1. Introduction
Urea
is a compound that is produced in the organism through a series of enzymatic reactions occurring in the cytoplasmic matrix and mitochondria. During this catabolic process, an excess of ammonia (NH
4+) is liberated during amino acid catabolism and protonates the conjugate base of a stronger acid (HCO
3−) in coupled reactions to produce urea, a less toxic compound which is subsequently transported by the blood to the kidneys and excreted in the urine [
1].
Urea is the main form of urinary nitrogen among the different nitrogenous constituents in the urine. The amount of urea is mainly influenced by the N-intake. The remaining nitrogen is found to be present in other compounds such as allantoin, hippuric acid, creatinine, creatine, uric acid, xanthine, hypoxanthine, free amino acid N, and ammonia [
2].
Urine has traditionally been considered as a waste product, and often contributes to environmental pollution by reducing nitrogen. Proper disposal and management are essential, particularly in places with wastewater containing urea and manure, which have the potential to be used as an alternate fertilizer for agricultural producers. This can lead to a positive trade balance by reducing the import of chemical fertilizers if the production process is performed at a large scale. However, if urea reacts with isocyanic acid, which is a product of urea decomposition, it can condensate to biuret, and eventually to triuret, and cyanuric acid, which are considered as undesirable impurities in urea-based fertilizers and also in urea-based selective catalytic reduction systems [
3].
According to Mikkelsen [
4], plant growth, protein synthesis, and internal N metabolism are affected by the presence of biuret and, although it can be mineralized by soil microorganisms, the process occurs at a slower rate than for urea. Equation (1) describes the condensation of urea into biuret. The stoichiometry reflects that the reaction is second order. In the case of small relative concentration variation, the rate can be expressed as
.
Urea hydrolysis by urease is required in order to obtain nitrogen urea derived for plants’ uptake. The action of urease enzyme in the hydrolysis of urea is described in the following reactions [
5]:
Generation of carbon dioxide and free volatile ammonia leads to the corresponding loss of nitrogen. Because this enzyme is ubiquitous in the environment, this process is very likely to occur. In agriculture, microorganisms present in solid excretory product and soil are responsible for enzymatic urea hydrolysis [
6]. This reaction leads to environmental pollution; as a result, it is essential to stabilize urea compounds contained in the liquid residue [
7].
Reactions of urea in acid or in alkali media are some of the possible means to achieve urea stabilization. The addition of acid prevents volatilization of ammonia by converting free ammonia into non-volatile ammonium. According to Equation (6), NH
3, NH
4+, and H
+ exist in equilibrium, which strongly depends on temperature, humidity, NH
4+ concentration, and pH. By decreasing the pH, the equilibrium is shifted to the left, which decreases the concentration of un-ionized ammonia in the liquid, attenuating the emission of NH
3. According to de Oliveira Vilela et al. [
8], at pH lower than 7, the H
+ ions increase the amount of nonvolatile NH
4+ while decreasing the quantity of volatile NH
3. This is the basic principle behind acidification of waste slurries to diminish emissions of NH
3 during storage. Accordingly, increasing the temperature affects the dissociation constant (K
d) and the fraction of un-ionized ammonia increases. Equation (7) represents the estimation of K
d for dilute aqueous solutions at 25 °C [
9]:
Urea can be also stabilized in alkali media to prevent enzymatic hydrolysis. Simha et al. reported the alkaline dehydration of fresh urine using MgO and MgO mixed with biochar, wheat bran, or calcium hydroxide at 50 °C. Co-substrates were used to facilitate the disintegration of the peptide layer over the surface of urine, which limited the evaporative removal of water. In this process, urine was dried to produce a solid fertilizer. A concentration of 2 g·L
−1 MgO was necessary to achieve saturation and to increase the pH to a value of 10 and to inhibit the enzyme-catalyzed hydrolytic degradation of urea [
10]. It was suggested to avoid higher temperatures, because this could lead to the reactivation of urease, as more than 40 °C decreased the saturation pH to <10.
