Electrochemical Properties of Pristine and Vanadium Doped LiFePO4 Nanocrystallized Glasses
Abstract
:1. Introduction
2. Materials and Methods
- Commercially purchased high-performance LiFePO4 (MTI), labelled C-LFP;
- Nanocrystallized LiFePO4 glass, labelled LFP;
- Nanocrystallized LiFe0.8V0.08PO4 glass, labelled LFVP.
3. Results
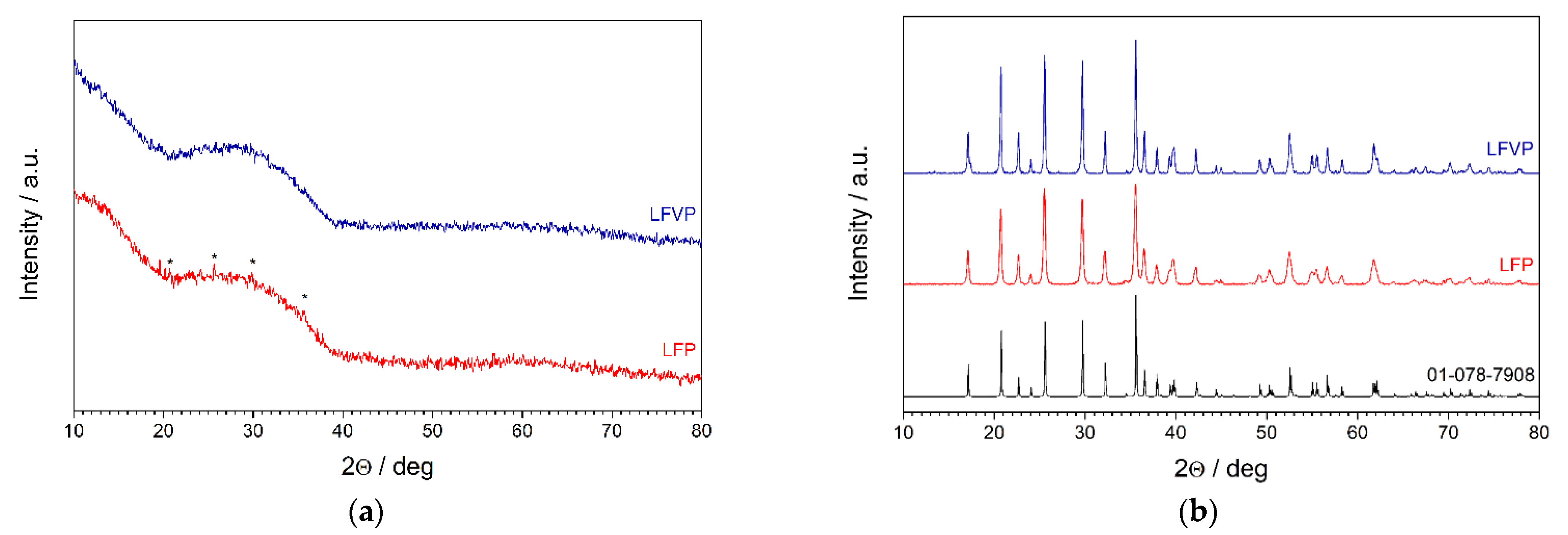
3.1. X-ray Diffractometry
3.2. Scanning Electron Microscopy
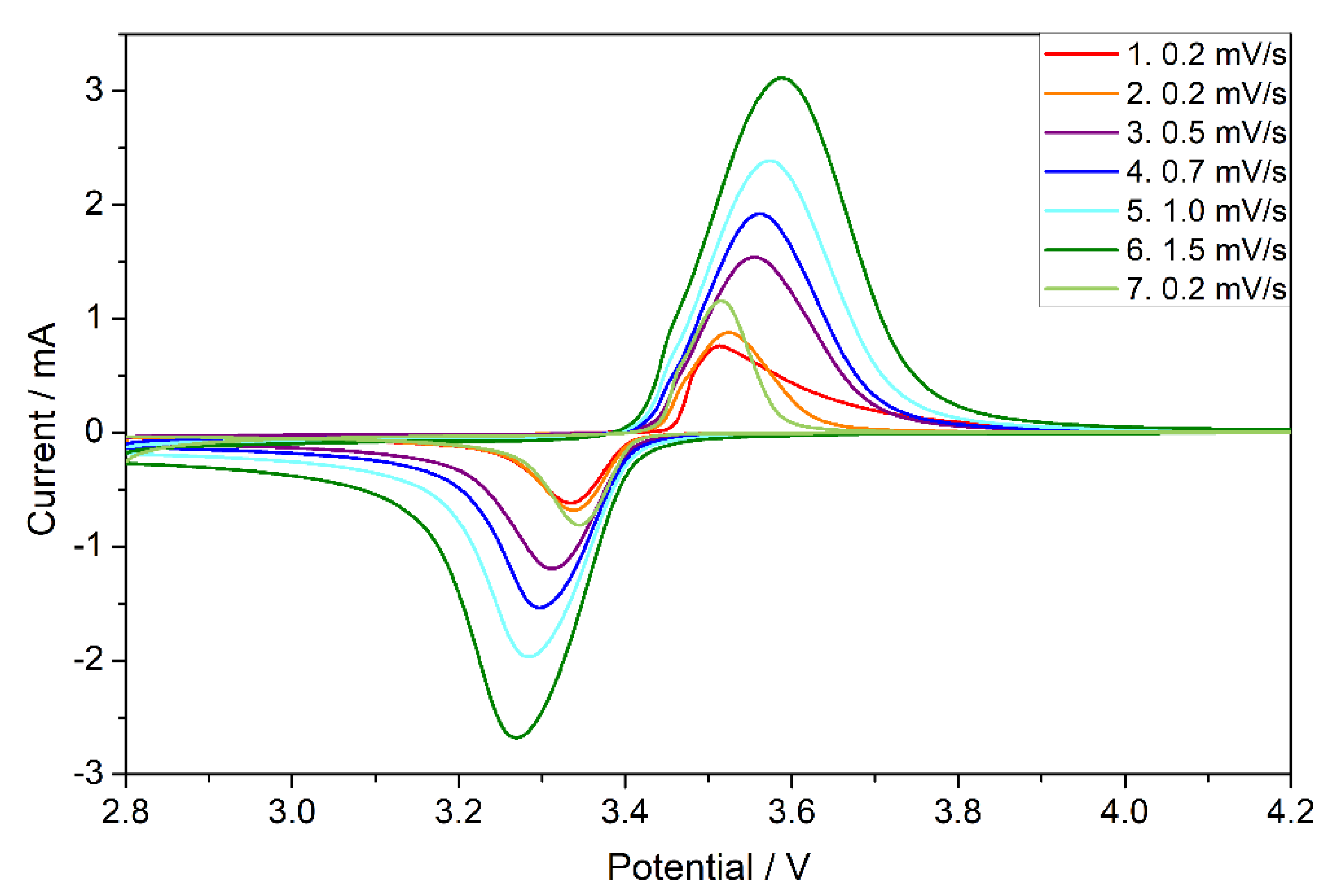
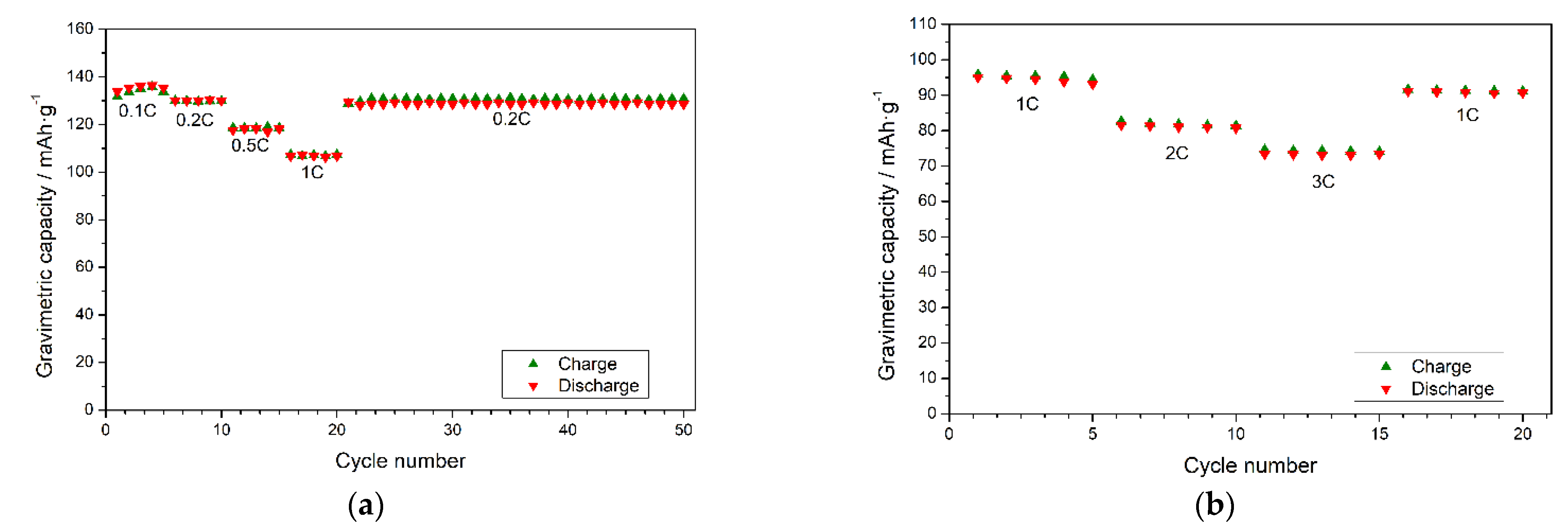
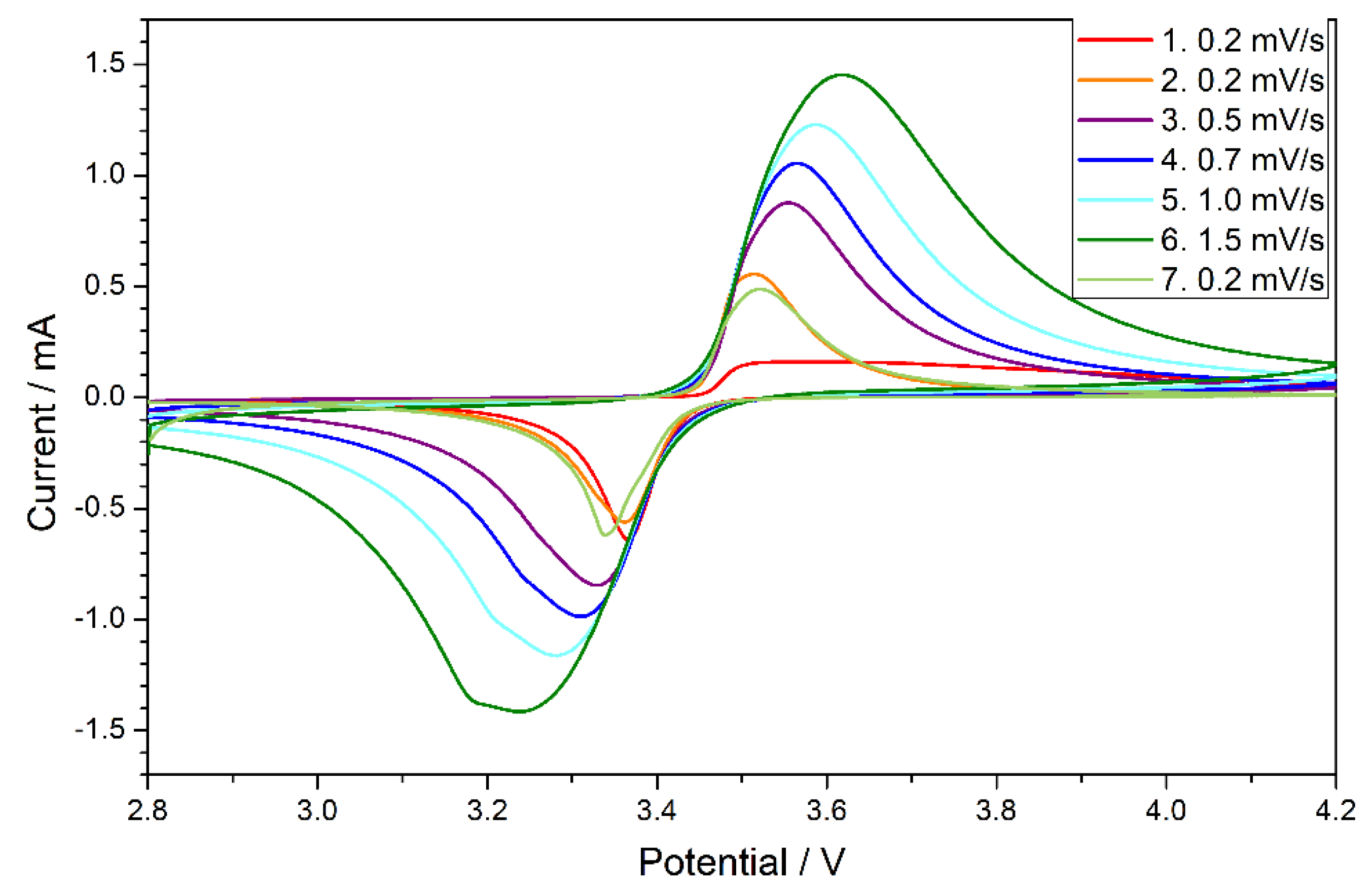
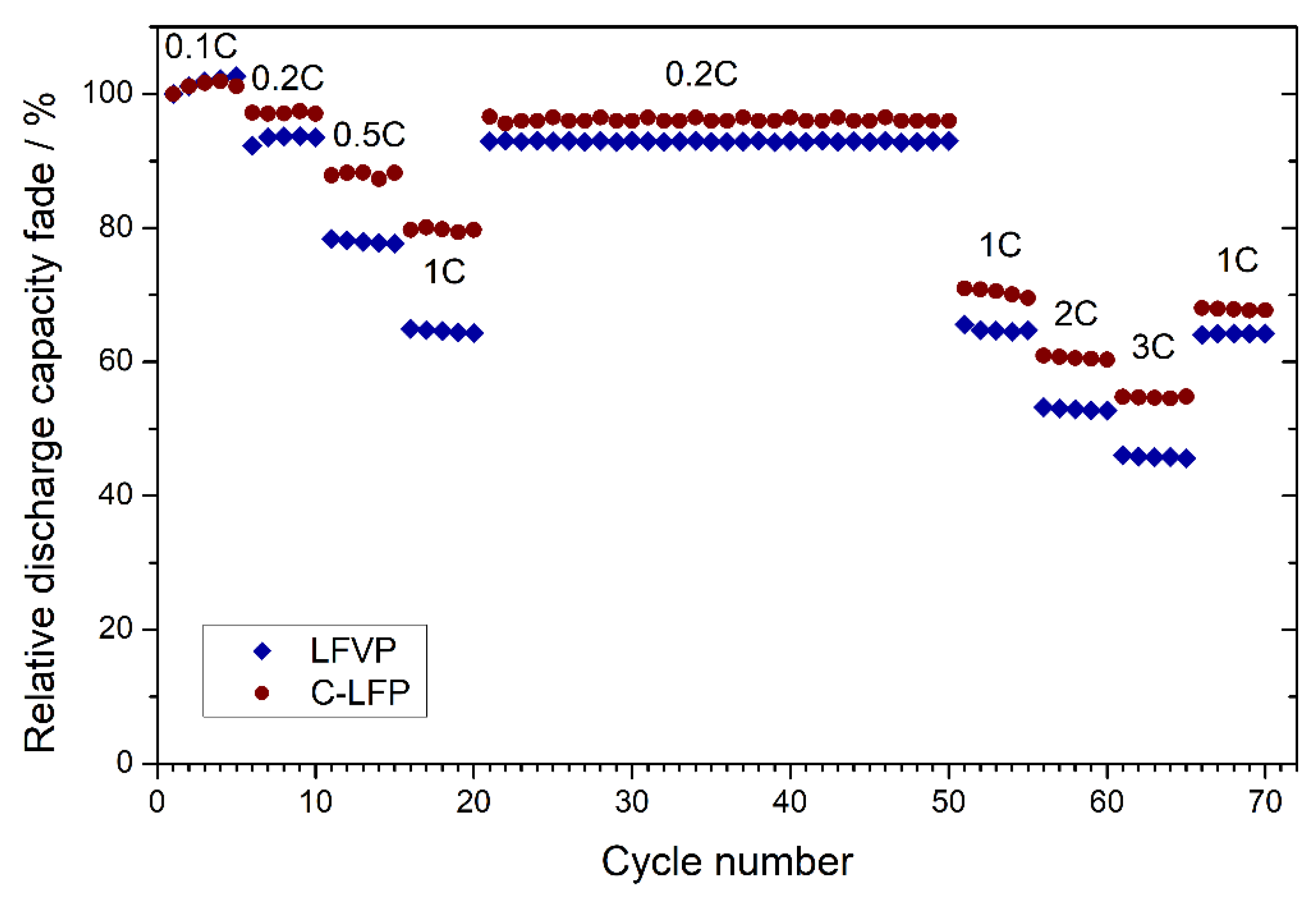
3.3. Electrochemical Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grey, C.P.; Hall, D.S. Prospects for lithium-ion batteries and beyond—A 2030 vision. Nat. Commun. 2020, 11, 6279. [Google Scholar] [CrossRef] [PubMed]
- Gür, T.M. Review of electrical energy storage technologies, materials and systems: Challenges and prospects for large-scale grid storage. Energy Environ. Sci. 2018, 11, 2696–2767. [Google Scholar] [CrossRef]
- Choi, D.; Shamim, N.; Crawford, A.; Huang, Q.; Vartanian, C.K.; Viswanathan, V.V.; Paiss, M.D.; Alam, M.J.E.; Reed, D.M.; Sprenkle, V.L. Li-ion battery technology for grid application. J. Power Sources 2021, 511, 230419. [Google Scholar] [CrossRef]
