FEC Additive for Improved SEI Film and Electrochemical Performance of the Lithium Primary Battery
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
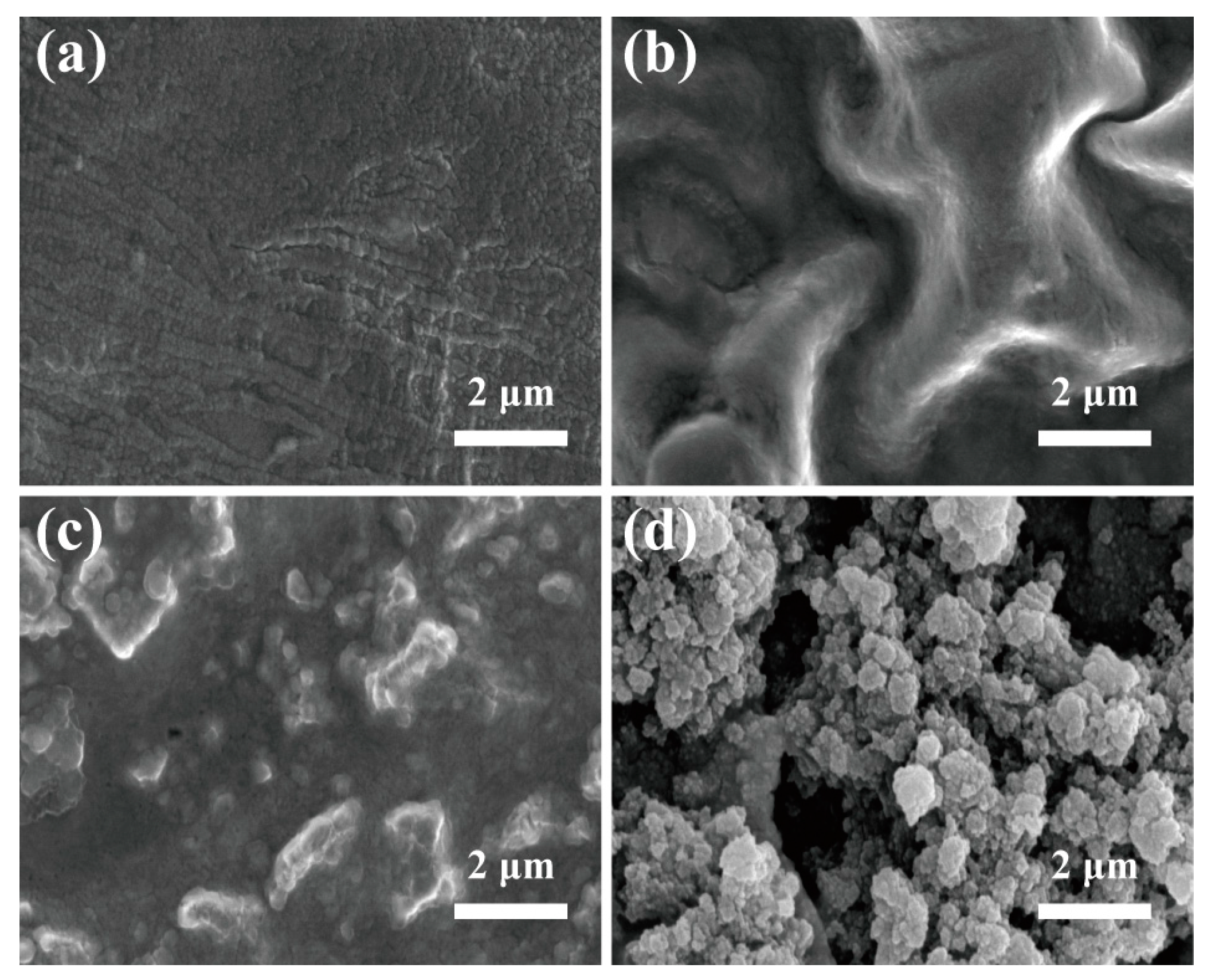
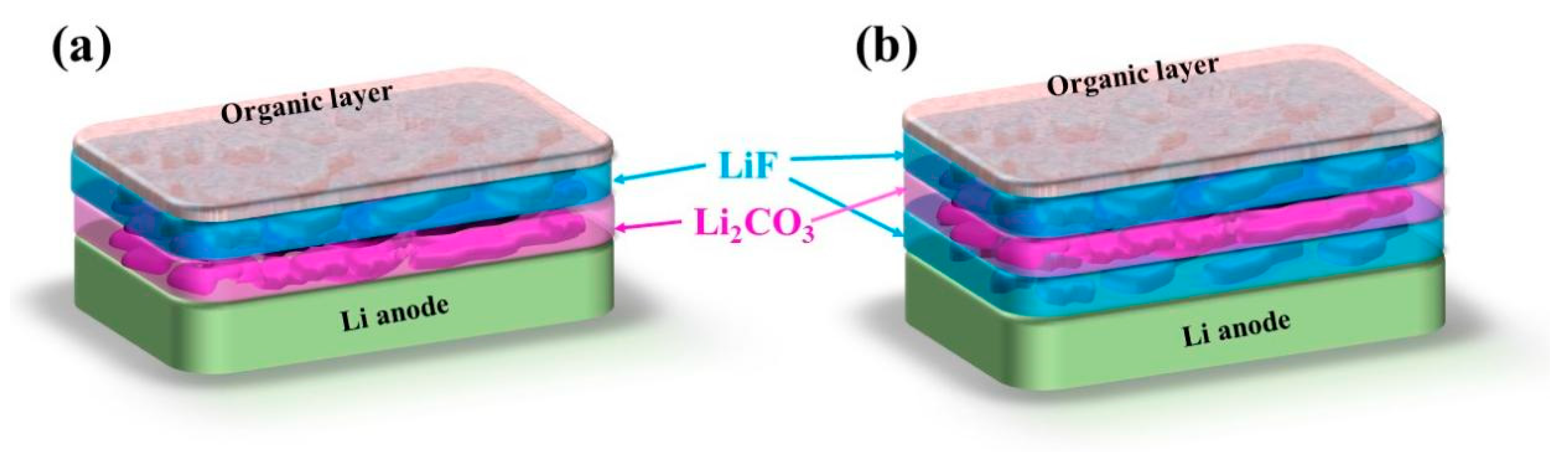
3.1. The Effect of Storage Temperature and Time on the SEI Film in Lithium Primary Batteries
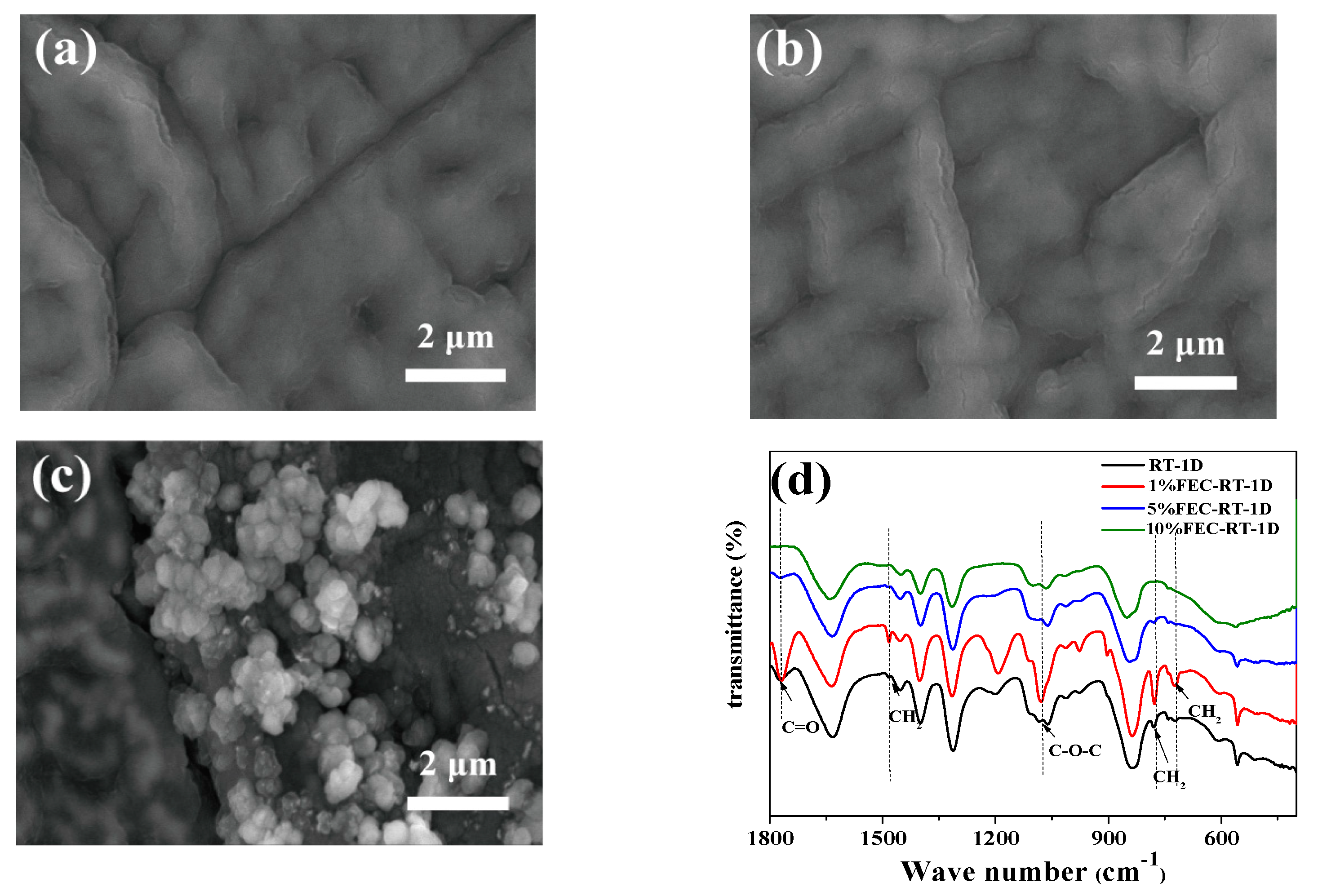
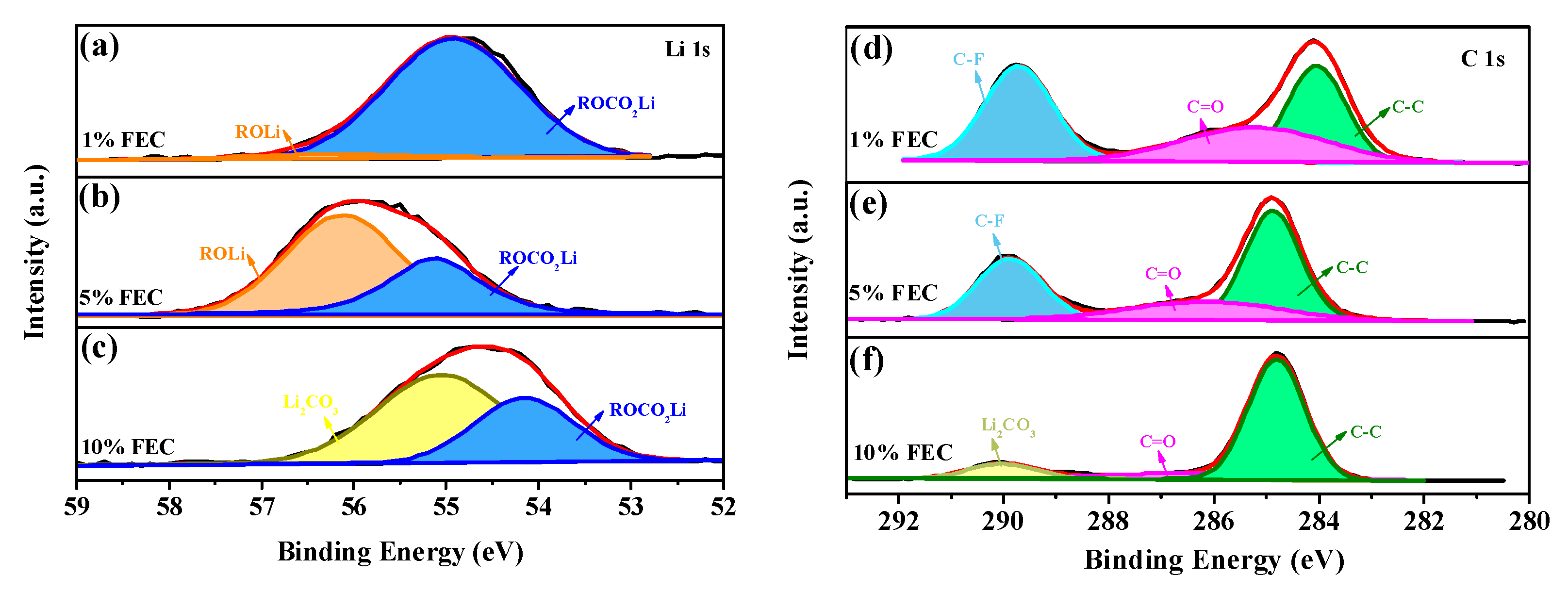
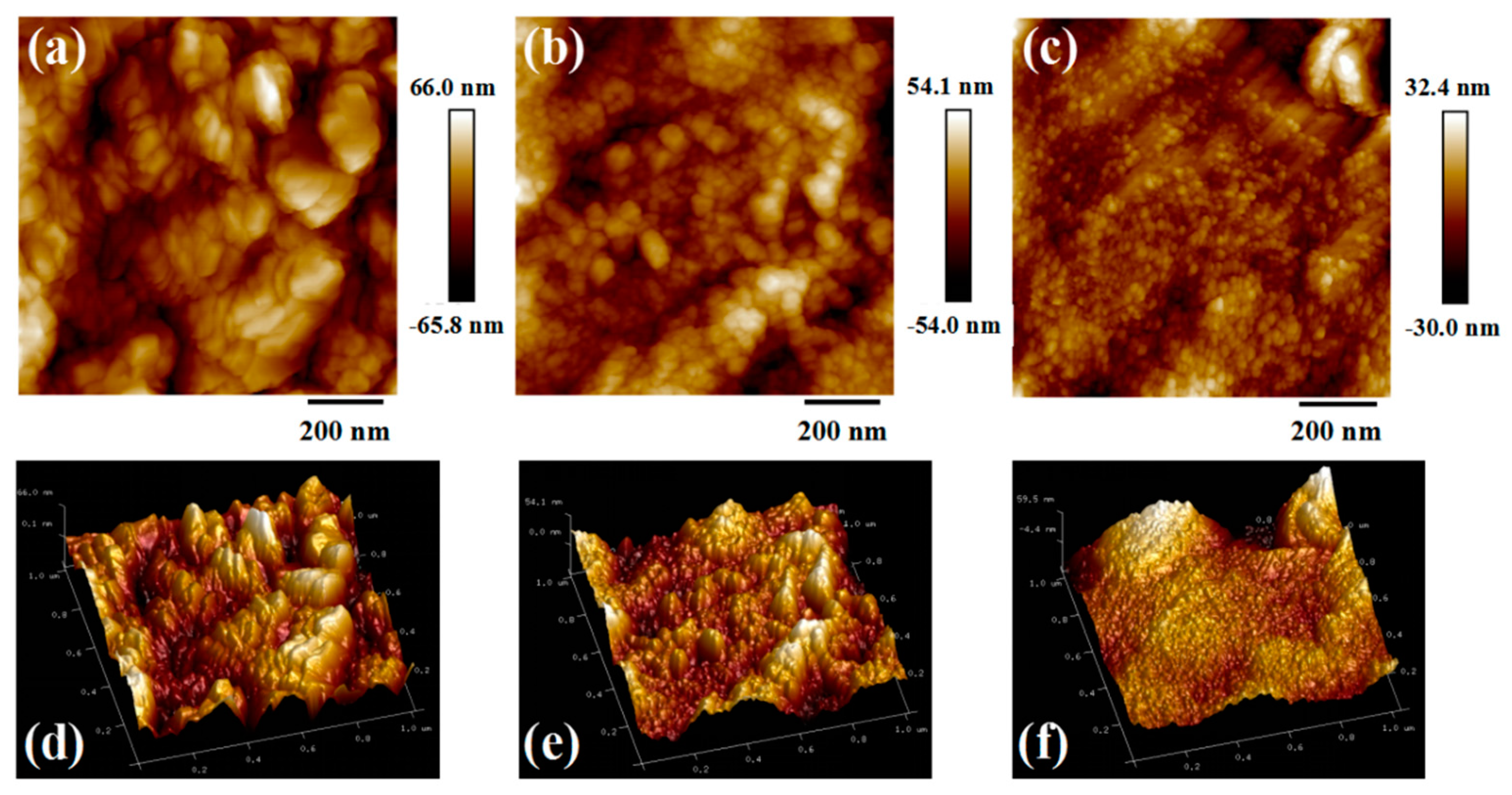
3.2. The Influence of FEC Additive on the SEI Film and Lithium Primary Battery Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raccichini, R.; Varzi, A.; Chakravadhanula, V.; Kübel, C.; Passerini, S. Boosting the power performance of multilayer graphene as lithium-ion battery anode via unconventional doping with in-situ formed Fe nanoparticles. Sci. Rep. 2016, 6, 23585. [Google Scholar] [CrossRef] [Green Version]
- Sun, P.-F.; Bai, P.-X.; Chen, Z.-F.; Su, H.; Yang, J.-X.; Xu, K.; Xu, Y.-H. A lithium-organic primary battery. Small 2020, 16, 1906462. [Google Scholar] [CrossRef]
- Karaolu, G.; Uzundal, C.-B.; Ulgut, B. Uneven discharge of metallic lithium causes increased voltage noise in Li/MnO2 primary batteries upon shorting. J. Electrochem. Soc. 2020, 167, 130534. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, Y.; Yan, X.-H.; Wang, B.-P.; Zou, Z.-L.; Han, F.-L.; Xue, T. Flexible and free-standing MnOx/reduced graphene oxide paper with excellent cycling stability for Li-ion battery anode. Bull. Mater. Sci. 2020, 43, 271. [Google Scholar] [CrossRef]
