Reburning of Animal Waste Based Biomass with Coals for NOx Reduction, Part II: Dairy Biomass (DB) and Coal–DB Blends
Abstract
:1. Introduction and Literature Review
2. Materials and Methods
2.1. Fuel Preparation and Characteristics
2.2. Experimental Setup
2.3. Operational Conditions
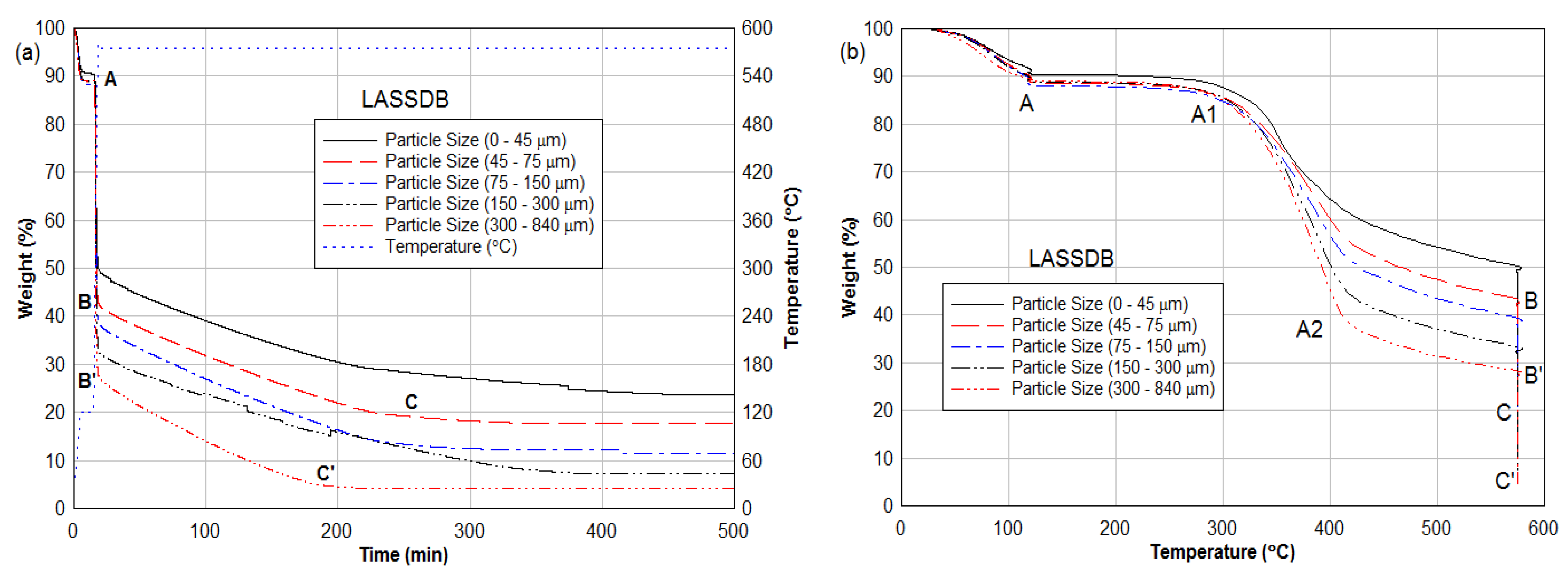
2.4. Size Distribution of Fuel Particles
2.5. Fuel-Nitrogen (N) Analysis
2.6. NOx Emission Relations
- (a)
- Heating value method
- (b)
- Respiratory Quotient (RQ) Method
3. Results and Discussion
3.1. Temperature Distribution within the Reactor
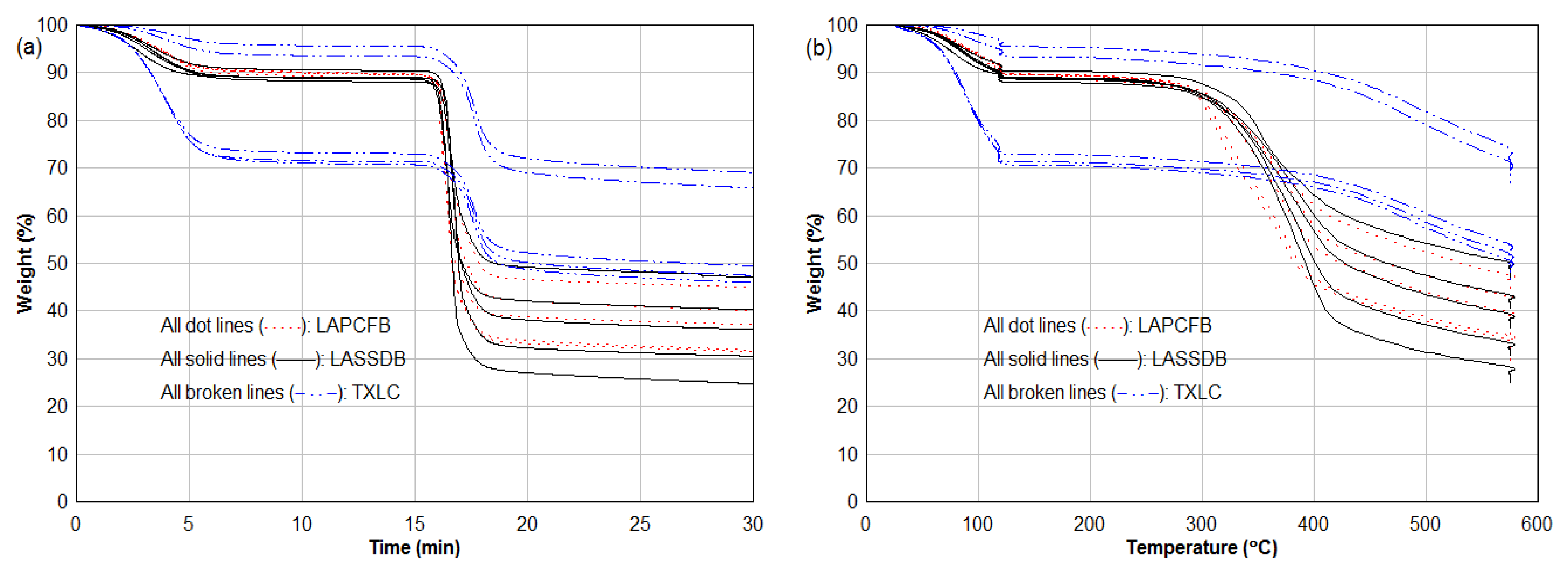
3.2. Thermogravimetric Analysis (TGA)
3.3. NOx Emission and % Reduction
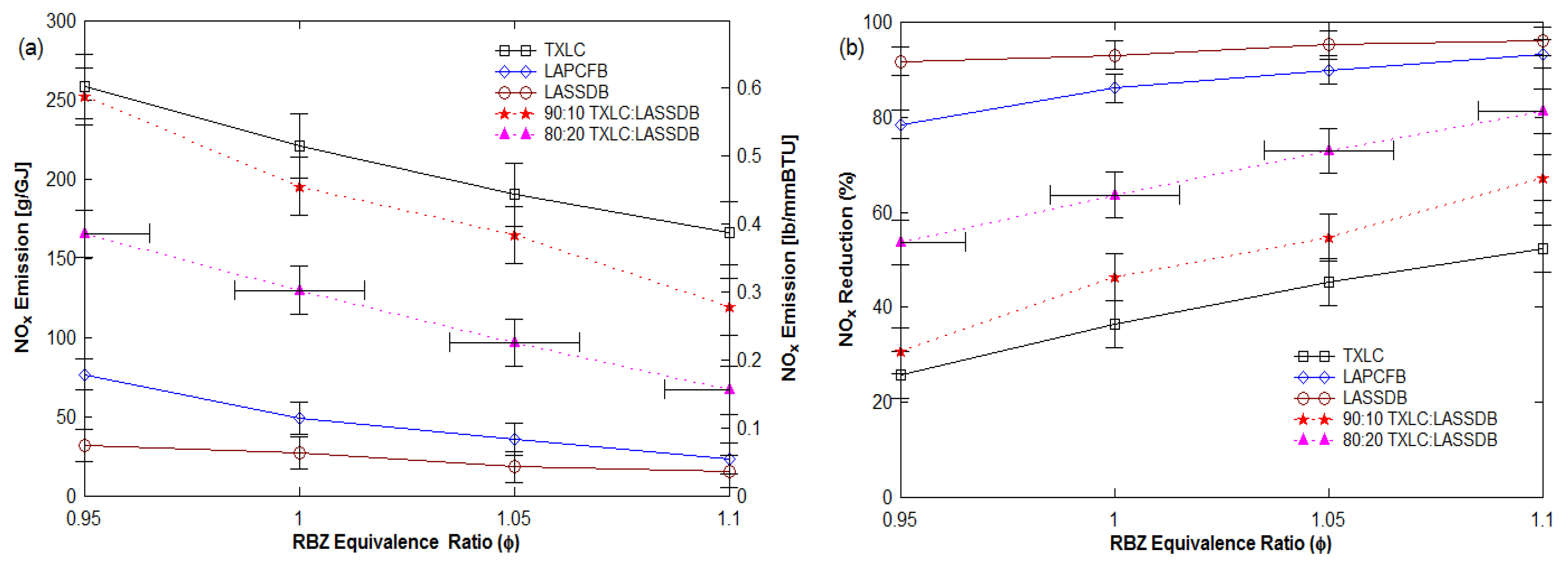
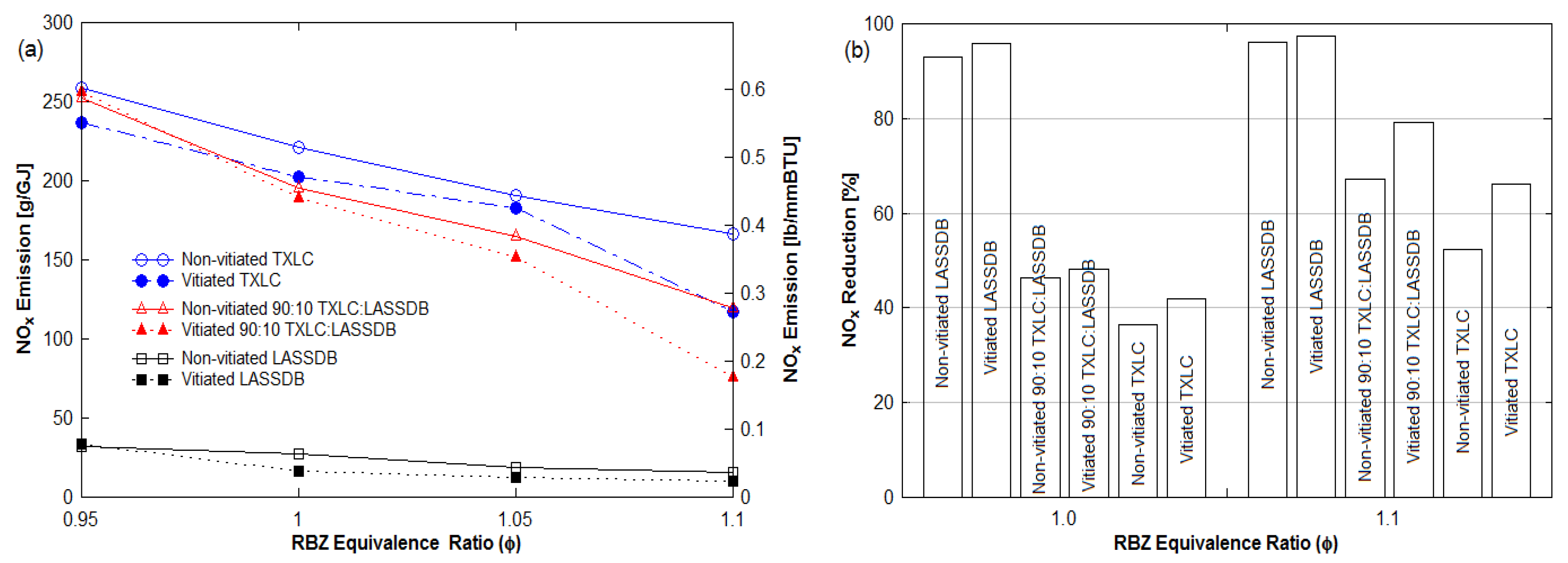
3.3.1. Effects of Reburn Equivalence Ratio (ERRBZ or ϕRBZ)
3.3.2. Effects of Reburn Fuel
3.3.3. Effects of Vitiated Reburn Air
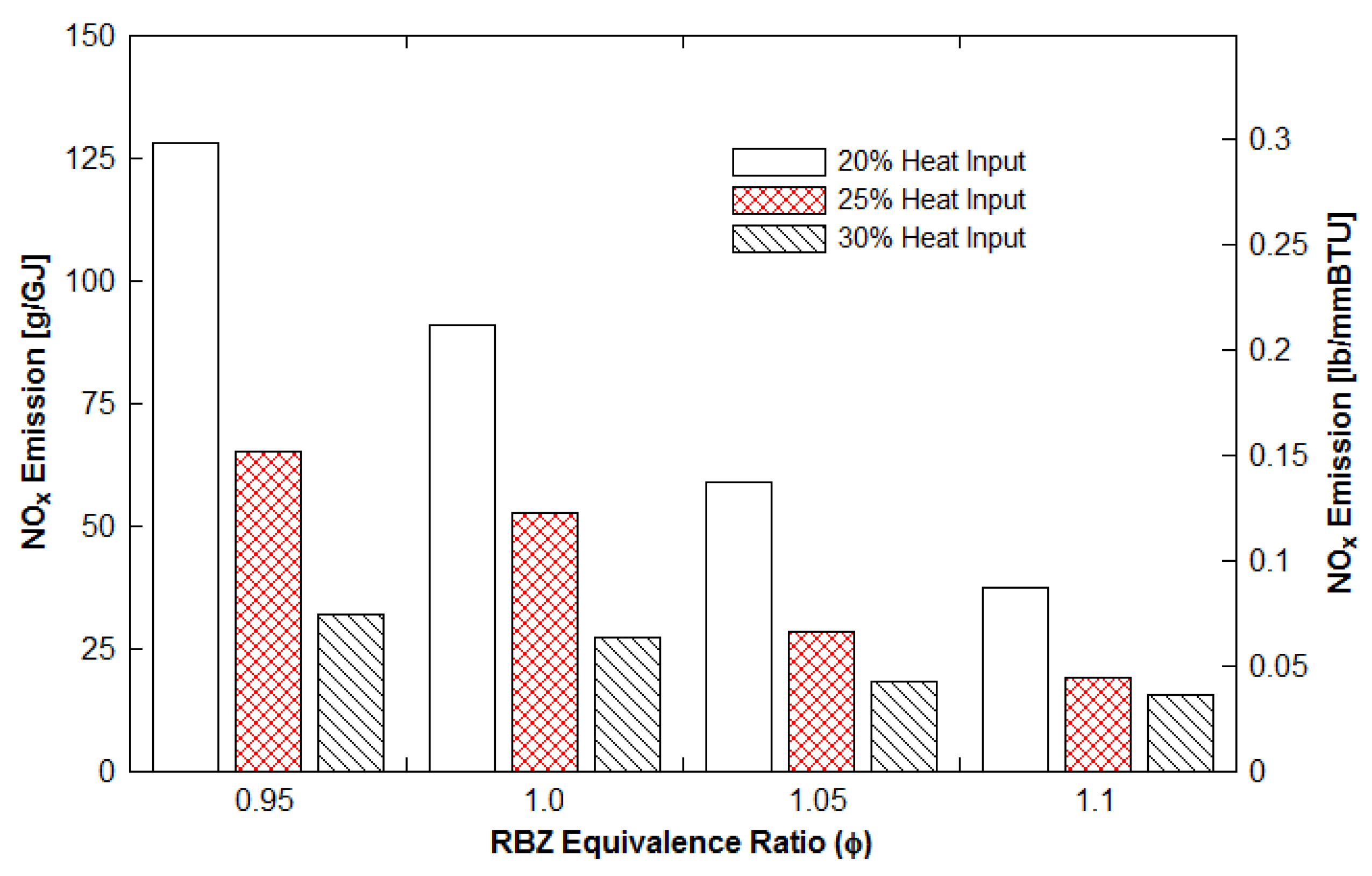
3.3.4. Effects of Reburn Heat Input
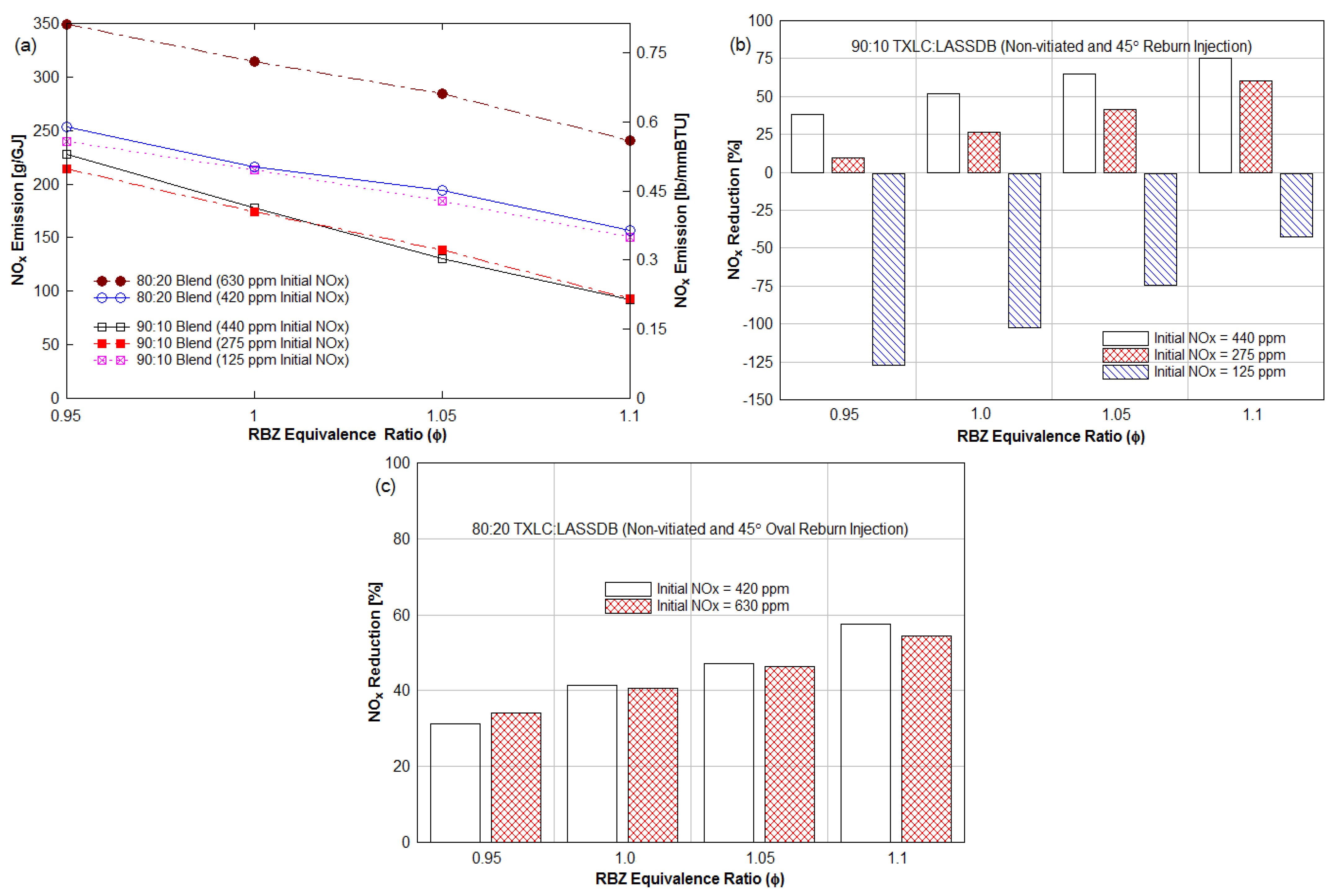
