Microwave Assisted Biodiesel Production Using Heterogeneous Catalysts
Abstract
:1. Introduction
2. Synthesis Techniques for Heterogeneous Catalysts
3. Microwave Assisted Transesterification Using Heterogeneous Catalysts
| Materials | Catalyst Preparation | Catalyst | Oil Used | Reaction Parameters | Yield | Ref. |
|---|---|---|---|---|---|---|
| Waste eggshells | Calcined at 800 °C for 4 h in air atmosphere (10 °C/min). | CaO | Palm olein oil | M/O: 18, catalyst: 15 wt.%, time: 4 min, temperature: -, mic. power: 900 W | Y = 96.7 | [36] |
| Corn cobs | First, activated carbon was obtained from corn cobs and then sulfonated by applying sulphonating agent. | Sulfonated activated carbon | Soybean oil | M/O: 6, catalyst: 20 wt.%, time: 20 min, temperature: -, mic. power: 0–600 W | Y = 88.7 | [37] |
| Waste shells of oyster and Pyramidella | Calcined at 900 °C for 2 h in air environment (10 °C/min) | CaO | Jatropha curcas oil | M/O: 15, catalyst: 4 wt.%, time: 5 min, temperature: -, mic. power: 800 W | Y = 93 | [45] |
| ZrO2-supported bamboo leaf ash | First, addition of ZrOCl solution to isopropanol solvent and water while stirring to get the precursor ZrO2 solution. Afterward, the mixing of the suspension of precursor solution and leaf ash accompanied by adding HCl and then refluxing for 4 h is needed to get ZrO2/ash composite. The mixture was then dried and calcined. | Silica-based material with acid properties of ZrO2 | Soybean oil | M/O: 15, catalyst:12 wt.%, time: 30 min, temperature: -, mic. assisted | Y > 90 | [46] |
| Cassava peel derived sulfonated acid catalyst | Initially, the biomass pyrolysis (slow) was carried out at 400 °C for 1 h. After that, the sulfonation for the catalyst functionalisation was executed by introducing SO3H groups into biochar to get biochar-based acid catalyst. The co-precipitation technique was applied for the synthesis of Fe3O4 magnetic nanoparticles. The nanomagnetic biocatalyst was synthesised by mixing magnetic nanoparticles and sulfonated biochar catalyst in demineralised H2O utilising an ultrasonicator at an ambient temperature for 8 h. Later, the nanomagnetic biocomposite was dried and stored in a desiccator. | weak hydroxyl and strong carboxylic and sulfonic acidic groups | Millettia pinnata seed oil | M/O: 11, catalyst: 3 wt.%, time: 45 min, temperature: -, mic. assisted | Y = 98.7 | [47] |
| Heteropolyacid catalyst | The catalyst was purchased as it is. | Cs2.5H0.5PW12O40 | Yellow horn oil | M/O: 12, catalyst: 1 wt.%, time: 10 min, temperature: -, mic. power: 500 W | Y = 96.22 | [38] |
