Effect of Tillage System and Organic Matter Management Interactions on Soil Chemical Properties and Biological Activity in a Spring Wheat Short-Time Cultivation
Abstract
:1. Introduction
2. Literature Review
3. Materials and Methods
3.1. Experiment Location
- T—
- tillage system (six variants):
- 1—
- skimming of the stubble field, pre-winter ploughing followed by seeder–cultivator unit in spring;
- 2—
- manure, skimming, pre-winter ploughing followed by seeder–cultivator unit in spring;
- 3—
- grubbing of the stubble field, grubbing followed by seeder–cultivator unit in spring;
- 4—
- grubbing of the stubble field combined with stubble catch crop, grubbing followed by seeder–cultivator unit in spring;
- 5—
- grubbing of the stubble field combined with stubble catch crop, sow ploughing followed by seeder–cultivator unit in spring;
- 6—
- manure, skimming combined with stubble catch crop, grubbing followed by seeder–cultivator unit in spring.
- S—
- stubble management (four variants):
- 1—
- leaving the shredded straw;
- 2—
- leaving shredded straw combined with EM application;
- 3—
- removing straw combined with EM application;
- 4—
- removing straw.
3.2. Soil Samples
3.3. Chemical Analyses
3.4. Microbiological Analyses
3.5. Soil Respiration Measurement
3.6. Economic Calculations
3.7. Data Analysis
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Williams, A.; van der Bom, F.; Young, A.J. Resilient and dynamic soil biology. In No-Till Farming Systems for Sustainable Agriculture: Challenges and Opportunities; Dang, Y.P., Dalal, R.C., Menzies, N.W., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 251–266. [Google Scholar]
- Sokolowski, A.C.; McCormick, B.P.; de Grazia, J.; Wolski, J.E.; Rodríguez, H.A.; Rodríguez-Frers, E.P.; Gagey, M.C.; Debelis, S.P.; Paladino, I.R.; Barrios, M.B. Tillage and no-tillage effects on physical and chemical properties of an Argiaquoll soil under long-term crop rotation in Buenos Aires, Argentina. Int. Soil Water Conserv. Res. 2020, 8, 185–194. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Amundson, R. Managing for soil carbon sequestration: Let’s get realistic. Glob. Chang. Biol. 2018, 25, 386–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woźniak, A.; Rachoń, L. Effect of Tillage Systems on the Yield and Quality of Winter Wheat Grain and Soil Properties. Agriculture 2020, 10, 405. [Google Scholar] [CrossRef]
- Blanco-Canqui, H.; Ruis, S.J. No-tillage and soil physical environment. Geoderma 2018, 326, 164–200. [Google Scholar] [CrossRef]
- Liu, Z.; Cao, S.; Sun, Z.; Wang, H.; Qu, S.; Lei, N.; He, J.; Dong, Q. Tillage effects on soil properties and crop yield after land reclamation. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Haruna, S.; Nkongolo, N. Effects of Tillage, Rotation and Cover Crop on the Physical Properties of a Silt-Loam Soil. Int. Agrophysics 2015, 29, 137–145. [Google Scholar] [CrossRef]
- Sandén, T.; Spiegel, H.; Stüger, H.-P.; Schlatter, N.; Haslmayr, H.-P.; Zavattaro, L.; Grignani, C.; Bechini, L.; Hose, T.; Molendijk, L.; et al. European long-term field experiments: Knowledge gained about alternative management practices. Soil Use Manag. 2018, 34, 167–176. [Google Scholar] [CrossRef]
- Dang, Y.P.; Page, K.L.; Dalal, R.C.; Menzies, N.W. No-till Farming Systems for Sustainable Agriculture: An Overview. In No-Till Farming Systems for Sustainable Agriculture: Challenges and Opportunities; Dang, Y.P., Dalal, R.C., Menzies, N.W., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 3–20. [Google Scholar]
- Gülser, F.; Salem, S.; Gülser, C. Changes in some soil properties of wheat fields under conventional and reduced tillage systems in Northern Iraq. Eurasian J. Soil Sci. 2020, 9, 314–320. [Google Scholar] [CrossRef]
- Nouri, A.; Lee, J.; Yoder, D.C.; Jagadamma, S.; Walker, F.R.; Yin, X.; Arelli, P. Management duration controls the synergistic effect of tillage, cover crop, and nitrogen rate on cotton yield and yield stability. Agric. Ecosyst. Environ. 2020, 301, 107007. [Google Scholar] [CrossRef]
- Coonan, E.; Richardson, A.E.; Kirkby, C.A.; Kirkegaard, J.; Amidy, M.; Strong, C.L. Soil fertility and nutrients mediate soil carbon dynamics following residue incorporation. Nutr. Cycl. Agroecosyst. 2019, 116, 205–221. [Google Scholar] [CrossRef]
- Amanullah; Khalid, S.; Imran; Khan, H.A.; Arif, M.; Altawaha, A.R.; Adnan, M.; Fahad, S.; Parmar, B. Organic Matter Management in Cereals Based System: Symbiosis for Improving Crop Productivity and Soil Health. Sustain. Agric. Rev. 2019, 29, 67–92. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of United Nations. FAO Cereal Supply and Demand Brief. 2021. Available online: http://www.fao.org/worldfoodsituation/csdb/en/ (accessed on 27 September 2021).