- Xu, G.-J.; Li, J.-D.; Wang, C.; Du, X.-F.; Lu, D.; Xie, B.; Wang, X.; Lu, C.-L.; Liu, H.-S.; Dong, S.-M.; et al. The formation/decomposition equilibrium of LiH and its contribution on anode failure in practical lithium metal batteries. Angew. Chem. Int. Ed. 2021, 60, 7770–7776. [Google Scholar] [CrossRef]
- Cheng, X.-B.; Zhang, R.; Zhao, C.-Z.; Zhang, Q. Toward safe lithium metal anode in rechargeable batteries: A review. Chem. Rev. 2017, 117, 10403–10473. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.-L.; Liu, S.-F.; Liu, B.; Wang, D.-H.; Zhong, Y.; Zhang, X.-Q.; Wang, X.-L.; Xia, X.-H.; Gu, C.-D.; Tu, J.-P. Bi-containing electrolyte enables robust and Li ion conductive solid electrolyte interphase for advanced lithium metal anodes. Front. Chem. 2020, 7, 952. [Google Scholar] [CrossRef]
- Lee, S.-H.; Hwang, J.-Y.; Ming, J.; Cao, Z.; Nguyen, H.-A.; Jung, H.-G.; Kim, J.; Sun, Y.-K. Toward the sustainable lithium metal batteries with a new electrolyte solvation chemistry. Adv. Energy Mater. 2020, 10, 2000567. [Google Scholar] [CrossRef]