| Potassium fluoride (KF) modification of hydrotalcite (Sorbacite®) | The solid reaction technique was used for the catalyst production. The mixture of potassium fluoride and hydrotalcite was milled and heated at a specified temperature for 2 h. KF content was differed from 5–30 wt.% in range. | KF modified hydrotalcite | Jatropha oil | M/O: 2–10, catalyst: 3.33, 5, 10 wt.%, time: 2–30 min, temperature: -, mic. power: 0–900 W | Y = 97 | [48] |
| KOH impregnated CaO catalyst | KOH impregnated calcium oxide (CaO) catalysts were synthesised by wet impregnation method | 20% KOH impregnation on CaO | Jatropha curcas oil | M/O: 8.42, catalyst: 3.17 wt.%, time: 67.9 min, temperature: -, mic. assisted | Y = 97.1 | [49] |
| Guinea fowl bone | Calcined at 900 °C for 5 h | β-Ca3(PO4)2 | Annona squamosa L. seed oil | M/O: 18, catalyst: 4 wt.%, time: 20 min, temperature: -, mic. power: 800 W | Y = 95.82 | [39] |
| Lignin based heterogeneous solid acid catalyst extracted from sugarcane bagasse | The alkaline pulping method was employed for lignin extraction from bagasse followed by the partial carbonisation in a furnace at 400 °C. Later, the product was mixed with conc. H2SO4 at a specified temperature for 2 h. | Sulfonic group, carboxyl group and hydroxyl group after effective chemical activation and sulfonation. | Waste cooking oil | M/O: 18, catalyst: 15 wt.%, time: 15 min, temperature: -, mic. assisted | Y = 89.19 | [50] |
| Alumina/silica loaded with potassium sodium tartrate | A one-step sol-gel method was employed for catalyst preparation. In the end, the catalyst was heat-treated at elevated temperature for 5 h. | Good dispersion of C4H4O6KNa on the support | Soybean oil | M/O: 13, catalyst: 8 wt.%, time: 45 min, temperature: 65 °C, mic. assisted | Y = 96.5 | [51] |
| A sulfonated-glucose solid acid catalyst | The incomplete carbonisation of a specific quantity of D-glucose was carried out while heating at 400 °C for 12 h under N2 gas. The product was ground and heated at 160 °C under inert atmosphere for 12 h in the presence of conc. H2SO4. Lastly, the suspension was further diluted with distilled water and the precipitate was acquired. | Glucose-SO3H catalyst | Non-edible palm fatty acid distillate | M/O: 12, catalyst: 3 wt.%, time: 15 min, temperature: 75 °C, mic. assisted | Y = 96 | [52] |
