Y.; Chávez-Fuentes, P. Biohydrogen production by Chlorella vulgaris and Scenedesmus obliquus immobilized cultivated in artificial wastewater under different light quality. AMB Express 2020, 10, 191. [Google Scholar] [CrossRef]
- Li, L.; Litao, Z.; Jianguo, L. Proteomic analysis of hydrogen production in Chlorella pyrenoidosa under nitrogen deprivation. Algal. Res. 2021, 53, 102143. [Google Scholar] [CrossRef]
- Grechanik, V.; Naidov, I.; Bolshakov, M.; Tsygankov, A. Photoautotrophic hydrogen production by nitrogen-deprived Chlamydomonas reinhardtii cultures. Int. J. Hydrog. Energy 2021, 46, 3565–3575. [Google Scholar] [CrossRef]
- Amaro, H.M.; Esquível, M.G.; Pinto, T.S.; Malcata, F.X. Hydrogen Production by Microalgae. In Natural and Artificial Photosynthesis: Solar Power as an Energy Source, 1st ed.; Razeghifard, R., Ed.; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 231–241. [Google Scholar]
- Oliveira, F.; Araujo, A.P.C.; Romao, B.B.; Cardoso, V.L.; Ferreira, J.S.; Batista, F.R.X. Hydrogen photo-production using Chlorella sp. through sulfur-deprived and hybrid system strategy. Chem. Eng. Trans. 2015, 43, 301–306. [Google Scholar]
- Jimenez-Llanos, J.; Ramirez-Carmona, M.; Rendon-Castrillon, L.; Ocampo-Lopez, C. Sustainable biohydrogen production by Chlorella sp. microalgae: A review. Int. J. Hydrog. Energy 2020, 45, 8310–8328. [Google Scholar] [CrossRef]
- Nagarajan, D.; Dong, C.D.; Chen, C.Y.; Lee, D.J.; Chang, J.S. Biohydrogen production from microalgae-Major bottlenecks and future research perspectives. Biotechnol. J. 2021, 16, e2000124. [Google Scholar] [CrossRef] [PubMed]
- Pongpadung, P.; Liu, J.; Yokthongwattana, K.; Techapinyawat, S.; Juntawong, N. Screening for hydrogen producing strains of green microalgae in phosphorus or sulfur deprived medium under nitrogen limitation. Sci. Asia 2015, 41, 97. [Google Scholar] [CrossRef] [Green Version]
- Rashid, N.; Lee, K.; Han, J.I.; Gross, M. Hydrogen production by immobilized Chlorella vulgaris: Optimizing pH, carbon source and light. Bioproc. Biosyst. Eng. 2013, 36, 867–872. [Google Scholar] [CrossRef]
- Alalayah, W.M.; Alhamed, Y.A.; Al-Zahrani, A.; Edris, G. Influence of culture parameters on biological hydrogen production using green algae Chlorella vulgaris. Rev. Chim. 2015, 66, 788–791. [Google Scholar]
- Melis, A. Green alga hydrogen production: Progress, challenges and prospects. Int. J. Hydrog. Energy 2002, 27, 1217–1228. [Google Scholar] [CrossRef]
- Tsygankov, A.A.; Kosourov, S.N.; Tolstygina, I.V.; Ghirardi, M.L.; Seibert, M. Hydrogen production by sulfur-deprived Chlamydomonas reinhardtii under photoautotrophic conditions. Int. J. Hydrog. Energy 2006, 31, 1574–1584. [Google Scholar] [CrossRef]
- Batyrova, K.; Gavrisheva, A.; Ivanova, E.; Liu, J.G.; Tsygankov, A. Sustainable hydrogen photoproduction by phosphorus-deprived marine green microalgae Chlorella sp. Int. J. Mol. Sci. 2015, 16, 2705–2716. [Google Scholar] [CrossRef]
- Rashid, N.; Lee, K.; Mahmood, Q. Bio-hydrogen production by Chlorella vulgaris under diverse photoperiods. Bioresour. Technol. 2011, 102, 2101–2104. [Google Scholar] [CrossRef]
