Abstract
Plastics are one of the basic construction materials with a wide range of various applications. One of their disadvantages is the problem of managing the waste they generate. Chemical recycling offers the possibility of liquefying polymeric waste and using it as fuel components. Existing technologies giving good quality products are expensive. The HT technology developed and described by the authors is cheaper and enables a high quality product to be obtained. The authors have shown that the quality of the received fuel components is influenced not only by the polymer waste processing technology, but also by the feedstock composition. The presented thermolysis technology not only enables more advanced recycling, but also gives the possibility of partial improvement of the product quality. A product with the best physico-chemical properties was obtained from a blend of PE:PP:PS used in the ratio 60:30:10. It was proved that diesel and petrol blends composed of a 5% v/v share of petrol and diesel fractions, obtained from thermolysis of plastics, meet the normative requirements of fuel quality standards.
1. Introduction
Polymers and plastics are an essential material in many areas of the economy, ranging from packaging, textiles and electronics to machinery and equipment components as well as various structures. This is due to their numerous advantages, such as low density, ease of shaping, high corrosion resistance and good mechanical strength. The potential of these materials is demonstrated by the constantly growing demand and output volumes. Comparing the change in the scale of plastics production over the last 70 years, it can be seen that in 1950 the world produced 1.5 million Mg of polymers per year, while in 2019 nearly 368 million Mg [1]. Due to the fact that plastics are so ubiquitous in people’s daily lives, they account for as much as 40% of the volume of waste generated and 10% of its mass. The ubiquity of these materials has led them to be chosen as the geological marker of the era of mankind (the Anthropocene) [2].
Polymeric materials also have their disadvantages, for example, compared to metals and their alloys, in most cases they are characterized by lower hardness, mechanical strength or heat resistance. However, the most important drawbacks include problems with recycling and disposal of used plastics. These problems have a global dimension, which only reinforces the need for solutions. Plastic waste recycling is increasing every year, but 2016 was the first year in which more plastic waste was recycled in Europe than was sent to landfill. In 2018, official EU treatment programs successfully collected 29.1 million Mg of plastic waste, but still almost 25% of plastic materials ended up in landfill [1].
Polyolefins (polyethylene PE and polypropylene PP) are the most commonly used polymers. The reasons for such popularization of these materials are their features such as: flexibility, transparency, low density, easy shaping and dyeing, low water vapor permeability, low chemical reactivity, thermoplasticity, high mechanical resistance and low price. This has become the reason for the use of these polymers in the production of foils, pipes, various types of containers, objects of everyday use, as well as disposable packaging: cups, plates, bottles, bags, boxes, etc. Therefore, polyolefins constitute about 70% of the plastic waste stream. Unfortunately, the useful life of plastic items is often very short—from 1 day to 3 years [3]. But the time of decomposition of these items remains disproportionately long—for example, the deterioration of a plastic bottle takes an average of 300–500 years [4]. Polymers appeared on the market in the 1950s. In such a short time it was impossible to adapt the enzymatic structures of microorganisms by evolution to the degradation of polymers [5]. As a result of this slow decomposition of polymeric materials, various compounds are released into the environment, including microplastic, which penetrates all parts of the biosphere and enters the digestive system of living beings [6]. It is estimated that more than half of the world’s population may have microplastics in their digestive tract [7]. The awareness of the risks posed by the growing production of plastic products is accompanied by the need to produce them due to the lack of natural alternatives to this type of material. Considering the scale of the problem, the development and dissemination of the best technologies for recycling and utilization of plastic waste becomes a priority.
The predominant form of recycling plastic waste is energy recovery through combustion. In 2018, a total of 29.1 million Mg of plastic waste was collected for recycling in the EU Member States together with Norway and Switzerland, of which: 24.9% went to landfill, 32.5% underwent other forms of recycling and 42.6% was combusted (energy recovery) [8]. The combustion of plastics results in the removal of this material from the economy and the irrevocable loss of material that could be used in other ways. The relatively high price of processing plastics and the lower quality of products made from recovered materials compared to their original counterparts are mainly responsible for the low recycling rate. Another reason is that in many countries the infrastructure for selective waste collection is still underdeveloped and there is low public awareness of the importance of recycling.
From the point of view of the quality of the product obtained, the best method for processing plastics is depolymerization, as the starting material, i.e., monomers, can be obtained from the used material. As a result, regardless of the number of processing cycles, a full quality product is obtained each time. This method is associated with increased energy input (e.g., higher temperature, use and recovery of solvents). Unfortunately, this technology is characterized by high monomer efficiency for only a few polymers, e.g., polymethyl methacrylate, polystyrene or polyethylene terephthalate [9,10]. Polyolefins depolymerize with low efficiency and are treated, among others, with material recycling resulting in regranulate. Material recycling is the most environmentally favorable waste management method [11] (the lowest amount of emitted CO2), however, the material loses quality with each successive processing cycle and, in order to improve the product quality, regranulate is mixed with a fresh batch of polymer. This results in products with poorer properties (compared to those obtained from the virgin polymer), which in the end lose their quality to such an extent that they cannot be recycled using this method.