- Jie, Y.-L.; Ren, X.-D.; Cao, R.-G.; Cai, W.-B.; Jiao, S.-H. Advanced liquid electrolytes for rechargeable Li metal batteries. Adv. Funct. Mater. 2020, 30, 1910777. [Google Scholar] [CrossRef]
- Xia, L.; Yu, L.-P.; Hu, D.; Chen, G.-Z. Electrolytes foe electrochemical energy storage. Mater. Chem. Front. 2017, 1, 584–618. [Google Scholar] [CrossRef]
- Young, J.; Kulick, P.-M.; Juran, T.-R.; Smeu, M. Comparative study of ethylene carbonated-based electrolyte decomposition at Li, Ca, and Al anode interfaces. ACS Appl. Energy Mater. 2019, 2, 1676–1684. [Google Scholar] [CrossRef]
- Yang, H.-J.; Guo, C.; Naveed, A.; Lei, J.-Y.; Yang, J.; Nuli, Y.-N.; Wang, L.J. Recent progress and perspective on lithium metal anode protection. Energy Storage Mater. 2018, 14, 199–221. [Google Scholar] [CrossRef]
- Zhou, Z.-X.; Feng, Y.-Y.; Wang, J.-L.; Liang, B.; Li, Y.-H.; Song, Z.-X.; Itkis, D.-M.; Song, J.-X. A robust, highly stretchable ion-conducive skin for stable lithium metal batteries. Chem. Eng. J. 2020, 396, 125254. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, J.-L.; Yang, Q.; Wang, S.-W.; Wang, W.-H.; Li, B.-H. Stable Cycling of high-voltage lithium-metal batteries enabled by high-concentration FEC-based electrolyte. ACS Appl. Mater. Interfaces 2020, 12, 22901–22909. [Google Scholar] [CrossRef]