- Khetkorn, W.; Rastogi, R.P.; Incharoensakdi, A.; Lindblad, P.; Madamwar, D.; Pandey, A.; Larroche, C. Microalgal hydrogen production—A review. Bioresour. Technol. 2017, 243, 1194–1206. [Google Scholar] [CrossRef]
- Kosourov, S.; Böhm, M.; Senger, M.; Berggren, G.; Stensjö, K.; Mamedov, F.; Lindblad, P.; Allahverdiyeva, Y. Photosynthetic hydrogen production: Novel protocols, promising engineering approaches and application of semi-synthetic hydrogenases. Physiol. Plant 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Ballester, D.; Jurado-Oller, J.L.; Fernandez, E. Relevance of nutrient media composition for hydrogen production in Chlamydomonas. Photosynth. Res. 2015, 125, 395–406. [Google Scholar] [CrossRef]
- Fakhimi, N.; Dubini, A.; Tavakoli, O.; González-Ballester, D. Acetic acid is key for synergetic hydrogen production in Chlamydomonas-bacteria co-cultures. Bioresour. Technol. 2019, 289, 121648. [Google Scholar] [CrossRef] [PubMed]
- Kosourov, S.; Jokel, M.; Aro, E.M.; Allahverdiyeva, Y. A new approach for sustained and efficient H2 photoproduction by: Chlamydomonas reinhardtii. Energy Environ. Sci. 2018, 11, 1431–1436. [Google Scholar] [CrossRef] [Green Version]
- Nagy, V.; Podmaniczki, A.; Vidal-Meireles, A.; Tengölics, R.; Kovács, L.; Rákhely, G.; Scoma, A.; Tóth, S.Z. Water-splitting-based, sustainable and efficient H2 production in green algae as achieved by substrate limitation of the Calvin–Benson–Bassham cycle. Biotechnol. Biofuels 2018, 11, 69. [Google Scholar] [CrossRef]
- Krishna, P.S.; Styring, S.; Mamedov, F. Photosystem ratio imbalance promotes direct sustainable H2 production in Chlamydomonas reinhardtii. Green Chem. 2019, 21, 4683–4690. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Zhang, J.; Zhao, L.; Xing, J.; Peng, L.; Kuang, T.; Rochaix, J.D.; Huang, F. Loss of algal Proton Gradient Regulation 5 increases reactive oxygen species scavenging and H2 evolution. J. Integr. Plant Biol. 2016, 58, 943–946. [Google Scholar] [CrossRef] [Green Version]
- Steinbeck, J.; Nikolova, D.; Weingarten, R.; Johnson, X.; Richaud, P.; Peltier, G.; Hermann, M.; Magneschi, L.; Hippler, M. Deletion of proton gradient regulation 5 (PGR5) and PGR5-Like 1 (PGRL1) proteins promote sustainable light-driven hydrogen production in Chlamydomonas reinhardtii due to increased PSII activity under sulfur deprivation. Front. Plant Sci. 2015, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Pinto, T.S.; Malcata, F.X.; Arrabaça, J.D.; Silva, J.M.; Spreitzer, R.J.; Esquivel, M.G. Rubisco mutants of Chlamydomonas reinhardtii enhance photosynthetic hydrogen production. Appl. Microbiol. Biotechnol. 2013, 97, 5635–5643. [Google Scholar] [CrossRef]
- Eilenberg, H.; Weiner, I.; Ben-Zvi, O.; Pundak, C.; Marmari, A.; Liran, O.; Wecker, M.S.; Milrad, Y.; Yacoby, I. The dual effect of a ferredoxin-hydrogenase fusion protein in vivo: Successful divergence of the photosynthetic electron flux towards hydrogen production and elevated oxygen tolerance. Biotechnol. Biofuels 2016, 9, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torzillo, G.; Scoma, A.; Faraloni, C.; Ena, A.; Johanningmeier, U. Increased hydrogen photoproduction by means of a sulfur-deprived Chlamydomonas reinhardtii D1 protein mutant. Int. J. Hydrog. Energy 2009, 34, 4529–4536. [Google Scholar] [CrossRef]