3.3.5. Effects of Initial NOx Concentration
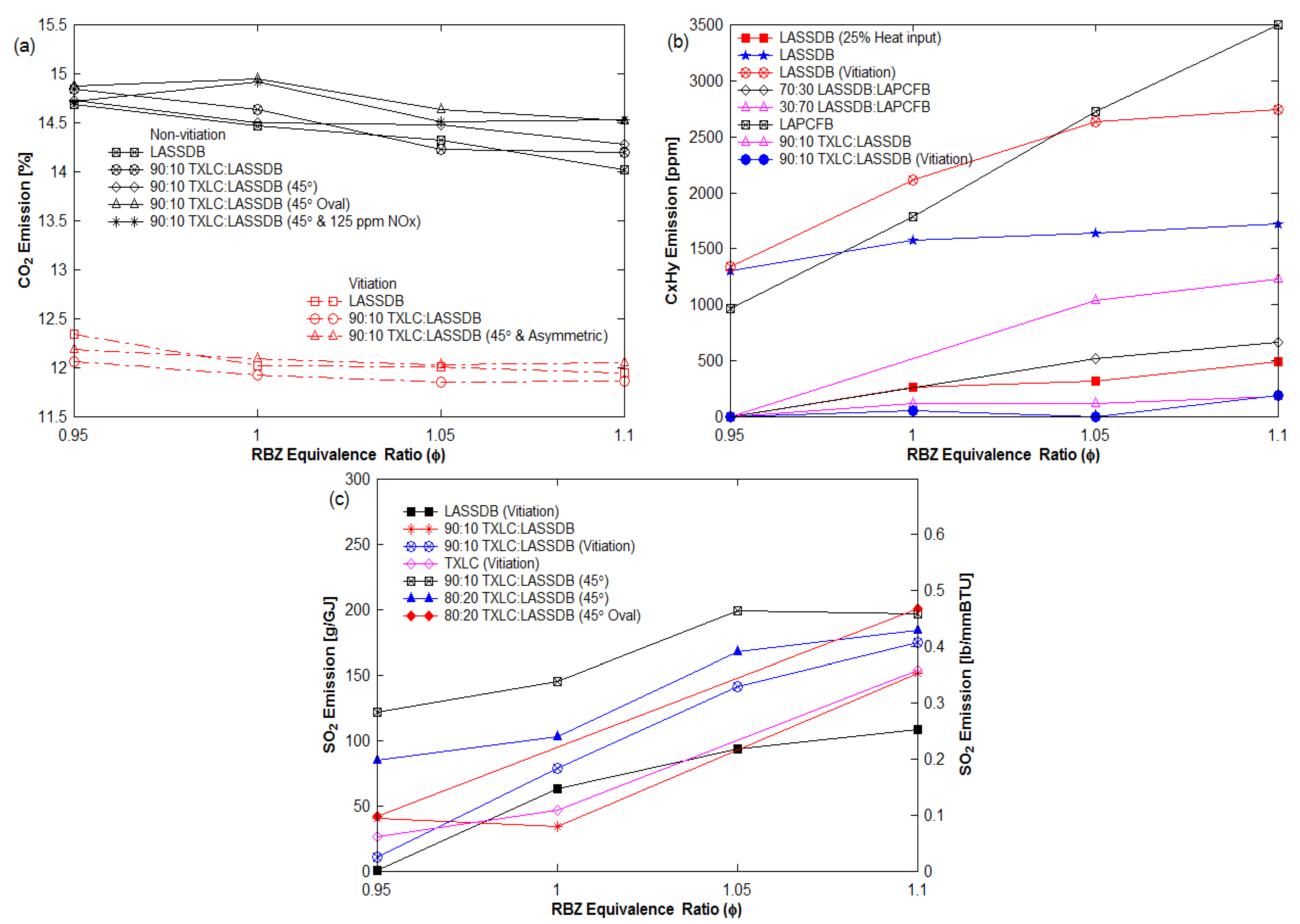
3.4. CO and Other Gas Emissions
3.4.1. CO Emission
3.4.2. CO-NO Emission Correlation
3.4.3. Other Emissions
3.5. Burnt Fraction (BF)
4. Summary and Conclusions
- The extent of NOx reduction was found to be strongly dependent on the ERRBZ, with a 96% reduction in fuel-rich combustion in the reburn zone with DB as the reburn fuel.
- NOx reduction increased with an increase in LASSDB proportion in reburn fuels, but ash % also increased. About 65 to 90% reduction is achieved for the 20% heat input with minimal ash production.
- Vitiated reburn air (12.5% O2) increased NOx reductions and % reduction is higher with higher ERRBZ.
- Results for the 45° upward injection provided the best NOx reduction.
- When the initial NOx was of the order of 125 ppm, negative NOx reductions (or NOx formations) took place with reburning.
- Optimum conditions are as follows: Initial NOx emissions higher than 275 ppm (or 127 g/GJ and 0.3 lb/MMBtu), 20% heat input by DB as the reburn fuel, and ERRBZ = 1.1.