- Lithourgidis, A.S.; Damalas, C.A.; Eleftherohorinos, I.G. Conservation Tillage: A Promising Perspective for Sustainable Agriculture in Greece. J. Sustain. Agric. 2009, 33, 85–95. [Google Scholar] [CrossRef]
- Moraru, P.; Rusu, T.; Paulette, L.; Buta, M.; Oroian, I.; Odagiu, A.; Clapa, D.; Cosma, S. Reducing Energy Consumption and Soil Conservation by Tillage System. ProEnviron. Promediu 2013, 6, 164–170. [Google Scholar]
- Moitzi, G.; Neugschwandtner, R.W.; Kaul, H.-P.; Wagentristl, H. Effect of tillage systems on energy input and energy efficiency for sugar beet and soybean under Pannonian climate conditions. Plant. Soil Environ. 2021, 67, 137–146. [Google Scholar] [CrossRef]
- Ribera, L.A.; Hons, F.M.; Richardson, J.W. An economic comparison between conventional and no-tillage farming systems in Burleson County Texas. Agron. J. 2004, 96, 415–424. [Google Scholar] [CrossRef]
- Jat, M.L.; Dagar, J.C.; Sapkota, T.B.; Singh, Y.; Govaerts, B.; Ridaura, S.L.; Saharawat, Y.S.; Sharma, R.K.; Tetarwal, J.P.; Jat, R.K.; et al. Climate Change and Agriculture: Adaptation Strategies and Mitigation Opportunities for Food Security in South Asia and Latin America. Adv. Agron. 2016, 137, 127–235. [Google Scholar]
- Šarauskis, E.; Buragienė, S.; Masilionytė, L.; Romaneckas, K.; Avižienytė, D.; Sakalauskas, A. Energy balance, costs and CO2 analysis of tillage technologies in maize cultivation. Energy 2014, 69, 227–235. [Google Scholar] [CrossRef]
- Fuentes-Llanillo, R.; Telles, T.S.; Junior, D.S.; de Melo, T.R.; Friedrich, T.; Kassam, A. Expansion of no-tillage practice in conservation agriculture in Brazil. Soil Tillage Res. 2020, 208, 104877. [Google Scholar] [CrossRef]
- Schjønning, P.; Jensen, J.L.; Bruun, S.; Jensen, L.S.; Christensen, B.T.; Munkholm, L.J.; Oelofse, M.; Baby, S.; Knudsen, L. The Role of Soil Organic Matter for Maintaining Crop Yields: Evidence for a Renewed Conceptual Basis. Adv. Agron. 2018, 150, 35–79. [Google Scholar] [CrossRef]
- Rusu, T. Energy efficiency and soil conservation in conventional, minimum tillage and no-tillage. Int. Soil Water Conserv. Res. 2014, 2, 42–49. [Google Scholar] [CrossRef] [Green Version]
- Gan, Y.; Siddique, K.H.; Turner, N.C.; Li, X.-G.; Niu, J.-Y.; Yang, C.; Liu, L.; Chai, Q. Ridge-Furrow Mulching Systems—An Innovative Technique for Boosting Crop Productivity in Semiarid Rain-Fed Environments. Adv. Agron. 2013, 118, 429–476. [Google Scholar] [CrossRef]
- Viaud, V.; Angers, D.A.; Parnaudeau, V.; Morvan, T.; Aubry, S.M. Response of organic matter to reduced tillage and animal manure in a temperate loamy soil. Soil Use Manag. 2010, 27, 84–93. [Google Scholar] [CrossRef]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The Role of Soil Microorganisms in Plant Mineral Nutrition—Current Knowledge and Future Directions. Front. Plant. Sci. 2017, 8, 1617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramesh, T.; Bolan, N.S.; Kirkham, M.B.; Wijesekara, H.; Kanchikerimath, M.; Rao, C.S.; Sandeep, S.; Rinklebe, J.; Ok, Y.S.; Choudhury, B.U.; et al. Soil organic carbon dynamics: Impact of land use changes and management practices: A review. Adv. Agron. 2019, 156, 1–107. [Google Scholar] [CrossRef]