The decomposition of urea in aqueous media was studied previously by Shaw and Bordeaux [
11], using the method of initial rates to estimate the entropy and activation energy at different values of acid concentration and ionic strength. They found that the reaction with respect to urea was of first order and that the presence of acid promoted the inhibition of urea decomposition, rather than acting as a catalyst. Additionally, reaction rates in water alone were lower than in acid due to higher ionic strength of the acid solution. Activation energies of 32.7 and 32.4 kcal were obtained using solutions of 50 mM H
2SO
4 and H
2O, respectively. They reported values of the rate constant between 2 × 10
−7 s
−1 (60 °C) and 4.15 × 10
−5 s
−1 (100 °C).
Urea is widely used in soil and foliar applications, providing benefits in agricultural production. The principal use of urea in agriculture is to provide nitrogen for soil fertilizer formulations. Fertilizers containing N, P, and K are important because these elements are in lower concentrations in soils and are also rapidly depleted once the plant is removed during the harvesting season. As a result, gradual addition of these elements to the soil helps to promote fertility. Some organic materials that are generally used to incorporate these required elements are manure, compost, and treated sewage sludge [
12]. However, the use of urea-derived compounds in agricultural production faces some challenges, mainly in terms of NH
3 volatilization, Nitrogen dioxide accumulation and phytotoxicity problems have been associated with adverse effects on seed germination and seedling growth in the presence of NH
3 [
13]. Some of the studies concerning the production of urea derivatives with application as fertilizers include research by Biskupsi et al. [
14] who reported a continuous process for the manufacturing of urea-superphosphate and phosphate fertilizers based on the decomposition of phosphate raw materials with urea solutions (1.5–4 mol) in acid media, and using 90% to 100% with respect of the stoichiometric requirement to achieve complete decomposition of the rock phosphate. They stressed the importance of the possibility of occurrence of exothermic reactions, which could represent a threat to the safety of the process and leading eventually to injury, loss of life, or damage to instruments and property. The main reactions that gave rise to a safety threat were the exothermic urea hydrolysis reaction, including neutralization of released ammonia in the presence of mineral acids (H
2SO
4 and H
3PO
4) and exothermic reactions that lead to the formation of amide compounds. Another study reported the reaction of H
3PO
4 with urea and ammonia to obtain solid and liquid concentrated fertilizers based on ammonium and urea polyphosphates containing N, P, and K through ammoniation and condensation of urea phosphate, which favored the separation of the impurities such as iron, aluminum, calcium, magnesia, and fluorine that were present in the liquid phase. Accordingly, the products obtained could be utilized after dissolution in water, by spraying, injection, sprinkling, or dilution as other common and commercial fertilizers [
15]. Additional works have reported the manufacturing of stable products containing mono and diurea sulfates free of sulfamic acid and ammonium sulfamate produced from the reaction between concentrated urea and sulfuric acid used in stoichiometric quantities [
16].
Thermal decomposition of urea (>130 °C) has been studied previously using different analytical methods, i.e., thermogravimetric analysis (TG), differential scanning calorimetry (DSC), ammonium ion-selective electrode (ISE), high performance liquid chromatography (HPLC), and Fourier transform-infrared (FT-IR) spectroscopy [
17]. It has been reported that this process can lead to a diversity of undesired intermediates and by-products, namely cyanic acid, ciamelide, cyanuric acid, ammelide, melamine, biuret, and triuret, among others [
18,
19,
20,
21]. For this reason, it is important to ensure that the proposed technologies for wastewater treatment containing urea avoid raising temperatures to higher levels. Taking this into consideration, we approach the stabilization of urea in acid media, modeling the reaction of urea with sulfuric acid and urea with phosphoric acid and estimating the enthalpy of the reaction and the adiabatic heat difference.
