Degradation of Lithium-Ion Batteries in an Electric Transport Complex
Abstract
:1. Introduction
1.1. Lead-Acid Battery
1.2. Nickel Cadmium Battery
1.3. Nickel-Metal Hydride Battery
2. Determination of the Most Efficient Traction Power Sources for Electric Vehicle Applications
2.1. Li-Ion Battery
2.1.1. Positive Electrodes
2.1.2. Negative Electrodes
2.1.3. Electrolyte
2.1.4. Separator
2.2. Comparison of Different Types of Batteries
- Compactness is a comparative characteristic that determines the weight and size properties to provide the specified parameters;
- Fast-charging process—the ability of the battery to be charged with the maximum currents for it in less than 2.5 h;
- Ease of disposal—the complexity of the technological process associated with the disposal or the impossibility of recovering useful chemical elements;
- Memory effect—a reversible loss of capacity that occurs in some types of electric batteries when the recommended charging mode is violated, in particular, when recharging an incompletely discharged battery;
- Permissible overcharge—a quantitative indication that determines the permissible value when the battery is charged over 100%;
- Depth of discharge (DOD)—the real amount (of the declared) energy that the battery can give without increasing the temperature.
- The distribution of quality indicators is shown in Table 2.
- high indicators of specific characteristics;
- high values of permissible charging and discharging currents;
- the ability to quickly charge;
- no need for maintenance;
- maximum service life;
- low self-discharge readings;
- lack of “memory effect”.
3. The Use of Lithium-Ion Batteries as the Most Promising Traction Power Sources
3.1. Processes on the Positive Electrode of the Li-Ion Battery
3.2. Negative Electrodes. Carbon Materials
3.3. Reversible Processes on Carbon Materials
- LiC72 + Li ↔ 2 LiC36(Stage 8) (stage 4)
- 3 LiC36 + Li ↔ 4 LiC27(Stage 4) (Stage 3)
- 2LiC27 + Li ↔ 3LiC18(Stage 3) (stage 2)
- 2LiC18 + Li ↔ 3LiC12(Stage 2) (Stage 2)
- 2LiC12 + Li ↔ LiC6(Stage 2) (Stage 1)
3.4. Determination of Parameters Affecting the Life of a Lithium-Ion Battery
4. Degradation Processes in a Lithium-Ion Battery
4.1. Chemical Degradation
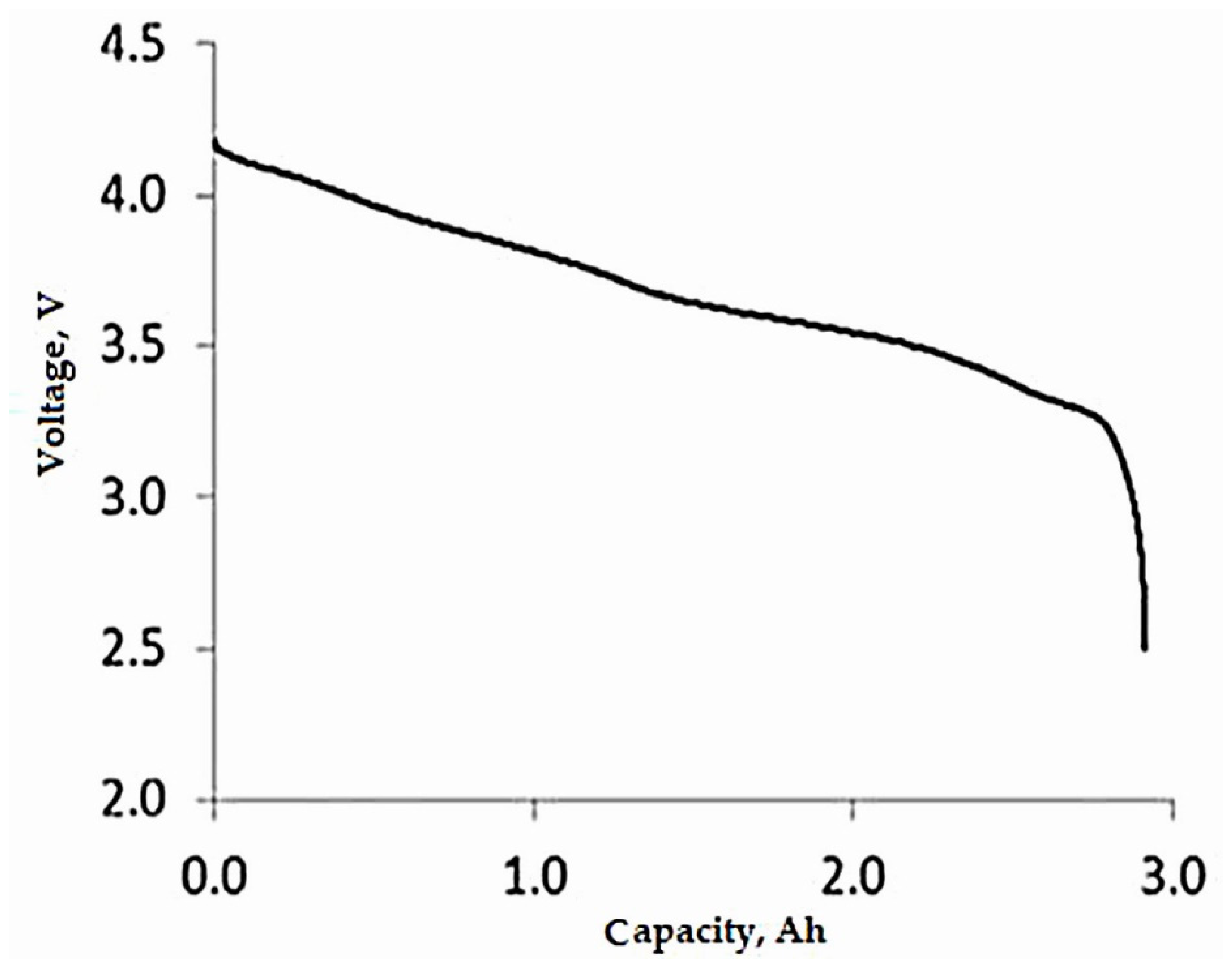
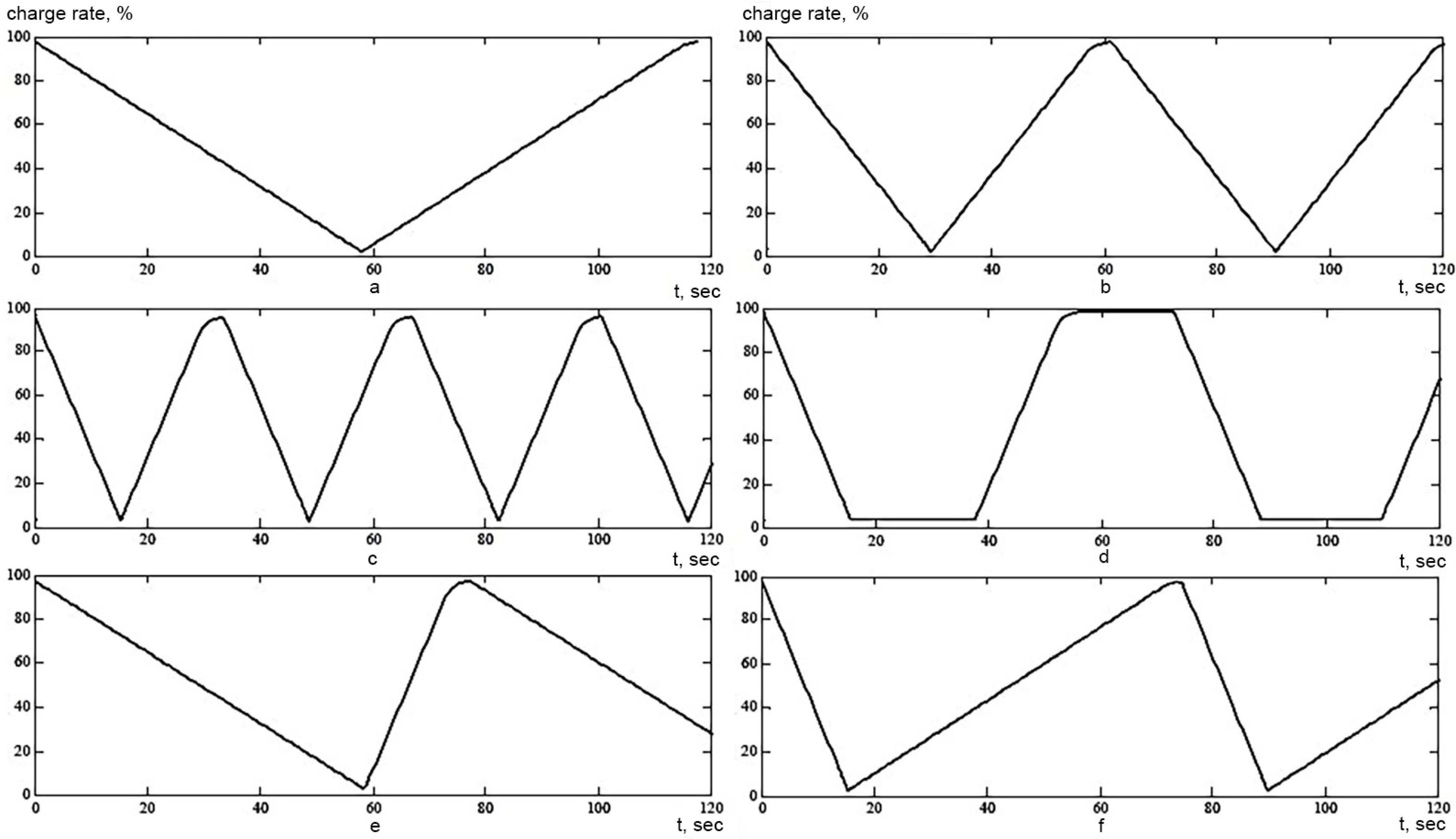
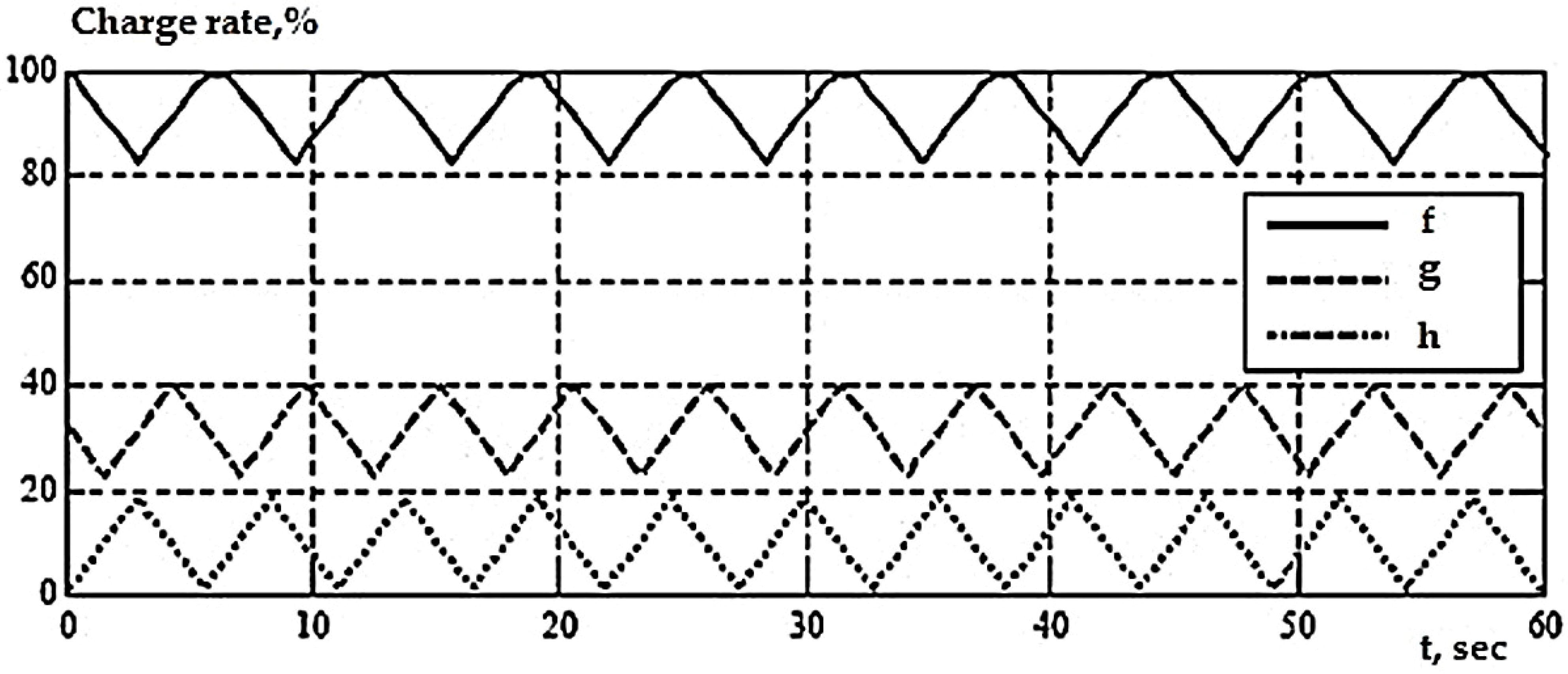
4.1.1. Influence of the Number of Cycles
- Charge with direct current, then with constant voltage (DC + PN), within 8 h and subsequent discharge up to 30% within 8 h;
- Charge PT + PN for 8 h and subsequent discharge up to 20% within 8 h;
- PT charge for 10 h and subsequent discharge up to 30% within 8 h;
- The charge of the PT within 10 h and the subsequent discharge up to 20% within 5 h.