- Scoma, A.; Krawietz, D.; Faraloni, C.; Giannelli, L.; Happe, T.; Torzillo, G. Sustained H2 production in a Chlamydomonas reinhardtii D1 protein mutant. J. Biotechnol. 2012, 157, 613–619. [Google Scholar] [CrossRef]
- Batyrova, K.; Hallenbeck, P.C. Hydrogen Production by a Chlamydomonas reinhardtii strain with inducible expression of photosystem II. Int. J. Mol. Sci. 2017, 18, 647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosourov, S.N.; Ghirardi, M.L.; Seibert, M. A truncated antenna mutant of Chlamydomonas reinhardtii can produce more hydrogen than the parental strain. Int. J. Hydrog. Energy 2011, 36, 2044–2048. [Google Scholar] [CrossRef]
- Xu, F.Q.; Ma, W.M.; Zhu, X.G. Introducing pyruvate oxidase into the chloroplast of Chlamydomonas reinhardtii increases oxygen consumption and promotes hydrogen production. Int. J. Hydrog. Energy 2011, 36, 10648. [Google Scholar] [CrossRef]
- Kruse, O.; Rupprecht, J.; Bader, K.P.; Thomas-Hall, S.; Schenk, P.M.; Finazzi, G.; Hankamer, B. Improved photobiological H2 production in engineered green algal cells. J. Biol. Chem. 2005, 280, 34170–34177. [Google Scholar] [CrossRef] [Green Version]
- Volgusheva, A.; Styring, S.; Mamedov, F. Increased photosystem II stability promotes H2 production in sulfur-deprived Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 2013, 110, 7223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oey, M.; Ross, I.L.; Stephens, E.; Steinbeck, J.; Wolf, J.; Radzun, K.A.; Kugler, J.; Ringsmuth, A.K.; Kruse, O.; Hankamer, B. RNAi knock-down of LHCBM1, 2 and 3 increases photosynthetic H2 production efficiency of the green alga Chlamydomonas reinhardtii. PLoS ONE 2013, 8, e61375. [Google Scholar] [CrossRef]
- Wu, S.X.; Yan, G.Y.; Xu, L.L.; Wang, Q.X.; Liu, X.L. Improvement of hydrogen production with expression of lba gene in chloroplast of Chlamydomonas reinhardtii. Int. J. Hydrog. Energy 2010, 35, 13419. [Google Scholar] [CrossRef]
- Noone, S.; Ratcliff, K.; Davis, R.; Subramanian, V.; Meuser, J.; Posewitz, M.C.; Ghirardi, M.L. Expression of a clostridial [FeFe]-hydrogenase in Chlamydomonas reinhardtii prolongs photo-production of hydrogen from water splitting. Algal. Res. 2017, 22, 116–121. [Google Scholar] [CrossRef] [Green Version]
- Kanygin, A.; Milrad, Y.; Thummala, C.; Reischneider, K.; Baker, P.; Pini, M.; Jacoby, I.; Redding, K.E. Rewriting photosynthesis, I-hydrogenase chimera that makes H2 in vivo. Energy Environ. Sci. 2020, 13, 2903. [Google Scholar] [CrossRef]
- Touloupakis, E.; Silva Benavides, A.M.; Cicchi, N.; Torzillo, G. Growth and hydrogen production of outdoor cultures of Synechocystis PCC 6803. Algal. Res. 2016, 16, 78–85. [Google Scholar] [CrossRef]
- Kosourov, S.; Murukesan, G.; Seibert, M.; Allahverdiyeva, Y. Evaluation of light energy to H2 energy conversion efficiency in thin films of cyanobacteria and green alga under photoautotrophic conditions. Algal. Res. 2017, 28, 253–263. [Google Scholar] [CrossRef]
