Valorization of Distillery Stillage for Bioenergy Production: A Review
Abstract
:1. Introduction
2. Generation of Distillery Stillage
| Parameters | Non-Process Waste | Process Waste | References | |||||
|---|---|---|---|---|---|---|---|---|
| Fermenter Cooling | Fermenter Cleaning | Condenser Cooling | Fermenter Wash | BottlingPlant | Fermentation Sludge | Thin Stillage | ||
| Color | Colorless | Colorless | Colorless | Colorless | Colorless | Dark brown | Dark brown | [18,19] |
| pH | 6.25 | 5.0–5.5 | 6.8–7.8 | 6 | 7.45 | 4.44 | 4.56 | [4,19] |
| Total solids (mg/L) | 1000–1300 | 1000–1500 | 700–900 | 550 | 400 | 5500 | 34,000 | [4,18] |
| Suspended solids (mg/L) | 220 | 400–600 | 180–200 | 300 | 100 | 4300 | 33,100 | [5,19] |
| COD (mg/L) | 500–1000 | 1200–1600 | 1200–1600 | 25 | 15 | 60,000–67,000 | 80,000–100,000 | [4,18] |
| BOD (mg/L) | 100–110 | 500–600 | 70–80 | 15 | 5 | 35,000–40,000 | 50,000–600,000 | [4,18] |
| VFA (mg/L) | 90–100 | 250–330 | 35–50 | - | - | 500–800 | 250–280 | [5,19] |
| Alkalinity (meq/L) | 300 | - | - | 40 | 80 | 6000 | 9860 | [18] |
| TP (mg/L) | 10–15 | 15-30 | 20 | - | - | 2500 | 2700 | [4] |
| PO4 3− (mg/L) | - | - | - | - | - | 1000 | 1000 | [5,18] |
| TN (mg/L) | 20–30 | 25–40 | 10–30 | - | - | 5000 | 7000 | [4] |
| NH4+ (mg/L) | - | - | - | - | - | 1100 | 2800 | [19] |
3. Processing of Distillery Stillage—Biomethane Production
3.1. Bioreactors and Operational Parameters
3.2. Effect of Polyphenols and Melanoidin on Biomethane Production
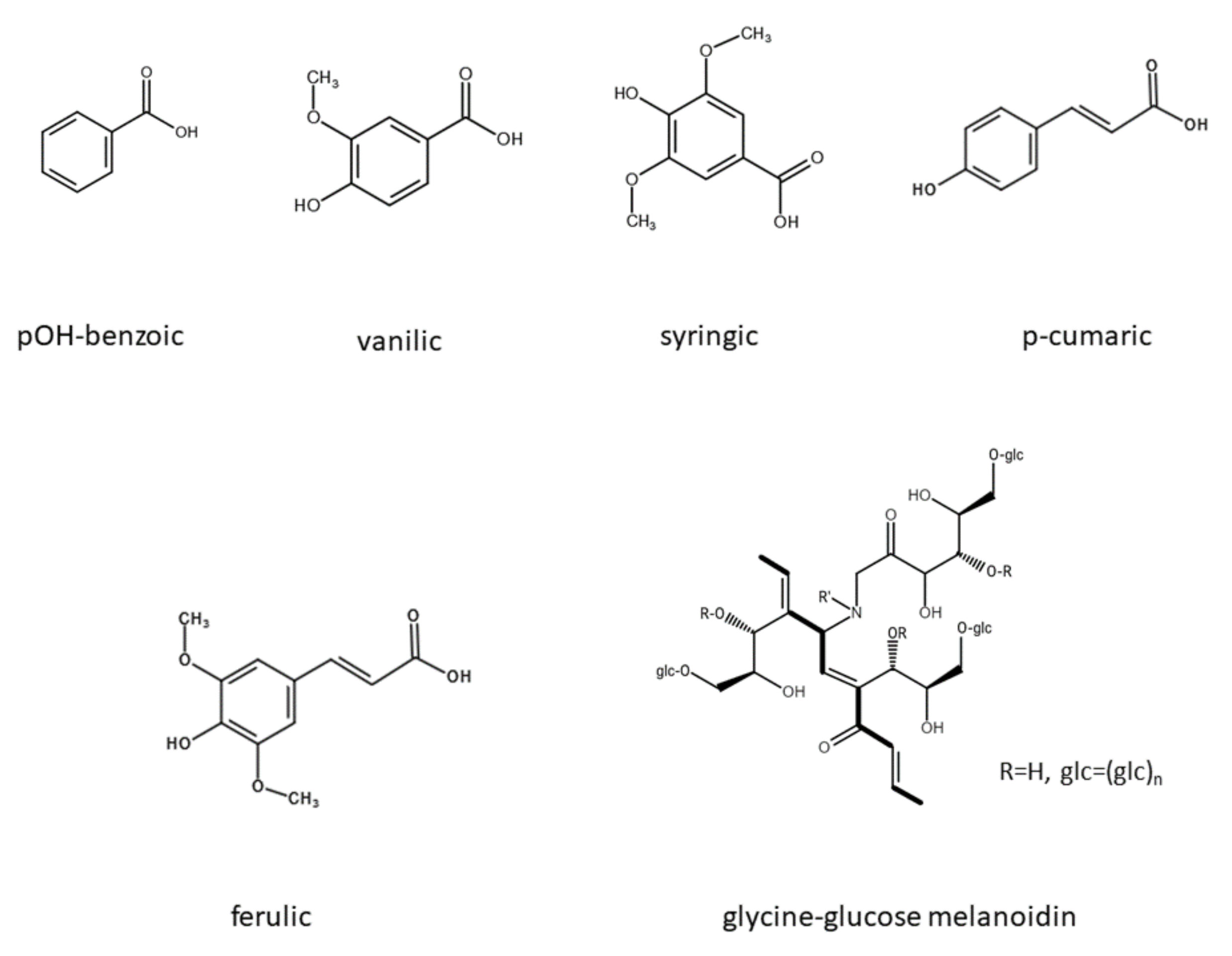
3.3. Pretreatment of Distillery Stillage
3.4. Post-Treatment of Distillery Stillage
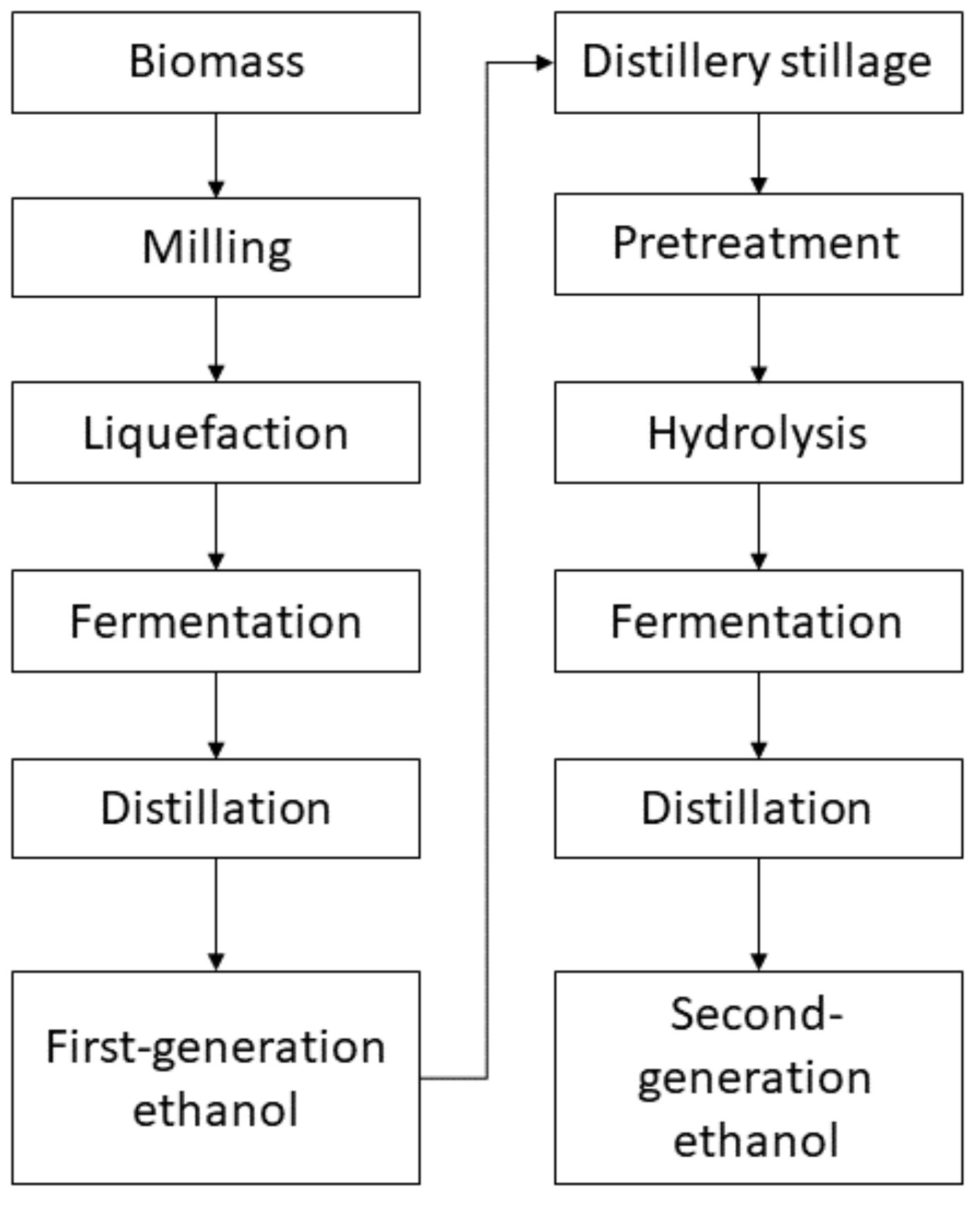
4. Processing of Distillery Stillage—Bioethanol Production
5. Processing of Distillery Stillage—Biohydrogen Production
| Organics in the Substrate (g COD/L) | Hydrogen Yield | References |
|---|---|---|
| 40.0 | 17.6 L/L of distillery waste | [85] |
| 38.0 | 9.17 mol/kg CODreduced | [86] |
| 52.0 | 12.2 mol/kg CODremoved | [87] |
| 40.0 | 172 mL/g CODremoved | [88] |
| 30.6 | 8.24 mL/g COD | [89] |
| 125.0 | 44.28 mL/g COD | [90] |
| 60.0 | 464 mL/g carbohydrate | [91] |
| 16.3 | 0.47 mol/mol carbohydrate | [92] |
6. Bioelectrochemical-Based Systems
| COD Removal (%) | Power Density (mW/m2) | References |
|---|---|---|
| 63.5 | 202.00 | [95] |
| 67.5 | 429.00 | [97] |
| 58.0 | 364.00 | [98] |
| 43.0 | 597.00 | [99] |
| 64.4 | 267.77 | [100] |
| 69.0–98.0 | 36.80–72.90 | [101] |
| 62.5 | 437.13 | [102] |
| 58.4–88.4 | 124.03 | [103] |
| 66.0–78.0 | 836.81 | [53] |
| 64.0 | 18.35 | [70] |
7. Conclusions and Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Tolmasquim, M.T. Bioenergy for the future. In Proceedings of the Conference on Biofuels: “An Option for a Less Carbon-Intensive Economy”, Rio de Janeiro, Brazil, 4–5 December 2007. [Google Scholar]
- Krzywonos, M.; Cibis, E.; Miskiewicz, T.; Ryznar-Luty, A. Utilization and biodegradation of starch stillage (distillery wastewater). Electron. J. Biotechnol. 2009, 12, 6–7. [Google Scholar] [CrossRef] [Green Version]
- Krzywonos, M. Forecast for transport biofuels in Poland in 2020–2030. Przemysł Chem. 2015, 1, 168–172. [Google Scholar] [CrossRef]
- Fito, J.; Tefera, N.; van Hulle, S.W.H. Sugarcane biorefineries wastewater: Bioremediation technologies for environmental sustainability. Chem. Biol. Technol. Agric. 2019, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Satyawali, Y.; Balakrishnan, M. Treatment of distillery effluent in a membrane bioreactor (MBR) equipped with mesh filter. Sep. Purif. Technol. 2008, 63, 278–286. [Google Scholar] [CrossRef]