- The BF increased with the increase in proportion of DB in the reburn fuels.
5. Future Work
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFT | Ash Fusion Temperature |
| AgB | Agricultural Waste based Biomass Fuels |
| AnB | Animal Waste based Biomass Fuels |
| As Rec. | As Received |
| B/A | Basic to acidic oxide ratio |
| CB | Cattle Biomass (or Cattle manure) |
| DAF | Dry Ash Free |
| DB | Dairy Biomass |
| EF | Energy Fraction |
| ER | Equivalence Ratio or ϕ |
| FB | Feedlot Biomass |
| FGR | Flue Gas Recirculation |
| HAPCFB | High Ash Partially Composted Feedlot Biomass |
| HEX | Heat Exchanger |
| HHV | Higher Heating Value |
| IDT | Initial Deformation Temperature |
| LAPCFB | Low Ash Partially Composted Feedlot Biomass |
| LASSDB | Low Ash Separated Solids Dairy Biomass |
| RBZ | Reburn Zone |
| RF | Reburn Fuel |
| RQ | Respiratory Quotient, {=CO2 moles/Stoichiometric O2 moles} |
| RZ | Reburn Zone |
| SATP | Standard Ambient Temperature and Pressure |
| SCFH | Standard Cubic Feet per Hour |
| SCR | Selective Catalytic Reduction |
| SMD | Sauter Mean Diameter |
| TAMU | Texas A&M University |
| TXLC | Texas Lignite Coal |
| UCF | Unit Carbon Formula |
| WYC | Wyoming Sub-bituminous Coal |
Appendix A. Estimation of Required NH3 Injection for Simulating NO and Reburn Fuel Flow Rates
Appendix A.1. Main Burner Modeling
Appendix A.2. Reburn Fuel Flow Rate
References
- Frazzitta, S.; Annamalai, K.; Sweeten, J. Performance of a Burner with Coal and Coal-Bio-Solid Fuel Blends. J. Propuls. Power 1999, 15, 181–186. [Google Scholar] [CrossRef]
- Johnson, D.K.; Engelhardt, D.A.; Harvilla, J. Clean Coal Technology—Reburning Technologies for the Control of Nitrogen Oxides Emissions from Coal—Fired Boilers; Topical Report Number 14; U.S. Department of Energy: Washington, DC, USA, 2007. Available online: www.fossil.energy.gov/programs/powersystems/publications/Clean_Coal_Topical_Reports/ (accessed on 14 October 2021).