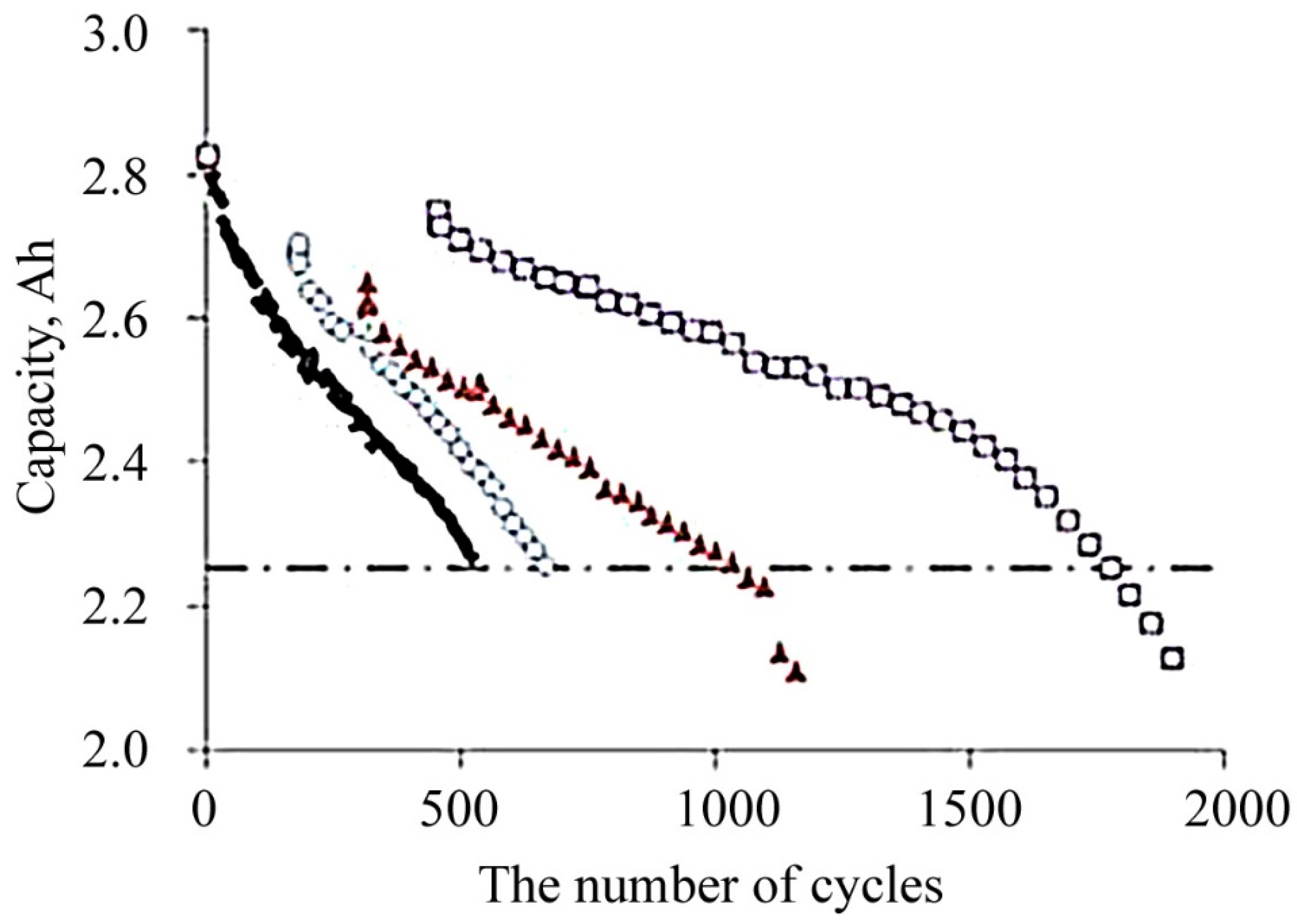
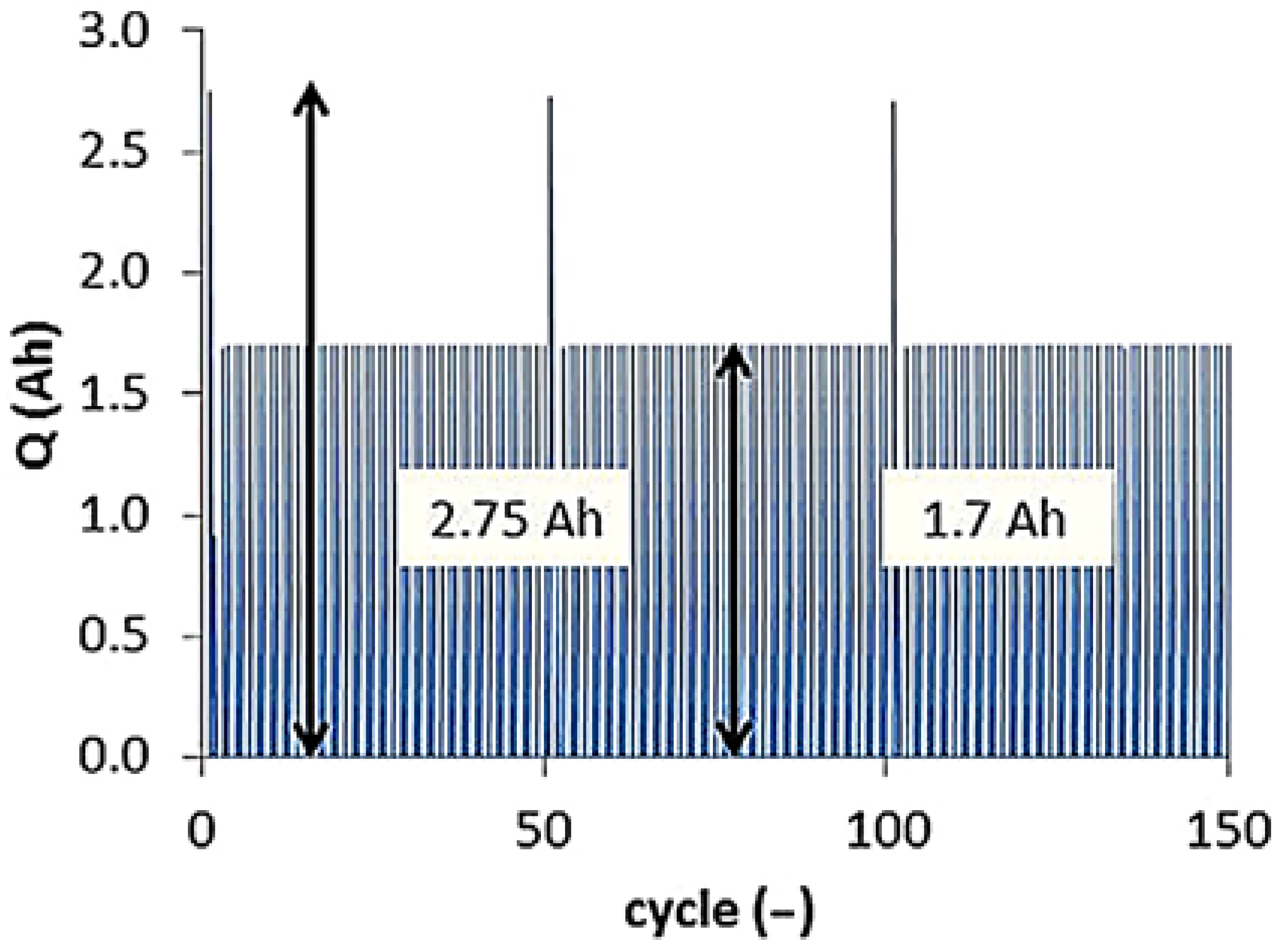
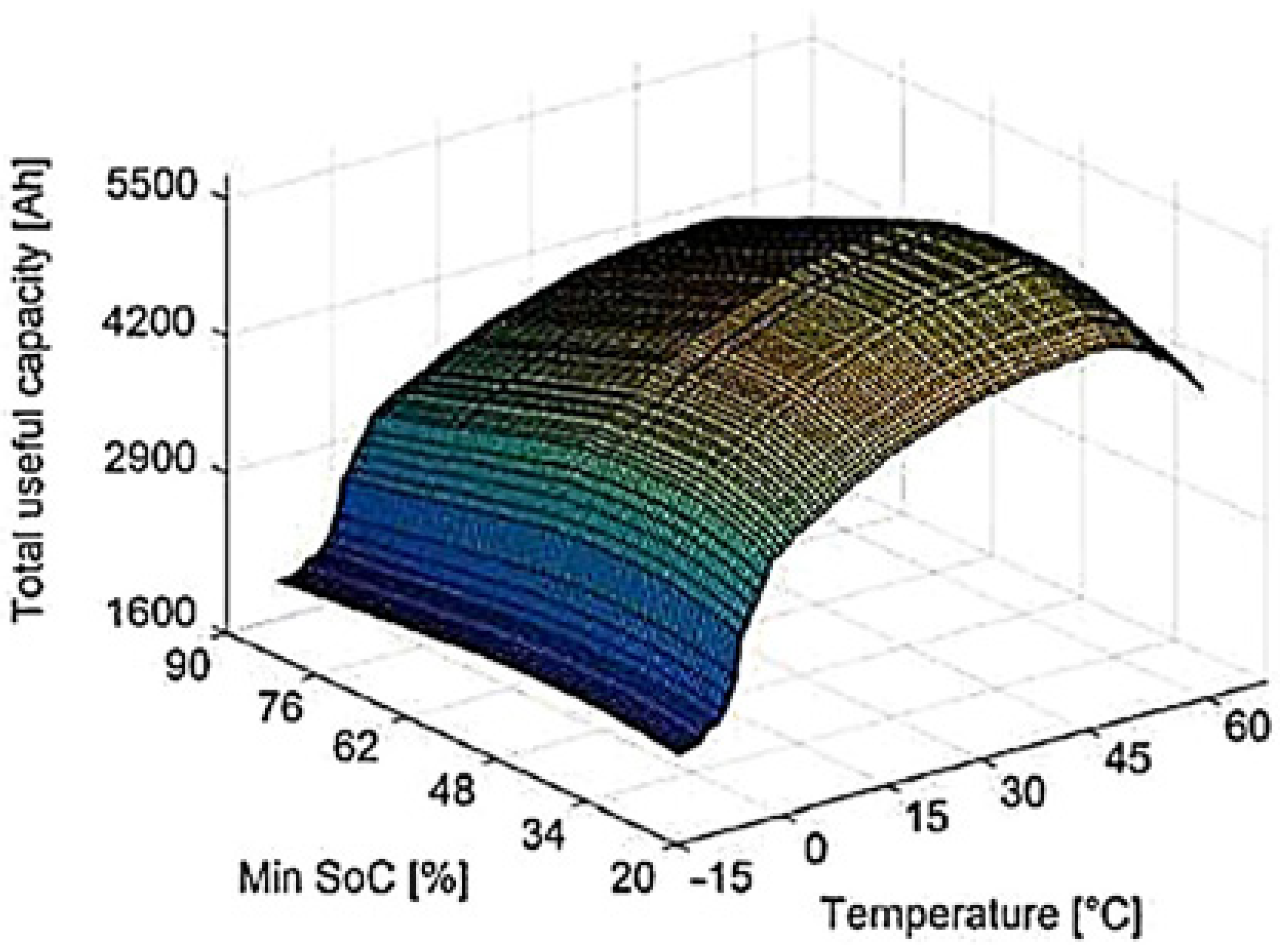
4.1.2. Influence of the Depth of Discharge
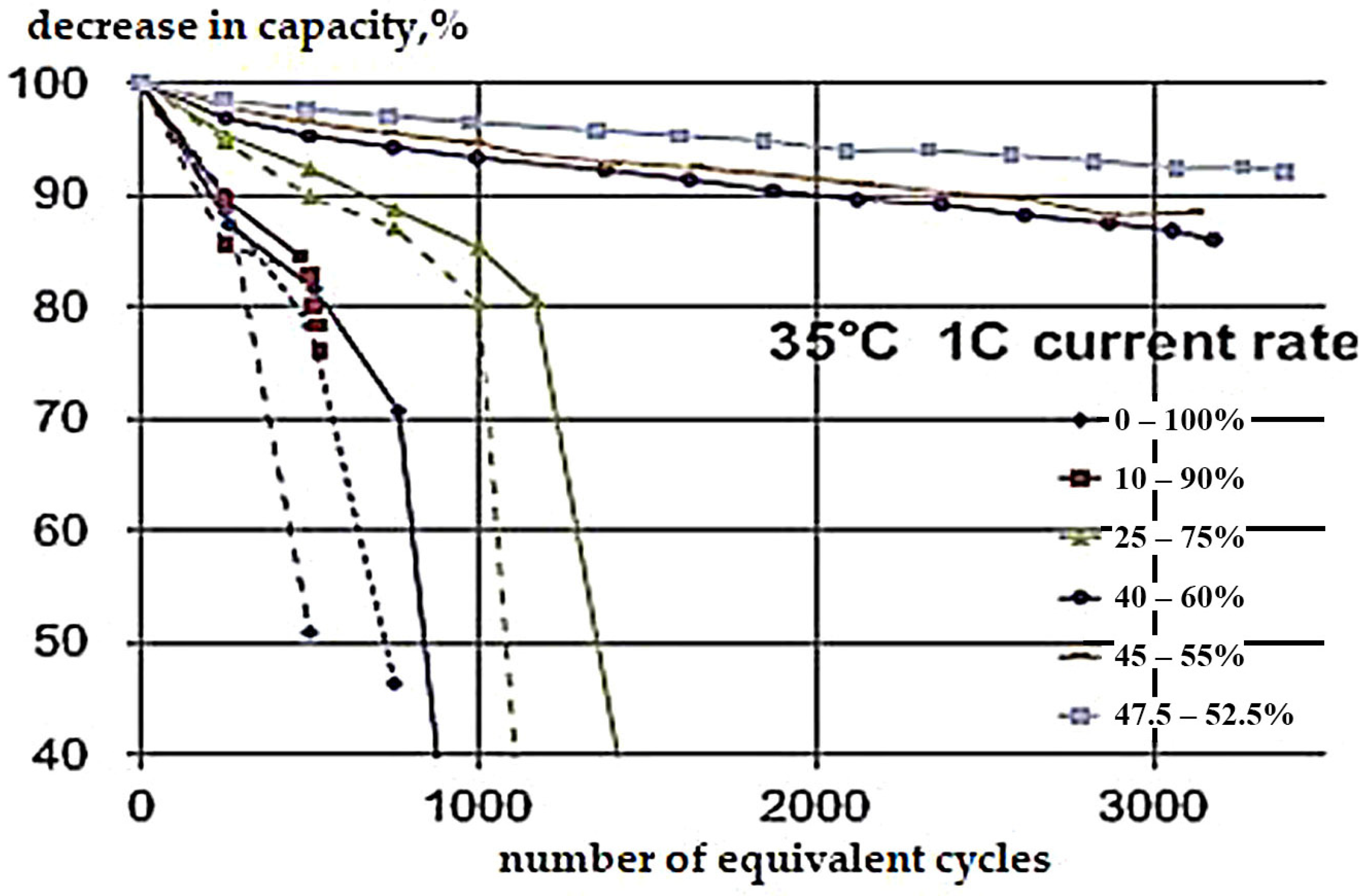