- Gunnarsdóttir, A.-B.; Vema, S.; Menkin, S.; Marbella, L.-E.; Grey, C.-P. Investigating the effect of a fluoroethylene carbonate additive on lithium deposition and the solid electrolyte interphase in lithium metal batteries using in situ NMR spectroscopy. J. Mater. Chem. A 2020, 8, 14975–14992. [Google Scholar] [CrossRef]
- Heiskanen, S.-K.; Lucht, B.-L. Fluorinated acetic anhydrides as electrolyte additives to improve cycling performance of the lithium metal anode. J. Electrochem. Soc. 2020, 167, 110506. [Google Scholar] [CrossRef]
- Zhang, X.-Q.; Cheng, X.-B.; Chen, X.; Yan, C.; Zhang, Q. Fluoroethylene carbonate additives to render uniform Li deposits in lithium metal batteries. Adv. Funct. Mater. 2017, 27, 1605989. [Google Scholar] [CrossRef]
- Bai, Y.; Zhou, Z.-Z.; Zhan, C.; Ma, L.; Yuan, Y.-F.; Wu, C.; Chen, M.-Z.; Chen, G.-H.; Ni, Q.; Wu, F.; et al. 3D hierarchical nano-flake/micro-flower iron fluoride with hydration water induced tunnels for secondary lithium battery cathodes. Nano Energy 2017, 32, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Chun, J.; Jo, C.; Sahgong, S.; Kim, M.-G.; Lim, E.; Kim, D.-H.; Hwang, J.; Kang, E.; Ryu, K.-A.; Jung, Y.-S.; et al. Ammonium fluoride mediated synthesis of anhydrous metal fluoride-mesoporous carbon nanocomposites for high-performance lithium ion battery cathodes. ACS Appl. Mater. Interfaces 2016, 8, 35180–35190. [Google Scholar] [CrossRef] [PubMed]