For contaminated materials or those made from recycled polyolefins (material with poorer performance properties), chemical recycling can be used. The lack of heteroatoms means that the liquid products obtained by chemical conversion can be further processed into hydrocarbons with various applications, including fuels. The liquefaction of polyolefins can be carried out by a number of methods. The most common is pyrolysis (thermal cracking) [12,13], which is most frequently carried out in an inert gas atmosphere (usually nitrogen) at temperatures in the range of 500–600 °C. Unfortunately, its product (pyrolysis oil) has no practical use as a fuel component because of the large mass range of hydrocarbons and high content of alkenes and long-chain alkanes (up to 50–80%) [14]. In order to improve its quality, it is necessary to separate long-chain alkanes and to process them by cracking, as well as to carry out hydrogenation [15]. By carrying out the pyrolysis process in the presence of a catalyst, it is possible to reduce the time and temperature of the process. An additional advantage is the changed composition of the liquid product—a smaller dispersion of hydrocarbon mass and a lower content of alkanes with long, straight chains. However, this process leads to the formation of large amounts of aromatic hydrocarbons (including polyaromatic hydrocarbons), and to the formation of significant amounts of carbon deposits on the surface of the catalyst, which deactivate it [16]. The amount of aromatic hydrocarbons and alkenes can be reduced by additional pyrolysis of polyolefin waste in the presence of hydrogen, so-called hydrocracking [17,18]. This process is usually carried out in the presence of a bifunctional catalyst, at high temperatures (300–450 °C) and high hydrogen pressure (2–15 MPa), which results in high capital costs [19].
Another option for liquefying polymer waste is metal-catalyzed hydrogenolysis [20]. The process is performed in the presence of a metal catalyst, under moderate temperature conditions (over 200 °C), high hydrogen pressure (over 2.2 MPa) and in the presence of a solvent [21]. This technology also generates high costs due to the high-pressure process and solvent waste recycling. Moreover, melt hydroconversion in the presence of catalyst [22] as a method of conversion of waste plastics is connected with high costs of equipment, because although it proceeds at a relatively low temperature of 250 °C, it is carried out under high hydrogen pressure of 2–5 MPa.
Very often, the preferred methods of plastic waste management result from legal and economic regulations, both domestic and international. The Directive on the promotion of energy from renewable sources (RED II) [23] existing in the European Union indicates and creates conditions for the wide use of waste treatment products as fuel components, both engine and heating fuels. There are many scientific studies on the conversion of waste plastics to liquid hydrocarbons. Most of them concern about using waste plastic conversion product as heavy fuel oil or as a raw material for refineries [24,25,26]. There are few reports in the literature on the quality of thermal conversion products of waste plastics and their blends with refinery fuels concerning the quality requirements for conventional fuels. For example, the authors of [27] synthesized high-density jet fuel from low-density polyethylene (LDPE) via catalyzed (with ZSM-5) microwave-induced degradation. Liquefaction was followed by a hydrotreating process conducted in a hydrogen atmosphere at a pressure of 0.5–8.3 MPa. The yield of liquid hydrocarbons was about 68%. The authors compared the chemical composition of liquid products with jet fuel (JP-5 navy fuel). It was found that the composition of hydrocarbons in the product from plastic waste is similar to the composition of JP-5, and the obtained product can be directly used as a fuel or additive for JP-5 navy fuel. However, the authors did not conduct comparative tests of the liquid products in terms of quality requirements for JP-5 fuel.
In turn, the work [28] describes the research results on the combustion, performance, and emissions of the blend of diesel with the hydrogenated product of polypropylene pyrolysis (HPPO) blended. The polypropylene pyrolysis was carried out using ZSM-5 as the catalyst. The hydrogenation of obtained oil was conducted under a hydrogen atmosphere at a pressure of 7.0 MPa, and in the presence of a catalyst (Ni on the ZSM-5). The HPPO product is characterized by physicochemical properties that match the EN 590 standard. The authors limited the research to only one polymer (PP) and one fuel, diesel. Moreover, both the liquefaction and hydrogenation processes were carried out with the use of catalysts and additionally, the hydrogenation was carried out under high pressure, which generates higher costs for the entire process.
In another study [29], the authors describe the possibility of obtaining gasoline fractions but only from low-density polyethylene due to catalytic microwave-assisted pyrolysis in the presence of NiO and HY zeolite as catalysts. Unfortunately, the work does not compare the product properties with the quality requirements for gasoline (EN 228). Some reviews of similar to HT technologies were published last years [30,31].
Literature information indicates that obtaining commercially valuable products of thermal decomposition of polyolefins (e.g., as components for gasoline or diesel oil) is connected with high costs (e.g., purchase of a catalyst, high- pressure equipment). This, in many cases, puts a question mark over the profitability of the technology.