- Reis, C.E.R.; Rajendran, A.; Hu, B. New technologies in value addition to the thin stillage from corn-to-ethanol process. Rev. Environ. Sci. Biotechnol. 2017, 16, 175–206. [Google Scholar] [CrossRef]
- Sankaran, K.; Premalatha, M.; Vijayasekaran, M.; Somasundaram, V.T. Distillery wastewater treatment through anaerobic digestion and phycoremediation—A green industrial approach. Renew. Sustain. Energy Rev. 2014, 37, 634–643. [Google Scholar] [CrossRef]
- McAloon, A.; Taylor, F.; Yee, W.; Ibsen, K.; Wooley, R. Determining the Cost of Producing Ethanol from Corn Starch and Lignocellulosic Feedstocks; National Renewable Energy Lab.: Golden, CO, USA, 2000. [Google Scholar]
- Kwiatkowski, J.R.; McAloon, A.J.; Taylor, F.; Johnston, D.B. Modeling the process and costs of fuel ethanol production by the corn dry-grind process. Ind. Crop. Prod. 2006, 23, 288–296. [Google Scholar] [CrossRef]
- Ganesan, V.; Rosentrater, K.A.; Muthukumarappan, K. Methodology to determine soluble content in dry grind ethanol coproduct streams. Appl. Eng. Agric. 2006, 22, 899–903. [Google Scholar] [CrossRef]
- M’Arimi, M.M.; Zhang, Y.; Götz, G.; Kiriamiti, K.H.; Geißen, S.-U. Antimicrobial colorants in molasses distillery wastewater and their removal technologies. Int. Biodeterior. Biodegrad. 2014, 87, 34–43. [Google Scholar] [CrossRef]
- Kaushik, A.; Basu, S.; Batra, V.; Balakrishnan, M. Fractionation of sugarcane molasses distillery wastewater and evaluation of antioxidant and antimicrobial characteristics. Ind. Crop. Prod. 2018, 118, 73–80. [Google Scholar] [CrossRef]
- Hayase, F. Recent development of 3-deoxyosone related Maillard reaction products. Food Sci. Technol. Res. 2000, 6, 79–86. [Google Scholar] [CrossRef]
- Amit; Ghosh, U.K. An approach for phycoremediation of different wastewaters and biodiesel production using microalgae. Environ. Sci. Pollut. Res. 2018, 25, 18673–18681. [Google Scholar] [CrossRef] [PubMed]
- Rufián-Henares, J.A.; Morales, F.J. Functional properties of melanoidins: In vitro antioxidant, antimicrobial and antihypertensive activities. Food Res. Int. 2007, 40, 995–1002. [Google Scholar] [CrossRef]
- Stauder, M.; Papetti, A.; Mascherpa, D.; Schito, A.M.; Gazzani, G.; Pruzzo, C.; Daglia, M. Antiadhesion and antibiofilm activities of high molecular weight coffee components against Streptococcus mutans. J. Agric. Food Chem. 2010, 58, 11662–11666. [Google Scholar] [CrossRef] [PubMed]
- Soto, M.L.; Moure, A.; Domínguez, H.; Parajó, J.C. Recovery, concentration and purification of phenolic compounds by adsorption: A review. J. Food Eng. 2011, 105, 1–27. [Google Scholar] [CrossRef]
- Kharayat, Y. Distillery wastewater: Bioremediation approaches. J. Integr. Environ. Sci. 2012, 9, 69–91. [Google Scholar] [CrossRef]
- Fortney, N.W.; Hanson, N.J.; Rosa, P.R.F.; Donohue, T.J.; Noguera, D.R. Diverse profile of fermentation by products from thin stillage. Front. Bioeng. Biotechnol. 2021, 9, 550. [Google Scholar] [CrossRef] [PubMed]
- Wilkie, A.C.; Riedesel, K.J.; Owens, J.M. Stillage characterization and anaerobic treatment of ethanol stillage from conventional and cellulosic feedstocks. Biomass Bioenergy 2000, 19, 63–102. [Google Scholar] [CrossRef]