- Paramesh, K.; Chandrasekhar, T. Improvement of photobiological hydrogen production in Chlorococcum minutum using various oxygen scavengers. Int. J. Hydrog. Energy 2020, 45, 7641–7646. [Google Scholar] [CrossRef]
- Su, D.; Qi, J.; Liu, X.; Wang, L.; Zhang, H.; Xie, H.; Huang, X. Enzyme-modulated anaerobic encapsulation of Chlorella cells allows switching from O2 to H2 production. Angew. Chem. Int. Ed. Engl. 2019, 58, 3992–3995. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Reyes, L.A.; Sánchez-Saavedra, M.P.; Valdez-Vazquez, I. Improvement of hydrogen production by reduction of the photosynthetic oxygen in microalgae cultures of Chlamydomonas gloeopara and Scenedesmus obliquus. Int. J. Hydrog. Energy 2015, 40, 7291–7300. [Google Scholar] [CrossRef]
- Chen, J.; Li, J.; Li, Q.; Wang, S.; Wang, L.; Liu, H.; Fan, C. Engineering a chemoenzymatic cascade for sustainable photobiological hydrogen production with green algae. Energy Environ. Sci. 2020, 13, 2064. [Google Scholar] [CrossRef]
- Scognamiglio, V.; Giardi, M.T.; Zappi, D.; Touloupakis, E.; Antonacci, A. Photoautotrophs–bacteria co-cultures: Advances, challenges and applications. Materials 2021, 14, 3027. [Google Scholar] [CrossRef] [PubMed]
- Ban, S.; Lin, W.; Wu, F.; Luo, J. Algal-bacterial cooperation improves algal photolysis-mediated hydrogen production. Bioresour. Technol. 2018, 251, 350–357. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Xi, L.; Sun, X.; Ge, B.; Liu, D.; Han, Z.; Pu, X.; Huang, F. Enhanced hydrogen production through co-cultivation of Chlamydomonas reinhardtii CC-503 and a facultative autotrophic sulfide-oxidizing bacterium under sulfurated conditions. Int. J. Hydrog. Energy 2018, 43, 15005–15013. [Google Scholar] [CrossRef]
- Fakhimi, N.; Tavakoli, O.; Marashi, S.-A.; Moghimi, H.; Mehrnia, M.R.; Dubini, A.; González-Ballester, D. Acetic acid uptake rate controls H2 production in Chlamydomonas-bacteria co-cultures. Algal. Res. 2019, 42, 101605. [Google Scholar] [CrossRef]
- Fakhimi, N.; Gonzalez-Ballester, D.; Fernández, E.; Galván, A.; Dubini, A. Algae-bacteria consortia as a strategy to enhance H2 production. Cells 2020, 9, 1353. [Google Scholar] [CrossRef]
- Hom, E.; Aiyar, P.; Schaeme, D.; Mittag, M.; Sasso, S. A chemical perspective on microalgal-microbial interactions. Trends Plant Sci. 2015, 20, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, J.L.; Nores, I.G.; Cuaresma, M.; Montero, Z.; Del Valle, M.G.; Vílchez, C. Impact of microalgae-bacteria interactions on the production of algal biomass and associated compounds. Mar. Drugs 2016, 14, 100. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Li, D.; Wang, Q.; Wu, S. Improved hydrogen production and biomass through the co-cultivation of Chlamydomonas reinhardtii and Bradyrhizobium japonicum. Int. J. Hydrog. Energy 2016, 41, 9276–9283. [Google Scholar] [CrossRef] [Green Version]
- Khosravitabar, F. Microalgal biohydrogen photoproduction: Scaling up challenges and the ways forward. J. Appl. Phycol. 2020, 32, 277–289. [Google Scholar] [CrossRef]
- Kosourov, S.N.; He, M.; Allahverdiyeva, Y.; Seibert, M. Immobilization of microalgae as a tool for efficient light utilization in H2 production and other biotechnology applications. In Microalgal Hydrogen Production; Royal Society of Chemistry: London, UK, 2018; pp. 355–384. [Google Scholar]