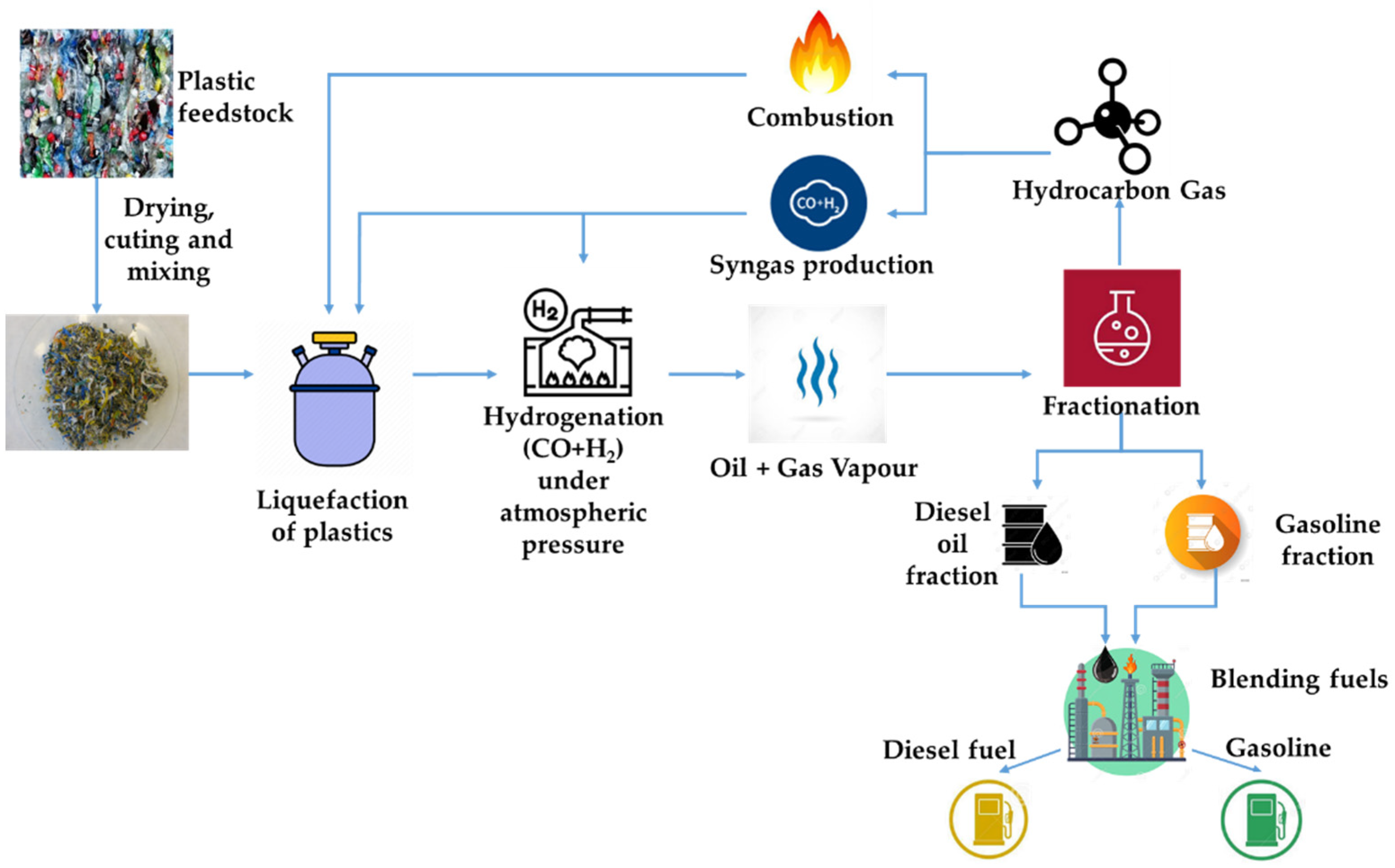
The Polish company Handerek Technologies, in cooperation with Łukasiewicz-Automotive Industry Institute, has developed a new technology (HT technology) for the conversion of waste plastics to hydrocarbon fractions with relatively short chains. The technology enables thermolysis and hydrogenation processes to be carried out under atmospheric pressure by using reactive distillation in a synthesis gas (H2 + CO) atmosphere as a carrier gas [32]. Performing liquefaction without catalysts and obtaining hydrocarbons with chain lengths from C4 to C22 and boiling points below 360 °C, without the need to separate and return to the process long chain alkanes, reduces process costs. In addition, avoiding increased pressure significantly reduces plant construction costs. The operational expenses can be further reduced by using non-condensable light hydrocarbon fractions for reactor heating or synthesis gas generation. The process diagram from raw material to fuel products is shown in Figure 1. It should be mentioned that, for example, thermolysis using the technology described above generates from 6 kg of waste plastics ca: 5 kg (6.3 dm3) of liquid product, 0.1 kg of solid phase (carbonizate) and 0.9 kg of gases of high-energy value.
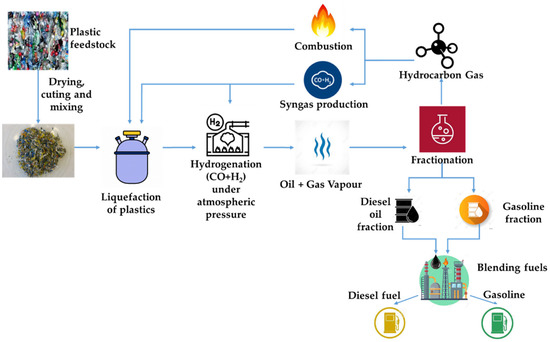
Figure 1.
The process diagram from raw material to fuel products.
The authors present the results of research on the influence of raw material composition on the quality of liquid hydrocarbon fractions obtained with the use of the developed technology and the possibility of using these fractions to compose engine fuels.