- Julien, C.M.; Mauger, A.; Zaghib, K.; Groult, H. Comparative Issues of Cathode Materials for Li-Ion Batteries. Inorganics 2014, 2, 132–154. [Google Scholar] [CrossRef] [Green Version]
- Zaghib, K.; Mauger, A.; Julien, C.M. Overview of olivines in lithium batteries for green transportation and energy storage. J. Solid State Electrochem. 2012, 16, 835–845. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Padhi, A.K.; Nanjundaswamya, K.; Goodenough, J.B. Phospho-olivines as Positive-Electrode Materials for Rechargeable Lithium Batteries. J. Electrochem. Soc. 1997, 144, 1188–1194. [Google Scholar] [CrossRef]
- Luntz, A.C.; McCloskey, B.D. Nonaqueous Li–Air Batteries: A Status Report. Chem. Rev. 2014, 114, 259–276. [Google Scholar] [CrossRef]
- Manthiram, A. A reflection on lithium-ion battery cathode chemistry. Nat. Commun. 2020, 11, 1550. [Google Scholar] [CrossRef]
- Amin, R.; Maier, J.; Balaya, P.; Chen, D.P.; Lin, C.T. Ionic and electronic transport in single crystalline LiFePO4 grown by optical floating zone technique. Solid State Ion. 2008, 179, 1683–1687. [Google Scholar] [CrossRef]
- Meethong, N.; Kao, Y.H.; Speakman, S.A.; Chiang, Y.M. Aliovalent substitutions in olivine lithium iron phosphate and impact on structure and properties. Adv. Funct. Mater. 2009, 19, 1060–1070. [Google Scholar] [CrossRef]