4.1.3. Effect of Charge–Discharge Currents
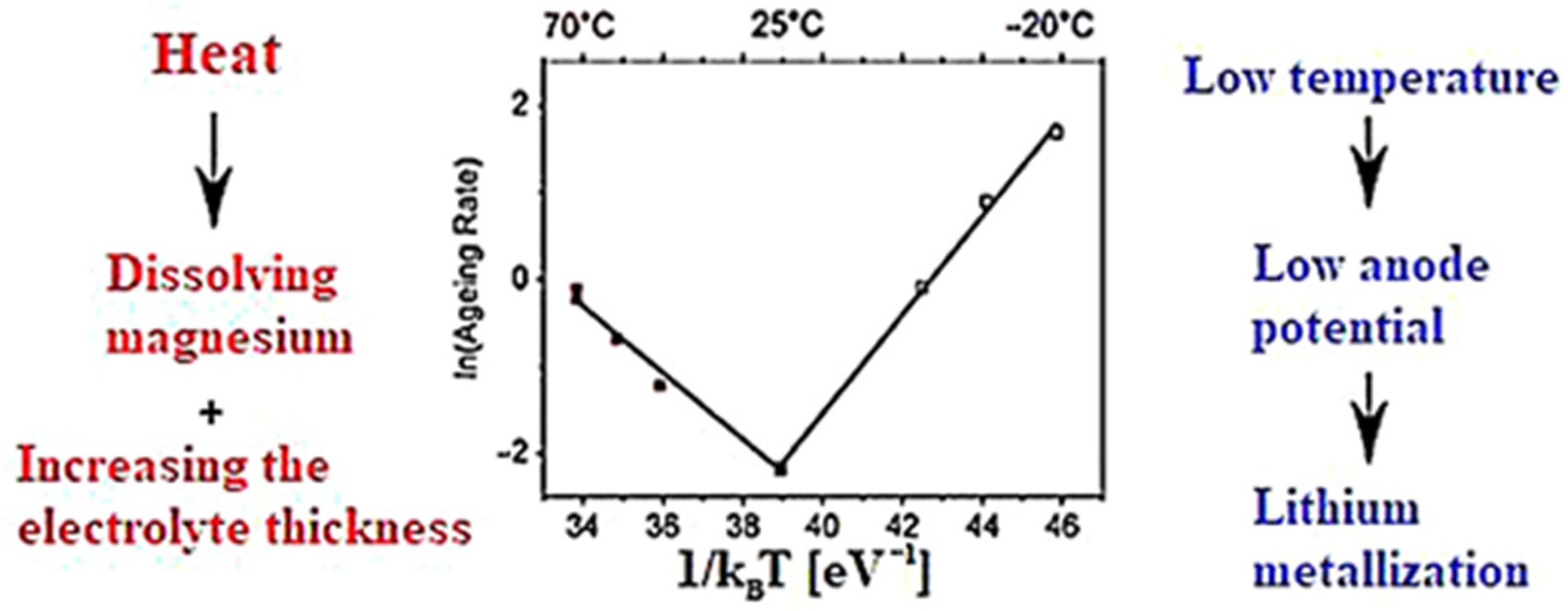
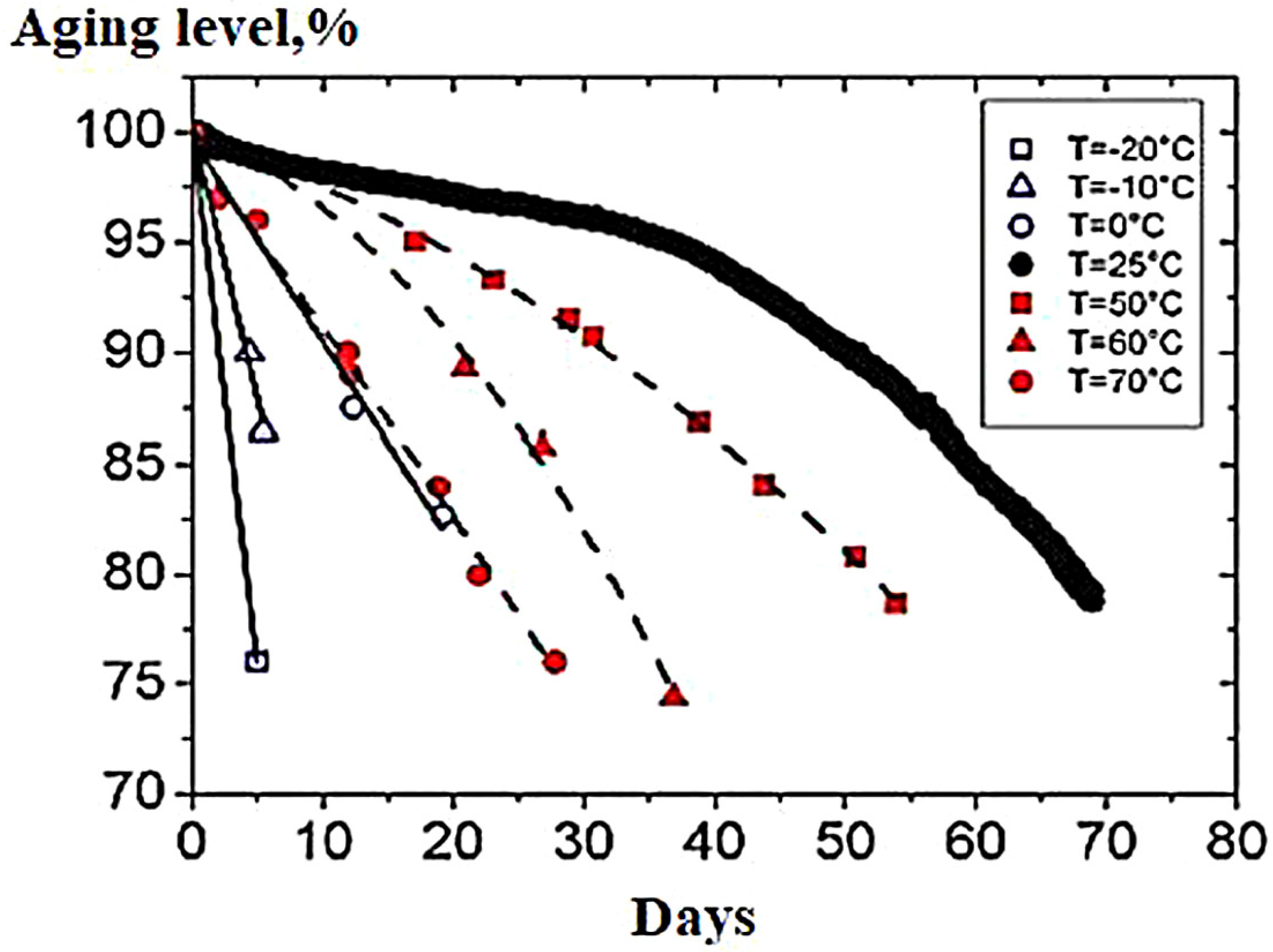
4.1.4. Influence of Temperature on Battery Life
- A—exponential coefficient;
- k is the Boltzmann constant.