- Owen, N.; Zhang, Q. Investigations of aluminum fluoride as a new cathode material for lithium-ion batteries. J. Appl. Electrochem. 2017, 47, 417–431. [Google Scholar] [CrossRef] [Green Version]
- Xia, H.; Wang, H.-L.; Xiao, W.; Lai, M.-O.; Lu, L. Thin film Li electrolytes for all-solid-state micro-batteries. Int. J. Surf. Sci. Eng. 2009, 3, 23–43. [Google Scholar] [CrossRef]
- Heiskanen, S.-K.; Kim, J.-J.; Lucht, B.-L. Generation and evolution of the solid electrolyte interphase of lithium-ion batteries. Joule 2019, 3, 2322–2333. [Google Scholar] [CrossRef]
- Eshkenazi, V.; Peled, E.; Burstein, L.; Golodnitsky, D. XPS analysis of the SEI formed on carbonaceous materials. Solid State Ion. 2004, 170, 83–91. [Google Scholar] [CrossRef]
- Luo, Q.; Chao, Y.-J.; Qu, B.; Ma, Z.-F.; Liao, X.-Z. Research technologies of solid electrolyte interphase in Li-ion batteries. Chin. J. Power Sources 2015, 2, 222–226. [Google Scholar]
- Wu, X.-J.; He, Z.-Y.; Wang, T.-Q.; Li, X.; Yang, D.-M.; Wang, Q.-S.; Li, Y. Effects of temperature on electrochemical impedance spectroscopy of the LiFePO4 battery. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; Volume 675, p. 012220. [Google Scholar]
- Zhang, Y.-Z.; Liu, Z.-H.; Wang, Z.; Jiang, Y.-P.; Wen, G.-W.; Gao, P.; Zhu, Y.-M. Electrochemical impedance spectroscopy study of lithium-rich material 0.5Li2MnO3·0.5LiNi1/3Co1/3Mn1/3O2 in the first two charge-discharge cycles. Electrochim. Acta 2019, 310, 136–145. [Google Scholar] [CrossRef]