- Adams, B.R.; Harding, N.S. Reburning using Biomass for NOx Control. Fuel Process. Technol. 1998, 54, 249–263. [Google Scholar] [CrossRef]
- Spliethoff, H.; Hein, K.R.G. Effect of Co-Combustion of Biomass on Emissions in Pulverized Fuel Furnaces. Fuel Process. Technol. 1998, 54, 189–205. [Google Scholar] [CrossRef]
- Arumugam, S. Nitrogen Oxides Emission Control through Reburning with Biomass in Coal-Fired Power Plants. Master’s Thesis, Mechanical Engineering, Texas A&M University, College Station, TX, USA, 2004. [Google Scholar]
- Yang, Y.B.; Naja, T.A.; Gibbs, B.M.; Hampartsoumian, E. Optimisation of Operating Parameters for NO Reduction by Coal Reburning in a 0.2 MW Furnace. J. Inst. Energy 1997, 70, 9–16. [Google Scholar]
- Brouwer, J. Cofiring Waste Biofuels and Coal for Emissions Reduction. Fuel Energy Abstr. 1995, 36, 437. [Google Scholar]
- Maly, P.M.; Zamansky, V.M.; Ho, L.; Payne, R. Alternative Fuel Reburning. Fuel 1999, 78, 327–334. [Google Scholar] [CrossRef]
- Zamansky, V.M.; Maly, P.M.; Lissianski, V.V.; Freeman, M.C. Advanced Biomass Reburning. Report. Department of Energy ad Agriculture. 2000. Available online: www.netl.doe.gov/publications/proceedings/98/98ps/pspa-5.pdf (accessed on 14 October 2021).
- Sweterlitsch, J.J.; Brown, R.C. Fuel Lean Biomass Reburning in Coal-Fired Boilers; Final Technical Report; DOE Award No: DE-FG26-00NT40811; Center for Sustainable Environmental Technologies, Iowa State University: Ames, IA, USA, 2002. [Google Scholar]
- Annamalai, K.; Sweeten, J.M.; Mathur, M.; Thien, B.; Wei, G.; Priyadarsan, S.; Arumugam, S.; Heflin, K. Co-Firing Coal: Feedlot and Litter Biomass (CFB and CLB) Fuels in Pulverized Fuel and Fixed Bed Burners; Final Report; National Energy Technology Laboratory, and Department of Energy: College Station, TX, USA, 2003. Available online: www.osti.gov/bridge/servlets/purl/822025-otOX2Z/native/822025.pdf (accessed on 14 October 2021).
- Storm, C.; Spliethoff, H.; Hein, K.R.G. Generation of a Gaseous Fuel by Pyrolysis of Biomass and Sewage Sludge for Use as Reburn Gas in Coal-Fired Boilers. Clean Air 2005, 6, 289–328. [Google Scholar] [CrossRef]
- Annamalai, K.; Goughnour, P.G.; Oh, H.; Arcot, V.U.; Sweeten, J.M. NOx and Hg Capture Using Coal, Feedlot Biomass (Cattle Manure) as Reburn Fuels—Task 2: Reburn Experiments for NOx and Hg Reduction; Final Report; College Staion: Mech Eng., Texas A&M; Texas Commission on Environment (TCEQ): Austin, TX, USA, 2006. [Google Scholar]
- Hall, R.E.; Miller, C.A.; Payne, R.; Yakushin, E.; Mespan, J. Application of Multifuel Reburn for NOx Control on a 300 MWe Boiler in Ukraine. In Proceedings of the Air & Waste Management Association’s Annual Meeting & Exhibition, Nashville, TN, USA, 23–28 June 1996; pp. 1–13. [Google Scholar]
- Flosom, B.A.; Engelhardt, D.A.; Moyeda, D.K.; Chang, S.S.; Janik, G.S.; Whelan, M.P. Gas Reburning and Advanced Gas Reburning for NOx Emissions Control. In Proceedings of the 90th Annual Meeting and Exhibition at Air and Waste Management Association, Toronto, ON, Canada, 8–13 June 1997. [Google Scholar]
- Lissianski, V.V.; Zamansky, V.M.; Maly, P.M. Effect of Metal-Containing Additives on NOx Reduction in Combustion and Reburning. Combust. Flame 2001, 125, 1118–1127. [Google Scholar] [CrossRef]
- Patsias, A.A.; Nimmo, W.; Gibbs, B.M.; Williams, P.T. Calcium-Based Sorbents for Simultaneous NOx/SOx Reduction in a Down-Fired Furnace. Fuel 2005, 84, 1864–1873. [Google Scholar] [CrossRef]
- Oh, H.; Annamalai, K.; Goughnour, P.G.; Thien, B.; Sweeten, J.M. Reburning Of Animal Waste Based Biomass With Coals For Nox Reduction, Part I: Feedlot Biomass (FB) And Coa:FB Blends. Energies 2021, in press. [Google Scholar]
- Liesa, F.; Alzueta, M.U.; Millera, A.; Bilbao, R. Influence of Reactant Mixing in a Laminar Flow Reactor: The Case of Gas Reburning. 1. Experimental Study. Ind. Eng. Chem. Res. 2007, 46, 3520–3527. [Google Scholar] [CrossRef]
- Oluwoye, I.; Zeng, Z.; Mosallanejada, S.; Altarawnehd, M.; Gore, J.; Dlugogorskif, B.G. Controlling NOx emission from boilers using waste polyethylene as reburning fuel. Chem. Eng. J. 2021, 411, 128427. [Google Scholar] [CrossRef]
- Li, S.; Xu, T.; Zhou, Q.; Tan, H.; Hui, S.; Hu, H. Optimization of Coal Reburning in a 1 MW Tangentially Fired Furnace. Fuel 2007, 86, 1169–1175. [Google Scholar] [CrossRef]
- Miller, C.A.; Touati, A.D.; Becker, J.; Wendt, J.O.L. NOx Abatement by Fuel-Lean Reburning: Laboratory Combustor and Pilot-Scale Package Boiler Results. In Symposium (International) on Combustion; Elsevier: Amsterdam, The Netherlands, 1998; pp. 3189–3195. [Google Scholar]
- Lawrence, B.; Annamalai, K.; Sweeten, J.M.; Heflin, K. Cofiring Coal and Dairy Biomass in a 29 kWt Furnace. J. Appl. Energy 2009, 86, 2359–2372. [Google Scholar] [CrossRef]
- Patumsawad, S.; Cliffe, K.R. Experimental Study on Fluidized Bed Combustion of High Moisture Municipal Solid Waste. Energy Convers. Manag. 2002, 43, 2329–2340. [Google Scholar] [CrossRef]
- Savolainen, K. Co-Firing of Biomass in Coal-Fired Utility Boilers. Appl. Energy 2003, 74, 369–381. [Google Scholar] [CrossRef]
- Sweeten, J.M.; Annamalai, K.; Thien, B.; McDonald, L.A. Co-Firing of Coal and Cattle Feedlot Biomass (FB) Fuels. Part I. Feedlot Biomass (Cattle Manure) Fuel Quality and Characteristics. Fuel 2003, 82, 1167–1182. [Google Scholar] [CrossRef]
- Thien, B.; Annamalai, K. Reduction of NO through Reburning with Coal and Feedlot Biomass. In Proceedings of the National Combustion Conference, Oakland, CA, USA, 25–27 March 2001. [Google Scholar]
- Annamalai, K.; Thien, B.; Sweeten, B. Co-Firing of Coal and Cattle Feedlot Biomass (FB) Part II. Performance Results from 30 kW (100,000) BTU/h Laboratory Scale Boiler Burner. Fuel 2003, 82, 1183–1193. [Google Scholar] [CrossRef]
- Annamalai, K.; Sweeten, J.; Freeman, M.; Mathur, M.; O’Dowd, W.; Walbert, G.; Jones, S. Co-Firing of Coal and Cattle Feedlot Biomass (FB) Fuels, Part III: Fouling Results from a 500,000 BTU/h Pilot Plant Scale Boiler Burner. Fuel 2003, 82, 1195–1200. [Google Scholar] [CrossRef]
- Annamalai, K.; Sweeten, J.M.; Martin, B. Pyrolysis, Ignition, and Fuel Characteristics of Coal, Feedlot Biomass, and Coal: Feedlot Biomass Blends; Final Report to Texas Commission on Environmental Quality (TCEQ). 2006. Available online: http://search.tceq.state.tx.us/ (accessed on 14 October 2021).