- Coonan, E.; Kirkby, C.A.; Kirkegaard, J.A.; Amidy, M.R.; Strong, C.L.; Richardson, A.E. Microorganisms and nutrient stoichiometry as mediators of soil organic matter dynamics. Nutr. Cycl. Agroecosyst. 2020, 117, 273–298. [Google Scholar] [CrossRef]
- Qin, J.; Liu, H.; Zhao, J.; Wang, H.; Zhang, H.; Yang, D.; Zhang, N. The Roles of Bacteria in Soil Organic Carbon Accumulation under Nitrogen Deposition in Stipa baicalensis Steppe. Microorganisms 2020, 8, 326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, R.; Li, W.; Dong, W.; Tian, Y.; Hu, C.; Liu, B. Tillage Changes Vertical Distribution of Soil Bacterial and Fungal Communities. Front. Microbiol. 2018, 9, 699. [Google Scholar] [CrossRef]
- Buerkert, A.; Joergensen, R.; Ludwig, B.; Schlecht, E. Nutrient and Carbon Fluxes in Terrestrial Agro-Ecosystems. In Marschners Mineral Nutrition of Higher Plants; Marschner, P., Ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2012; pp. 473–482. [Google Scholar]
- Powlson, D.; Bhogal, A.; Chambers, B.; Coleman, K.; Macdonald, A.; Goulding, K.; Whitmore, A. The potential to increase soil carbon stocks through reduced tillage or organic material additions in England and Wales: A case study. Agric. Ecosyst. Environ. 2012, 146, 23–33. [Google Scholar] [CrossRef]
- Stockfisch, N.; Forstreuter, T.; Ehlers, W. Ploughing effects on soil organic matter after twenty years of conservation tillage in Lower Saxony, Germany. Soil Tillage Res. 1999, 52, 91–101. [Google Scholar] [CrossRef]
- Afanador-Barajas, L.N.; Navarro-Noya, Y.E.; Luna-Guido, M.L.; Dendooven, L. Impact of a bacterial consortium on the soil bacterial community structure and maize (Zea mays L.) cultivation. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Javaid, A. Effects of Biofertilizers Combined with Different Soil Amendments on Potted Rice Plants. Chil. J. Agric. Res. 2011, 71, 157–163. [Google Scholar] [CrossRef] [Green Version]
- Kotwica, K.; Jaskulska, I.; Gałęzewski, L.; Jaskulski, D.; Lamparski, R. Spring wheat yield in short-term monoculture depending on the tillage method, use of organic matter and a biostimulant. Acta Sci. Pol. Agric. 2014, 13, 19–28. [Google Scholar]
- PN-ISO 10390. Chemical and Agricultural Analysis—Determining Soil pH; Polish Standards Committee: Warszawa, Poland, 1997. [Google Scholar]
- PN-R-04023. Chemical and Agricultural Analysis—Determination of the Content of Available Phosphorus in Mineral Soils; Polish Standards Committee: Warszawa, Poland, 1996. [Google Scholar]
- PN-R-04022. Chemical and Agricultural Analysis—Determination of the Content Available Potassium in Mineral Soils; Polish Standards Committee: Warszawa, Poland, 1996. [Google Scholar]
- PN-R-04020. Chemical and Agricultural Analysis. Determination of the Content Available Magnesium; Polish Standards Committee: Warszawa, Poland, 1994. [Google Scholar]