Stream effluents from wastewater originated in farms and companies that focus on agricultural production are characterized for containing urea, organic matter, and high values of oxidizable organic matter. These effluents have to be treated before final disposal in order to remove colloidal suspended solids and improve water quality. Particularly, in the case of effluents that contain urea, an additional benefit can be explored if the sedimentation allows the formation of compounds with added value such as fertilizers. Additionally, the separated water can be recycled in the premises of the company and used in operations that do not require high levels of purity. Efficient use of water resources is important because critical drought periods have limited the supply of water, leading to an increment in operational costs. The proposal of a technology that promotes the reuse of water while favoring the formation and separation of high value-added compounds from the wastewater is a solution approach that will benefit this type of company. In this research, we aimed to study the reaction of urea with mineral acids to support urea stabilization by describing the mathematical modeling of such a process and estimating the enthalpy of the exothermic reaction and the adiabatic heat difference, which is particularly important for the safety of the process. We also performed sedimentation tests to evaluate the rate of settlement using H3PO4 and H2SO4 to promote the formation of a coagulated solid phase that can be used as a valuable organic nitrogen fertilizer.
1.1. Role of Sulfur in Plants
Sulfur is one of the chemical elements required in large amounts by plants (>1000 mg·kg
−1); however, in order to be absorbed through the root system it is required to be in the form of sulfate. The reaction of urea with sulfuric acid leads to the generation of ammonium sulfate, a compound that is generally used as fertilizer containing 21% of N and 24% of S, which is normally incorporated within the irrigation water by the drip system and also through direct soil application. It is quickly absorbed by the plant and can be used alone or in a mixture combined with other fertilizers. Once absorbed by the roots, it allows sulfur to be incorporated into the structure of o-acetylserine, which eventually contributes to the formation of L-cysteine by a complex variety of isoforms that are present in the cytosol, chloroplasts, and mitochondria [
22]. The most significant sources of S are ammonium sulfate, single superphosphate, and potassium sulfate. Sulfur concentration in plant tissues varies between 0.1 and 0.5% [
23]. For most common crops, SO
4−2 concentrations of 3–5 ppm are sufficient, except for rapeseed/canola, alfalfa, and broccoli, which require higher concentration. Deficiency of sulfur in the soil has been associated with reduced plant growth, occurrence of uniform yellowing of leaf tissue due to a lack of chlorophyll on younger leaves, reduction in N and P fixation by affecting nodule development and function, and accumulation of nonprotein N as NH
2 and NO
3− in leaves. This affects the optimum N:S ratio needed for effective N use by rumen microorganisms and reduces food quality [
24].
Sulfur compounds are also used as fungicides, particularly to prevent fungal spores from germinating, and against powdery mildew, rose black spot, rusts, and other diseases. During the nitrification process, the conversion of NH
4+ to NO
3− liberates H
+ and reduces the alkalinity of the soil. This can be explained thorough Equations (8)–(10). It can be seen that ammonium sulfate generates double the amount of H
+ in comparison to urea or ammonium nitrate and promotes acidification. As a result, this creates a favorable environment to keep other elements in solution, such as P, Fe, Zn, B, Cu, and Mn, especially in cases of alkaline soils, and increases its availability and further absorption.
1.2. Importance of Phosphorous in Plant
Diffusion and mass flow contribute to the transport of P from the soil to the root surface. The main role of P in the plant is to participate in membrane transport, and energy storage and transfer. After plants perform the photosynthesis process, carbohydrates and phosphate compounds are required for plant growth and the reproductive process. Phosphorous is present in ATP and ADP molecules, which actively participate in biosynthesis of proteins, phospholipids, and nucleic acids. When P is in low concentrations, growth and development is restricted [
24]. Experiments in the field have been performed in soil with a low concentration of phosphorus. In such cases, several N, P, NP, and NPS fertilizers were applied. It was observed that a very low yield was obtained in the control treatment and the treatment with nitrogen alone. However, when phosphorus was applied, seeds’ yield increased dramatically. The best results were obtained with a mixture of urea, elemental sulfur, and a natural phosphate obtained from a marine sediment, followed by ammonium phosphate [
25].