2. Materials and Methods
Waste polymer materials (polyethylene, polypropylene and polystyrene—PS), selected directly from municipal waste, were used as input material for the reactor. The material, after being dried roughly to a moisture content of 15–20% and mechanically separated from impurities, was cut into fragments of approx. (3–5) mm × (15–30) mm in size. Two polymer blends were prepared: the first with PE and PP in a 60:40 weight ratio, the second with PE:PP:PS in a 60:30:10 ratio.
The liquefaction process was carried out in batches in a reactor filled with Raschig rings made of aluminum. The process was conducted under atmospheric pressure in a stream of synthesis gas (H2:CO 2:1 v/v) as a carrier gas at a flow rate of 4 dm3/min. The reactor was filled with cold substrate (10 kg) mixed with rings and heated at 4 °C/min until the right temperature was reached. The process was carried out non-isothermally within the temperature range in the reactor: 450 °C in the lower part and 360 °C in the upper part. The reactor load was 1 kg of polymer per 10 dm3 of Raschig rings.
The hydrogenation of thermolysis products was carried out in a reactor filled with a catalyst (platinum and palladium deposited on alumina), under atmospheric pressure, at 360 °C, in an atmosphere of synthesis gas (H2:CO 2:1 v/v) as a hydrogenating agent, flowing at a rate of 2 dm3/min. The execution time of the whole process (liquefaction and hydrogenation) was 120 min. The scheme of construction of the test stand and the principle of operation of the liquefaction reactor were described in [22].
The liquid fractions obtained from both types of feedstock mixtures were filtered to remove solids and distilled into a petrol fraction (boiling up to 190 °C) and a diesel fraction (boiling above 190 °C). They were then subjected to physicochemical analysis. The degree of hydrogenation was examined by the bromine number titrimetric method. For this purpose, 0.4 g of the sample was weighed into a conical flask, 20 cm3 of 0.1 n KBr+KBrO3 solution and 50 cm3 of acetic acid were added and the mixture was kept in a dark place for 15 min. After this time, 20 cm3 of a 10% water solution of KI was added to the flask and was titrated with 0.1 n sodium thiosulphate solution in the presence of starch solution until the mixture was discolored. The bromine number was calculated from the formula:
where: V0—volume of thiosulphate solution consumed for titration of the blank sample [cm3], V1—volume of thiosulphate solution consumed for titration of the test sample [cm3], m—product mass [g].
The fractional composition of the distilled fractions was determined according to EN ISO 3405:2012 using Herzog’s HDA 620 normal distillation apparatus. The flash point for diesel fractions was determined by the closed cup method according to EN ISO 2719:2016-08 using the Pensky–Martens apparatus manufactured by Herzog. The chemical composition of the fraction was determined by gas chromatography with a mass detector using the Agilent GC 6890N apparatus with a 5973N detector. Designation conditions:
- Column—RESTEK Rxi-1ms with dimensions 30 m × 0.250 mm × 0.25 μm;
- injection chamber temperature—250 °C;
- oven temperature program—from 100 °C, 10 °C/min. increments up to 320 °C, then isothermal conditions for 10 min;
- split—1:100;
- gas—helium with a flow rate of 1 cm3/min;
- source temperature—230 °C.
Physicochemical properties of fuels were tested in an accredited laboratory using methods specified in quality standards for individual fuels: in the case of petrol—EN 228+A1:2017-06, EN 228+A1:2017-06/Ap1:2017-11 (Fuels for motor vehicles—Unleaded petrol—Requirements and test methods), and in the case of diesel—EN 590+A1:2017-06, EN 590+A1:2017-06/Ap1:2017-11 (Fuels for motor vehicles—Diesel—Requirements and test methods). Table 1 and Table 2 summarize the tested characteristics of the individual fuels and the test methods applied.

Table 1.
List of methods applicable for petrol testing.

Table 2.
List of methods applicable for diesel fuel testing.
3. Results
In their previous works, the authors conducted research on the liquefaction of polyethylene/polypropylene blends using HT technology. The polymers were placed in a thermolysis reactor at different mass ratios to each other. With the temperature at the top of the reactor set at 360 °C, only hydrocarbon vapors whose boiling point was equal to or lower than the set temperature at the top of the reactor left the thermolysis reactor. Investigations of the obtained liquid products showed that the optimal composition of the raw material in terms of fuel requirements was that obtained from a blend in which the PE:PP ratio was 60:40. Therefore, such a blend was used in the experiment described in this paper. After liquefaction and hydrogenation, the obtained liquid product (broad fraction) was distilled into a petrol fraction (with boiling point up to 190 °C) and a diesel fraction (with boiling point above 190 °C). The petrol fraction accounted for about 28% m/m of the broad fraction. It was found that the petrol fraction had a density of 0.737 g/cm3, was poorly hydrogenated under process conditions (bromine number value 28.9 g/100 g) and had a high alkene content (48.48%), determining its susceptibility to ageing. Alkanes constitute 42.8% and aromatic compounds 0.00% of its composition. The lack of aromatic hydrocarbons in this fraction does not make it possible to obtain a high octane number. Research on the composition and physicochemical properties of this fraction allowed us to conclude that the product obtained from thermolysis of polyolefins is not promising as a source of high quality petrol component, mainly due to the lack of high-octane aromatic hydrocarbons.