- Touloupakis, E.; Rontogiannis, G.; Silva Benavides, A.M.; Cicchi, B.; Ghanotakis, D.F.; Torzillo, G. Hydrogen production by immobilized Synechocystis sp. PCC 6803. Int. J. Hydrog. Energy 2016, 41, 15181–15186. [Google Scholar] [CrossRef]
- Tsygankov, A.; Kosourov, S. Microbial BioEnergy: Hydrogen production, advances in photosynthesis and respiration. In Immobilization of Photosynthetic Microorganisms for Efficient Hydrogen Production; Zannoni, D., De Philippis, R., Eds.; Springer Science & Business Media: Dordrecht, The Netherlands, 2014; pp. 321–347. [Google Scholar]
- Laurinavichene, T.V.; Fedorov, A.S.; Ghirardi, M.L.; Seibert, M.; Tsygankov, A.A. Demonstration of sustained hydrogen photoproduction by immobilized, sulfur-deprived Chlamydomonas reinhardtii cells. Int. J. Hydrog. Energy 2006, 31, 659–667. [Google Scholar] [CrossRef]
- Kosourov, S.N.; Seibert, M. Hydrogen photoproduction by nutrient-deprived Chlamydomonas reinhardtii cells immobilized within thin alginate films under aerobic and anaerobic conditions. Biotechnol. Bioeng. 2009, 102, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Maswanna, T.; Lindblad, P.; Maneeruttanarungroj, C. Improved biohydrogen production by immobilized cells of the green alga Tetraspora sp. CU2551 incubated under aerobic condition. J. Appl. Phycol. 2020, 32, 2937–2945. [Google Scholar] [CrossRef]
- Melis, A.; Zhang, L.; Forestier, M.; Ghirardi, M.L.; Seibert, M. Sustained photobiological hydrogen gas production upon reversible inactivation of oxygen evolution in the green alga Chlamydomonas reinhardtii. Plant Physiol. 2000, 122, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.-Z.; Ge, Y.-M.; Xia, S.-Y.; Sun, J.-Y.; Mu, J. Photoautotrophic hydrogen production by Chlorella pyrenoidosa without sulfur deprivation. Int. J. Hydrog. Energy 2016, 41, 8427–8432. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Wang, Y.; Chen, M.; Zhuang, X.; Wang, C.; Wang, J.; Hu, Z. Improved photobio-H2 production regulated by artificial miRNA targeting psbA in green microalga Chlamydomonas reinhardtii. Biotechnol. Biofuels 2018, 11, 36. [Google Scholar] [CrossRef] [Green Version]
- Ben-Zvi, O.; Dafni, E.; Feldman, Y.; Yacoby, I. Re-routing photosynthetic energy for continuous hydrogen production in vivo. Biotechnol. Biofuels 2019, 12, 266. [Google Scholar] [CrossRef]
- Hwang, J.H.; Kim, H.C.; Choi, J.A.; Abou-Shanab, R.A.; Dempsey, B.A.; Regan, J.M.; Kim, J.R.; Song, H.; Nam, I.H.; Kim, S.N.; et al. Photoautotrophic hydrogen production by eukaryotic microalgae under aerobic conditions. Nat. Commun. 2014, 5, 3234. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.-H.; Lee, M.; Kang, E.H.; Lee, W.H. Renewable algal photo H2 production without S control using acetate enriched fermenter effluents. Int. J. Hydrog. Energy 2021, 46, 1740–1751. [Google Scholar] [CrossRef]
- Sirawattanamongkol, T.; Maswanna, T.; Maneeruttanarungroj, C. A newly isolated green alga Chlorella sp. KLSc59: Potential for biohydrogen production. J. Appl. Phycol. 2020, 32, 2927–2936. [Google Scholar] [CrossRef]