- Li, W.; Song, B.; Manthiram, A. High-voltage positive electrode materials for lithium-ion batteries. Chem. Soc. Rev. 2017, 46, 3006–3059. [Google Scholar] [CrossRef] [PubMed]
- Ojczyk, W.; Marzec, J.; Świerczek, K.; Zając, W.; Molenda, M.; Dziembaj, R.; Molenda, J. Studies of selected synthesis procedures of the conducting LiFePO4-based composite cathode materials for Li-ion batteries. J. Power Sources 2007, 173, 700–706. [Google Scholar] [CrossRef]
- Wilcox, J.D.; Doeff, M.M.; Marcinek, M.; Kostecki, R. Factors Influencing the Quality of Carbon Coatings on LiFePO4. J. Electrochem. Soc. 2007, 154, A389–A395. [Google Scholar] [CrossRef]
- Julien, C.M.; Zaghib, K.; Mauger, A.; Massot, M.; Ait-Salah, A.; Selmane, M.; Gendron, F. Characterization of the carbon coating onto LiFePO4 particles used in lithium batteries. J. Appl. Phys. 2006, 100, 063511. [Google Scholar] [CrossRef]
- Pietrzak, T.K.; Garbarczyk, J.E.; Gorzkowska, I.; Wasiucionek, M.; Nowinski, J.L.; Gierlotka, S.; Jozwiak, P. Correlation between electrical properties and microstructure of nanocrystallized V2O5-P2O5 glasses. J. Power Sources 2009, 194, 73–80. [Google Scholar] [CrossRef]