- Chandrasiri, K.-W.-D.-K.; Nguyen, C.-C.; Zhang, Y.-Z.; Parimalam, B.-S.; Lucht, B.-L. Systematic investigation of alkali metal ions as additives for graphite anode in propylene carbonate based electrolytes. Electrochim. Acta 2017, 20, 285–291. [Google Scholar] [CrossRef]
- Lang, J.-L.; Long, Y.-Z.; Qu, J.-L.; Luo, X.-Y.; Wei, H.-H.; Huang, K.; Zhang, H.-T.; Qi, L.-H.; Zhang, Q.-F.; Li, Z.-C.; et al. One-pot solution coating of high quality LiF layer to stabilize Li metal anode. Energy Storage Mater. 2018, 16, 85–90. [Google Scholar] [CrossRef]
- Nie, M.-Y.; Abraham, D.-P.; Chen, Y.-J.; Bose, A.; Lucht, B.-L. Anode solid electrolyte interphase (SEI) of lithium-ion battery characterized by microscopy and spectroscopy. J. Phys. Chem. C 2014, 117, 254–263. [Google Scholar]
- Marroquín-Cardona, A.; Deng, Y.; Garcia-Mazcorro, J.-F.; Johnson, N.-M.; Mitchell, N.-J.; Tang, L.; Robinson, A.; Taylor, J.-F.; Wang, J.-S.; Phillips, T.-D. Characterization and safety of uniform particle size novasil clay as a potential aflatoxin enterosorbent. Appl. Clay Sci. 2011, 54, 248–257. [Google Scholar] [CrossRef] [Green Version]