3. Discussion
One of the main problems present in agricultural production is the disposal of organic waste. Wastewater contains an average of 5% dry matter, which is in the form of fine particles’ dispersion. Dry matter is produced by excrement and has a major share in the high COD value, which has to be reduced. Very often, the treatment is performed on the premises of the company; however, problems can arise when the capacity of the treatment plant is at critical levels, particularly during periods of drought and clean water deficit, which are factors that present a real threat to its efficiency. An alternative could be the construction of an upstream separation plant, where streams with higher chemical demand of oxygen could be reduced to the standard required values through coagulation and sedimentation. As a result, the liquid phase with reduced COD values could be partially recycled and purified at the treatment plant. Recent periods of severe drought have caused a sharp drop in groundwater levels and thus of its reserves. This has been a problem for farms which frequently rely on using water sources coming from their own wells, thus forcing them to use water from public sources and increasing operating costs. The reduction in the COD level allows the company to avoid operating at critical limits of its capacity, and decreases the load on the wastewater treatment plant and consumption of incoming clean water. The coagulated solid phase can be drained and used as a valuable organic nitrogen fertilizer.
Several sources of nitrogenous and phosphorous fertilizer are used in agriculture including ammonia, urea, ammonium nitrate, phosphonitrate, ammonium sulfate, anhydrous ammonia, potassium nitrate, di-ammonium phosphate, and monoammonium phosphate. They differ not only in the content of N or K, but also in the efficiency, as not all is used and assimilated equally by the plant. Ammonia, for example, has a N content of 82% but because it is in gas phase, only between 30% and 50% is approximately absorbed by the plant as the rest is lost through volatilization. By comparison, ammonium sulfate has a N content of 21% but has an efficiency between 70% and 85%. Efficiency values represent the kg of biomass produced per kg of nutrient applied. These values can be improved by means of fractional fertilization as fewer amounts of nutrients are lost through washing or soil drainage. Another factor that should be taken into account is the cation exchange capacity of the soil. Soil colloid has a negative charge; as a result, it has affinity towards cations and repels anions according to the number of positive charges of the ion. In the case of heavy rain, nitrates tend to be leached from the soil or drained in the case of soil saturation leading to loss of nitrogen, which is not the case when nitrogen is incorporated in the fertilizer as ammonium nitrate, di-ammonium phosphate, or monoammonium phosphate. Accordingly, it is important to know that there is no unique fertilizer that will solely improve the efficiency of a crop. Several factors, such as season, daily temperatures, soil properties, humidity, type of crop, soil salinity, pests, and diseases will determine the efficiency. Running tests in a control zone and through several rows placed on different parts of the field for assessing the crop response to different fertilizer rates and nutrient combinations allows farmers to conduct research on their own soil and fields for that particular season of the year and conditions.
Another factor that influences the fertilizer efficiency is the plant growth stage at which it is applied. It is important to consider the use of fertilizer from the sprout phase through the vegetative phase and before the flowering to have better assimilation of nutrients. This implies the use of fractional fertilization adding different quantities of nutrients according to the physicochemical characterization of the soil (pH, salinity and nutrients concentration, microbial diversity in the soil etc.) and environmental parameters (temperature, humidity, etc.).
The stabilization of urea from wastewater also has the added benefit of contributing significantly to the reduction in the value of COD. It also prevents the growth of microorganisms due to biofilm and planktonic growth [
27]. Additionally, the implementation of this type of technologies would reduce the consumption of pure water by recycling the separated liquid. After acid stabilization of urea and further neutralization with alkali, the solid phase has the potential to be used as a valuable nitrogen fertilizer. Part of the separated liquid with a low COD value of about 60% can be recycled and used as rinsing water in the washing of floors. This will reduce the load on the wastewater treatment plant and the consumption of incoming clean water, and help to solve problems related to dry seasons.