The fraction boiling above 190 °C is characterized by a good degree of hydrogenation (bromine number value of 7.2 g/100 g), a flash point of 63 °C and a cloud point of 15 °C. These results indicate a lower content of alkenes than the petrol fraction, a relatively high content of alkanes with long carbon chains and a low content of light diesel components (kerosine). However, this fraction is promising as a good diesel component.
Consideration of the selection of a waste plastic that could be a source of octane-raising compounds led to polystyrene. The styrene obtained from its thermolysis is a compound that could increase the aromatic hydrocarbon content in the liquid product and thus increase the octane number. The boiling point of this compound is 145 °C, which in the result of distillation means it would remain in the petrol fraction. Therefore, in order to improve properties of the petrol fraction, an experiment was performed in which 10% of waste PP was replaced by PS. The mixture prepared as described above was thermolyzed and hydrogenated under similar process conditions as for PE/PP mixture. The obtained liquid product was distilled into petrol fractions (boiling to 190 °C) and diesel fractions (boiling above 190 °C); the appearance of both fractions is presented in Figure 2.

Figure 2.
Liquid products from thermolysis of a mixture of plastics (PE, PP and PS): (1) HTG—petrol fraction, (2) HTD—diesel fraction.
It was concluded that the new raw material composition increased the efficiency of the petrol fraction (boiling in the range 25–190 °C), which accounted for 40.6% m/m of the broad fraction (liquid product). The bromine value of this fraction was 29.3 g/100 g, the alkane content—24.89% and the alkene content—34.54%. The use of polystyrene in the feedstock increased the content of aromatic hydrocarbons (increasing the octane number) in the petrol fraction to 35.68%. The obtained results allow to state that the product of thermolysis of polyolefins with participation of polystyrene makes it possible to obtain a petrol component of the quality equal to that of standard petrol components manufactured in refinery technologies.
The obtained diesel fraction contains a small amount of unsaturated compounds, as evidenced by the bromine number value (4.10 g/100 g). The low content of alkenes makes it possible to easily stabilize this fraction with antioxidant additives. This fraction is also characterized by a high flash point (72 °C) and a relatively high cloud point (17 °C). The values of these parameters indicate low content of low boiling components of diesel fuel and higher content of hydrocarbons with longer carbon chains. The distillation start temperature of 170 °C and evaporation of 85% of the sample to 340 °C are within the range accepted for standard diesel fuel. Thus, it can be concluded that the tested fraction can be a good diesel component.
In order to study the influence of the obtained components from the thermolysis of PE, PP and PS blends (designated as HTG—petrol fraction, HTD—diesel fraction) on fuel quality, their 5% (v/v) blends with various fuels were prepared. Base petrol (G0) and base diesel fuels (DF0) devoid of any additives and biocomponents, as well as commercial petrol (G1) and commercial diesel (DF1) containing the full package of additives, including biocomponents, were selected for testing. The initial fuels and their blends were tested in an accredited laboratory to check the compliance of their properties with the requirements specified in quality standards. Test results for petrols are presented in the Table 3, where red color indicates results not achieving the requirements of the standards.

Table 3.
Petrol test results.
The data presented in the Table 3 indicate that the initial G0 fuel does not meet all standard requirements, which is most likely due to the lack of additives. In general, the addition of HTG, due to the low content of the lowest boiling components of petrol (a significant part of them passed into the gas phase), causes a slight increase in fuel density, a decrease in vapor pressure and volatility index, as well as a decrease in the content of low boiling fractions determined by the distillation method. Due to the presence of a considerable amount of unsaturated compounds in this fraction, its introduction into petrols resulted in an increase in alkenes content. On the other hand, the absence of oxygenates in HTG resulted in a slight decrease in the content of these compounds in blends. A slight decrease in octane number values was also observed after inserting the investigated fraction into blends. In general, however, it can be stated that the addition of 5% HTG fraction to petrols does not adversely affect fuel quality, and even in the case of the distillation end temperature of raw petrol, improves this parameter to a value meeting the requirements of the standard. Commercial petrol with 5% v/v HTG meets the quality requirements of the quality standard.
Table 4 summarizes the results of the tests of the properties of diesel fuels and influence of 5% of HTD diesel fraction.

Table 4.
Diesel fuel test results.
As the results of testing diesel blends with 5% HTD fraction (Table 4) indicate, the introduction of HTD causes a slight decrease in density, which is most likely related to the higher content of light hydrocarbons in this fraction.
HTD fraction does not contain esters, therefore its addition to diesel reduces FAME content in the blend with commercial diesel. Due to the content of unsaturated hydrocarbons, the tested plastic fraction is characterized by lower oxidative stability than base diesel and its blending with this fuel results in lower values of this parameter. However, as indicated by blending with commercial diesel, the introduction of a package of additives (including antioxidants) stabilizes the fuel to such an extent that under test conditions the value of the parameter reflecting oxidative stability does not change. The fraction extracted from polymer waste causes an increase in the CFPP, as can be seen for base diesel. However, for commercial fuel, in the summer period, the magnitude of this parameter remains unchanged. The results of the conducted research allow to conclude that 5% v/v additive of HTD–diesel fuel fraction to conventional fuel does not change the examined properties to such an extent that the fuel no longer meets the examined standard requirements.
