Experimental Research on the Law of Energy Conversion during CO2 Sequestration in Coal
Abstract
:1. Introduction
2. Adsorption Theories
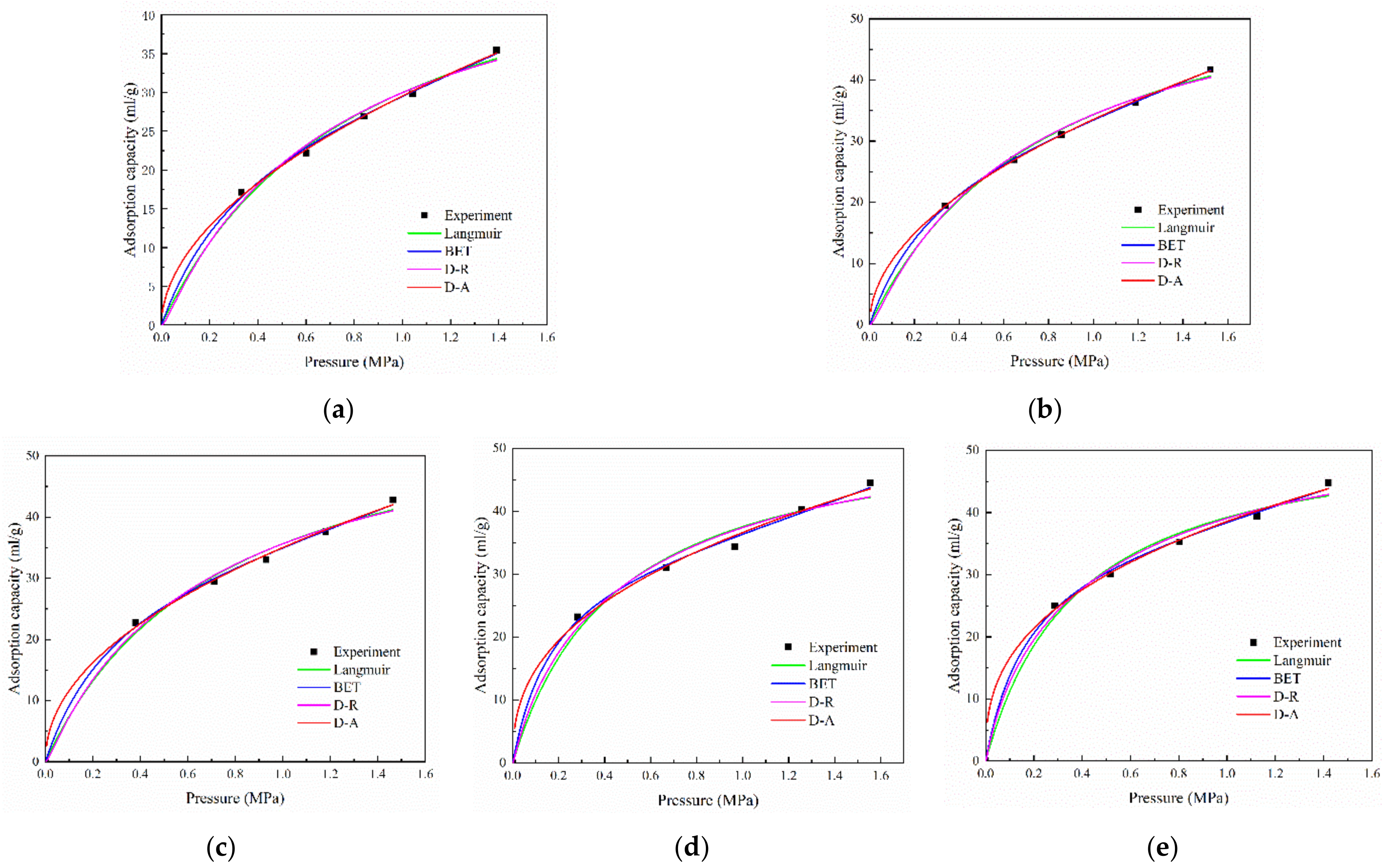
2.1. Adsorption Models
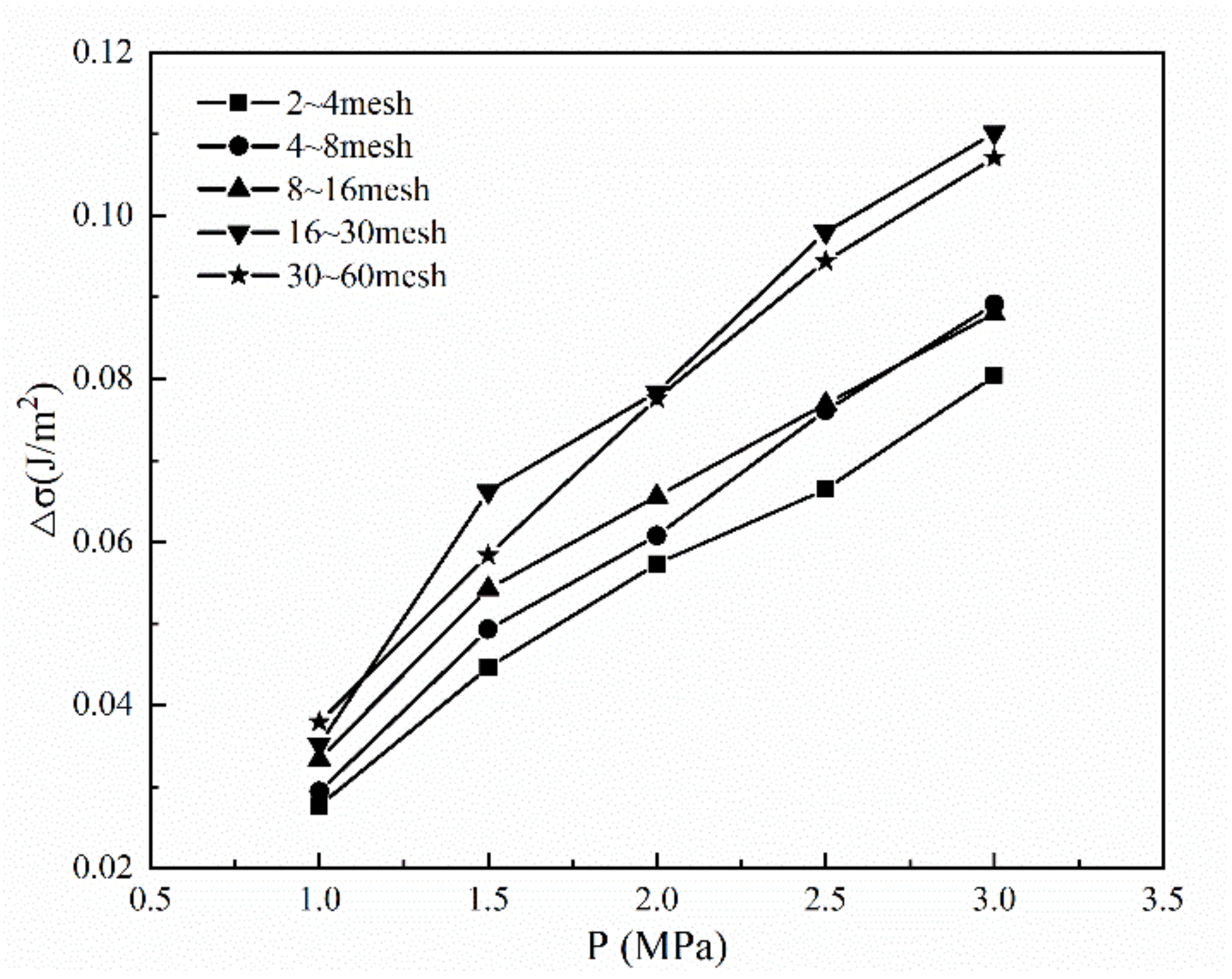
2.2. Surface Free Energy
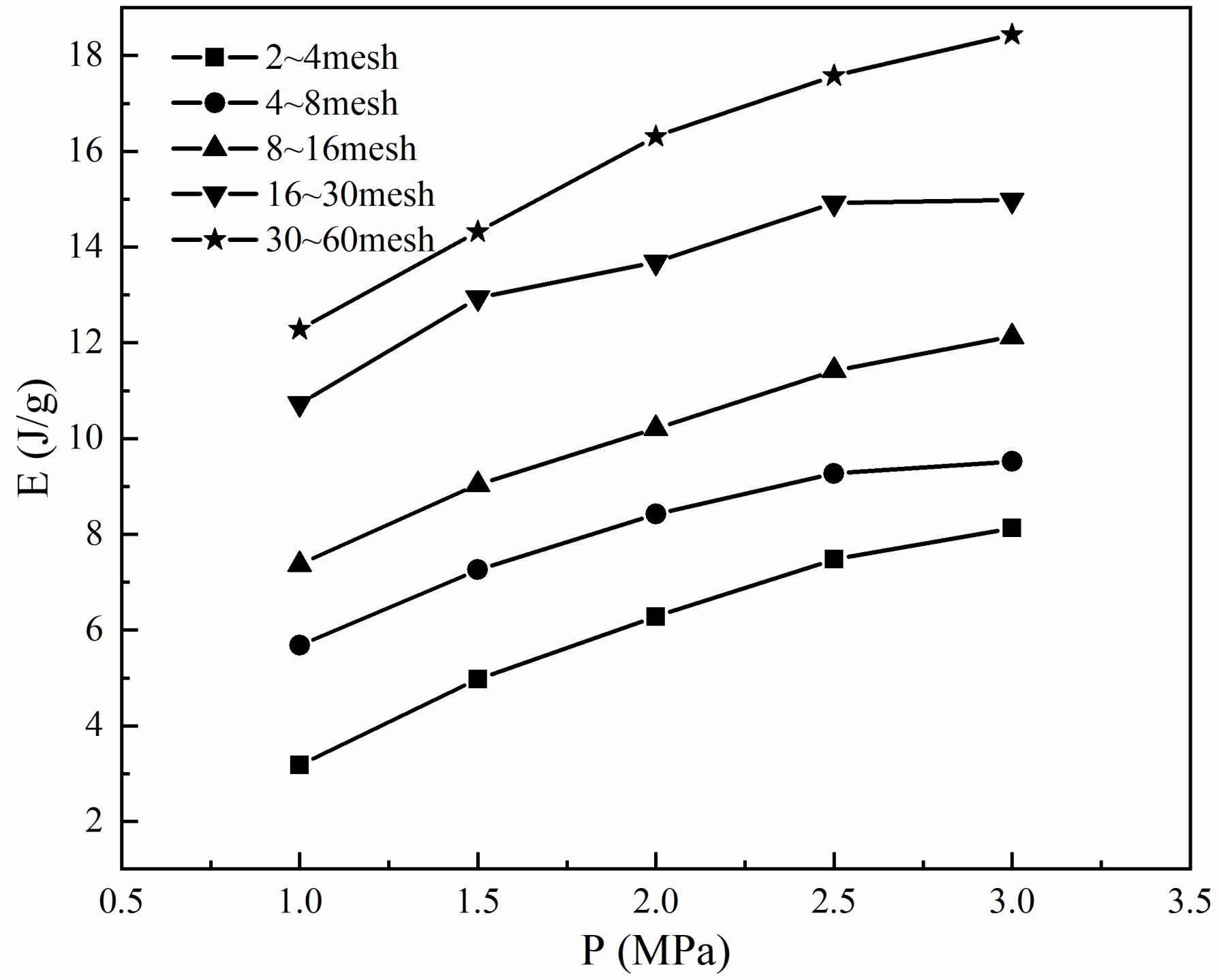
2.3. Heat Energy
3. Experiments
3.1. Samples Preparation
3.2. Experimental Setup
3.3. Adsorption Experiment Procedure
3.4. Experimental Data Calibration
4. Results and Discussion
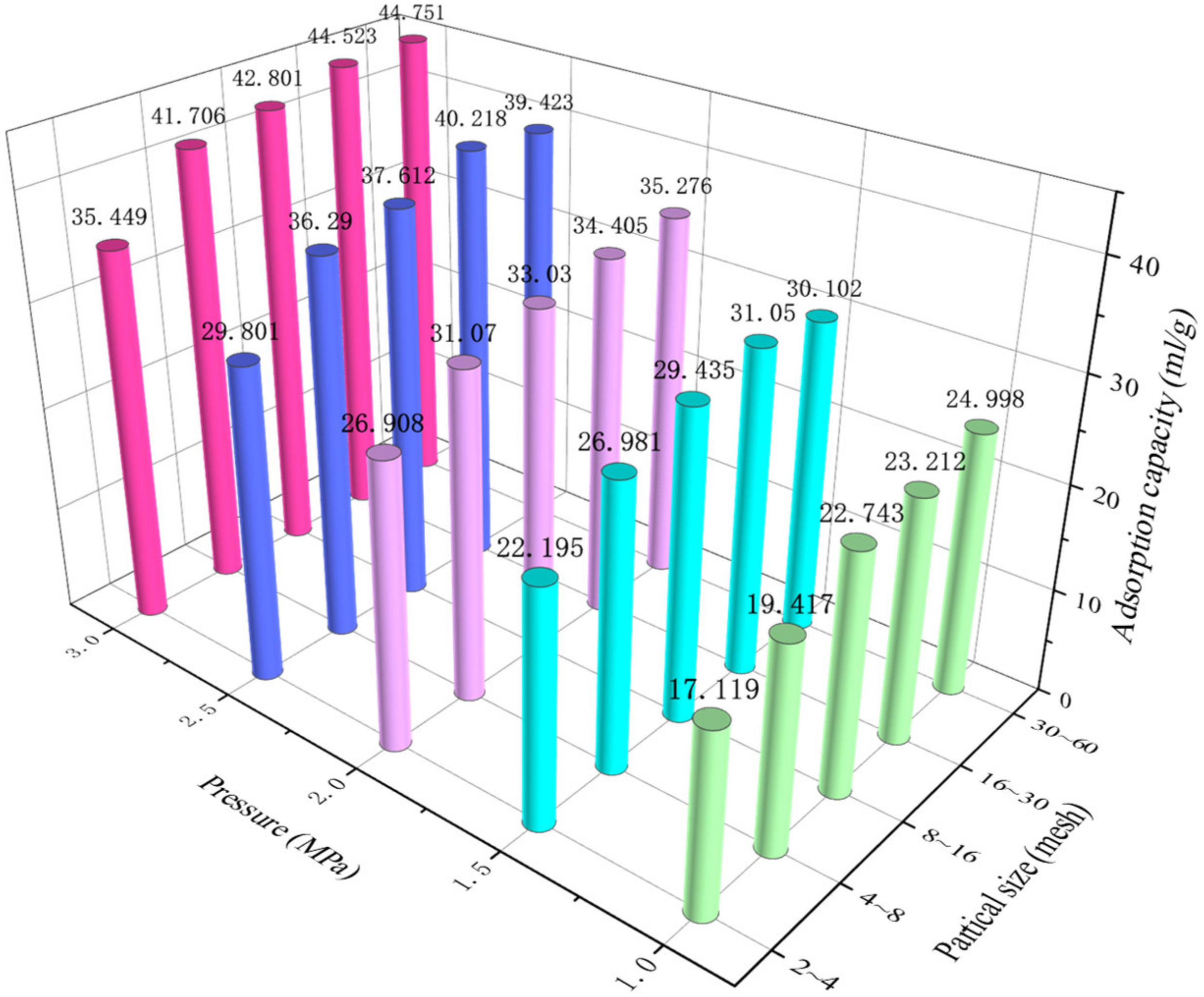
4.1. Adsorption Capacity and Temperature
4.2. Adsorption Characteristics
4.3. Law of Energy Conversion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drexler, S.; Silveira, T.M.G.; Belli, G.D.; Couto, P. Experimental study of the effect of carbonated brine on wettability and oil displacement for EOR application in the