- Atlas, R.M. Handbook of Microbiological Media, 4th ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2010. [Google Scholar]
- Crawford, D.L.; Lynch, J.M.; Whipps, J.M.; Ousley, M.A. Isolation and Characterization of Actinomycete Antagonists of a Fungal Root Pathogen. Appl. Environ. Microbiol. 1993, 59, 3899–3905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farm Accountancy Data Network. Available online: http://fadn.pl/en/ (accessed on 23 October 2021).
- Statistica, Data Analysis Software System, version 12; TIBCO Software Inc.: Palo Alto, CA, USA, 2019. Available online: https://www.tibco.com/products/data-science(accessed on 28 September 2021).
- Hawes, C.; Alexander, C.J.; Begg, G.S.; Iannetta, P.P.M.; Karley, A.J.; Squire, G.R.; Young, M. Plant Responses to an Integrated Cropping System Designed to Maintain Yield Whilst Enhancing Soil Properties and Biodiversity. Agronomy 2018, 8, 229. [Google Scholar] [CrossRef] [Green Version]
- Sørensen, C.G.; Halberg, N.; Oudshoorn, F.W.; Petersen, B.M.; Dalgaard, R. Energy inputs and GHG emissions of tillage systems. Biosyst. Eng. 2014, 120, 2–14. [Google Scholar] [CrossRef]
- Parcerisas, L.; Dupras, J. From mixed farming to intensive agriculture: Energy profiles of agriculture in Quebec, Canada, 1871–2011. Reg. Environ. Change 2018, 18, 1047–1057. [Google Scholar] [CrossRef]
- Özpinar, S.; Çay, A. Effects of minimum and conventional tillage systems on soil properties and yield of winter wheat (Triticum aestivum L.) in clay-loam in Çanakkale region. Turk. J. Agric. For. 2005, 29, 9–18. [Google Scholar]
- Chen, J.; Zhu, R.; Zhang, Q.; Kong, X.; Sun, D. Reduced-tillage management enhances soil properties and crop yields in a alfalfa-corn rotation: Case study of the Songnen Plain, China. Sci. Rep. 2019, 9, 17064. [Google Scholar] [CrossRef]
- Varvel, G.E.; Wilhelm, W. No-tillage increases soil profile carbon and nitrogen under long-term rainfed cropping systems. Soil Tillage Res. 2011, 114, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Han, S.H.; An, J.Y.; Hwang, J.; Bin Kim, S.; Park, B.B. The effects of organic manure and chemical fertilizer on the growth and nutrient concentrations of yellow poplar (Liriodendron tulipifera Lin.) in a nursery system. For. Sci. Technol. 2016, 12, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Kaur, K.; Kapoor, K.K.; Gupta, A.P. Impact of organic manures with and without mineral fertilizers on soil chemical and biological properties under tropical conditions. J. Plant. Nutr. Soil Sci. 2005, 168, 117–122. [Google Scholar] [CrossRef]
- Furtak, K.; Gajda, A.M. Activity and Variety of Soil Microorganisms Depending on the Diversity of the Soil Tillage System. In Sustainability of Agroecosystems; de Oliveira, A.B., Ed.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Jaskulska, I.; Romaneckas, K.; Jaskulski, D.; Gałęzewski, L.; Breza-Boruta, B.; Dębska, B.; Lemanowicz, J. Soil Properties after Eight Years of the Use of Strip-Till One-Pass Technology. Agronomy 2020, 10, 1596. [Google Scholar] [CrossRef]