4. Discussion and Conclusions
The chemical recycling of waste plastics by liquefying them is one of the forms of utilization of this raw material stream. The literature research carried out allows us to conclude that there is no relatively cheap method of converting these raw materials into high-quality products. The existing technologies, which provide products suitable for use as fuel fractions, are based on the process of catalytic liquefaction and subsequent high-pressure hydrogenation. The developed HT technology offers the possibility to liquefy plastics without a catalyst and conduct the catalytic hydrogenation process under atmospheric pressure (in a synthesis gas atmosphere), thus lowering the process costs. It is suspected that the possibility of carrying out hydrogenation under atmospheric pressure may be due to the catalytic effect of carbon monoxide present in the synthesis gas. At the same time, a product with desirable functional properties is obtained from the waste with high efficiency.
The composition of the liquid products obtained depends not only on the conditions of the decomposition process, but also on the type of raw materials used. In the literature, there is little information on the composition of the feedstock in order to obtain a liquid product with the best possible properties, allowing them to be used for the composition of motor fuels. In a study [33], it was found that thermal cracking of polypropylene gives a higher yield of light products corresponding to petrol, while polyethylene gives heavier fractions similar to those found in diesel. Studies, carried out by the authors of this article, indicate that polyolefins themselves do not provide a good enough product for fuel applications. It is necessary to admix the raw material with polystyrene, which makes it possible to obtain aromatic compounds responsible for the octane number in the petrol fraction. In the research conducted, the best quality of the liquid fraction for fuel applications was obtained for the PE:PP:PS blend with the mutual weight ratio of individual polymers 60:30:10. This kind of raw material blend for manufacturing fuel components is protected by the patent application. The liquid product obtained from the above waste polymer blend, separated by distillation into petrol and diesel fractions, can be used to compose motor fuels. Petrol/diesel blends containing about 5% v/v of respective fractions obtained by thermolysis of waste plastics meet normative requirements for the fuels in question.
As is well known, the dominant form of plastic waste management is still landfilling due to the lower cost of disposal compared to recycling. In the case of recycling, depending on its form, there are various types of capital costs related to waste collection, transport and logistics, segregation, material recovery, washing, cutting, water recycling, etc. [34]. Depending on the region and country, the cost of each step can vary from US $65 to $400/ton. For example, the costs associated with just collecting waste in the US can range from $50 to $150/ton, while landfilling costs from $25 to $150/ton [35]. Most of these costs, especially waste collection, transport and logistics, are unavoidable and borne by the producers of this waste. Only a dozen percent of the entire plastic waste stream can be selected for material recycling (re-granulation). The materials can be recycled in this way only several times due to the progressive degradation of the polymer in each subsequent recycling cycle (the influence of the extrusion temperature). The rest of the waste, unsuitable for material recycling, may end up in landfills, be chemically recycled, or incinerated.
It is estimated that the cost of producing heating oil (and, therefore, fuel of lower quality) from waste plastics in the pyrolysis process is approximately $1.13/kg, which is over 50% more than in the case of market fuels [24]. The authors of [36,37] also point to the high costs of recycling waste plastics. Therefore, there is a need for efficient and cost-effective technologies to recycle these materials. Such possibilities are offered by HT technology. Calculations indicate that with the current price of the raw material for chemical recycling of $50/ton, the cost of producing engine fuel components using this technology is at most $450 per m3. Already in the current economic conditions, this is less than the market price of motor fuels ($470 per m3). These low production costs of fuel components result, among other things, from the relatively low energy consumption of the technology. The process of liquefying plastic waste in the developed technology consumes about 0.67 kWh of energy. By adding all heat losses from the process, the energy demand was assumed to be about 0.97 kWh per kilogram of processed plastic (mixture of PE, PP and PS in the ratio of 60%:30%:10%, respectively). On the other hand, 1 kg of liquid hydrocarbons obtained in this process contains an average of 11.5 kWh. And what’s more, the hydrogenation process is an exothermic reaction. Since the hydrogenation process occurs at atmospheric pressure, the only energy-consuming factor is the fan cooling the hydrogenation reactor with air. In the scale of the entire process carried out in a continuous mode, these costs are negligible.
Under the provisions in the RED II Directive [23], recycled carbon fuels (and, therefore, also from waste plastics) can contribute to the implementation of policy objectives on the diversification of energy sources and the reduction of emissions in the transport sector. The condition is that these fuels meet the relevant minimum threshold for reducing greenhouse gas emissions. Work is currently underway on setting the threshold for reducing GHG emissions for these fuels. For example, in the case of biofuels, the threshold is 65% for installations put into operation after 1 January 2021. From the preliminary calculations made for HT technology, the reduction in GHG emissions relative to the fossil counterpart is about 70%. Moreover, to increase the use of renewable energy in the transport sector, by 2030, fuel suppliers in EU countries will have to provide at least a 14% share (the so-called “minimum share”) of renewable energy in the final energy consumption in this sector. Recycled carbon fuels may be taken into account for the calculation of this share. This is one of the reasons for looking for relatively cheap technologies that enable obtaining high-quality fuel components.
The RED II Directive indicates that fuel fractions obtained from waste plastics can be used instead of biocomponents and included in the National Indicating Target. Thereby, it would be possible to improve properties of e.g., commercial diesel fuel (in particular resistance to oxidation) by eliminating fatty acid esters from its composition.