One of the main nutrients needed for the nutrition of cultivated plants is nitrogen, which is supplied to the soil in the form of organic compounds or as inorganic soluble nitrogen salts. It is important to keep in mind that inorganic nitrogen fertilizer supplies the cultivated plant with the necessary nitrogen, but can also negatively affect the soil structure by reducing its ability to bind the necessary nutrients. For this reason, there has been a recent increase in interest in organic nitrogen fertilizers. However, natural sources of nitrogen organic fertilizers are limited, and for this reason an effort to obtain new sources for organic nitrogen fertilizers is recommended. Additionally, this type of organic fertilizer has the potential to be applied as biostimulants, and to increase the plant resistance to immunity to a wide range of harmful pathogens, and also as biological insecticides. Successful attempts were previously reported by our research group, using an organo-nitrogenous fertilizer (enzymatic hydrolysate) in lettuce plants. Considerable differences were found in the yields and nitrate concentration in comparison to a control crop (unfertilized soil) and an available commercial fertilizer (blend of ammonium nitrate and urea in a 1:1 ratio based on nitrogen content). This had a positive effect on the growth and development of the tested lettuce plants with a considerable higher value as a foodstuff, taking into account the low nitrates content obtained in the plant treated with the enzymatic hydrolysate [
28].
4. Conclusions
The proposed reaction system has the potential to be used in places where urine is disposed in the wastewater. The farm industry is one example, in the special case that the liquid residues originated from the sanitization and washing of floors that contained urea. The reactions studied contribute to the production of ammonium sulfate and ammonium dihydrogen phosphate. The proposed reaction of urea with sulfuric acid and phosphoric acid also yields multiple compounds, such as ammonium hydrogen sulfate, amidosulfonic acid, or urea phosphate. We focused our analysis on the modeling of ammonium sulfate and ammonium dihydrogen phosphate due to their importance as fertilizers. However, the presence of other multiple compounds does not cause nitrogen losses, which is the key parameter for application in agriculture. Those compounds can be used to prepare a balanced solution containing N, S, and K, and complement fertilizer mixtures tailored to the characteristics of the particular soil and crop to provide the missing nutritional elements, and also based on additional characteristics such as pH, organic matter, and texture. Proper understanding and quantification of how the nutrients are diffused and transformed in the soil, including by the action of enzymes and microorganisms, i.e., mineralization, reduction, and oxidation, is essential for a sustainable agricultural management.
Enrichment of the resulting fertilizer with phosphorus could be also approached by performing firstly alkaline hydrolysis of the feedstock, followed by adjust of the pH to a level suitable for the use of proteolytic enzymes by neutralization with phosphoric or nitric acid. At the end of both phases of the hydrolysis, the pH could be adjusted to a value close to 7; with this combination, the resulting hydrolysate would also be enriched in potassium. Additionally, further derived products based on phosphorus in the form of polyphosphate can be also considered to analyze the most suitable form in terms of applicability to the plant.