- Duangjan, K.; Nakkhuntho, W.; Pekkoh, J.; Pumas, C. Comparision of hydrogen production in microalgae under autotrophic mixotrophic media. Bot. Lith. 2017, 23, 169–177. [Google Scholar]
- Zhang, L.; He, M.; Liu, J.; Li, L. Role of the mitochondrial alternative oxidase pathway in hydrogen photoproduction in Chlorella protothecoides. Planta 2015, 241, 1005–1014. [Google Scholar] [CrossRef]
- Shetty, P.; Boboescu, I.Z.; Pap, B.; Wirth, R.; Kovacs, K.L.; Bíro, T.; Futo, Z.; White, R.A.; Maroti, G. Exploitation of algal-bacterial consortia in combined biohydrogen generation and wastewater treatment. Front. Energy Res. 2019, 7. [Google Scholar] [CrossRef]
- Chader, S.; Hacene, H.; Agathos, S.N. Study of hydrogen production by three strains of Chlorella isolated from the soil in the Algerian Sahara. Int. J. Hydrog. Energy 2009, 34, 4941–4946. [Google Scholar] [CrossRef]
- Giannelli, L.; Torzillo, G. Hydrogen production with the microalga Chlamydomonas reinhardtii grown in a compact tubular photobioreactor immersed in a scattering light nanoparticle suspension. Int. J. Hydrog. Energy 2012, 37, 16951–16961. [Google Scholar] [CrossRef]
- Faraloni, C.; Ena, A.; Pintucci, C.; Torzillo, G. Enhanced hydrogen production by means of sulfur-deprived Chlamydomonas reinhardtii cultures grown in pretreated olive mill wastewater. Int. J. Hydrog. Energy 2011, 36, 5920–5931. [Google Scholar] [CrossRef]
- Song, W.; Rashid, N.; Choi, W.; Lee, K. Biohydrogen production by immobilized Chlorella sp. using cycles of oxygenic photosynthesis and anaerobiosis. Bioresour. Technol. 2011, 102, 8676–8681. [Google Scholar] [CrossRef]
- Zhu, X.-G.; Long, S.P.; Ort, D.R. What is the maximum efficiency with which photosynthesis can convert solar energy into biomass? Curr. Opin. Biotechnol. 2008, 19, 153–159. [Google Scholar] [CrossRef]
- Melis, A. Solar energy conversion efficiencies in photosynthesis: Minimizing the chlorophyll antennae to maximize efficiency. Plant Sci. 2009, 177, 272–280. [Google Scholar] [CrossRef]
- Finazzi, G.; Barbagallo, R.P.; Bergo, E.; Barbato, R.; Forti, G. Photoinhibition of Chlamydomonas reinhardtii in State 1 and State 2: Damages to the photosynthetic apparatus under linear and cyclic electron flow. J. Biol. Chem. 2001, 276, 22251. [Google Scholar] [CrossRef] [Green Version]
- Antal, T.K.; Krendeleva, T.E.; Laurinavichene, T.V.; Makarova, W.; Ghirardi, M.L.; Rubin, A.B.; Tsygankov, A.A.; Seibert, M. The dependence of algal H2 production on photosystem II and O2 consumption activities in sulphur-deprived Chlamydomonas reinhardtii cells. Biochem. Biophys. Acta 2003, 1607, 153–160. [Google Scholar]
- Burlacot, A.; Sawyer, A.; Cuiné, S.; Auroy-Tarrago, P.; Blangy, S.; Happe, T.; Peltiera, G. Flavodiiron-mediated O2 photoreduction links H2 production with CO2 fixation during the anaerobic induction of photosynthesis. Plant Physiol. 2018, 177, 1639–1649. [Google Scholar] [CrossRef] [Green Version]
- Antal, T.K.; Kukarskikh, G.P.; Volgusheva, A.A.; Krendeleva, T.E.; Tyystjärvi, E.; Rubin, A.B. Hydrogen photoproduction by immobilized S-deprived Chlamydomonas reinhardtii: Effect of light intensity and spectrum, and initial medium pH. Algal. Res. 2016, 17, 38–45. [Google Scholar] [CrossRef]