4.2. Mechanical Damage
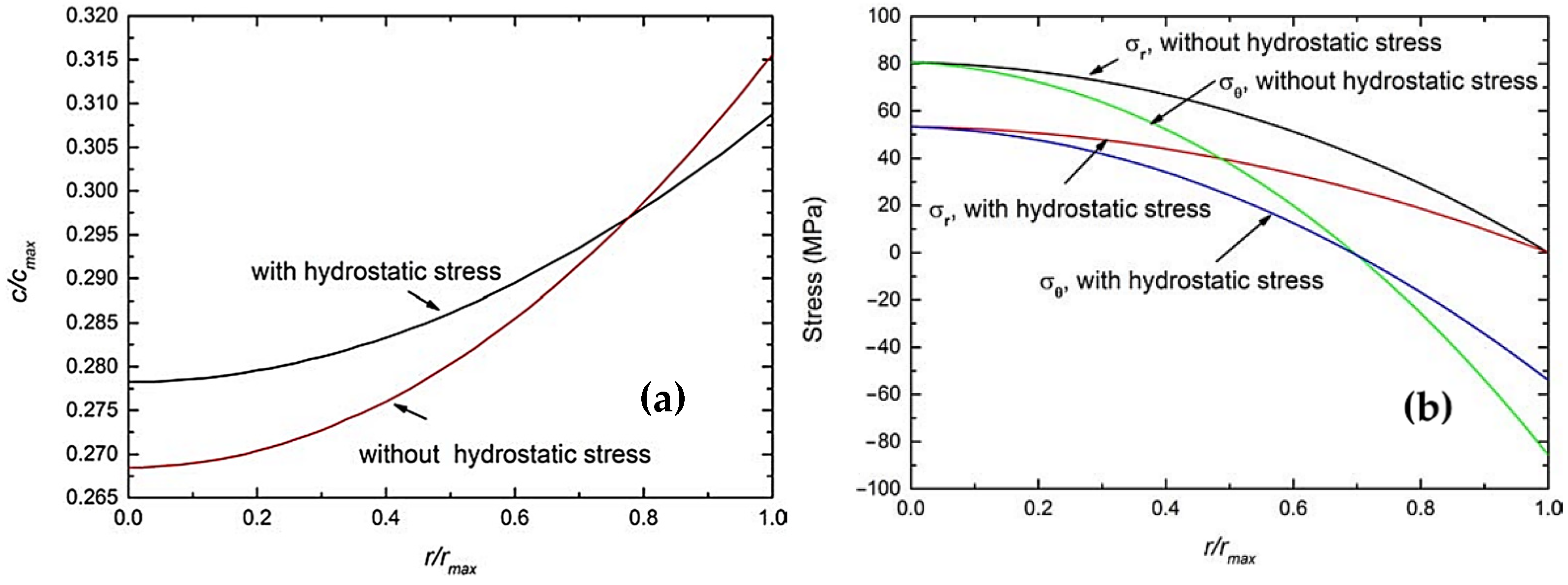
4.2.1. Effect of Elastic Stiffening
4.2.2. Effects of External Mechanical Loading
5. Discussions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lin, C.; Tang, A.; Wang, W. A review of SOH estimation methods in lithium-ion batteries for Electric Vehicle Applications. Energy Procedia 2015, 75, 1920–1925. [Google Scholar] [CrossRef] [Green Version]
- Naumann, M.; Karl, R.C.; Truong, C.N.; Jossen, A.; Hesse, H.C. Lithium-ion battery cost analysis in PV-household application. Energy Procedia 2015, 73, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Thomann, M.; Popescu, F. Estimating the effect of domestic load and renewable supply variability on battery capacity requirements for decentralized microgrids. Procedia Comput. Sci. 2014, 32, 715–722. [Google Scholar] [CrossRef]
- Ormston, T.; Maleville, L.; Tran, V.D.; Lucas, L.; Van Der Pols, K.; Denis, M.; Mardle, N. Lithium Ion Battery Management Strategies for European Space Operations Centre Missions. In Proceedings of the SpaceOps 2014 Conference, Pasadena, CA, USA, 5–9 May 2014; pp. 1–18. [Google Scholar]
- Yagües-Gomà, M.; Olivella-Rosell, P.; Villafafila-Robles, R.; Sumper, A. Ageing of Electric Vehicle Battery considering mobility needs for urban areas. In Proceedings of the International Conference on Renewable Energies and Power Quality (ICREPQ’14), Cordoba, Spain, 8–10 April 2014; pp. 1019–1024. [Google Scholar]
- Xia, B.; Wang, S.; Tian, Y.; Sun, W.; Xu, Z.; Zheng, W. Experimental research on the linixcoymnzo2 lithium-ion battery characteristics for model modification of SOC estimation. Inf. Technol. J. 2014, 13, 2395–2403. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Jiang, J.; Zhang, C.; Wang, L.Y.; Zheng, L. Robustness of SOC estimation algorithms for EV lithium-ion batteries against modeling errors and measurement noise. Math. Probl. Eng. 2015, 2015, 719490. [Google Scholar] [CrossRef]
- Barcellona, S.; Brenna, M.; Foiadelli, F.; Longo, M.; Piegari, L. Analysis of ageing effect on Li-polymer batteries. Sci. World J. 2015, 2015, 979321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleischer, C.; Waag, W.; Bai, Z.; Sauer, D.U. Adaptive on-line state-of-available-power prediction of lithium-ion batteries. J. Power Electron. 2013, 13, 516–527. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Li, K.; Jiang, Y.; Lv, Q.; Shang, L.; Sun, Y. Large-scale battery system development and user-specificdriving behavior analysis for emerging electric-drive vehicles. Energies 2011, 4, 758–779. [Google Scholar] [CrossRef]
- He, Z.; Gao, M.; Wang, C.; Wang, L.; Liu, Y. Adaptive State of charge estimation for Li-ion batteries based on an unscented Kalman filter with an enhanced battery model. Energies 2013, 6, 4134–4151. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Xia, B.; Wang, M.; Sun, W.; Xu, Z. Comparison study on two model-based adaptive algorithms for SOC estimation of lithium-ion batteries in electric vehicles. Energies 2014, 7, 8446–8464. [Google Scholar] [CrossRef] [Green Version]
- Tseng, K.-H.; Liang, J.-W.; Chang, W.; Huang, S.-C. Regression models using fully discharged voltage and internal resistance for state of health estimation of lithium-ion batteries. Energies 2015, 8, 2889–2907. [Google Scholar] [CrossRef] [Green Version]
- Sepasi, S.; Roose, L.; Matsuura, M. Extended Kalman filter with a fuzzy method for accurate battery pack state of charge estimation. Energies 2015, 8, 5217–5233. [Google Scholar] [CrossRef]
- Wang, T.; Pei, L.; Wang, T.; Lu, R.; Zhu, C. On-board state-of-health estimation at a wide ambient temperature range in lithium-ion batteries. Energies 2015, 8, 8467–8481. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Tian, B.; Lin, W.; Ji, B.; Li, J.; Pan, H. Analysis and prediction of the discharge characteristics of the lithium–ion battery based on the Grey System theory. IET Power Electron. 2015, 8, 2361–2369. [Google Scholar] [CrossRef] [Green Version]
- Qing, D.; Huang, J.; Sun, W. SOH estimation of lithium-ion batteries for electric vehicles. In Proceedings of the 31st International Symposium on Automation and Robotics in Construction and Mining (ISARC), Sydney, Australia, 9–11 July 2014; pp. 913–916. [Google Scholar]
- Gyan, P.; Aubret, P.; Hafsaoui, J.; Sellier, F.; Bourlot, S.; Zinola, S.; Badin, F. Experimental Assessment of Battery Cycle Life within the Simstock Research Program. Oil Gas Sci. Technol. Rev. d’IFP Energies Nouv. 2013, 68, 137–147. [Google Scholar] [CrossRef] [Green Version]
- Leng, F.; Tan, C.M.; Pecht, M. Effect of temperature on the aging rate of Li ion battery operating above room temperature. Sci. Rep. 2015, 5, 12967. [Google Scholar] [CrossRef] [Green Version]
- Hafsaoui, J.; Sellier, F. Electrochemical model and its parameters identification tool for the follow up of batteries ageing. World Electr. Veh. J. 2010, 4, 386–395. [Google Scholar] [CrossRef]
- Fleckenstein, M.; Bohlen, O.; Bäker, B. Aging effect of temperature gradients in Li-ion cells experimental and simulative investigations and the consequences on Thermal Battery Management. World Electr. Veh. J. 2012, 5, 322–333. [Google Scholar] [CrossRef] [Green Version]
- De Breucker, S.; Engelen, K.; D’hulst, R.; Driesen, J. Impact of current ripple on Li-Ion Battery ageing. World Electr. Veh. J. 2013, 6, 532–540. [Google Scholar] [CrossRef] [Green Version]
- Grolleau, S.; Delaille, A.; Gualous, H. Predicting lithium-ion battery degradation for efficient design and management. World Electr. Veh. J. 2013, 6, 549–554. [Google Scholar] [CrossRef] [Green Version]
- Christensen, A.; Adebusuyi, A. Using on-board electrochemical impedance spectroscopy in battery management systems. World Electr. Veh. J. 2013, 6, 793–799. [Google Scholar] [CrossRef] [Green Version]
- Prada, E.; Di Domenico, D.; Creff, Y.; Sauvant-Moynot, V. Towards advanced BMS algorithms development for (p)hev and EV by use of a physics-based model of Li-Ion Battery Systems. World Electr. Veh. J. 2013, 6, 807–818. [Google Scholar] [CrossRef] [Green Version]
- Uddin, K.; Perera, S.; Widanage, W.; Marco, J. Characterising Li-ion battery degradation through the identification of perturbations in Electrochemical Battery Models. World Electr. Veh. J. 2015, 7, 76–84. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.-J.; Zeng, X.-F.; Wan, T.-L. Real-time measurement of lithium-ion batteries’ state-of-charge based on air-coupled ultrasound. AIP Adv. 2019, 9, 085116. [Google Scholar] [CrossRef]
- Varini, M.; Campana, P.E.; Lindbergh, G. A semi-empirical, electrochemistry-based model for Li-ion battery performance prediction over lifetime. J. Energy Storage 2019, 25, 100819. [Google Scholar] [CrossRef]
- Uddin, K.; Somerville, L.; Barai, A.; Lain, M.; Ashwin, T.R.; Jennings, P.; Marco, J. The impact of high-frequency-high-current perturbations on film formation at the negative electrode-electrolyte interface. Electrochim. Acta 2017, 233, 1–12. [Google Scholar] [CrossRef]
- Uddin, K.; Gough, R.; Radcliffe, J.; Marco, J.; Jennings, P. Techno-economic analysis of the viability of residential photovoltaic systems using lithium-ion batteries for energy storage in the United Kingdom. Appl. Energy 2017, 206, 12–21. [Google Scholar] [CrossRef]