Brazilian Pre-Salt reservoirs. Energy Sources Part A Recovery Util. Environ. Eff. 2021, 43, 3282–3296. [Google Scholar] [CrossRef]
- Aoife, F.; Beatrice, M.S.; Tomislav, P.; Natasa, M.; Duic, N. A review of developments in technologies and research that have had a direct measurable impact on sustainability considering the Paris agreement on climate change. Renew. Sustain. Energy Rev. 2017, 68, 835–839. [Google Scholar]
- Anderson, T.R.; Hawkins, E.; Jones, P.D. CO2, the greenhouse effect and global warming: From the pioneering work of Arrhenius and Callendar to today’s Earth System Models. Endeavour 2016, 40, 178–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aminu, M.; Nabavi, S.A.; Rochelle, C.A.; Manovic, V. A review of developments in carbon dioxide storage. Appl. Energy 2017, 208, 1389–1419. [Google Scholar] [CrossRef] [Green Version]
- Agency, I.E.; Ebrary, I. Energy Technology Perspectives. In 2010, Scenarios & Strategies to 2050. Sourceoecd Energy. 2010. Available online: https://www.oecd-ilibrary.org/energy/energy-technology-perspectives-2010_energy_tech-2010-en (accessed on 30 November 2021).
- Hu, B.; Zhai, H. The cost of carbon capture and storage for coal-fired power plants in China. Int. J. Greenh. Gas Control 2017, 65, 23–31. [Google Scholar] [CrossRef]
- Zhao, X.; Liao, X.; He, L. The evaluation methods for CO2 storage in coal beds, in China. J. Energy Inst. 2016, 89, 389–399. [Google Scholar] [CrossRef]
- Maa, B.; Ab, A.; Ml, A.; Si, A. CO2-wettability of low to high rank coal seams: Implications for carbon sequestration and enhanced methane recovery. Fuel 2016, 181, 680–689. [Google Scholar]