- Mikulski, D.; Kłosowski, G.; Menka, A.; Koim-Puchowska, B. Microwave-assisted pretreatment of maize distillery stillage with the use of dilute sulfuric acid in the production of cellulosic ethanol. Bioresour. Technol. 2019, 278, 318–328. [Google Scholar] [CrossRef]
- Tian, Z.; Mohan, G.R.; Ingram, L.; Pullammanappallil, P. Anaerobic digestion for treatment of stillage from cellulosic bioethanol production. Bioresour. Technol. 2013, 144, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, R.; Massé, D.I.; Singh, G. A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresour. Technol. 2013, 143, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Schnürer, A.; Nordberg, Å. Ammonia, a selective agent for methane production by syntrophic acetate oxidation at mesophilic temperature. Water Sci. Technol. 2008, 57, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Procházka, J.; Dolejš, P.; Máca, J.; Dohányos, M. Stability and inhibition of anaerobic processes caused by insufficiency or excess of ammonia nitrogen. Appl. Microbiol. Biotechnol. 2012, 93, 439–447. [Google Scholar] [CrossRef]
- Moestedt, J.; Nordell, E.; Schnürer, A. Comparison of operating strategies for increased biogas production from thin stillage. J. Biotechnol. 2014, 175, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Hu, C.; Zhang, D.; Dai, L.; Duan, N. Impact of a high ammonia-ammonium-pH system on methane-producing archaea and sulfate-reducing bacteria in mesophilic anaerobic digestion. Bioresour. Technol. 2017, 245, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Huertas, J.K.; Quipuzco, L.; Hassanein, A.; Lansing, S. Comparing hydrogen sulfide removal efficiency in a field-scale digester using microaeration and iron filters. Energies 2020, 13, 4793. [Google Scholar] [CrossRef]
- Erdirencelebi, D.; Kucukhemek, M. Control of hydrogen sulphide in full-scale anaerobic digesters using iron (III) chloride: Performance, origin and effects. Water SA 2018, 44, 176–183. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Herrera, J.; Martínez, A.I.; Sentandreu, R. Determination of the stability of protein pools from the cell wall of fungi. Res. Microbiol. 2002, 153, 373–378. [Google Scholar] [CrossRef]
- Lee, P.H.H.; Bae, J.; Kim, J.; Chen, W.-H. Mesophilic anaerobic digestion of corn thin stillage: A technical and energetic assessment of the corn-to-ethanol industry integrated with anaerobic digestion. J. Chem. Technol. Biotechnol. 2011, 86, 1514–1520. [Google Scholar] [CrossRef]
- Schaefer, S.H.; Sung, S. Retooling the ethanol industry: Thermophilic anaerobic digestion of thin stillage for methane production and pollution prevention. Water Environ. Res. 2008, 80, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Oosterkamp, M.J.; Bauer, S.; Ibáñez, A.B.; Méndez-García, C.; Hong, P.; Cann, I.; Mackie, R.I. Identification of methanogenesis and syntrophy as important microbial metabolic processes for optimal thermophilic anaerobic digestion of energy cane thin stillage. Bioresour. Technol. Rep. 2019, 7, 100254. [Google Scholar] [CrossRef]