- Li, S.; Wu, A.; Deng, S.; Pan, W.P. Effect of co-combustion of chicken litter and coal on emissions in a laboratory-scale fluidized bed combustor. Fuel Process. Technol. 2008, 89, 7–12. [Google Scholar] [CrossRef]
- Qian, X. Statistical Analysis and Evaluation of the Advanced Biomass and Natural Gas Co-Combustion Performance. Ph.D. Dissertation, Morgan State University, Baltimore, Maryland, 2019. [Google Scholar]
- Annamalai, K.; Sweeten, B. Reburn System with Feedlot Biomass. US Patent #6,973,883, 13 December 2005. Available online: http://www.patentstorm.us/patents/6973883-fulltext.html (accessed on 14 October 2021).
- Sweeten, J.M. Composting Manure and Sludge; Texas Agricultural Extension Service: College Station, TX, USA, 2008; Publication L-2289. [Google Scholar]
- Eseltine, D.; Thanapal, S.S.; Annamalai, K.; Ranjan, D. Torrefaction of Woody Biomass Using Inert and Non-Inert Gases. Fuel 2013, 113, 379–388. [Google Scholar] [CrossRef]
- Annamalai, K.; Puri, I.K. Combustion Science and Engineering; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Boca Raton, FL, USA, 2007. [Google Scholar]
- Annamalai, K.; Thanapal, S.; Ranjan, D. Ranking renewable and fossil fuels on global warming potential using respiratory quotient (RQ) Concept. J. Combust. 2018, 2018, 1270708. [Google Scholar] [CrossRef] [Green Version]
- Meesuk, S.; Cao, J.P.; Sato, K.; Hoshino, A. Nitrogen Conversion of Pig Compost during Pyrolysis. J. Chem. Eng. Jpn. 2013. [Google Scholar] [CrossRef]
- Soyuz, P. Reburn Studies for Hg and NOX Reduction in Coal Fired Burners. Ph.D. Thesis, Texas A&M University, Mechanical Engineering, College Station, TX, USA, 2007. Posthumous. [Google Scholar]
- Wargadalam, V.J.; Loffler, G.; Winter, F.; Hofbauer, H. Homogeneous Formation of NO and N2O from the Oxidation of HCN and NH3 at 600–1000 °C. Combust. Flame 2000, 120, 465–478. [Google Scholar] [CrossRef]
- Roberge, B. Effect of Varying the Combustion Parameters on the Emissions of Carbon Monoxide and Nitrogen Oxides in the Exhaust Gases from Propane Fueled Vehicles. Appl. Occup. Environ. Hyg. 2000, 15, 421–428. [Google Scholar] [CrossRef] [PubMed]


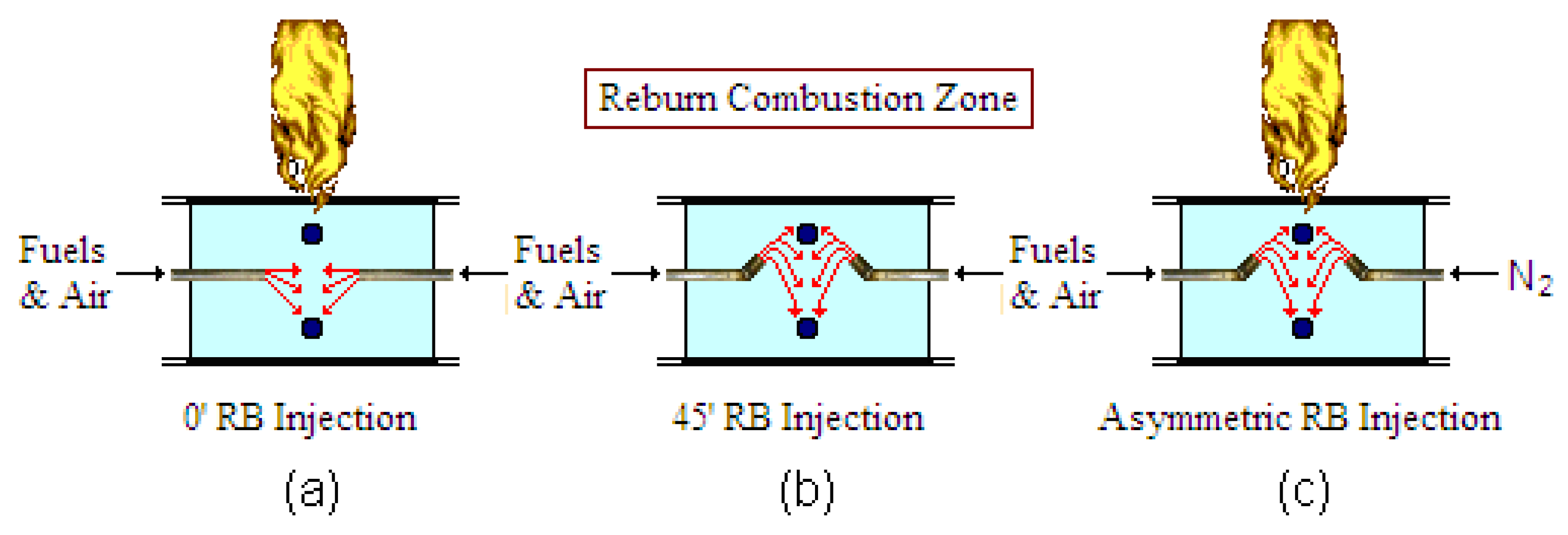




| Ref. | Boiler Capacity | Reburn Fuel (Heat Input) | Fuel Particle Size (Reburn Temp.) | Reburn SR (Main SR) | Residence Time | Max. NOx Reduction (Baseline NOx) | Conclusion |
|---|---|---|---|---|---|---|---|
| [3] | 265 MW + OFA | Wood (15%) | 80% 800 μm (1900 K) | 0.9 (1.05) | 0.3–1.2 s | 60% (~1300 ppm) | Wood was successfully used as a reburn fuel on NOx reduction using a cyclone fired boiler. |
| [4] | Electric furnace + OFA | CH4, synthetic gas, pyrolysis gas of coal | Gas (1300–1700 K) | 0.65–1.15 (0.65–1.15) | 1.2–3 s | 88% (NA) | Pyrolysis gas was an effective reburn fuel. Longer residence time reduced NOx emissions. |
| [5] | 30 kW | CB, coals, Coal:CB blends (30%) | 50–70% 100 μm (1500 K) | 0.83–1.0 (1.05) | 0.4–0.9 s | 62% (600 ppm) | Circular jet/flat spray reburn nozzles were tested, and CB resulted in higher NOx reduction than coal. |
| [6] | 200 kW + OFA and AGR | Bituminous coals (10–35%) | 75% 63 μm (1600 K) | 0.83–1.0 (1.0–1.18) | 0.12–0.84 s | 65% (600 ppm) | NOx reduction was strongly dependent on the reburn SR. The optimum reburn residence time was 0.45 s. |
| [7] | 38 kW + OFA | Wood (10%) | 100% 1680 μm (1700 K) | 0.85–1.05 (1.15) | 0.4 s | 60% (200–500 ppm) | Wood was an effective reburn fuel for a small-scale pulverized coal-fired furnace. |
| [8] | 300 kW + OFA and AGR | Biomass, coals, CRDF (10–20%) | 70% 75 μm (1700 K) | 0.88–0.99 (1.1) | 0.4–0.7 s | 91% (AR) (200–1300 ppm) | Advance gas reburning (AGR) by the addition of N-agent with promoters greatly increased NOx reduction. |