- Talapatra, A. A study on the carbon dioxide injection into coal seam aiming at enhancing coal bed methane (ECBM) recovery. J. Pet. Explor. Prod. Technol. 2020, 10, 1965–1981. [Google Scholar] [CrossRef] [Green Version]
- Sripada, P.; Khan, M.M.; Ramasamy, S.; Trivedi, J.; Gupta, R. Influence of coal properties on the CO2 adsorption capacity of coal gasification residues. Energy Sci. Eng. 2018, 6, 321–335. [Google Scholar] [CrossRef] [Green Version]
- Ramasamy, S.; Sripada, P.P.; Khan, M.M.; Tian, S.; Trivedi, J.; Gupta, R. Adsorption Behavior of CO2 in Coal and Coal Char. Energy Fuels 2014, 28, 5241–5251. [Google Scholar] [CrossRef]
- Wu, D.; Liu, X.; Sun, K.; Xiao, X.; Xin, L. Experiments on supercritical CO2 adsorption in briquettes. Energy Sources Part A Recovery Util. Environ. Eff. 2018, 41, 1–7. [Google Scholar] [CrossRef]
- Sw, A.; Zja, B.; Cda, C. Molecular simulation of coal-fired plant flue gas competitive adsorption and diffusion on coal. Fuel 2019, 239, 87–96. [Google Scholar]
- Zhang, L.; Ren, T.; Naj, A. Influences of temperature and moisture on coal sorption characteristics of a bituminous coal from the Sydney Basin, Australia. Int. J. Oil 2014, 8, 62–81. [Google Scholar] [CrossRef]
- Likar, J.; Tajnik, T. Analysis of CO2 adsorption in different lytotypes of lignite. Acta Chim. Slov. 2013, 60, 221–227. [Google Scholar] [PubMed]
- Chaback, J.J.; Morgan, D.; Dan, Y. Sorption Irreversibilities and Mixture Compositional Behavior During Enhanced Coal Bed Methane Recovery Processes. In Proceedings of the SPE Gas Technology Symposium, Calgary, AB, Canada, 28 April 1996; Volume 4, pp. 431–438. Available online: https://onepetro.org/SPEGTS/proceedings-abstract/96GTS/All-96GTS/SPE-35622-MS/59682 (accessed on 30 November 2021).