- Andalib, M.; Hafez, H.; Elbeshbishy, E.; Nakhla, G.; Zhu, J. Treatment of thin stillage in a high-rate anaerobic fluidized bed bioreactor (AFBR). Bioresour. Technol. 2012, 121, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Sayedin, F.; Kermanshahi-Pour, A.; He, S. Anaerobic digestion of thin stillage of corn ethanol plant in a novel anaerobic baffled reactor. Waste Manag. 2018, 78, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, A.; Kennedy, K.J. Anaerobic digestion of corn ethanol thin stillage in batch and by high-rate down-flow fixed film reactors. Water Sci. Technol. 2012, 66, 1834–1841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dereli, R.K.; van der Zee, F.P.; Heffernan, B.; Grelot, A.; van Lier, J.B. Effect of sludge retention time on the biological performance of anaerobic membrane bioreactors treating corn-to-ethanol thin stillage with high lipid content. Water Res. 2014, 49, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Melamane, X.L.; Tandlich, R.; Burgess, J.E. Treatment of wine distillery wastewater by high rate anaerobic digestion. Water Sci. Technol. 2007, 56, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Wolmarans, B.; de Villiers, G.H. Start-up of a UASB effluent treatment plant on distillery wastewater. Water SA 2002, 28, 63. [Google Scholar] [CrossRef] [Green Version]
- Blonskaja, V.; Menert, A.; Vilu, R. Use of two-stage anaerobic treatment for distillery waste. Adv. Environ. Res. 2003, 7, 671–678. [Google Scholar] [CrossRef]
- Cabrera-Díaz, A.; Reyes, I.P.; Merencio, D.O.; Lebrero, R.; Zaiat, M. Anaerobic digestion of sugarcane vinasse through a methanogenic UASB reactor followed by a packed bed reactor. Appl. Biochem. Biotechnol. 2017, 183, 1127–1145. [Google Scholar] [CrossRef]
- Wu, B.; Lin, R.; Kang, X.; Deng, C.; Xia, A.; Dobson, A.D.W.; Murphy, J.D. Graphene addition to digestion of thin stillage can alleviate acidic shock and improve biomethane production. ACS Sustain. Chem. Eng. 2020, 8, 13248–13260. [Google Scholar] [CrossRef]
- Pereira, M.C.; Oliveira, D.A.; Hill, L.E.; Zambiazi, R.C.; Borges, C.D.; Vizzotto, M.; Mertens-Talcott, S.; Talcott, S.; Gomes, C.L. Effect of nanoencapsulation using PLGA on antioxidant and antimicrobial activities of guabiroba fruit phenolic extract. Food Chem. 2018, 240, 396–404. [Google Scholar] [CrossRef]
- Elalami, D.; Carrere, H.; Abdelouahdi, K.; Garcia-Bernet, D.; Peydecastaing, J.; Vaca-Medina, G.; Oukarroum, A.; Zeroual, Y.; Barakat, A. Mild microwaves, ultrasonic and alkaline pretreatments for improving methane production: Impact on biochemical and structural properties of olive pomace. Bioresour. Technol. 2020, 299, 122591. [Google Scholar] [CrossRef]
- Fedorak, P.M.; Hrudey, S.E. The effects of phenol and some alkyl phenolics on batch anaerobic methanogenesis. Water Res. 1984, 18, 361–367. [Google Scholar] [CrossRef]
- Chapleur, O.; Mazeas, L.; Godon, J.-J.; Bouchez, T. Asymmetrical response of anaerobic digestion microbiota to temperature changes. Appl. Microbiol. Biotechnol. 2015, 100, 1445–1457. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, F.; Cabrol, L.; Carballa, M.; Donoso-Bravo, A.; Cruz, L.; Ruiz-Filippi, G.; Chamy, R.; Lema, J. Relationship between phenol degradation efficiency and microbial community structure in an anaerobic SBR. Water Res. 2013, 47, 6739–6749. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.; Edyvean, R. Inhibition of biogas production and biodegradability by substituted phenolic compounds in anaerobic sludge. J. Hazard. Mater. 2008, 160, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Chandra, R. Biodegradation of organic compounds of molasses melanoidin (MM) from biomethanated distillery spent wash (BMDS) during the decolourisation by a potential bacterial consortium. Biodegradation 2012, 23, 609–620. [Google Scholar] [CrossRef]