- Allison, S.D.; Czimczik, C.I.; Treseder, K.K. Microbial activity and soil respiration under nitrogen addition in Alaskan boreal forest. Glob. Chang. Biol. 2008, 14, 1156–1168. [Google Scholar] [CrossRef] [Green Version]
- Jia, X.; Cao, H.; Jiang, L.; Yuan, J.; Zheng, S. Comparison of heat output and CO2 respiration to assess soil microbial activity: A case of ultisol soil. Plant Soil Environ. 2018, 64, 470–478. [Google Scholar]
- Mühlbachová, G.; Růžek, P.; Kusá, H.; Vavera, R.; Káš, M. Winter Wheat Straw Decomposition under Different Nitrogen Fertilizers. Agriculture 2021, 11, 83. [Google Scholar] [CrossRef]
- Thomsen, I.K.; Christensen, B.T. Yields of wheat and soil carbon and nitrogen contents following long-term incorporation of barley straw and ryegrass catch crops. Soil Use Manag. 2004, 20, 432–438. [Google Scholar] [CrossRef]
- Blanco-Canqui, H.; Lal, R. No-Tillage and Soil-Profile Carbon Sequestration: An On-Farm Assessment. Soil Sci. Soc. Am. J. 2008, 72, 693–701. [Google Scholar] [CrossRef] [Green Version]
- Higa, T. Effective microorganisms: A biotechnology for mankind. In Proceedings of the 1st International Conference on Kyusei Nature Farming, KhonKaen, Thailand, 19–21 October 1989; Parr, J.F., Hornick, S.B., Whitman, S.E., Eds.; USA Department of Agriculture: Washington, DC, USA, 1991; pp. 8–14. [Google Scholar]
- Dos Santos, L.F.; Lana, R.P.; Da Silva, M.C.; Veloso, T.G.; Kasuya, M.C.M.; Ribeiro, K.G. Effective microorganisms inoculant: Diversity and effect on the germination of palisade grass seeds. An. Acad. Bras. Cienc. 2020, 92, e20180426. [Google Scholar] [CrossRef] [Green Version]
- Szymanek, M.; Dziwulska-Hunek, A.; Zarajczyk, J.; Michałek, S.; Tanaś, W. The Influence of Red Light (RL) and Effective Microorganism (EM) Application on Soil Properties, Yield, and Quality in Wheat Cultivation. Agronomy 2020, 10, 1201. [Google Scholar] [CrossRef]
- Hu, C.; Qi, Y. Long-term effective microorganisms application promote growth and increase yields and nutrition of wheat in China. Eur. J. Agron. 2013, 46, 63–67. [Google Scholar] [CrossRef]
- Mi, Y.; Zhao, X.; Liu, F.; Sun, C.; Sun, Z.; Liu, L. Changes in soil quality, bacterial community and anti-pepper Phytophthora disease ability after combined application of straw and multifunctional composite bacterial strains. Eur. J. Soil Biol. 2021, 105, 103329. [Google Scholar] [CrossRef]
- Glenk, K.; Shrestha, S.; Topp, C.F.; Sánchez, B.; Iglesias, A.; Dibari, C.; Merante, P. A farm level approach to explore farm gross margin effects of soil organic carbon management. Agric. Syst. 2017, 151, 33–46. [Google Scholar] [CrossRef]
- Cook, S.; Nicholson, F.; Kindred, D.; Bhogal, A.; Roques, S.; Kerley, J.; Twining, S.; Brassington, T.; Gladders, P.; Balshaw, H.; et al. Straw Incorporation Review; Technical Report No. 81; Home-Grown Cereals Authority: Warwickshire, UK, 2013. [Google Scholar] [CrossRef]


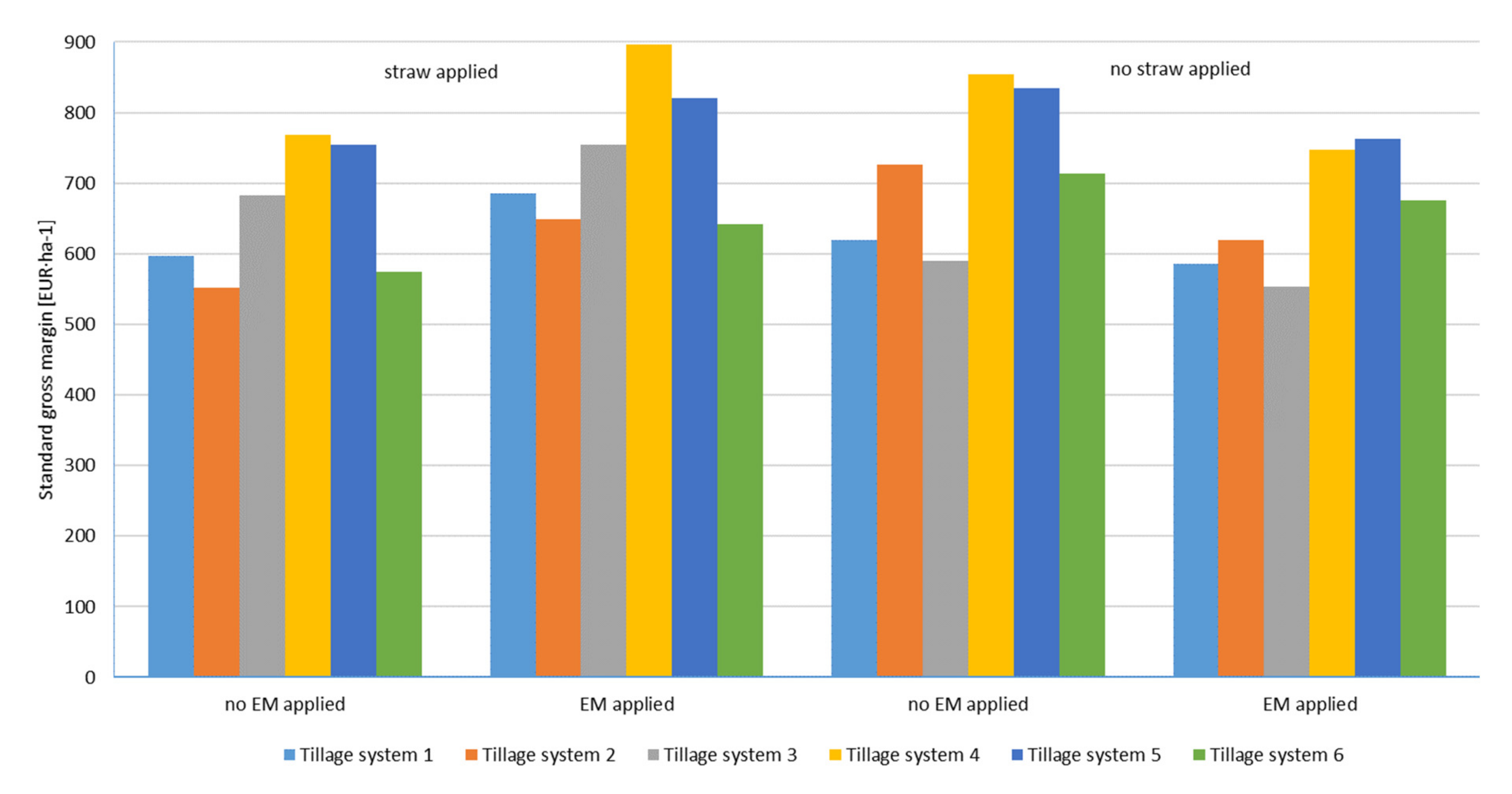
| Factor T Variants | Factor S Variants | Mean | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||||||