- Mai, S.-W.; Xu, M.-Q.; Wang, Y.-T.; Liao, X.-L.; Lin, H.-B.; Li, W.-S. Methylene Methanedisulfonate (MMDS) as a novel SEI forming additive on anode for lithium ion batteries. Int. J. Electrochem. Sci. 2014, 9, 6294–6304. [Google Scholar]
- Wang, D.; Zhang, W.; Zheng, W.-T.; Cui, X.-Q.; Rojo, T.; Zhang, Q. Towards high-safe lithium metal anodes: Suppressing lithium dendrites via tuning surface energy. Adv. Sci. 2017, 4, 1600168. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liang, Q.-W.; Wang, G.; Liu, D.-D.; Xiong, X.-H. Grain-boundary-rich artificial SEI layer for high-rate lithium metal anodes. Adv. Funct. Mater. 2021. [Google Scholar] [CrossRef]
- Lei, W.-Y.; Liu, Y.-Y.; Jiao, X.-X.; Zhang, C.-F.; Xiong, S.-Z.; Li, B.; Song, J.-X. Improvement of cycling phosphorus-based anode with LiF-rich solid electrolyte interphase for reversible lithium storage. ACS Appl. Energy Mater. 2019, 2, 2699–2707. [Google Scholar] [CrossRef]
- Huang, Z.-H.; Li, X.; Wang, Q.-S.; Duan, Q.-L.; Li, Y.; Li, L.-N.; Wang, Q.-S. Experimental investigation on thermal runaway propagation of large format lithium-ion battery modules with two cathodes. Int. J. Heat Mass Transf. 2021, 172, 121077. [Google Scholar] [CrossRef]
- Liu, Q.; Cresce, A.; Schroeder, M.; Xu, K.; Mu, D.-B.; Wu, B.-R.; Shi, L.-L.; Wu, F. Insight on lithium metal anode interphasial chemistry: Reduction mechanism of cyclic ether solvent and SEI film formation. Energy Storage Mater. 2019, 17, 366–373. [Google Scholar] [CrossRef]