- Strasser, R.; Srivastava, A.; Govindjee. Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria. Photochem. Photobiol. 1995, 61, 32–42. [Google Scholar] [CrossRef]
- Faraloni, C.; Torzillo, G. Xanthophyll cycle induction by anaerobic conditions under low light in Chlamydomonas reinhardtii. J. Appl. Phycol. 2013, 25, 1457–1471. [Google Scholar] [CrossRef]
- Pongpadung, P.; Zhang, L.; Sathasivam, R.; Yokthongwattana, K.; Juntawon, N.; Liu, J. Stimulation of hydrogen photoproduction in Chlorella sorokiniana subjected to simultaneous nitrogen limitation and sulfur- and/or phosphorus-deprivation. J. Pure Appl. Microbiol. 2018, 12, 1719–1727. [Google Scholar] [CrossRef]
- Torzillo, G.; Chini Zittelli, G. Tubular photobioreactors. Products and biorefinery design. In Algal Biorefineries; Prokop, A., Bajpai, R.K., Zappi, M.E., Eds.; Springer International Publishing: Cham, Switzerland, 2015; Volume 2. [Google Scholar]
- Scoma, A.; Giannelli, L.; Faraloni, C.; Torzillo, G. Outdoor H2 production in a 50-L tubular photobioreactor by means of a sulfur-deprived culture of the microalga Chlamydomonas reinhardtii. J. Biotechnol. 2012, 157, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Lindblad, P.; Fuente, D.; Borbe, F.; Cicchi, B.; Conejero, J.A.; Couto, N.; Čelešnik, H.; Diano, M.; Dolinar, M.; Esposito, S.; et al. CyanoFactory, a European consortium to develop technologies needed to advance cyanobacteria as chassis for production of chemicals and fuels. Algal. Res. 2019, 41, 101510. [Google Scholar] [CrossRef]
- Siebert, M.; Torzillo, G. Microalgal Hydrogen Production: Achievements and Perspectives; The Royal Society of Chemistry: Cambridge, UK, 2018. [Google Scholar]
- James, B.D.; Baum, G.N.; Perez, J.; Baum, K.N. Technoeconomic Analysis of Photoelectrochemical (PEC) Hydrogen Production; (US DOE Contract no. GS-10F-009J); Directed Technologies Inc.: Arlington, VA, USA, 2009. [Google Scholar]
- Show, K.-Y.; Yan, Y.; Zong, C.; Guo, N.; Chang, J.-S.; Lee, D.-J. State of the art and challenges of biohydrogen from microalgae. Bioresour. Technol. 2019, 289, 121747. [Google Scholar] [CrossRef]
- Silva Benavides, A.M.; Campos Rudin, M.; Villalobos, N.; Touloupakis, E.; Torzillo, G. Growth and hydrogen production by three Chlamydomonas strains cultivated in a commercial fertilizer. Int. J. Hydrog. Energy 2019, 44, 9849–9855. [Google Scholar] [CrossRef]
- Frowijn, L.S.F.; van Sark, W.G.J.H.M. Analysis of photon-driven solar-to-hydrogen production methods in the Netherlands, Sustain. Energy Technol. Assess. 2021, 48, 101631. [Google Scholar]
- Perrine, Z.; Negi, S.; Sayre, R. Optimization of photosynthetic light energy utilization by microalgae. Algal. Res. 2012, 1, 134–142. [Google Scholar] [CrossRef] [Green Version]
- Cazzaniga, S.; Dall’Osto, L.; Szaub, J.; Ballottari, M.; Purton, S.; Bassi, R. Domestication of the green alga Chlorella sorokiniana: Reduction of antenna size improves light-use efficiency in a photobioreactor. Biotechnol. Biofuels 2014, 7, 157–170. [Google Scholar] [CrossRef] [Green Version]