The HT technology under development requires further improvements and research. According to the authors, it is still necessary to check the properties of fuel compositions with a higher content of hydrocarbon fractions obtained in HT technology. This will make it possible to determine whether these fractions can be used, for example, in amounts of 10% or as so-called drop-in fuels. However, at the current research stage it can be concluded that the liquid product from HT technology can be a fully-fledged fuel component.
The aforementioned economic profitability of processing waste into fuel components also translates into environmental benefits an additional advantage of HT technology. In this technology, it is possible to recycle waste unsuitable for material recycling due to impurities or lack of good separation of individual groups of polymeric materials. This provides an opportunity to increase the efficiency of plastic waste management and reduce its quantity in landfills. The technology described is fully compatible with a closed-circuit economy and shows no waste, only unused raw materials.
Author Contributions
Conceptualization, A.M., A.H. and K.B.; methodology, A.M., A.H. and M.P.; formal analysis, A.M. and K.B.; investigation, A.M., A.H. and M.P.; writing—original draft preparation, A.M. and M.P.; writing—review and editing, M.P. and K.B.; supervision, K.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Plastics—Facts 2020—Report Plastics Europe. The Association of Plastics Manufacturers in Europe. Available online: https://www.plasticseurope.org/en/resources/publications/4312-plastics-facts-2020 (accessed on 21 May 2021).
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ncube, L.K.; Ude, A.U.; Ogunmuyiwa, E.N.; Zulkifli, R.; Beas, I.N. An Overview of Plastic Waste Generation and Management in Food Packaging Industries. Recycling 2021, 6, 12. [Google Scholar] [CrossRef]
- Jalaluddin, M. Use of Plastic Waste in Civil Constructions and Innovative Decorative Material (Eco-Friendly). MedCrave Online J. Civ. Eng. 2017, 3, 00082. [Google Scholar] [CrossRef] [Green Version]
- Mueller, R.J. Biological degradation of synthetic polyesters—Enzymes as potential catalysts for polyester recycling. Process. Biochem. 2006, 41, 2124–2128. [Google Scholar] [CrossRef]
- Al-Jaibachi, R.; Cuthbert, R.N.; Callaghan, A. Up and away: Ontogenic transference as a pathway for aerial dispersal of microplastics. Biol. Lett. 2018, 14, 20180479. [Google Scholar] [CrossRef] [Green Version]
- Harvey, F.; Watts, J. Microplastics Found in Human Stools for the First Time. The Guardian. 22 October 2018. Available online: https://www.theguardian.com/environment/2018/oct/22/microplastics-found-in-human-stools-for-the-first-time (accessed on 31 May 2021).
- Plastics—The Facts 2019—Report Plastics Europe. The Association of Plastics Manufacturers in Europe. Available online: https://www.plasticseurope.org/application/files/9715/7129/9584/FINAL_web_version_Plastics_the_facts2019_14102019.pdf (accessed on 25 May 2021).
- Balema, V.P.; Hlova, I.Z.; Carnahan, S.L.; Seyedi, M.; Dolotko, O.; Rossini, A.J.; Luzinov, I. Depolymerization of polystyrene under ambient conditions. New J. Chem. 2021, 45, 2935–2938. [Google Scholar] [CrossRef]
- Kang, M.J.; Yu, H.J.; Jegal, J.; Kim, H.S.; Cha, H.G. Depolymerization of PET into terephthalic acid in neutral media catalyzed by the ZSM-5 acidic catalyst. Chem. Eng. J. 2020, 398, 125655. [Google Scholar] [CrossRef]
- Rubel, H.; Follette, C.; Meyer zum Felde, A.; Appathurai, S.; Benedi Díaz, M.; Jung, U. A Circular Solution to Plastic Waste. 15 July 2019. Available online: https://www.bcg.com/publications/2019/plastic-waste-circular-solution (accessed on 6 September 2021).