- Pietrzak, T.K.; Garbarczyk, J.E.; Wasiucionek, M.; Gorzkowska, I.; Nowinski, J.L.; Gierlotka, S. Electrical properties vs. microstructure of nanocrystallized V2O5–P2O5 glasses—An extended temperature range study. Solid State Ion. 2011, 192, 210–214. [Google Scholar] [CrossRef]
- Pietrzak, T.K.; Wasiucionek, M.; Michalski, P.P.; Kaleta, A.; Garbarczyk, J.E. Highly conductive cathode materials for Li-ion batteries prepared by thermal nanocrystallization of selected oxide glasses. Mater. Sci. Eng. B 2016, 213, 140–147. [Google Scholar] [CrossRef]
- Garbarczyk, J.E.; Pietrzak, T.K.; Wasiucionek, M.; Kaleta, A.; Dorau, A.; Nowiński, J.L. High electronic conductivity in nanostructured materials based on lithium-iron-vanadate-phosphate glasses. Solid State Ion. 2015, 272, 53–59. [Google Scholar] [CrossRef]
- Pietrzak, T.K.; Wasiucionek, M.; Garbarczyk, J.E. Towards Higher Electric Conductivity and Wider Phase Stability Range via Nanostructured Glass-Ceramics Processing. Nanomaterials 2021, 11, 1321. [Google Scholar] [CrossRef]
- Pietrzak, T.K.; Wasiucionek, M.; Gorzkowska, I.; Nowiński, J.L.; Garbarczyk, J.E. Novel vanadium-doped olivine-like nanomaterials with high electronic conductivity. Solid State Ion. 2013, 251, 40–46. [Google Scholar] [CrossRef]