- Klintberg, A.; Klintberg, E.; Fridholm, B.; Kuusisto, H.; Wik, T. Statistical modeling of OCV-curves for aged battery cells. IFAC PapersOnLine 2017, 50, 2164–2168. [Google Scholar] [CrossRef]
- Casals, L.C.; Amante García, B.; Canal, C. Second Life batteries lifespan: Rest of useful life and environmental analysis. J. Environ. Manag. 2019, 232, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Allafi, W.; Uddin, K.; Zhang, C.; Mazuir Raja Ahsan Sha, R.; Marco, J. On-line scheme for parameter estimation of nonlinear lithium ion battery equivalent circuit models using the simplified refined instrumental variable method for a modified wiener continuous-time model. Appl. Energy 2017, 204, 497–508. [Google Scholar] [CrossRef]
- Narayan, N.; Papakosta, T.; Vega-Garita, V.; Qin, Z.; Popovic-Gerber, J.; Bauer, P.; Zeman, M. Estimating battery lifetimes in solar home system design using a practical modelling methodology. Appl. Energy 2018, 228, 1629–1639. [Google Scholar] [CrossRef]
- Uddin, K.; Jackson, T.; Widanage, W.D.; Chouchelamane, G.; Jennings, P.A.; Marco, J. On the possibility of extending the lifetime of lithium-ion batteries through optimal V2G facilitated by an integrated vehicle and smart-grid system. Energy 2017, 133, 710–722. [Google Scholar] [CrossRef]
- Ashwin, T.R.; McGordon, A.; Jennings, P.A. Electrochemical modelling of li-ion battery pack with constant voltage cycling. J. Power Sources 2017, 341, 327–339. [Google Scholar] [CrossRef]
- Worwood, D.; Kellner, Q.; Wojtala, M.; Widanage, W.D.; McGlen, R.; Greenwood, D.; Marco, J. A new approach to the internal thermal management of cylindrical battery cells for automotive applications. J. Power Sources 2017, 346, 151–166. [Google Scholar] [CrossRef]
- Richardson, R.R.; Osborne, M.A.; Howey, D.A. Gaussian process regression for forecasting battery state of health. J. Power Sources 2017, 357, 209–219. [Google Scholar] [CrossRef]
- Ashwin, T.R.; Barai, A.; Uddin, K.; Somerville, L.; McGordon, A.; Marco, J. Prediction of battery storage ageing and solid electrolyte interphase property estimation using an electrochemical model. J. Power Sources 2018, 385, 141–147. [Google Scholar] [CrossRef]
- Vega-Garita, V.; Hanif, A.; Narayan, N.; Ramirez-Elizondo, L.; Bauer, P. Selecting a suitable battery technology for the photovoltaic Battery Integrated Module. J. Power Sources 2019, 438, 227011. [Google Scholar] [CrossRef]
- Rücker, F.; Bremer, I.; Linden, S.; Badeda, J.; Sauer, D.U. Development and evaluation of a battery lifetime extending charging algorithm for an Electric Vehicle Fleet. Energy Procedia 2016, 99, 285–291. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Sun, F.; Xiong, R.; He, H. A novel dual H infinity filters based battery parameter and state estimation approach for Electric Vehicles Application. Energy Procedia 2016, 103, 375–380. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, R.; Yang, D.; Tang, X.; Chen, Z. Remaining useful life prediction of lithium-ion battery based on discrete wavelet transform. Energy Procedia 2017, 105, 2053–2058. [Google Scholar] [CrossRef]
- Grün, T.; Stella, K.; Wollersheim, O. Impacts on load distribution and ageing in lithium-ion home storage systems. Energy Procedia 2017, 135, 236–248. [Google Scholar] [CrossRef]
- Du, J.; Zhang, W.; Zhang, C.; Zhou, X. Battery remaining useful life prediction under coupling stress based on support vector regression. Energy Procedia 2018, 152, 538–543. [Google Scholar] [CrossRef]
- Tang, X.; Yao, K.; Zou, C.; Liu, B.; Gao, F. Predicting battery aging trajectory via a migrated aging model and Bayesian Monte Carlo Method. Energy Procedia 2019, 158, 2456–2461. [Google Scholar] [CrossRef]
- Wei, J.; Dong, G.; Chen, Z. Model-based fault diagnosis of lithium-ion battery using strong tracking extended Kalman filter. Energy Procedia 2019, 158, 2500–2505. [Google Scholar] [CrossRef]
- Vonsien, S.; Madlener, R. Economic modeling of the economic efficiency of li-ion battery storage with a special focus on residential PV systems. Energy Procedia 2019, 158, 3964–3975. [Google Scholar] [CrossRef]
- Singh, P.; Khare, N.; Chaturvedi, P.K. Li-ion battery ageing model parameter: SEI layer analysis using magnetic field probing. Eng. Sci. Technol. Int. J. 2018, 21, 35–42. [Google Scholar] [CrossRef]
- Torchio, M.; Magni, L.; Braatz, R.D.; Raimondo, D.M. Optimal health-aware charging protocol for lithium-ion batteries: A fast model predictive control approach. IFAC PapersOnLine 2016, 49, 827–832. [Google Scholar] [CrossRef]
- Tang, L.; Rizzoni, G.; Cordoba-Arenas, A. Battery life extending charging strategy for plug-in Hybrid Electric Vehicles and battery electric vehicles. IFAC PapersOnLine 2016, 49, 70–76. [Google Scholar] [CrossRef]
- Rechkemmer, S.K.; Zhang, W.; Sawodny, O. Modeling of a permanent magnet synchronous motor of an e-scooter for simulation with Battery Aging Model. IFAC PapersOnLine 2017, 50, 4769–4774. [Google Scholar] [CrossRef]
- Mohajer, S.; Sabatier, J.; Lanusse, P.; Cois, O. A fractional-order electro-thermal aging model for lifetime enhancement of lithium-ion batteries. IFAC PapersOnLine 2018, 51, 220–225. [Google Scholar] [CrossRef]
- Kong, X.R.; Bonakdarpour, A.; Wetton, B.T.; Wilkinson, D.P.; Gopaluni, B. State of Health Estimation for lithium-ion batteries. IFAC PapersOnLine 2018, 51, 667–671. [Google Scholar] [CrossRef]
- Chu, H.; Zheng, Q.; Guo, L.; Gao, B. Acceleration velocity trajectory optimization of intelligent evs using Battery Life Model. IFAC PapersOnLine 2018, 51, 285–289. [Google Scholar] [CrossRef]
- Mohajer, S.; Lanusse, P.; Sabatier, J.; Cois, O. Design of a model-based fractional-order controller for optimal charging of batteries. IFAC PapersOnLine 2018, 51, 97–102. [Google Scholar] [CrossRef]
- Pózna, A.I.; Hangos, K.M.; Magyar, A. Design of experiments for Battery Aging Estimation. IFAC PapersOnLine 2018, 51, 386–391. [Google Scholar] [CrossRef]
- Yuan Zou, J.F.; Zhang, X. Quantifying Electric Vehicle Battery’s ohmic resistance increase caused by degradation from on-board data. IFAC PapersOnLine 2019, 52, 297–302. [Google Scholar] [CrossRef]
- Pian, C.; Liu, J.; Zhao, H.; Zhang, L. Transient thermal characteristic analysis and charging state estimation of lithium batteries for automated guided vehicle during discharge. Therm. Sci. 2019, 23, 2731–2739. [Google Scholar] [CrossRef] [Green Version]
- Beneš, M.; Fučík, R.; Havlena, V.; Klement, V.; Kolář, M.; Polívka, O.; Solovský, J.; Strachota, P. An efficient and robust numerical solution of the full-order multiscale model of lithium-ion battery. Math. Probl. Eng. 2018, 2018, 3530975. [Google Scholar] [CrossRef] [Green Version]
- Zhou, D.; Yin, H.; Xie, W.; Fu, P.; Lu, W. Research on online capacity estimation of power battery based on EKF-GPR model. J. Chem. 2019, 2019, 5327319. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Dai, S.; Wang, J.; Shan, Z.; Min, J. Switch Matrix Algorithm for series lithium battery pack equilibrium based on derived acceleration information Gauss-Seidel. Math. Probl. Eng. 2019, 2019, 5159497. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Zhang, X.; Zhou, X.; Guo, B.; Yang, J. A novel model for lithium-ion battery aging quantitative analysis based on pseudo two-dimension expressions. Int. J. Electrochem. Sci. 2019, 14, 3180–3203. [Google Scholar] [CrossRef]