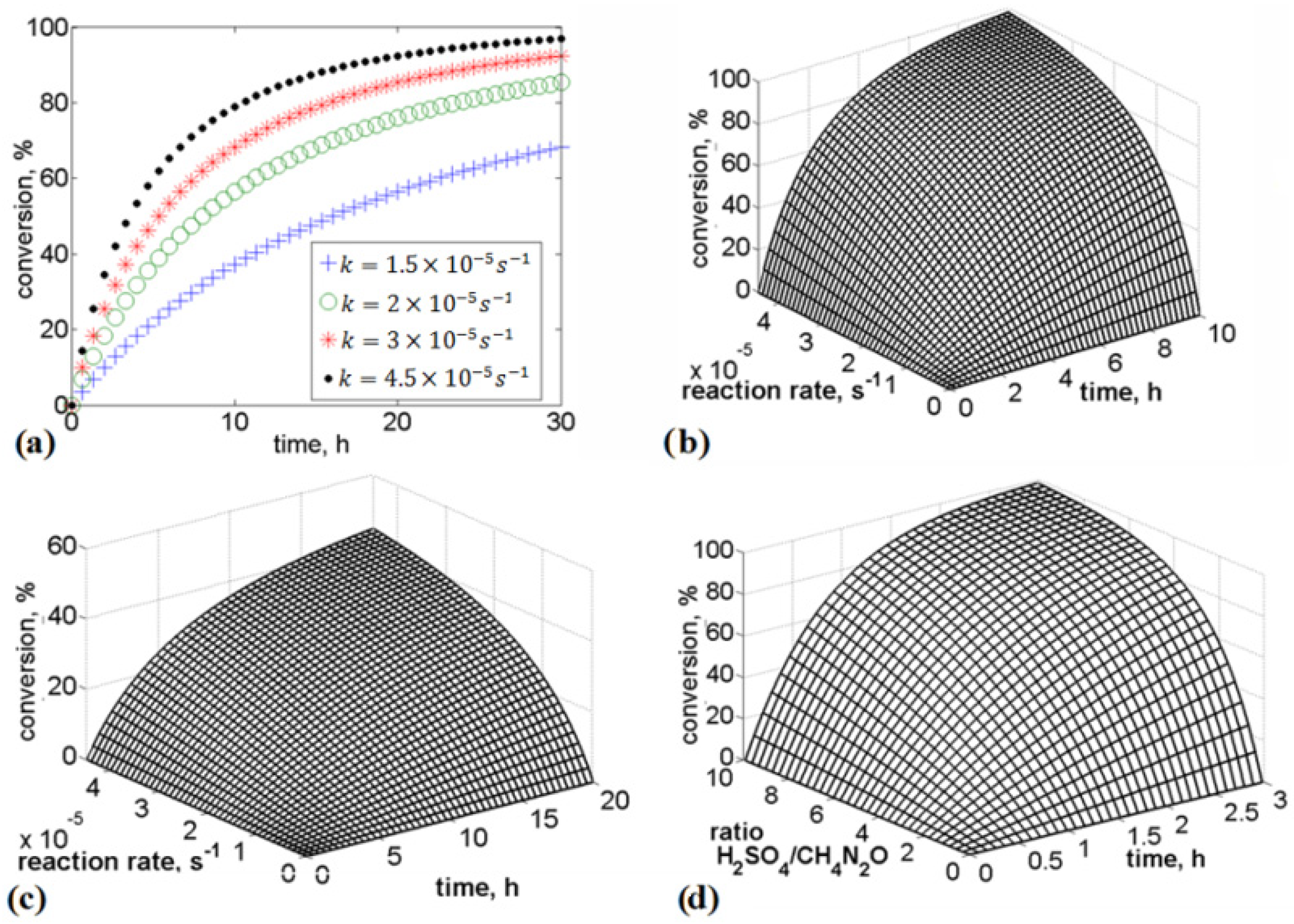
In the present study, we analyzed urea stabilization in acid media. Particular attention was given to the use of sulfuric acid and phosphoric acid. The kinetic rate law is a function of the intensive properties of the reagents, namely, temperature, pressure, and concentration. As the reaction rate is strongly dependent on the concentration of reacting species, it is common to express the reaction rate as a function of conversion. Numerical analysis was performed to evaluate the influence of the rate of addition of reagents, rate constants, and time on urea conversion. It was observed that the rate of urea conversion was affected by the rate of acid addition. The required time for urea conversion was reduced when the initial concentration of acid was several times higher than the initial concentration of urea.
An additional benefit of urea stabilization by acid media, followed by the neutralization step, could be the significant increase in the economic efficiency of the current production in the farm industry, from the ecological and economic point of view, due to the generation of a fertilizer containing nitrogen that can be used on the same farm, and the potential reuse of the treated wastewater containing urea.