| [9] | 300 kW + OFA | Wood, waste, walnut shell, NG (10–25%) | 55% 75 μm (1700 K) | 0.84–0.99 (1.1) | 0.82 s | 72% (400–900 ppm) | NO reductions: NG walnut shell furniture waste willow wood |
| [10] | 35 kW | Switchgrass, alfalfa (4–23%) | 75% 1000 μm (1600 K) | 1.05–1.4 | 0.81 s | 70% (500 ppm) | High N-containing fuel was successfully used as a reburn fuel in a fuel-lean combustion. |
| [11] | 30 kW | FB, LB (30%) | 70–90% 75 μm (1500 K) | 0.91–1.0 (1.05) | 1 s | 80% (600 ppm) | FB and litter biomass (LB) were successfully used for co-firing, reburning and gasification. |
| [12] | 30 kW + OFA | Pyrolysis gas of sewage sludge, sawdust, etc. (40%) | Gas (1600 K) | 0.75–1.15 (1.15) | 2 s | 89% (1000–1200 mg/m3) | Pyrolysis gas from biomass used as a reburn fuel contributed high NOx reduction and the net CO2 reduction. |
| [13] | 30 kW | CB, coals, Coal:CB blends (30%) | 60–90% 150 μm (1500 K) | 0.87–1.0 (1.05) | 0.6–0.7 s | 95% (400 ppm) | CB as a reburn fuel significantly reduced NOx emissions. NOx reductions strongly depended on the reburn equivalence ratio. |
| [14] | 300 MW + OFA and FGR | NG, oil, coal (15–20%) | 80% 44 μm (1800 K) | 0.9 | NA | 65% (600 ppm) | High NOx reductions with fine fuel particles. |
| [15] | 105 MW + OFA and AGR | NG (10–20%) | Gas (1200–1400 K) | 0.9 | NA | 70% (0.62 lb/MMBtu) | AGR by the injection of N-agent significantly improved NOx reduction and CO emissions. |
| [16] | 300 kW + OFA | NG (5–25%) | Gas (1700 K) | 0.83–1.05 (1.1) | 0.82 s | 66% (600 ppm) | Reburning with alkali compounds was effective in NOx reduction. |
| [17] | 80 kW + OFA | Carboxylic salts + C3H8 (9–20%) | NA (1400 K) | 0.86–1.03 (1.05) | NA | 80% (NA) | The addition of carboxylic salts to propane as a reburn fuel improved the reburn process. |
| [18] | 30 kW | FB, Coal:FB Blends (30%) | 60% 113 μm | (0.95–1.15) | 0.6–0.7 s | 95% (400 ppm) | Feedlot Biomass as reburn fuel with NG as main fuel |
| [19] | Laminar flow reactor | Synthetic gas (CH4 + C2H6) | Gas (900–1500 K) | 0.6–2.0 | 0.8 s | 50% (850 ppm) | Fuel mixing was effective for reburning due to the change in local stoichiometry and residence time. |
| [20] | Entrained bed reactor | Polyethylene | 150–355 μm | 0.8–1.2 | 0.45 s (total 1 s) | up to 82% | 1000–1200 °C |
| [21] | 1 MW + LNB and OFA | Coals (15–25%) | Micronized Dmedian: 11–54 μm (1600 K) | 0.85–0.95 (1.05) | NA | 80% (775–820 ppm) | Micronized fuels resulted in little increase in NOx reduction. |
| [22] | 17 kW and 0.9 MW | NG (7–25%) | Gas (800–1100 K) | 1.2 (1.2–1.3) | NA | 60% (250–2600 ppm) | The fuel-lean reburning is an effective method to reduce NOx for lab- and pilot-scale boilers. |
| Contents | TXLC | LAPCFB | LASSDB | ||||||
|---|---|---|---|---|---|---|---|---|---|
| As Rec. | Dry | DAF | As Rec. | Dry | DAF | As Rec. | Dry | DAF | |
| Moisture | 38.34 | 0 | 0 | 19.64 | 0 | 0 | 25.26 | 0 | 0 |
| Ash | 11.46 | 18.59 | 0 | 16.50 | 20.53 | 0 | 14.93 | 19.97 | 0 |
| Volatile Matter | 24.79 | 40.20 | 49.38 | 52.33 | 65.11 | 81.94 | 46.86 | 62.70 | 78.35 |
| Fixed Carbon | 25.41 | 41.21 | 50.62 | 11.54 | 14.36 | 18.06 | 12.95 | 17.33 | 21.65 |
| HHV, kJ/kg (BTU/lb) | 14,290 (6145) | 23,170 (9960) | 28,465 (12,240) | 13,265 (5705) | 16,505 (7095) | 20,773 (8930) | 12,845 (5520) | 17,180 (7385) | 21,475 (9230) |
| Carbon, C | 37.18 | 60.30 | 74.07 | 33.79 | 42.05 | 52.91 | 35.20 | 47.09 | 58.84 |
| Hydrogen, H | 2.12 | 3.44 | 4.23 | 3.65 | 4.55 | 5.72 | 3.12 | 4.17 | 5.21 |
| Nitrogen, N | 0.68 | 1.11 | 1.35 | 1.97 | 2.45 | 3.08 | 1.93 | 2.58 | 3.22 |
| Oxygen, O | 9.61 | 15.58 | 19.14 | 23.94 | 29.78 | 37.49 | 19.15 | 25.62 | 32.01 |
| Sulfur, S | 0.61 | 0.98 | 1.21 | 0.51 | 0.64 | 0.79 | 0.43 | 0.57 | 0.71 |
| TXLC | LAPCFB | LASSDB | |
|---|---|---|---|
| Stoich. Air: Fuel Ratio (A:F) | 4.63 | 4.14 | 4.32 |
| Stoich. O2: Fuel Ratio (O:F) | 0.97 | 0.87 | 0.91 |
| As Rec. HHV, kJ/kg (BTU/lb) | 14,290 (6145) | 13,265 (5705) | 12,845 (5520) |