- Tao, G.; Dong, Z.; Chen, W.; Zengchao, F. Energy variation in coal samples with different particle sizes in the process of adsorption and desorption—ScienceDirect. J. Pet. Sci. Eng. 2020, 188, 106932. [Google Scholar]
- Fan, Y.; Deng, C.; Zhang, X.; Li, F.; Wang, X.; Qiao, L. Numerical study of CO2-enhanced coalbed methane recovery. Int. J. Greenh. Gas Control 2018, 76, 12–23. [Google Scholar] [CrossRef]
- Cai, Y.; Pan, Z.; Liu, D.; Zheng, G.; Tang, S.; Connell, L.; Yao, Y.; Zhou, Y. Effects of pressure and temperature on gas diffusion and flow for primary and enhanced coalbed methane recovery. Energy Explor. Exploit. 2014, 32, 601–620. [Google Scholar] [CrossRef] [Green Version]
- Hao, J.; Wen, H.; Ma, L.; Fei, J.; Ren, L. Theoretical Derivation of a Prediction Model for CO2 Adsorption by Coal. ACS Omega 2021, 6, 13275–13283. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Ripepi, N. High pressure supercritical carbon dioxide adsorption in coal: Adsorption model and thermodynamic characteristics. J. CO2 Util. 2017, 18, 189–197. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Cui, Y.J.; Liu, B.; Li, S.G.; Song, W.L.; Lin, W.G. Supercritical Pure Methane and CO2 Adsorption on Various Rank Coals of China: Experiments and Modeling. Energy Fuels 2011, 25, 1891–1899. [Google Scholar] [CrossRef]
- Zang, J.; Wang, K.; Zhao, Y. Evaluation of gas sorption-induced internal swelling in coal. Fuel 2015, 143, 165–172. [Google Scholar] [CrossRef]
- Day, S.; Fry, R.; Sakurovs, R.; Weir, S. Swelling of Coals by Supercritical Gases and Its Relationship to Sorption. Energy Fuels 2010, 24, 2777–2783. [Google Scholar] [CrossRef]
- Qiao, L.; Deng, C.; Fan, Y. Numerical simulation study on CO2 storage in coalbed. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 41, 1005–1011. [Google Scholar] [CrossRef]
- Jia, J.; Sang, S.; Cao, L.; Liu, S. Characteristics of CO2/supercritical CO2 adsorption-induced swelling to anthracite: An experimental study. Fuel 2018, 216, 639–647. [Google Scholar]
- Perera, M.; Ranjith, P.G.; Viete, D.R. Effects of gaseous and super-critical carbon dioxide saturation on the mechanical properties of bituminous coal from the Southern Sydney Basin. Appl. Energy 2013, 110, 73–81. [Google Scholar] [CrossRef]
- Majewska, Z.; Zietek, J. Changes of acoustic emission and strain in hard coal during gas sorption–desorption cycles. Int. J. Coal Geol. 2007, 70, 305–312. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Dubinin, M.M.; Isirikyan, A.A.; Sarakhov, A.I.; Serpinskii, V.V. Energy of adsorption of gases and vapors on microporous adsorbents. Bull. Acad. Sci. USSR Div. Chem. Sci. 1968, 17, 1599–1606. [Google Scholar] [CrossRef]
- Dubinin, M.M.; Radushkevich, L.V. Equation of the characteristic curve of activated charcoal. Chem. Zentr. 1947, 1, 875–890. [Google Scholar]
- Dubinin, M.M.; Astakhov, V.A. Description of Adsorption Equilibria of Vapors on Zeolites over Wide Ranges of Temperature and Pressure. Adv. Chem. 1971, 44, 69–85. [Google Scholar] [CrossRef]