- Peña, M.; Coca, M.; González, G.; Rioja, R.; García, M. Chemical oxidation of wastewater from molasses fermentation with ozone. Chemosphere 2003, 51, 893–900. [Google Scholar] [CrossRef]
- Cämmerer, B.; Jalyschkov, V.; Kroh, L.W. Carbohydrate structures as part of the melanoidin skeleton. Int. Congr. Ser. 2002, 1245, 269–273. [Google Scholar] [CrossRef]
- Kaushik, A.; Basu, S.; Raturi, S.; Batra, V.; Balakrishnan, M. Recovery of antioxidants from sugarcane molasses distillery wastewater and its effect on biomethanation. J. Water Process. Eng. 2018, 25, 205–211. [Google Scholar] [CrossRef]
- Nayak, J.K.; Amit; Ghosh, U.K. An innovative mixotrophic approach of distillery spent wash with sewage wastewater for biodegradation and bioelectricity generation using microbial fuel cell. J. Water Process. Eng. 2018, 23, 306–313. [Google Scholar] [CrossRef]
- Prasad, R.K.; Srivastava, S. Electrochemical degradation of distillery spent wash using catalytic anode: Factorial design of experiments. Chem. Eng. J. 2009, 146, 22–29. [Google Scholar] [CrossRef]
- Apollo, S.; Onyango, M.S.; Ochieng, A. An integrated anaerobic digestion and UV photocatalytic treatment of distillery wastewater. J. Hazard. Mater. 2013, 261, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Siles, J.; García-García, I.; Martín, A. Integrated ozonation and biomethanization treatments of vinasse derived from ethanol manufacturing. J. Hazard. Mater. 2011, 188, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Padoley, K.; Saharan, V.K.; Mudliar, S.; Pandey, R.; Pandit, A.B. Cavitationally induced biodegradability enhancement of a distillery wastewater. J. Hazard. Mater. 2012, 219–220, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Sangave, P.C.; Gogate, P.R.; Pandit, A.B. Ultrasound and ozone assisted biological degradation of thermally pretreated and anaerobically pretreated distillery wastewater. Chemosphere 2007, 68, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Melamane, X.; Tandlich, R.; Burgess, J. Anaerobic digestion of fungally pre-treated wine distillery wastewater. Afr. J. Biotechnol. 2007, 6, 1990–1993. [Google Scholar] [CrossRef]
- Romero, C.; Brenes, M.; García, P.; García, A.; Garrido, A. Polyphenol changes during fermentation of naturally black olives. J. Agric. Food Chem. 2004, 52, 1973–1979. [Google Scholar] [CrossRef]
- Tsioulpas, A.; Dimou, D.; Iconomou, D.; Aggelis, G. Phenolic removal in olive oil mill wastewater by strains of Pleurotus spp. in respect to their phenol oxidase (laccase) activity. Bioresour. Technol. 2002, 84, 251–257. [Google Scholar] [CrossRef]
- Kumar, P.; Chandra, R. Decolourisation and detoxification of synthetic molasses melanoidins by individual and mixed cultures of Bacillus spp. Bioresour. Technol. 2006, 97, 2096–2102. [Google Scholar] [CrossRef] [PubMed]
- Santal, A.R.; Singh, N.; Saharan, B.S. Biodegradation and detoxification of melanoidin from distillery effluent using an aerobic bacterial strain SAG5 of Alcaligenes faecalis. J. Hazard. Mater. 2011, 193, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Mohana, S.; Desai, C.; Madamwar, D. Biodegradation and decolourization of anaerobically treated distillery spent wash by a novel bacterial consortium. Bioresour. Technol. 2007, 98, 333–339. [Google Scholar] [CrossRef]