- Shin, W.-S.; Lee, B.; Jeong, B.-R.; Chang, Y.K. Truncated light-harvesting chlorophyll antenna size in Chlorella vulgaris improves biomass productivity. J. Appl. Phycol. 2016, 28, 3193–3202. [Google Scholar] [CrossRef]
- Hu, G.-R.; Fan, Y.; Zhen, Y.-L.; Xu, F.; Zhang, L.; Li, F.-L. Photoprotection capacity of microalgae improved by regulating the antenna size of high-harvesting complexes. J. Appl. Phycol. 2020, 32, 1027–1039. [Google Scholar] [CrossRef]





| Microalgal Species | Growth Mode | H2 Production | References |
|---|---|---|---|
| C. reinhardtii | TAP | 25 μmol/mgChl/h | [29] |
| Chlorella sp. AARL G014 | TAP-S | 0.49 mmol/mgChl/h | [75] |
| C. reinhardtii CC-503 | TAP co-culture | 255 mmol/mgChl | [55] |
| Chlorella vulgaris strains YSL01 | BBM-EDTANa2 | 1.9 mL/L | [72] |
| Chlorella lewinii KU201 | TAP-S | 13.03 mL/L | [18] |
| Chlorella sp. IOAC707S | TAP-NaCl | 38.00 mL/L | [38] |
| Chlorella sorokiniana KU204 | TAP-P | 69.00 mL/L | [18] |
| Chlorella protothecoides | TAP-NS | 82.50 mL/L | [76] |
| Chlorella sorokiniana KU204 | TAP-S | 89.64 mL/L | [18] |
| Chlorella pyrenoidosa | TCP + DCMU | 93.86 mL/L | [69] |
| C. reinhardtii Stm6 | TAP-S | 540 mL/L | [41] |
| C. reinhardtii C3 | TAP | 3.0 mL/L/d | [31] |
| C. reinhardtii (HS-14) | TAP | 20 mL/L/d | [71] |
| Immobilized Chlorella vulgaris | Artificial wastewater-S | 39.1 mL/L/d | [11] |
| Chlorella vulgaris MACC360 | TAP co-culture | 56.0 mL/L/d | [77] |
| Immobilized Scenedesmus obliquus | Artificial wastewater-S | 204.8 mL/L/d | [11] |
| Chlorella salina Mt | TAP-S | 0.5 mL/L/h | [78] |
| C. reinhardtii CC124 | TAP-S | 0.6 mL/L/h | [79] |
| C. reinhardtii CC-124 | TAP-S | 3.3 mL/L/h | [80] |
| C. reinhardtii pgr5/pgrl1 | TAP-S | 7.0 mL/L/h | [33] |
| C. reinhardtii L159I-N230Y | TAP-S | 11.1 mL/L/h | [37] |
| Chlorella vulgaris BEIJ (G-120) | HM + glucose | 5.0 mL/L/h. | [8] |
| Immobilized Chlorella vulgaris NIER-10003 | MA-S + glucose | 238 mL/L/h | [81] |
| Chlorella sorokiniana | 150 of acetate/Cl− ratio | 0.33 mmol/L/min | [73] |
| C. reinhardtii | 150 of acetate/Cl− ratio | 0.38 mmol/L/min | [73] |
| C. reinhardtii (ΨH1) | TAP | 3.6 mL/L/h | [46] |
| C. reinhardtti | HSM + O2 absorbent | 2.58 mL/L/h | [30] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Touloupakis, E.; Faraloni, C.; Silva Benavides, A.M.; Torzillo, G. Recent Achievements in Microalgal Photobiological Hydrogen Production. Energies 2021, 14, 7170. https://doi.org/10.3390/en14217170
Touloupakis E, Faraloni C, Silva Benavides AM, Torzillo G. Recent Achievements in Microalgal Photobiological Hydrogen Production. Energies. 2021; 14(21):7170. https://doi.org/10.3390/en14217170
Chicago/Turabian StyleTouloupakis, Eleftherios, Cecilia Faraloni, Ana Margarita Silva Benavides, and Giuseppe Torzillo. 2021. "Recent Achievements in Microalgal Photobiological Hydrogen Production" Energies 14, no. 21: 7170. https://doi.org/10.3390/en14217170
APA StyleTouloupakis, E., Faraloni, C., Silva Benavides, A. M., & Torzillo, G. (2021). Recent Achievements in Microalgal Photobiological Hydrogen Production. Energies, 14(21), 7170. https://doi.org/10.3390/en14217170