| HHV in kJ per kg Stoich. Air (BTU/lb) | 3090 (1330) | 3210 (1380) | 2975 (1280) |
| HHV in kJ per kg Stoich.O2 (BTU/lb) | 14,710 (6325) | 15,275 (6565) | 14,170 (6090) |
| DAF HHV, kJ/kg (BTU/lb) | 28,465 (12,240) | 20,775 (8930) | 21,474 (9232) |
| Ash Loading kg/GJ | 8.02 | 12.44 | 11.62 |
| VM-HHV, kJ/kg, Equations in Ref [35,36] | 24,055 | 18,130 | 18,355 |
| VM heat contribution, % | 42 | 72 | 67 |
| VM HHV/DAF Fuel HHV | 0.845 | 0.873 | 0.855 |
| Chemical Formula | CH0.68 N0.02 O0.19 S0.006 | CH1.28 N0.05 O0.53 S0.006 | CH1.06 N0.05 O0.41 S0.005 |
| CFr, DAF | 0.74 | 0.53 | 0.59 |
| M, DAF | 0.74 | 22.68 | 20.5 |
| CFr Bl/HHVBl (GJ/kg) | 16.21 | 17.18 | 17.72 |
| RQRF | 0.85 | 0.75 | 0.79 |
| RQBl; see Ref [37] for blends with EFRF = 0.3 | 0.62 | 0.59 | 0.60 |
| Compositions | TXLC | LAPCFB | LASSDB |
|---|---|---|---|
| Silicon, SiO2 | 48.72 | 20.78 | 31.36 |
| Aluminum, Al2O3 | 16.04 | 4.94 | 2.89 |
| Titanium, TiO2 | 0.85 | 0.22 | 0.2 |
| Iron, Fe2O3 | 7.44 | 1.71 | 1.62 |
| Calcium, CaO | 11.70 | 21 | 26.4 |
| Magnesium, MgO | 1.93 | 7.54 | 7.47 |
| Sodium, Na2O | 0.29 | 5.26 | 2.28 |
| Potassium, K2O | 0.61 | 14.6 | 6.9 |
| Phosphorus, P2O5 | 0.1 | 13.77 | 6.01 |
| Sulfur, SO3 | 10.80 | 4.47 | 4.72 |
| Chlorine, Cl | 0.01 | 5.07 | 0.92 |
| Carbon dioxide, CO2 | 0.08 | 0.59 | 9.49 |
| Basic oxides/Acidic oxides, B/A | 0.34 | 2.46 | 1.47 |
| Properties | TXLC | LAPCFB | LASSDB |
|---|---|---|---|
| Reducing atmosphere | |||
| Initial deformation temperature, IDT or AFT, °F (°C) | 2111 (1155) | 2126 (1165) | 2153 (1178) |
| Softening temperature, ST, °F (°C) | 2150 (1175) | 2143 (1170) | 2169 (1187) |
| Hemispherical temperature, HT, °F (°C) | 2181 (1195) | 2148 (1175) | 2175 (1191) |
| Flow temperature, FT, °F (°C) | 2190 (1200) | 2156 (1180) | 2181 (1194) |
| Oxidizing atmosphere | |||
| Initial deformation temperature, IDT or AFT, °F (°C) | 2238 (1225) | 2124 (1160) | 2190 (1199) |
| Softening temperature, ST, °F (°C) | 2256 (1235) | 2186 (1200) | 2198 (1203) |
| Hemispherical temperature, HT, °F (°C) | 2276 (1245) | 2146 (1175) | 2201 (1205) |
| Flow temperature, FT, °F (°C) | 2310 (1265) | 2154 (1180) | 2206 (1208) |
| Mean Dia. (μm) | TXLC (w%) | LASSDB (w%) |
|---|---|---|
| 1596 | 0.015 | 0.260 |
| 1015 | 0.02 | 0.841 |
| 570 | 5.595 | 21.815 |
| 225 | 37.986 | 31.451 |
| 113 | 34.204 | 22.880 |
| 60 | 14.857 | 9.602 |
| 22.5 | 7.320 | 13.110 |
| Mean size by mass (μm) | 166.84 | 242.24 |
| SMD (μm) | 94.72 | 88.84 |
| Reburn Fuel | Ash [%] | Combustibles [%] | Burnt Fraction [%] |
|---|---|---|---|
| LASSDB | 62.91 | 37.09 | 85.29 |
| 80:20 TXLC:LASSDB | 43.77 | 56.23 | 70.39 |
| 90:10 TXLC:LASSDB | 40.46 | 59.54 | 65.78 |
| TXLC | 38.91 | 61.09 | 64.15 |
| LAPCFB | 69.94 | 30.06 | 88.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, H.; Annamalai, K.; Sweeten, J.M.; Heflin, K. Reburning of Animal Waste Based Biomass with Coals for NOx Reduction, Part II: Dairy Biomass (DB) and Coal–DB Blends. Energies 2021, 14, 8076. https://doi.org/10.3390/en14238076
Oh H, Annamalai K, Sweeten JM, Heflin K. Reburning of Animal Waste Based Biomass with Coals for NOx Reduction, Part II: Dairy Biomass (DB) and Coal–DB Blends. Energies. 2021; 14(23):8076. https://doi.org/10.3390/en14238076
Chicago/Turabian StyleOh, Hyukjin, Kalyan Annamalai, John M. Sweeten, and Kevin Heflin. 2021. "Reburning of Animal Waste Based Biomass with Coals for NOx Reduction, Part II: Dairy Biomass (DB) and Coal–DB Blends" Energies 14, no. 23: 8076. https://doi.org/10.3390/en14238076
APA StyleOh, H., Annamalai, K., Sweeten, J. M., & Heflin, K. (2021). Reburning of Animal Waste Based Biomass with Coals for NOx Reduction, Part II: Dairy Biomass (DB) and Coal–DB Blends. Energies, 14(23), 8076. https://doi.org/10.3390/en14238076