- Sirianuntapiboon, S.; Zohsalam, P.; Ohmomo, S. Decolorization of molasses wastewater by Citeromyces sp. WR-43-6. Process. Biochem. 2004, 39, 917–924. [Google Scholar] [CrossRef]
- Asgher, M.; Bhatti, H.N.; Ashraf, M.; Legge, R. Recent developments in biodegradation of industrial pollutants by white rot fungi and their enzyme system. Biodegradation 2008, 19, 771–783. [Google Scholar] [CrossRef]
- Li, W.; Li, X.; Zeng, K. Aerobic biodegradation kinetics of tannic acid in activated sludge system. Biochem. Eng. J. 2009, 43, 142–148. [Google Scholar] [CrossRef]
- Lin, J.; Reddy, M.; Moorthi, V.; Qoma, B.E. Bacterial removal of toxic phenols from an industrial effluent. Afr. J. Biotechnol. 2008, 7, 2232–2238. [Google Scholar]
- Kanimozhi, R.; Vasudevan, N. Effect of organic loading rate on the performance of aerobic SBR treating anaerobically digested distillery wastewater. Clean Technol. Environ. Policy 2014, 16, 467–476. [Google Scholar] [CrossRef]
- Anupama, S.; Pradeep, N.V.; Hampannavar, U.S. Anaerobic followed by aerobic treatment approaches for Spentwash using MFC and RBC. Sugar Tech 2013, 15, 197–202. [Google Scholar] [CrossRef]
- Sundararaman, S.; Kumar, J.L.; Kumar, N.G. Reduction of COD and decolourisation of UASB spent wash using E-MBR. Res. J. Pharm. Technol. 2015, 8, 845–848. [Google Scholar] [CrossRef]
- Gupta, R.; Satyawali, Y.; Batra, V.S.; Balakrishnan, M. Submerged membrane bioreactor using fly ash filters: Trials with distillery wastewater. Water Sci. Technol. 2008, 58, 1281–1284. [Google Scholar] [CrossRef] [PubMed]
- Deschamps, L.; Merlet, D.; Lemaire, J.; Imatoukene, N.; Filali, R.; Clément, T.; Lopez, M.; Theoleyre, M.-A. Excellent performance of anaerobic membrane bioreactor in treatment of distillery wastewater at pilot scale. J. Water Process. Eng. 2021, 41, 102061. [Google Scholar] [CrossRef]
- Travieso, L.; Benítez, F.; Sánchez, E.; Borja, R.; León, M.; Raposo, F.; Rincon, B. Assessment of a microalgae pond for post-treatment of the effluent from an anaerobic fixed bed reactor treating distillery wastewater. Environ. Technol. 2008, 29, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.G.; Ghangrekar, M.M. Comprehensive review on treatment of high-strength distillery wastewater in advanced physico-chemical and biological degradation pathways. Int. J. Environ. Sci. Technol. 2019, 16, 527–546. [Google Scholar] [CrossRef]
- Lasik, M.; Gumienna, M.; Szambelan, K.; Czarnecki, Z. Water and energy saving bioprocess for bioethanol production from corn grain applying stillage liquid part recirculation. Afr. J. Biotechnol. 2013, 12, 5950–5955. [Google Scholar] [CrossRef] [Green Version]
- Pejin, D.; Mojovic, L.; Grujic, O.; Pejin, J.; Rakin, M. The bioethanol production with the thin stillage recirculation. Chem. Ind. Chem. Eng. Q. 2009, 15, 49–52. [Google Scholar] [CrossRef]
- Gumienna, M.; Lasik, M.; Szambelan, K.; Czarnecki, Z. Reduction of water consumption in bioethanol production from triticale by recycling the stillage liquid phase. Acta Sci. Pol. Technol. Aliment. 2012, 10, 467–474. [Google Scholar]
- Mikulski, D.; Kłosowski, G. Efficiency of dilute sulfuric acid pretreatment of distillery stillage in the production of cellulosic ethanol. Bioresour. Technol. 2018, 268, 424–433. [Google Scholar] [CrossRef]
- Mikulski, D.; Kłosowski, G. Hydrotropic pretreatment on distillery stillage for efficient cellulosic ethanol production. Bioresour. Technol. 2020, 300, 122661. [Google Scholar] [CrossRef]