- Pietrzak, T.K.; Maciaszek, M.; Nowiński, J.L.; Ślubowska, W.; Ferrari, S.; Mustarelli, P.; Wasiucionek, M.; Wzorek, M.; Garbarczyk, J.E. Electrical properties of V2O5 nanomaterials prepared by twin rollers technique. Solid State Ion. 2012, 225, 658–662. [Google Scholar] [CrossRef]
- Pietrzak, T.K.; Kruk-Fura, P.E.; Mikołajczuk, P.J.; Garbarczyk, J.E. Syntheses and nanocrystallization of NaF–M2O3–P2O5 NASICON-like phosphate glasses (M = V, Ti, Fe). Int. J. Appl. Glas. Sci. 2020, 11, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Johnson, I.D.; Ashton, T.E.; Blagovidova, E.; Smales, G.J.; Lübke, M.; Baker, P.J.; Corr, S.A.; Darr, J.A. Mechanistic insights of Li+ diffusion within doped LiFePO4 from Muon Spectroscopy. Sci. Rep. 2018, 8, 4114. [Google Scholar] [CrossRef]
- Kaleta, A.; Dłużewski, P.; Wasiucionek, M.; Pietrzak, T.K.; Nowiński, J.L.; Michalski, P.P.; Garbarczyk, J.E. TEM studies on thermally nanocrystallized vanadium-containing glassy analogs of LiFePO4 olivine. Mater. Charact. 2017, 127, 214–221. [Google Scholar] [CrossRef]
- Gadomski, K.; Pietrzak, T.K. Faculty of Physics, Warsaw University of Technology, Koszykowa 75, 00-662 Warsaw, Poland (manuscript in preparation).