- Mureddu, M.; Facchini, A.; Damiano, A. A statistical approach for modeling the aging effects in Li-Ion Energy Storage Systems. IEEE Access 2018, 6, 42196–42206. [Google Scholar] [CrossRef]
- Zhu, X.; Revilla, R.I.; Jaguemont, J.; Van Mierlo, J.; Hubin, A. Insights into cycling aging of LiNi0.80Co0.15Al0.05O2 cathode induced by surface inhomogeneity: A post-mortem analysis. J. Phys. Chem. C 2019, 123, 30046–30058. [Google Scholar] [CrossRef]
- Quesne-Turin, A.; Flahaut, D.; Croguennec, L.; Vallverdu, G.; Allouche, J.; Charles-Blin, Y.; Chotard, J.-N.; Ménétrier, M.; Baraille, I. Surface reactivity of li2mno3: First-principles and experimental study. ACS Appl. Mater. Interfaces 2017, 9, 44222–44230. [Google Scholar] [CrossRef] [Green Version]
- Harting, N.; Schenkendorf, R.; Wolff, N.; Krewer, U. State-of-health identification of lithium-ion batteries based on Nonlinear Frequency Response Analysis: First steps with machine learning. Appl. Sci. 2018, 8, 821. [Google Scholar] [CrossRef] [Green Version]
- Dambone Sessa, S.; Tortella, A.; Andriollo, M.; Benato, R. Li-ion battery-flywheel hybrid storage system: Countering Battery Aging during a grid frequency regulation service. Appl. Sci. 2018, 8, 2330. [Google Scholar] [CrossRef] [Green Version]
- Uddin, K.; Perera, S.; Widanage, W.; Somerville, L.; Marco, J. Characterising lithium-ion battery degradation through the identification and tracking of electrochemical battery model parameters. Batteries 2016, 2, 13. [Google Scholar] [CrossRef]
- Canals Casals, L.; Amante García, B. Second-life batteries on a gas turbine power plant to provide area regulation services. Batteries 2017, 3, 10. [Google Scholar] [CrossRef] [Green Version]
- Kandasamy, N.; Badrinarayanan, R.; Kanamarlapudi, V.; Tseng, K.; Soong, B.-H. Performance analysis of machine-learning approaches for modeling the charging/discharging profiles of stationary battery systems with Non-Uniform Cell Aging. Batteries 2017, 3, 18. [Google Scholar] [CrossRef] [Green Version]
- El Ghossein, N.; Sari, A.; Venet, P. Lifetime prediction of lithium-ion capacitors based on accelerated aging tests. Batteries 2019, 5, 28. [Google Scholar]
- Müller, D.; Dufaux, T.; Birke, K.P. Model-based investigation of porosity profiles in graphite anodes regarding sudden-death and second-life of lithium ion cells. Batteries 2019, 5, 49. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Lyu, C. Decomposition study of degradation reasons for licoo2-based 14500 lithium-ion batteries using a nondestructive method. IEEE Access 2018, 6, 44417–44432. [Google Scholar] [CrossRef]
- Burzyński, D.; Kasprzyk, L. The operation and capacity fade modelling of the lithium-ion cell for electric vehicles. E3S Web Conf. 2019, 108, 01017. [Google Scholar] [CrossRef] [Green Version]
- Dudley, G.; Blake, R.; Lucas, L. Mars Express Lithium Ion batteries performance analysis. E3S Web Conf. 2017, 16, 06002. [Google Scholar] [CrossRef] [Green Version]
- Canals Casals, L.; Igualada, L.; Corchero, C. The effect of building energy management systems on Battery Aging. E3S Web Conf. 2018, 61, 00014. [Google Scholar] [CrossRef]
- Jinlei, S.; Lei, P.; Ruihang, L.; Qian, M.; Chuanyu, T.; Tianru, W. Economic Operation Optimization for 2nd use batteries in Battery Energy Storage Systems. IEEE Access 2019, 7, 41852–41859. [Google Scholar] [CrossRef]
- Lai, X.; Qiao, D.; Zheng, Y.; Yi, W. A novel screening method based on a partially discharging curve using a genetic algorithm and back-propagation model for the Cascade Utilization of retired lithium-ion batteries. Electronics 2018, 7, 399. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Jiang, J.; Zhang, L.; Liu, S.; Wang, L.; Loh, P. A generalized SOC-OCV model for lithium-ion batteries and the SOC estimation for LNMCO Battery. Energies 2016, 9, 900. [Google Scholar] [CrossRef] [Green Version]
- Nájera, J.; Moreno-Torres, P.; Lafoz, M.; de Castro, R.M.; Arribas, J.R. Approach to hybrid energy storage systems dimensioning for urban electric buses regarding efficiency and battery aging. Energies 2017, 10, 1708. [Google Scholar] [CrossRef] [Green Version]
- Barcellona, S.; Piegari, L. Lithium ion battery models and parameter identification techniques. Energies 2017, 10, 2007. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Li, J.; Zhang, Y.; Bao, G.; Ge, X.; Li, P. A hierarchical optimal operation strategy of hybrid energy storage system in distribution networks with high photovoltaic penetration. Energies 2018, 11, 389. [Google Scholar] [CrossRef] [Green Version]
- De Hoog, J.; Jaguemont, J.; Abdel-Monem, M.; Van Den Bossche, P.; Van Mierlo, J.; Omar, N. Combining an electrothermal and impedance aging model to investigate thermal degradation caused by fast charging. Energies 2018, 11, 804. [Google Scholar] [CrossRef] [Green Version]
- Chin, C.; Gao, Z.; Chiew, J.; Zhang, C. Nonlinear temperature-dependent state model of cylindrical LiFePO4 battery for open-circuit voltage, terminal voltage and state-of-charge estimation with extended Kalman filter. Energies 2018, 11, 2467. [Google Scholar] [CrossRef] [Green Version]
- De Sutter, L.; Berckmans, G.; Marinaro, M.; Smekens, J.; Firouz, Y.; Wohlfahrt-Mehrens, M.; Van Mierlo, J.; Omar, N. Comprehensive aging analysis of volumetric constrained lithium-ion pouch cells with high concentration silicon-alloy anodes. Energies 2018, 11, 2948. [Google Scholar] [CrossRef] [Green Version]
- Fang, Q.; Wei, X.; Lu, T.; Dai, H.; Zhu, J. A state of health estimation method for lithium-ion batteries based on voltage relaxation model. Energies 2019, 12, 1349. [Google Scholar] [CrossRef] [Green Version]
- Xia, B.; Chen, G.; Zhou, J.; Yang, Y.; Huang, R.; Wang, W.; Lai, Y.; Wang, M.; Wang, H. Online parameter identification and joint estimation of the State of charge and the state of health of lithium-ion batteries considering the degree of polarization. Energies 2019, 12, 2939. [Google Scholar] [CrossRef] [Green Version]
- Noh, T.-W.; Ahn, J.-H.; Lee, B.K. Cranking capability estimation algorithm based on modeling and online update of model parameters for Li-ion SLI Batteries. Energies 2019, 12, 3365. [Google Scholar] [CrossRef] [Green Version]
- Somakettarin, N.; Pichetjamroen, A. Characterization of a practical-based Ohmic series resistance model under life-cycle changes for a lithium-ion battery. Energies 2019, 12, 3888. [Google Scholar] [CrossRef] [Green Version]
- Burzyński, D.; Pietracho, R.; Kasprzyk, L.; Tomczewski, A. Analysis and modeling of the wear-out process of a lithium-nickel-manganese-cobalt cell during cycling operation under constant load conditions. Energies 2019, 12, 3899. [Google Scholar] [CrossRef] [Green Version]
- Venugopal, P.; Vigneswaran, T. State-of-health estimation of Li-ion batteries in electric vehicle using INDRNN under variable load condition. Energies 2019, 12, 4338. [Google Scholar] [CrossRef] [Green Version]