| Content | Wz | Content | Wz | Content | Wz | Content | Wz | ||
| Organic Carbon—OC (C g kg−1 d.m. soil) | |||||||||
| 1 | 16.7 | 1.00 | 30.8 | 1.02 | 22.9 | 0.99 | 17.1 | 0.98 | 21.9 |
| 2 | 25.9 | 1.04 | 24.7 | 1.06 | 28.4 | 1.02 | 20.6 | 1.06 | 24.9 |
| 3 | 12.7 | 1.02 | 28.8 | 1.02 | 18.4 | 0.98 | 16.5 | 0.98 | 19.1 |
| 4 | 31.1 | 1.04 | 21.5 | 1.06 | 21.1 | 1.01 | 25.0 | 1.00 | 24.7 |
| 5 | 32.1 | 1.03 | 18.9 | 1.03 | 21.5 | 1.01 | 24.6 | 1.00 | 24.3 |
| 6 | 31.1 | 1.05 | 21.5 | 1.06 | 20.8 | 1.01 | 25.0 | 1.00 | 24.6 |
| Mean | 24.9 | 24.4 | 22.2 | 21.5 | |||||
| Total Nitrogen—TN (N g kg−1 d.m. soil) | |||||||||
| 1 | 1.43 | 0.96 | 2.89 | 1.01 | 1.97 | 0.96 | 1.46 | 0.96 | 1.94 |
| 2 | 2.22 | 1.00 | 2.21 | 1.12 | 3.10 | 1.24 | 1.84 | 1.08 | 2.34 |
| 3 | 1.08 | 1.03 | 2.49 | 1.04 | 1.74 | 0.92 | 1.41 | 0.97 | 1.68 |
| 4 | 2.61 | 0.95 | 2.05 | 1.07 | 1.77 | 0.99 | 2.59 | 0.96 | 2.25 |
| 5 | 3.29 | 0.83 | 1.93 | 0.91 | 1.97 | 0.89 | 2.82 | 1.09 | 2.50 |
| 6 | 1.55 | 1.09 | 3.07 | 1.06 | 1.83 | 1.04 | 2.21 | 1.02 | 2.16 |
| Mean | 2.03 | 2.44 | 2.06 | 2.05 | |||||
| Factor T Variants * | Factor S Variants | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Mean | 1 | 2 | 3 | 4 | Mean | |
| autumn | spring | |||||||||
| N-NH4 (mg kg−1) | ||||||||||
| 1 | 15.6 | 13.3 | 15.1 | 16.5 | 15.1 | 21.0 | 21.4 | 21.4 | 15.6 | 19.9 |
| 2 | 21.4 | 21.1 | 21.8 | 22.4 | 21.7 | 28.0 | 28.4 | 27.5 | 27.4 | 27.8 |
| 3 | 17.3 | 16.3 | 16.6 | 19.0 | 17.3 | 19.4 | 20.8 | 20.0 | 17.2 | 19.3 |
| 4 | 17.1 | 15.0 | 15.1 | 16.7 | 16.0 | 27.3 | 28.2 | 26.5 | 26.4 | 27.1 |
| 5 | 16.1 | 14.7 | 14.8 | 18.0 | 15.9 | 27.3 | 27.8 | 27.4 | 26.4 | 27.2 |
| 6 | 19.5 | 18.9 | 19.8 | 21.1 | 19.8 | 30.5 | 33.6 | 28.2 | 28.2 | 30.1 |
| Mean | 17.8 | 16.5 | 17.2 | 19.0 | 25.6 | 26.7 | 25.2 | 23.5 | ||
| N-NO3 (mg kg−1) | ||||||||||
| 1 | 24.8 | 16.7 | 26.4 | 28.1 | 24.0 | 11.9 | 12.2 | 10.9 | 10.5 | 11.4 |
| 2 | 32.5 | 30.3 | 30.5 | 33.8 | 31.8 | 26.1 | 26.3 | 25.8 | 24.3 | 25.6 |
| 3 | 26.6 | 20.5 | 30.8 | 31.7 | 27.4 | 13.6 | 14.9 | 13.7 | 11.6 | 13.5 |
| 4 | 20.9 | 20.9 | 21.9 | 22.5 | 21.5 | 23.5 | 23.9 | 22.5 | 20.4 | 22.6 |
| 5 | 21.3 | 20.7 | 21.9 | 23.2 | 21.8 | 23.5 | 24.0 | 24.7 | 19.5 | 22.9 |
| 6 | 24.1 | 23.9 | 25.6 | 25.8 | 24.9 | 26.3 | 27.2 | 26.9 | 24.2 | 26.1 |
| Mean | 25.0 | 22.2 | 26.2 | 27.5 | 20.8 | 21.4 | 20.7 | 18.4 | ||
| TMN | ||||||||||
| 1 | 40.4 | 30.0 | 41,5 | 44.7 | 39.1 | 33.0 | 33.7 | 32.3 | 26.0 | 31.2 |
| 2 | 53.9 | 51.6 | 52.3 | 56.2 | 53.5 | 54.1 | 54.8 | 53.2 | 51.6 | 53.4 |
| 3 | 43.9 | 36.8 | 47.5 | 50.7 | 44.7 | 33.0 | 35.8 | 33.7 | 28.8 | 32.8 |
| 4 | 38.0 | 35.9 | 37.0 | 39.2 | 37.5 | 50.8 | 52.1 | 49.0 | 46.8 | 49.7 |
| 5 | 37.4 | 35.3 | 36.6 | 41.2 | 37.6 | 50.8 | 51.8 | 52.1 | 45.9 | 50.7 |
| 6 | 43.5 | 42.8 | 45.4 | 46.9 | 44.7 | 56.7 | 60.8 | 55.1 | 52.3 | 56.2 |
| Mean | 42.9 | 38.7 | 43.4 | 46.5 | 46.4 | 48.1 | 45.9 | 41.9 | ||
| LSD0.05 for: Factor T = 8.7; Factor S = 4.2; Interaction T/S = n.s.; S/T = n.s. ** | LSD0.05 for: Factor T = 8.5; Factor S = 3.9 Interaction T/S = n.s. S/T = n.s. | |||||||||