- Yan, C.; Cheng, X.-B.; Tian, Y.; Chen, X.; Zhang, X.-Q.; Li, W.-J.; Huang, J.-Q.; Zhang, Q. Dual-layered film protected lithium metal anode to enable dendrite-free lithium deposition. Adv. Mater. 2018, 30, 1707629. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Li, P.; Tang, Z.; Liu, J.; Zhang, S.; Zhou, Y.; Tian, X. FEC Additive for Improved SEI Film and Electrochemical Performance of the Lithium Primary Battery. Energies 2021, 14, 7467. https://doi.org/10.3390/en14227467
Zhou X, Li P, Tang Z, Liu J, Zhang S, Zhou Y, Tian X. FEC Additive for Improved SEI Film and Electrochemical Performance of the Lithium Primary Battery. Energies. 2021; 14(22):7467. https://doi.org/10.3390/en14227467
Chicago/Turabian StyleZhou, Xuan, Ping Li, Zhihao Tang, Jialu Liu, Shaowei Zhang, Yingke Zhou, and Xiaohui Tian. 2021. "FEC Additive for Improved SEI Film and Electrochemical Performance of the Lithium Primary Battery" Energies 14, no. 22: 7467. https://doi.org/10.3390/en14227467
APA StyleZhou, X., Li, P., Tang, Z., Liu, J., Zhang, S., Zhou, Y., & Tian, X. (2021). FEC Additive for Improved SEI Film and Electrochemical Performance of the Lithium Primary Battery. Energies, 14(22), 7467. https://doi.org/10.3390/en14227467