- Worwood, D.; Algoo, R.; McGlen, R.J.; Marco, J.; Greenwood, D. A study into different cell-level cooling strategies for cylindrical lithium-ion cells in automotive applications. Int. J. Powertrains 2018, 7, 199. [Google Scholar] [CrossRef]
- Fan, J.; Zou, Y.; Zhang, X.; Guo, H. A novel state of health estimation method for lithium-ion battery in electric vehicles. J. Phys. Conf. Ser. 2019, 1187, 022014. [Google Scholar] [CrossRef]
- Han, X.; Feng, X.; Ouyang, M.; Lu, L.; Li, J.; Zheng, Y.; Li, Z. A comparative study of charging voltage curve analysis and state of health estimation of lithium-ion batteries in Electric Vehicle. Automot. Innov. 2019, 2, 263–275. [Google Scholar] [CrossRef] [Green Version]
- Harting, N.; Wolff, N.; Röder, F.; Krewer, U. State-of-health diagnosis of lithium-ion batteries using nonlinear frequency response analysis. J. Electrochem. Soc. 2019, 166, A277. [Google Scholar] [CrossRef]
- Hildebrand, S.; Rheinfeld, A.; Friesen, A.; Haetge, J.; Schappacher, F.M.; Jossen, A.; Winter, M. Thermal analysis of lini0.4co0.2mn0.4o2/mesocarbon microbeads cells and electrodes: State-of-charge and state-of-health influences on reaction kinetics. J. Electrochem. Soc. 2018, 165, A104. [Google Scholar] [CrossRef] [Green Version]
- Osara, J.; Bryant, M. A thermodynamic model for lithium-ion battery degradation: Application of the degradation-entropy generation theorem. Inventions 2019, 4, 23. [Google Scholar] [CrossRef] [Green Version]
- Hinz, H. Comparison of lithium-ion battery models for simulating storage systems in distributed power generation. Inventions 2019, 4, 41. [Google Scholar] [CrossRef] [Green Version]
- Kuo, T.J.; Lee, K.Y.; Chiang, M.H. Development of a neural network model for SOH of LiFePO4 batteries under different aging conditions. IOP Conf. Ser. Mater. Sci. Eng. 2019, 486, 012083. [Google Scholar] [CrossRef]
- Wu, Y.; Li, W.; Wang, Y.; Zhang, K. Remaining useful life prediction of lithium-ion batteries using neural network and bat-based particle filter. IEEE Access 2019, 7, 54843–54854. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Liao, C.; Zhang, W. Research on multiple states joint estimation algorithm for Electric Vehicles Under Charge Mode. IEEE Access 2018, 6, 40143–40153. [Google Scholar] [CrossRef]
- Yu, Q.-Q.; Xiong, R.; Wang, L.-Y.; Lin, C. A comparative study on open circuit voltage models for lithium-ion batteries. Chin. J. Mech. Eng. 2018, 31, 65. [Google Scholar] [CrossRef] [Green Version]
- Savard, C.; Pietrac, L.; Venet, P.; Sari, A.; Niel, E. Comparing lithium-ion battery architecture performances with Colored Petri Net. SN Appl. Sci. 2019, 1, 1691. [Google Scholar] [CrossRef] [Green Version]
- Schimpe, M.; Von Kuepach, M.E.; Naumann, M.; Hesse, H.C.; Smith, K.; Jossen, A. Comprehensive modeling of temperature-dependent degradation mechanisms in lithium iron phosphate batteries. J. Electrochem. Soc. 2018, 165, A181. [Google Scholar] [CrossRef] [Green Version]
- Shkrob, I.A.; Rodrigues, M.-T.F.; Dees, D.W.; Abraham, D.P. Fast charging of Li-ion cells: Part II. nonlinear contributions to cell and electrode polarization. J. Electrochem. Soc. 2019, 166, A3305–A3313. [Google Scholar] [CrossRef] [Green Version]
- Somakettarin, N.; Pichetjamroen, A. A study on modeling of effective series resistance for lithium-ion batteries under life cycle consideration. In Proceedings of the IOP Conference Series: Earth and Environmental Science International Conference on Smart Power & Internet Energy Systems, Melbourne, Australia, 25–27 April 2019; Volume 322, p. 012008. [Google Scholar]
- Chen, Z.; Xue, Q.; Xiao, R.; Liu, Y.; Shen, J. State of Health Estimation for lithium-ion batteries based on fusion of autoregressive moving average model and Elman Neural Network. IEEE Access 2019, 7, 102662–102678. [Google Scholar] [CrossRef]
- Zheng, X.; Deng, X. State-of-health prediction for lithium-ion batteries with multiple gaussian process regression model. IEEE Access 2019, 7, 150383–150394. [Google Scholar] [CrossRef]
- Gantenbein, S.; Schönleber, M.; Weiss, M.; Ivers-Tiffée, E. Capacity fade in lithium-ion batteries and cyclic aging over various state-of-charge ranges. Sustainability 2019, 11, 6697. [Google Scholar] [CrossRef] [Green Version]
- Karlsen, H.; Dong, T.; Yang, Z.; Carvalho, R. Temperature-dependence in battery management systems for electric vehicles: Challenges, criteria, and solutions. IEEE Access 2019, 7, 142203–142213. [Google Scholar] [CrossRef]
- Shchurov, N.I.; Myatezh, S.V.; Malozyomov, B.V.; Shtang, A.A.; Martyushev, N.V.; Klyuev, R.V.; Dedov, S.I. Determination of Inactive Powers in a Single-Phase AC Network. Energies 2021, 14, 4814. [Google Scholar] [CrossRef]
- Kalogiannis, T.; Jaguemont, J.; Omar, N.; Van Mierlo, J.V.; Van den Bossche, P.V. A comparison of internal and external preheat methods for NMC Batteries. World Electr. Veh. J. 2019, 10, 18. [Google Scholar] [CrossRef] [Green Version]
- Diao, W.; Kulkarni, C.; Pecht, M. Development of an Informative Lithium-Ion Battery Datasheet. Energies 2021, 14, 5434. [Google Scholar] [CrossRef]
- Novák, P.; Scheifele, W.; Winter, M.; Haas, O. Graphite electrodes with tailored porosity for rechargeable ion-transfer batteries. J. Power Sources 1997, 68, 267–270. [Google Scholar] [CrossRef]
- Gnanaraj, J.S.; Cohen, Y.S.; Levi, M.D.; Aurbach, D. The effect of pressure on the electroanalytical response of graphite anodes and licoo2 cathodes for Li-Ion Batteries. J. Electroanal. Chem. 2001, 516, 89–102. [Google Scholar] [CrossRef]
- Shim, J.; Striebel, K.A. Effect of electrode density on cycle performance and irreversible capacity loss for natural graphite anode in lithium-ion batteries. J. Power Sources 2003, 119–121, 934–937. [Google Scholar] [CrossRef] [Green Version]
- Shim, J.; Striebel, K.A. The dependence of natural graphite anode performance on electrode density. J. Power Sources 2004, 130, 247–253. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W. Effects of external mechanical loading on stress generation during lithiation in li-ion battery electrodes. Electrochim. Acta 2015, 185, 28–33. [Google Scholar] [CrossRef] [Green Version]
- Yen, J.-Y.; Hwu, J.-G. Enhancement of silicon oxidation rate due to tensile mechanical stress. Appl. Phys. Lett. 2000, 76, 1834–1835. [Google Scholar] [CrossRef]
- Sanchez, J.; Fullea, J.; Andrade, C.; de Andres, P.L. Hydrogen inα-iron: Stress and Diffusion. Phys. Rev. B 2008, 78, 014113. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Rajasekhara, S.; Goodenough, J.B.; Zhou, F. Monodisperse porous LiFePO4 microspheres for a high power Li-ion battery cathode. J. Am. Chem. Soc. 2011, 133, 2132–2135. [Google Scholar] [CrossRef]
- Chung, S.-Y.; Blocking, J.T.; Andersson, A.S.; Chiang, Y.-M. Electronically conductive phospho-olivines as lithium storage electrodes. Nat. Mater. 2002, 1, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Meethong, N.; Kao, Y.-H.; Speakman, S.A.; Chiang, Y.-M. Aliovalent substitutions in olivine lithium iron phosphate and impact on structure and properties. Adv. Funct. Mater. 2009, 19, 1060–1070. [Google Scholar] [CrossRef]