- Davis, L.; Jeon, Y.-J.; Svenson, C.; Rogers, P.; Pearce, J.; Peiris, P. Evaluation of wheat stillage for ethanol production by recombinant Zymomonas mobilis. Biomass Bioenergy 2005, 29, 49–59. [Google Scholar] [CrossRef]
- Das, D. Hydrogen production by biological processes: A survey of literature. Int. J. Hydrogen Energy 2001, 26, 13–28. [Google Scholar] [CrossRef]
- Puyol, D.; Batstone, D.J.; Hülsen, T.; Astals, S.; Peces, M.; Krömer, J. Resource recovery from wastewater by biological technologies: Opportunities, challenges, and prospects. Front. Microbiol. 2017, 7, 2106. [Google Scholar] [CrossRef] [Green Version]
- Singh, H.; Das, D. Biofuels from microalgae: Biohydrogen. Green Energy Technol. 2018, 2018, 201–228. [Google Scholar] [CrossRef]
- Laurinavichene, T.; Tekucheva, D.; Laurinavichius, K.; Tsygankov, A. Utilization of distillery wastewater for hydrogen production in one-stage and two-stage processes involving photofermentation. Enzym. Microb. Technol. 2018, 110, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Roy, S.; Das, D. Comparative evaluation of the hydrogen production by mixed consortium, synthetic co-culture and pure culture using distillery effluent. Bioresour. Technol. 2015, 198, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Balachandar, G.; Varanasi, J.L.; Singh, V.; Singh, H.; Das, D. Biological hydrogen production via dark fermentation: A holistic approach from lab-scale to pilot-scale. Int. J. Hydrogen Energy 2020, 45, 5202–5215. [Google Scholar] [CrossRef]
- Searmsirimongkol, P.; Rangsunvigit, P.; Leethochawalit, M.; Chavadej, S. Hydrogen production from alcohol distiller wastewater containing high potassium and sulfate using an anaerobic sequencing batch reactor. Int. J. Hydrogen Energy 2011, 36, 12810–12821. [Google Scholar] [CrossRef]
- Cheng, H.-H.; Whang, L.-M.; Chung, M.-C.; Chan, K.-C. Biological hydrogen and methane production from bagasse bioethanol fermentation residues using a two-stage bioprocess. Bioresour. Technol. 2016, 210, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.N.; Pugalenthi, V.; Vaidya, A.N.; Ghosh, P.C.; Mudliar, S.N. Kinetics of nano-catalysed dark fermentative hydrogen production from distillery wastewater. Energy Procedia 2014, 54, 417–430. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.; Ghosh, S.; Das, D. Improvement of hydrogen production with thermophilic mixed culture from rice spent wash of distillery industry. Int. J. Hydrogen Energy 2012, 37, 15867–15874. [Google Scholar] [CrossRef]
- Lazaro, C.Z.; Varesche, M.B.A.; Silva, E. Sequential fermentative and phototrophic system for hydrogen production: An approach for Brazilian alcohol distillery wastewater. Int. J. Hydrogen Energy 2015, 40, 9642–9655. [Google Scholar] [CrossRef]
- Hiremath, S.G.; Joshi, S.G. Roadmap to distillery spent wash treatment and use of soft computing techniques. Evol. Intell. 2020, 1–15. [Google Scholar] [CrossRef]
- Geetha, K.; Raj, S.A. Biomass-electrochemical integrated system for distillery wastewater treatment with electricity generation using anaerobic mixed consortium in microbial fuel cells. Int. J. Environ. Waste Manag. 2015, 15, 217. [Google Scholar] [CrossRef]
- Samsudeen, N.; Radhakrishnan, T.; Matheswaran, M. Bioelectricity production from microbial fuel cell using mixed bacterial culture isolated from distillery wastewater. Bioresour. Technol. 2015, 195, 242–247. [Google Scholar] [CrossRef]