- Bezergianni, S.; Dimitriadis, A.; Faussone, G.C.; Karonis, D. Alternative Diesel from Waste Plastics. Energies 2017, 10, 1750. [Google Scholar] [CrossRef] [Green Version]
- Idumah, C.I. Recent advancements in thermolysis of plastic solid wastes to liquid fuel. J. Therm. Anal. Calorim. 2021, 1–14. [Google Scholar] [CrossRef]
- Lopez, G.; Artetxe, M.; Amutio, M.; Bilbao, J.; Olazar, M. Thermochemical routes for the valorization of waste polyolefinic plastics to produce fuels and chemicals. A review. Renew. Sustain. Energy Rev. 2017, 73, 346–368. [Google Scholar] [CrossRef]
- Thahir, R.; Altway, A.; Juliastuti, S.R.; Susianto. Production of liquid fuel from plastic waste using integrated pyrolysis method with refinery distillation bubble cap plate column. Energy Rep. 2019, 5, 70–77. [Google Scholar] [CrossRef]
- Akubo, K.; Nahil, M.A.; Williams, P.T. Aromatic fuel oils produced from the pyrolysis-catalysis of polyethylene plastic with metal-impregnated zeolite catalysts. J. Energy Inst. 2019, 92, 195–202. [Google Scholar] [CrossRef]
- Gala, A.; Catalan-Martínez, D.; Guerrero, M.; Serra, J.M. Simulation-assisted design of a catalytic hydrogenation reactor for plastic pyrolysis fuels. Fuel 2021, 287, 119400. [Google Scholar] [CrossRef]
- Munir, D.; Piepenbreier, F.; Usman, M.R. Hydrocracking of a plastic mixture over various micro-mesoporous composite zeolites. Powder Technol. 2017, 316, 542–550. [Google Scholar] [CrossRef]
- Munir, D.; Irfan, M.F.; Usman, M.R. Hydrocracking of virgin and waste plastics: A detailed review. Renew. Sustain. Energy Rev. 2018, 90, 490–515. [Google Scholar] [CrossRef]
- Zhang, F.; Zeng, M.; Yappert, R.D.; Sun, J.; Lee, Y.H.; LaPointe, A.M.; Peters, B.; Abu-Omar, M.M.; Scott, S.L. Polyethylene upcycling to long-chain alkylaromatics by tandem hydrogenolysis/aromatization. Science 2020, 370, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rivera, K.L.; Huber, G.W. Catalytic Hydrogenolysis of Polyolefins into Alkanes. ACS Cent. Sci. 2021, 7, 17–19. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Kots, P.A.; Vance, B.C.; Danielson, A.; Vlachos, D.G. Plastic waste to fuels by hydrocracking at mild conditions. Sci. Adv. 2021, 7, eabf8283. [Google Scholar] [CrossRef] [PubMed]
- Directive (EU) 2018/2001 of the European Parliament and of the Council of 11 December 2018 on the Promotion of the Use of Energy from Renewable Sources. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv:OJ.L_.2018.328.01.0082.01.ENG&toc=OJ:L:2018:328:TOC (accessed on 30 June 2021).
- Fivga, A.; Dimitriou, I. Pyrolysis of plastic waste for production of heavy fuel substitute: A techno-economic assessment. Energy 2018, 149, 865–874. [Google Scholar] [CrossRef]
- Kasar, P.; Sharma, D.K.; Ahmaruzzaman, M. Thermal and catalytic decomposition of waste plastics and its co-processing with petroleum residue through pyrolysis process. J. Clean. Prod. 2020, 265, 121639. [Google Scholar] [CrossRef]
- Papari, S.; Bamdad, H.; Berruti, F. Pyrolytic Conversion of Plastic Waste to Value-Added Products and Fuels: A Review. Materials 2021, 14, 2586. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, H. Synthesis of high-density jet fuel from plastics via catalytically integral processes. RSC Adv. 2016, 6, 6154–6163. [Google Scholar] [CrossRef]
- Mangesh, V.L.; Padmanabhan, S.; Tamizhdurai, P.; Narayanan, S.; Ramesh, A. Combustion and emission analysis of hydrogenated waste polypropylene pyrolysis oil blended with diesel. J. Hazard. Mater. 2020, 386, 121453. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Liu, S.; Huang, Y.; Liu, S.; Zhou, N.; Peng, P.; Wang, Y.; Chen, P.; Ruan, R. Catalytic microwave-assisted pyrolysis of plastic waste over NiO and HY for gasoline-range hydrocarbons production. Energy Convers. Manag. 2019, 196, 1316–1325. [Google Scholar] [CrossRef]
- Sarker, M.; Rashid, M.M. Mixture of LDPE, PP and PS Waste Plastics into Fuel by Thermolysis Process. Int. J. Eng. Technol. Res. 2013, 1, 1–16. [Google Scholar]
- Eze, W.U.; Umunakwe, R.; Obasi, H.C.; Ugbaja, M.I.; Uche, C.C.; Madufor, I.C. Plastics waste management; A review of pyrolysis technology. Clean Technol. Recycl. 2021, 1, 50–69. [Google Scholar] [CrossRef]
- Matuszewska, A.; Hańderek, A.; Biernat, K.; Bukrejewski, P. Thermolytic Conversion of Waste Polyolefins into Fuels Fraction with the Use of Reactive Distillation and Hydrogenation with the Syngas Under Atmospheric Pressure. Energy Fuels 2019, 33, 1363–1371. [Google Scholar] [CrossRef]
- Lamar, Y.R.; Noboa, J.; Torres Miranda, A.S.; Streitwieser, D.A. Conversion of PP, HDPE and LDPE Plastics into Liquid Fuels and Chemical Precursors by Thermal Cracking. J. Polym. Environ. 2021, 29, 3842–3853. [Google Scholar] [CrossRef]
- Nanda, S.; Berruti, F. Thermochemical conversion of plastic waste to fuels: A review. Environ. Chem. Lett. 2021, 19, 123–148. [Google Scholar] [CrossRef]
- Kunwar, B.; Cheng, H.N.; Chandrashekaran, S.R.; Sharma, B.K. Plastics to fuel: A review. Renew. Sustain. Energy Rev. 2016, 54, 421–428. [Google Scholar] [CrossRef]
- Schyns, Z.O.G.; Shaver, M.P. Mechanical Recycling of Packaging Plastics: A Review. Macromol. Rapid Commun. 2021, 42, 2000415. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, M.S.; Oasmaa, A.; Pihkola, H.; Deviatkin, I.; Tenhunen, A.; Mannila, J.; Minkkinen, H.; Pohjakallio, M.; Laine-Ylijoki, J. Pyrolysis of plastic waste: Opportunities and challenges. J. Anal. Appl. Pyrolysis 2020, 152, 104804. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).