| Factor T Variants * | Factor S Variants | Mean | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||||||
| Content | Wz | Content | Wz | Content | Wz | Content | Wz | ||
| Phosphorus—Pa (mg kg−1 d.m. soil) | |||||||||
| 1 | 185.0 | 1.00 | 189.6 | 0.99 | 187.8 | 0.98 | 187.9 | 0.99 | 187.6 |
| 2 | 193.2 | 1.11 | 206.8 | 1.11 | 213.1 | 1.10 | 202.9 | 1.09 | 204.0 |
| 3 | 189.2 | 0.99 | 188.3 | 0.99 | 188.1 | 0.98 | 196.3 | 0.99 | 190.5 |
| 4 | 182.7 | 0.98 | 186.3 | 0.98 | 179.9 | 0.97 | 183.2 | 0.97 | 183.0 |
| 5 | 193.2 | 0.99 | 181.2 | 0.98 | 178.5 | 0.97 | 184.2 | 0.97 | 184.3 |
| 6 | 207.9 | 1.11 | 206.8 | 1.11 | 202.9 | 1.09 | 203.1 | 1.09 | 205.2 |
| Mean | 191.9 | 193.2 | 191.7 | 192.9 | |||||
| Potassium—Ka (mg kg−1 d.m. soil) | |||||||||
| 1 | 387.5 | 1.11 | 388.1 | 1.10 | 366.2 | 1.01 | 358.9 | 1.03 | 375.2 |
| 2 | 421.3 | 1.22 | 418.1 | 1.21 | 389.4 | 1.11 | 392.3 | 1.11 | 405.3 |
| 3 | 389.1 | 1.10 | 384.9 | 1.08 | 328.9 | 1.01 | 359.8 | 1.03 | 365.7 |
| 4 | 392.6 | 1.09 | 379.9 | 1.08 | 348.9 | 1.00 | 347.2 | 1.00 | 367.1 |
| 5 | 384.5 | 1.08 | 379.3 | 1.08 | 349.1 | 0.99 | 348.2 | 1.00 | 365.3 |
| 6 | 429.6 | 1.22 | 425.8 | 1.20 | 396.2 | 1.11 | 388.0 | 1.11 | 409.9 |
| Mean | 400.7 | 396.0 | 363.1 | 365.7 | |||||
| Magnesium—Mg (mg kg−1 d.m. soil) | |||||||||
| 1 | 42.3 | 0.99 | 48.9 | 0.99 | 39.2 | 0.98 | 43.5 | 0.98 | 43.5 |
| 2 | 48.6 | 1.03 | 47.2 | 1.02 | 44.9 | 1.01 | 50.3 | 1.03 | 47.7 |
| 3 | 48.2 | 0.99 | 47.4 | 0.99 | 52.3 | 0.99 | 42.3 | 0.99 | 47.5 |
| 4 | 49.3 | 0.98 | 50.1 | 0.99 | 43.5 | 0.97 | 41.2 | 0.98 | 46.0 |
| 5 | 42.5 | 0.99 | 46.2 | 0.99 | 47.5 | 0.98 | 44.4 | 0.98 | 45.1 |
| 6 | 42.1 | 1.03 | 45.2 | 1.04 | 48.2 | 1.03 | 43.2 | 1.01 | 44.7 |
| Mean | 45.5 | 47.5 | 45.9 | 44.1 | |||||
| pH | |||||||||
| 1 | 7.2 | 0.96 | 7.4 | 0.96 | 7.3 | 0.98 | 7.6 | 1.02 | 7.4 |
| 2 | 7.5 | 0.96 | 7.6 | 0.98 | 7.5 | 0.99 | 7.5 | 0.98 | 7.5 |
| 3 | 7.6 | 0.99 | 7.6 | 1.00 | 7.5 | 1.02 | 7.5 | 1.02 | 7.5 |
| 4 | 7.6 | 0.99 | 7.6 | 1.01 | 7.6 | 1.03 | 7.4 | 1.00 | 7.5 |
| 5 | 7.5 | 0.99 | 7.6 | 0.99 | 7.5 | 1.02 | 7.5 | 1.01 | 7.5 |
| 6 | 7.6 | 0.99 | 7.6 | 0.99 | 7.5 | 1.00 | 7.4 | 0.99 | 7.5 |
| Mean | 7.5 | 7.6 | 7.5 | 7.5 | |||||
| Factor T Variants * | Factor S Variants | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Mean | |
| Bacteria (106 cfu g−1) | |||||
| 1 | 40.5 | 48.0 | 35.2 | 34.1 | 39.4 |
| 2 | 78.5 | 93.0 | 68.3 | 59.7 | 74.9 |
| 3 | 45.3 | 40.9 | 41.5 | 42.9 | 42.7 |
| 4 | 69.5 | 72.5 | 51.5 | 49.2 | 60.7 |
| 5 | 56.3 | 57.9 | 49.0 | 51.0 | 53.6 |
| 6 | 89.3 | 92.8 | 75.8 | 79.6 | 84.4 |
| Mean | 63.3 | 67.5 | 53.5 | 52.7 | |
| LSD0.05 for: Factor T = 6.4; Factor S = 8.7; Interaction T/S = 12.7; S/T = 13.4 ** | |||||
| Actinobacteria (105 cfu g−1) | |||||
| 1 | 30.4 | 30.4 | 26.6 | 19.7 | 26.8 |
| 2 | 30.1 | 30.1 | 26.3 | 19.5 | 26.5 |
| 3 | 25.6 | 34.6 | 21.1 | 20.8 | 25.5 |
| 4 | 27.6 | 29.0 | 30.7 | 25.8 | 28.3 |
| 5 | 29.8 | 28.1 | 29.4 | 27.7 | 28.8 |