- Ziolkowska, D.A.; Jasinski, J.B.; Hamankiewicz, B.; Korona, K.P.; Wu, S.-H.; Czerwinski, A. In Situ XRD and TEM Studies of Sol-Gel-Based Synthesis of LiFePO4. Cryst. Growth Des. 2016, 16, 5006–5013. [Google Scholar] [CrossRef]




| ID | Capacity @ 0.1C | Capacity @ 1C | Capacity @ 3C |
|---|---|---|---|
| C-LFP | 137.4 | 108.3 | 74.4 |
| LFP | 26.6 | 17.3 | 12.7 |
| LFVP | 97.1 | 60.0 | 44.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frąckiewicz, J.E.; Pietrzak, T.K.; Boczar, M.; Buchberger, D.A.; Wasiucionek, M.; Czerwiński, A.; Garbarczyk, J.E. Electrochemical Properties of Pristine and Vanadium Doped LiFePO4 Nanocrystallized Glasses. Energies 2021, 14, 8042. https://doi.org/10.3390/en14238042
Frąckiewicz JE, Pietrzak TK, Boczar M, Buchberger DA, Wasiucionek M, Czerwiński A, Garbarczyk JE. Electrochemical Properties of Pristine and Vanadium Doped LiFePO4 Nanocrystallized Glasses. Energies. 2021; 14(23):8042. https://doi.org/10.3390/en14238042
Chicago/Turabian StyleFrąckiewicz, Justyna E., Tomasz K. Pietrzak, Maciej Boczar, Dominika A. Buchberger, Marek Wasiucionek, Andrzej Czerwiński, and Jerzy E. Garbarczyk. 2021. "Electrochemical Properties of Pristine and Vanadium Doped LiFePO4 Nanocrystallized Glasses" Energies 14, no. 23: 8042. https://doi.org/10.3390/en14238042