- Anacleto, J.; Pereira, M.G. From free expansion to abrupt compression of an ideal gas. Eur. J. Phys. 2009, 30, 177–183. [Google Scholar] [CrossRef]
- De Silva, P.N.K.; Ranjith, P.G.; Choi, S.K. A study of methodologies for CO2 storage capacity estimation of coal. Fuel 2012, 91, 1–15. [Google Scholar] [CrossRef]
- Meng, Z.; Liu, S.; Li, G. Adsorption capacity, adsorption potential and surface free energy of different structure high rank coals. J. Pet. Sci. Eng. 2016, 146, 856–865. [Google Scholar] [CrossRef]



| Coal Sample | Mad/% | Ash/% | Ad/% | VM/% | FC/% | FCd/% | Coal Type |
|---|---|---|---|---|---|---|---|
| San-Yuan Zhongneng | 0.70 | 12.02 | 12.01 | 12.19 | 75.09 | 74.92 | Lean coal |
| Coal Sample | BET Specific Area/ (m2·g−1) | Pore Volume/(×10−4ml·g−1) | Specific Surface Area/(m2·g−1) | ||||
|---|---|---|---|---|---|---|---|
| <10 nm | 10~100 nm | 100~1000 nm | <10 nm | 10~100 nm | 100~1000 nm | ||
| San-Yuan Zhongneng | 0.3784 | 3.78 | 10.49 | 17.94 | 0.3478 | 0.1289 | 0.0501 |
| Device | Model | Scope | Precision | Manufacturer |
|---|---|---|---|---|
| Temperature sensor | SIN-WZP-PT100 | −50~500 °C | ±(0.15 + 0.002 °C) | Hangzhou Sinomeasure Automation Technology Co. Ltd., Hangzhou, China. |
| Temperature converter module | SIN-ST-500 | −40~85 °C | ≤0.1%FS | |
| Paperless recorder | SIN-R200D | −200.0~650.0 °C | ±0.2%FS |
| Model | Langmuir | BET | D-R | D-A | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | VL | PL | R2 | A | R2 | D | V0 | R2 | D | V0 | n | R2 | |
| 2~4 mesh | 54.96 | 0.83 | 0.992 | 15.37 | 31.30 | 0.998 | 0.13 | 43.60 | 0.991 | 0.52 | 72.17 | 1.0 | 0.998 |
| 4~8 mesh | 62.84 | 0.83 | 0.995 | 16.54 | 34.69 | 0.999 | 0.13 | 49.97 | 0.995 | 0.51 | 79.18 | 1.0 | 0.999 |
| 8~16 mesh | 62.10 | 0.74 | 0.990 | 18.56 | 36.19 | 0.998 | 0.12 | 51.24 | 0.990 | 0.48 | 80.79 | 1.0 | 0.997 |
| 16~30 mesh | 54.46 | 0.45 | 0.980 | 31.59 | 34.07 | 0.997 | 0.09 | 49.17 | 0.984 | 0.39 | 71.79 | 1.0 | 0.993 |
| 30~60 mesh | 54.24 | 0.38 | 0.988 | 34.54 | 36.62 | 0.998 | 0.08 | 50.98 | 0.992 | 0.37 | 74.37 | 1.0 | 0.997 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, T.; Deng, C.; Han, Q. Experimental Research on the Law of Energy Conversion during CO2 Sequestration in Coal. Energies 2021, 14, 8079. https://doi.org/10.3390/en14238079
Gao T, Deng C, Han Q. Experimental Research on the Law of Energy Conversion during CO2 Sequestration in Coal. Energies. 2021; 14(23):8079. https://doi.org/10.3390/en14238079
Chicago/Turabian StyleGao, Tao, Cunbao Deng, and Qing Han. 2021. "Experimental Research on the Law of Energy Conversion during CO2 Sequestration in Coal" Energies 14, no. 23: 8079. https://doi.org/10.3390/en14238079
APA StyleGao, T., Deng, C., & Han, Q. (2021). Experimental Research on the Law of Energy Conversion during CO2 Sequestration in Coal. Energies, 14(23), 8079. https://doi.org/10.3390/en14238079