| 6 | 32.4 | 40.7 | 27.1 | 25.6 | 31.5 |
| Mean | 29.3 | 32.1 | 26.9 | 23.2 | |
| LSD0.05 for: Factor T = 3.9; Factor S = 4.2; Interaction T/S = 6.5; S/T = 6.4 | |||||
| Fungi (104 cfu g−1) | |||||
| 1 | 21.6 | 22.7 | 18.1 | 12.6 | 18.7 |
| 2 | 26.8 | 28.1 | 22.4 | 15.6 | 23.2 |
| 3 | 20.0 | 23.3 | 18.0 | 14.7 | 19.0 |
| 4 | 21.5 | 26.6 | 20.0 | 17.0 | 21.3 |
| 5 | 20.7 | 19.4 | 19.6 | 20.3 | 20.0 |
| 6 | 23.1 | 25.5 | 23.7 | 22.5 | 23.7 |
| Mean | 22.3 | 24.3 | 20.3 | 17.1 | |
| LSD0.05 for: Factor T = 1.3; Factor S = 3.0; Interaction T/S = 3.7; S/T = 4.4 | |||||
| Factor T Variants * | Factor S Variants | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Mean | |||||
| count | Wk | count | Wk | count | Wk | count | Wk | ||
| 1 | 43.7 | 1.21 | 51.2 | 1.41 | 38.0 | 1.05 | 36.2 | 1.00 | 42.3 |
| 2 | 81.8 | 2.26 | 96.3 | 2.66 | 71.1 | 1.96 | 61.8 | 1.71 | 77.8 |
| 3 | 48.1 | 1.32 | 44.5 | 1.23 | 43.8 | 1.21 | 45.1 | 1.27 | 45.4 |
| 4 | 72.5 | 2.0 | 75.7 | 2.09 | 54.8 | 1.51 | 51.9 | 1.43 | 63.7 |
| 5 | 59.5 | 1.62 | 61.0 | 1.66 | 52.1 | 1.44 | 53.9 | 1.49 | 56.7 |
| 6 | 92.8 | 2.56 | 97.1 | 2.68 | 78.7 | 2.17 | 82.4 | 2.27 | 87.7 |
| Mean | 66.4 | 71.0 | 56.5 | 55.2 | |||||
| LSD0.05 for: Factor T = 6.4; Factor S = 8.6; Interaction T/S = 12.7; S/T = 13.3 ** | |||||||||
| Factor T Variants * | Factor S Variants | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Mean | |
| 1 | 0.450 | 0.480 | 0.150 | 0.144 | 0.306 |
| 2 | 0.570 | 0.540 | 0.380 | 0.395 | 0.471 |
| 3 | 0.440 | 0.465 | 0.165 | 0.160 | 0.307 |
| 4 | 0.433 | 0.514 | 0.163 | 0.148 | 0.315 |
| 5 | 0.423 | 0.485 | 0.160 | 0.200 | 0.317 |
| 6 | 0.555 | 0.519 | 0.398 | 0.390 | 0.465 |
| Mean | 0.479 | 0.501 | 0.236 | 0.239 | |
| LSD0.05 for: Factor T = 0.109. Factor S = 0.223; Interaction T/S = 0.149; S/T = 0.237 ** | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Breza-Boruta, B.; Kotwica, K.; Bauza-Kaszewska, J. Effect of Tillage System and Organic Matter Management Interactions on Soil Chemical Properties and Biological Activity in a Spring Wheat Short-Time Cultivation. Energies 2021, 14, 7451. https://doi.org/10.3390/en14217451
Breza-Boruta B, Kotwica K, Bauza-Kaszewska J. Effect of Tillage System and Organic Matter Management Interactions on Soil Chemical Properties and Biological Activity in a Spring Wheat Short-Time Cultivation. Energies. 2021; 14(21):7451. https://doi.org/10.3390/en14217451
Chicago/Turabian StyleBreza-Boruta, Barbara, Karol Kotwica, and Justyna Bauza-Kaszewska. 2021. "Effect of Tillage System and Organic Matter Management Interactions on Soil Chemical Properties and Biological Activity in a Spring Wheat Short-Time Cultivation" Energies 14, no. 21: 7451. https://doi.org/10.3390/en14217451
APA StyleBreza-Boruta, B., Kotwica, K., & Bauza-Kaszewska, J. (2021). Effect of Tillage System and Organic Matter Management Interactions on Soil Chemical Properties and Biological Activity in a Spring Wheat Short-Time Cultivation. Energies, 14(21), 7451. https://doi.org/10.3390/en14217451