- Hannan, M.A.; Hoque, M.M.; Hussain, A.; Yusof, Y.; Ker, P.J. State-of-the-art and Energy Management System of lithium-ion batteries in electric vehicle applications: Issues and recommendations. IEEE Access 2018, 6, 19362–19378. [Google Scholar] [CrossRef]
- Megahed, S.; Scrosati, B. Lithium-ion rechargeable batteries. J. Power Sources 1994, 51, 79–104. [Google Scholar] [CrossRef]
- Winter, M.; Besenhard, J.O.; Spahr, M.E.; Novak, P. Cheminform abstract: Insertion electrode materials for rechargeable lithium batteries. Mater. Sci. Adv. Materials. 1998, 10, 725–763. [Google Scholar] [CrossRef]
- Baughman, R.H.; Zakhidov, A.A.; de Heer, W.A. Carbon nanotubes—The route toward applications. Science 2002, 297, 787–792. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zeng, H.C.; Lee, J.Y. Highly reversible lithium storage in porous SNO2 nanotubes with coaxially grown carbon nanotube overlayers. Adv. Mater. 2006, 18, 645–649. [Google Scholar] [CrossRef]
- Chan, C.; Peng, H.; Liu, G. High-performance lithium battery anodes using silicon nanowires. Nat. Nanotechnol. 2008, 3, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Dias, F.B.; Plomp, L.; Veldhuis, J.B.J. Trends in polymer electrolytes for secondary lithium batteries. J. Power Sources 2000, 88, 169–191. [Google Scholar] [CrossRef]
- Meyer, W.H. Polymer electrolytes for lithium-ion batteries. Adv. Mater. 1998, 10, 439–448. [Google Scholar] [CrossRef]
- Fergus, J.W. Ceramic and polymeric solid electrolytes for lithium-ion batteries. J. Power Sources 2010, 195, 4554–4569. [Google Scholar] [CrossRef]
- Zhang, S.S. A review on the separators of liquid electrolyte li-ion batteries. J. Power Sources 2007, 164, 351–364. [Google Scholar] [CrossRef]
- Zhou, W.; Hao, F.; Fang, D. The effects of elastic stiffening on the evolution of the stress field within a spherical electrode particle of lithium-ion batteries. Int. J. Appl. Mech. 2013, 5, 1350040. [Google Scholar] [CrossRef]
- Karabelli, D.; Birke, K.P. Feasible Energy Density Pushes of Li-Metal vs. Li-Ion Cells. Appl. Sci. 2021, 11, 7592. [Google Scholar] [CrossRef]
- Savla, N.; Khanna, N.; Pandit, S.; Jung, S.P.; Campus, D.; Pandit, S.; Jung, S.P. Microbially-powered Electrochemical Systems Coupled with Membrane-based Technology for Sustainable Desalination and Efficient Wastewater Treatment. J. Korean Soc. Environ. Eng. 2020, 42, 360–380. [Google Scholar] [CrossRef]
- Kanevskii, L.S.; Dubasova, V.S. Degradation of Lithium-Ion batteries and how to fight it: A review. Russ. J. Electrochem. 2005, 41, 1–16. [Google Scholar] [CrossRef]
- Hussain, S.; Yang, X.; Aslam, M.K.; Shaheen, A.; Javed, M.S.; Aslam, N.; Aslam, B.; Liu, G.; Qiao, G. Robust TiN nanoparticles polysulfide anchor for Li–S storage and diffusion pathways using first principle calculations. Chem. Eng. J. 2020, 391, 123595. [Google Scholar] [CrossRef]
- Hussaina, S.; JabbarKhanb, A.; Arshad, M.; Javed, M.S.; Ahmad, A.; Shoaib, S.; Shah, A.; Khan, M.R.; Akram, S.; Ali, Z.S.; et al. Charge storage in binder-free 2D-hexagonal CoMoO4 nanosheets as a redox active material for pseudocapacitors. Ceram. Int. 2021, 47, 8659–8667. [Google Scholar] [CrossRef]
- Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Slater, P.; Stolkin, R.; Walton, A.; Christensen, P.; Heidrich, O.; Lambert, S.; et al. Recycling lithium-ion batteries from electric vehicles. Nature 2019, 575, 75–86. [Google Scholar] [CrossRef] [Green Version]
- Xiaotu, M.; Azhari, L.; Wang, Y. Li-ion battery recycling challenges. Chem 2021, 11, 2843–2847. [Google Scholar] [CrossRef]
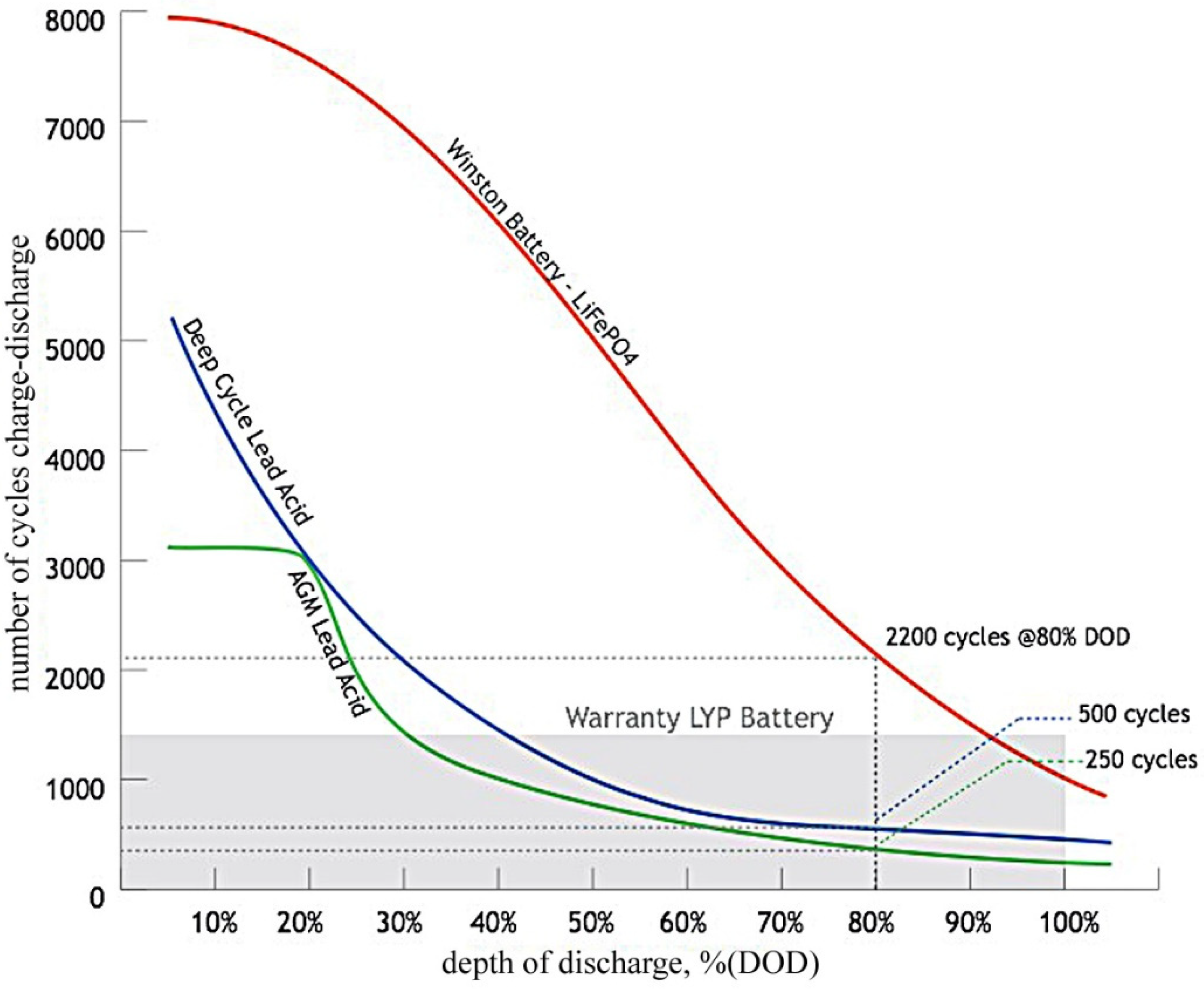

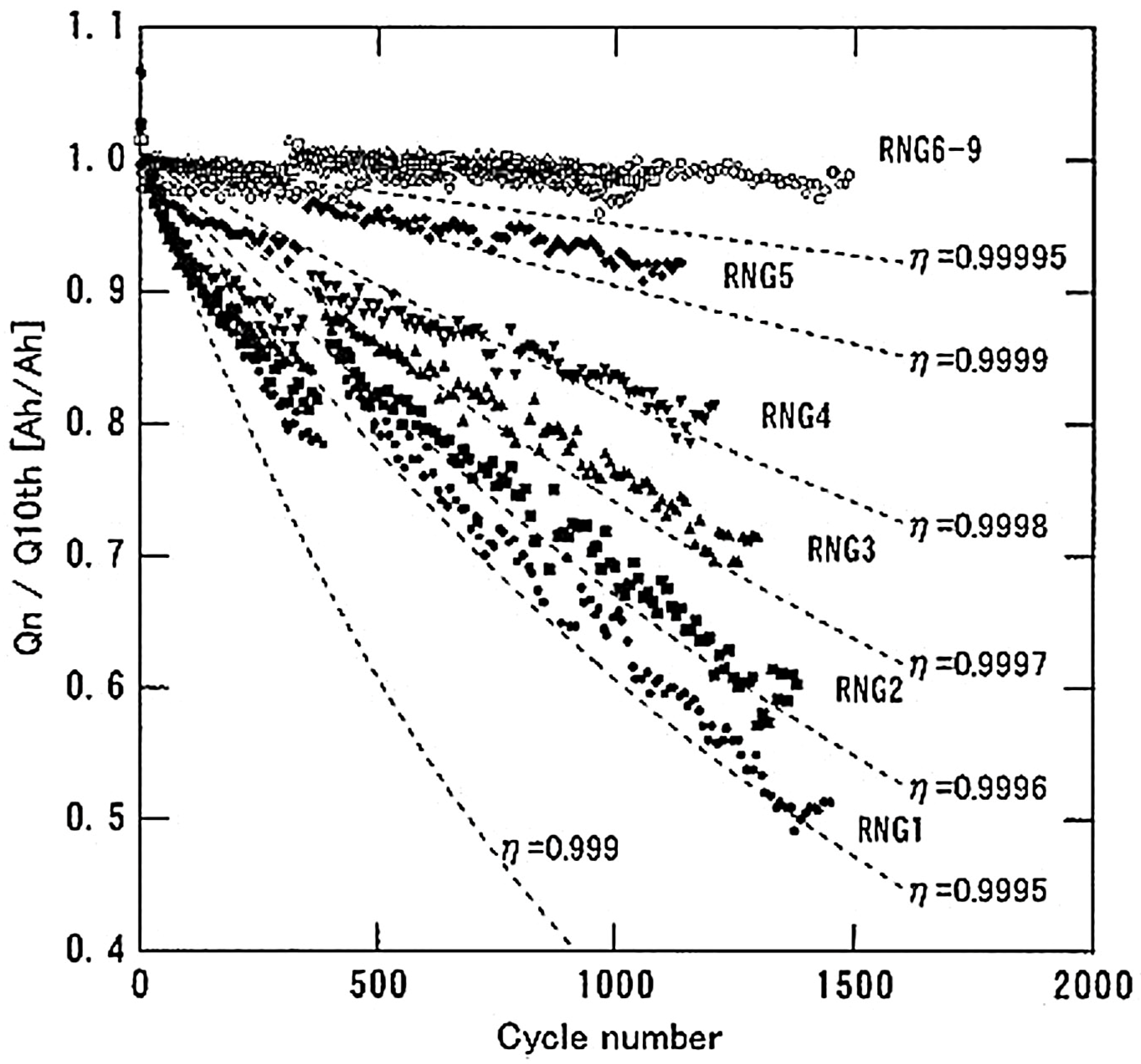

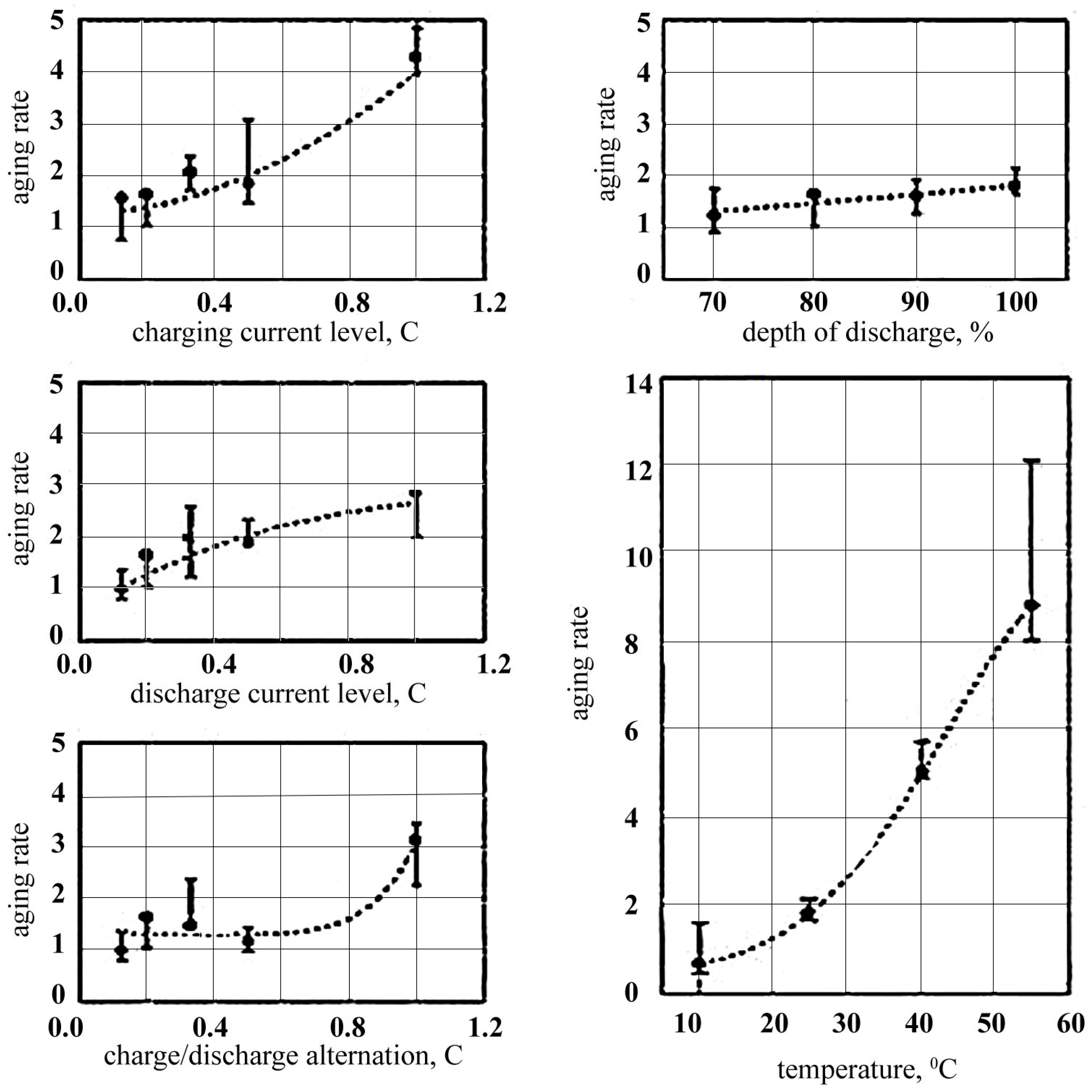





| Battery Parameters | Lead Acid | Nickel-Cadmium | Nickel Metal Hydride | Li-Ion |
|---|---|---|---|---|
| Battery rated voltage, V | 2 | 1.2 | 1.2 | 3.7 |
| Specific energy consumption, Wh/kg | 30–40 | 40–60 | 30–80 | 90–140 |
| Specific power, W/kg | 180 | 150 | 250–1000 | 1800 |
| Average charge time, hour | more than 10 | 8 | 6 | 2 |
| Number of discharge/charge cycles (service life) | 500–800 | 2000 | 800 | 2000 |
| Average self-discharge per month, % | 4 | twenty | thirty | 7 |
| Average cost per kWh, $ | 150 | 400–800 | 250 | 450 |
| Comparison Parameter | Lead Acid | Nickel-Cadmium | Nickel Metal Hydride | Li-Ion |
|---|---|---|---|---|
| Compactness | - | + | + | + |
| Fast-charging process | - | + | + | + |
| Ease of disposal | - | - | + | + |
| Shelf life is more than 3 years | + | + | - | + |
| Memory effect | - | + | + | - |
| Permissible recharge | High | Average | Short | Very low |
| Depth of discharge (DOD) | 50% | 50–80% | 50–85% | 80% |
| Service intervals | 3–6 months | 30–60 days | 60–90 days | Not regulated |
| Chemical Formula of the Cathode Material | Manufacturer Country | Company Manufacturer |
|---|---|---|
| LiCoO2 | Japan, Tokyo | Nippon Chemical Industry Co.; Simimoto Co. |
| USA, Clearwater Loop | OMG | |
| Germany, Darmstadt | Merck KGaA | |
| Belgium, Brussels | Umicore | |
| China | Shanghai Shanshan Science & Technology Co. | |
| LiNi1-yCOyO2 | Japan, Tokyo | Simimoto Co.; Seimi Chemical Co |
| Germany, Darmstadt | Merck KGaA | |
| LiMn2O4 | Japan, Tokyo | Mitsui Mining & Smelting Co. Ltd. |
| USA, Philadelphia | FMC Corp | |
| Germany, Darmstadt | Merck KGaA |
| Type (Formula) of Electrochemical System, Cathode/Anode materials | Specific Energy Consumption (Wh/kg) | Resource, (The Number of Discharge Charge Cycles Per 1 C Discharge Depth 80% | Allowable Charge/Discharge Rates in Multiples of the Rated Capacity C—(Hourly Discharge Current) | Operating Temperature Range without the Use of Passive or Active Temperature Compensation Systems, °C |
|---|---|---|---|---|
| LiCoO2/C | 150–190/ | <200 | 0.5 C/1 C | −15… +50/ |
| LiMn2O4/C | 135 | <1500 | 2 C/5 C | −30…+50 |
| LiFePO4/C | 125 | <2000 | 2 C/5 C | −30…+50 |
| Discharge Depth, % | Number of Cycles | Capacity Used (Total for All Time), Aph |
|---|---|---|
| The State of Charge is 100%, Charge Current Is 0.5 C, Discharge Current Is 1 C | ||
| 100 | 550 | 1360 |
| 75 | 650 | 1360 |
| 50 | 1070 | 1480 |
| 25 | 1840 | 1370 |
| Discharge Depth 100%, Charge Current Is 0.5 C, Discharge Current Is 1 C | ||
| 100 | 550 | 1360 |
| 90 | 660 | 1510 |
| 80 | 900 | 1920 |
| Partial Discharge, Charge Current Is 0.25 C, Discharge Current Is C/2 | ||
| 100 | 1300 | 2230 |
| 75 | 2220 | 3610 |
| 60 | 2500 | 4130 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shchurov, N.I.; Dedov, S.I.; Malozyomov, B.V.; Shtang, A.A.; Martyushev, N.V.; Klyuev, R.V.; Andriashin, S.N. Degradation of Lithium-Ion Batteries in an Electric Transport Complex. Energies 2021, 14, 8072. https://doi.org/10.3390/en14238072
Shchurov NI, Dedov SI, Malozyomov BV, Shtang AA, Martyushev NV, Klyuev RV, Andriashin SN. Degradation of Lithium-Ion Batteries in an Electric Transport Complex. Energies. 2021; 14(23):8072. https://doi.org/10.3390/en14238072
Chicago/Turabian StyleShchurov, Nickolay I., Sergey I. Dedov, Boris V. Malozyomov, Alexander A. Shtang, Nikita V. Martyushev, Roman V. Klyuev, and Sergey N. Andriashin. 2021. "Degradation of Lithium-Ion Batteries in an Electric Transport Complex" Energies 14, no. 23: 8072. https://doi.org/10.3390/en14238072