- Mohanakrishna, G.; Mohan, S.V.; Sarma, P. Bio-electrochemical treatment of distillery wastewater in microbial fuel cell facilitating decolorization and desalination along with power generation. J. Hazard. Mater. 2010, 177, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Samsudeen, N.; Radhakrishnan, T.K.; Matheswaran, M. Performance comparison of triple and dual chamber microbial fuel cell using distillery wastewater as a substrate. Environ. Prog. Sustain. Energy 2015, 34, 589–594. [Google Scholar] [CrossRef]
- Sonawane, J.; Marsili, E.; Ghosh, P.C. Treatment of domestic and distillery wastewater in high surface microbial fuel cells. Int. J. Hydrogen Energy 2014, 39, 21819–21827. [Google Scholar] [CrossRef]
- Sonawane, J.; Gupta, A.; Ghosh, P.C. Multi-electrode microbial fuel cell (MEMFC): A close analysis towards large scale system architecture. Int. J. Hydrogen Energy 2013, 38, 5106–5114. [Google Scholar] [CrossRef]
- Mohanakrishna, G.; Mohan, S.K. Carbon based nanotubes and nanopowder as impregnated electrode structures for enhanced power generation: Evaluation with real field wastewater. Appl. Energy 2012, 95, 31–37. [Google Scholar] [CrossRef]
- Lin, C.-W.; Wu, C.-H.; Huang, W.-T.; Tsai, S.-L. Evaluation of different cell-immobilization strategies for simultaneous distillery wastewater treatment and electricity generation in microbial fuel cells. Fuel 2015, 144, 1–8. [Google Scholar] [CrossRef]
- Quan, X.; Tao, K.; Mei, Y.; Jiang, X. Power generation from cassava alcohol wastewater: Effects of pretreatment and anode aeration. Bioprocess Biosyst. Eng. 2014, 37, 2325–2332. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yang, P.; Guo, Y.; Zhang, K. Electricity generation during wastewater treatment: An approach using an AFB-MFC for alcohol distillery wastewater. Desalination 2011, 276, 373–378. [Google Scholar] [CrossRef]

| Anaerobic Digestion Technology | Organic Loading Rate | Hydraulic Retention Time (Days) | Biogas Yield | Methane Yield | References |
|---|---|---|---|---|---|
| CSTR | 1.6–3.5 g VS/(L.d) | 25–40 | 1.67–2.39 L/(L.d) | 0.45–1.41 L CH4/(L·d) 0.12–0.63 L CH4/g VS | [31] |
| CSTR | 3.2–7.6 g COD/(L.d) | 12–30 | - | 0.46–0.62 L CH4/g VS | [32] |
| AFBR | 29 kg COD/(m3·d) | 3.5 | 15.8 L/(L.d) | 160 L CH4/d | [34] |
| Conventional ABRHybrid ABR | 1.1–1.8 kg COD/(m3·d) 1.0–3.5 kg COD/(m3·d) | 4.2–11.0 | - | 0.14–0.24 L CH4/g COD 0.29–0.31 L CH4/g COD | [35] |
| DSFF | 1.2–11.6 g COD/(L·d) | 4.2–20.0 | 0.7–3.8 L/d | 0.43–2.05 L CH4/(L·d) | [36] |
| AnMBRs | 6.1–8.3 kg COD/(m3·d) | 10–12 | - | 16.9–22.6 L/d 0.26–0.29 L CH4/kg COD | [37] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zielińska, M.; Bułkowska, K.; Mikucka, W. Valorization of Distillery Stillage for Bioenergy Production: A Review. Energies 2021, 14, 7235. https://doi.org/10.3390/en14217235
Zielińska M, Bułkowska K, Mikucka W. Valorization of Distillery Stillage for Bioenergy Production: A Review. Energies. 2021; 14(21):7235. https://doi.org/10.3390/en14217235
Chicago/Turabian StyleZielińska, Magdalena, Katarzyna Bułkowska, and Wioleta Mikucka. 2021. "Valorization of Distillery Stillage for Bioenergy Production: A Review" Energies 14, no. 21: 7235. https://doi.org/10.3390/en14217235
APA StyleZielińska, M., Bułkowska, K., & Mikucka, W. (2021). Valorization of Distillery Stillage for Bioenergy Production: A Review. Energies, 14(21), 7235. https://doi.org/10.3390/en14217235