| Potassium hydroxide (KOH) impregnated alumina catalysts | Wet impregnation method was employed for KOH/γ-Al2O3 catalysts preparation using the γ-Al2O3 support. The powdery material was heat-treated in a tube furnace under the air environment at the specified time and temperature. | K2O | Soybean oil | M/O: 12, catalyst: 3 wt.%, time: 35 min, temperature: 65 °C, mic. assisted | Y > 95 | [53] |
| Ionic liquid (IL)-microwave heating | - | - | Wet Nannochloropsis sp. biomass | M/O: 4, catalyst: -, time: 14 min, temperature: -, mic. assisted, IL ratio maintained at 1:0.5 | Y = 42.22 | [54] |
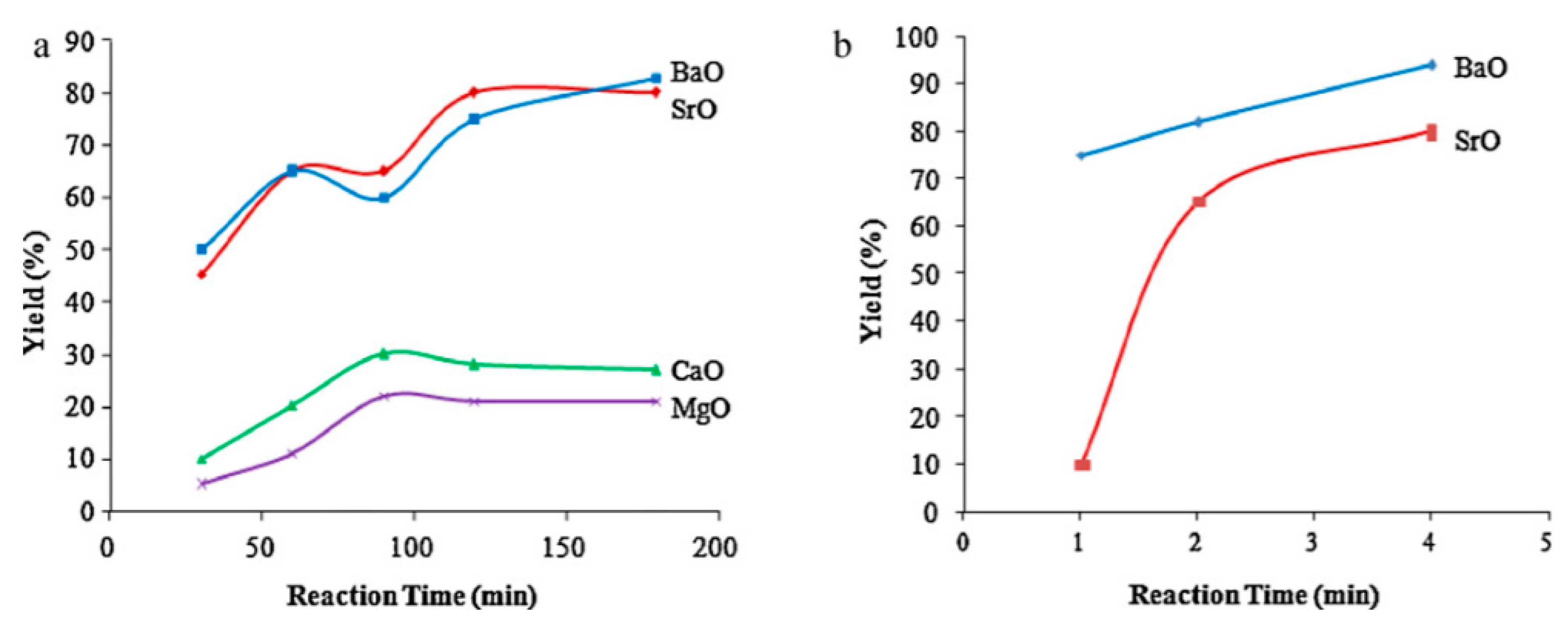
| Strontium Oxide Agglomerates Depositing upon Titanium Plate | Strontium oxide powder deposited on a titanium plate picked as the support because of the possible formation of SrTiO3 at their interface after heat treatment | SrO/TiO2_P | Olive oil | M/O: 6, catalyst: 3 wt.%, time: 4 min, temperature: -, mic. assisted | C = 87.7 | [55] |
| Metal Oxide catalysts | Barium oxide | BaO | Camelina sativa oil | M/O: 9, catalyst: 1.5 wt.%, time: 4 min, temperature: -, mic. assisted | Y = 94 | [42] |
| The propyl-SO3H amorphous SiO2 | The sol-gel technique was employed for the preparation of the propyl-SO3H (10 wt.%)/silica. The specified quantity of tetraethyl orthosilicate was added in ethanol and kept for stirring for 15 min at 45 °C. Next, a specific quantity of acetic acid aqueous solution (pH:5) was put on the mixture, followed by 3-mercaptopropyltrimethoxy silane and hydrogen peroxide. The solution was continuously mixed at 45 °C overnight under reflux followed by the increase in temperature up to 80 °C and remained same for 4 h. The gel was formed by heating up to 100 °C and finally dried. | Amorphous SiO2 loaded with 10 wt.% of sulfonic groups | Soybean oil | Substrate/tert-butyl-methyl ether:1/10, catalyst: 1 wt.%, time: -, temperature: -, mic. power: 20 W | - | [56] |
| Heterogeneous base catalyst-calcium diglyceroxide | A specific quantity of calcined CaO was put into the glass reactor carrying methanol and glycerol. The resulting product was stirred at 60 °C for 3 h that lead to the formation of CaDG. | CaDG as a heterogeneous base catalyst | Waste cooking oil | M/O: 7.46, catalyst: 1.03 wt.%, time: 15 min, temperature: 62 °C, mic. assisted | Y = 94.86 | [43] |
| Pork bone derived natural hydroxyapatite | Calcination at 900 °C for 2 h | β-Ca3(PO4)2 | Jatropha Curcas oil | M/O: 18, catalyst: 4 wt.%, time: 5 min, temperature: -, mic. power: 800 W | C = 94 | [40] |
| KF-Modified Natural Halloysite | The solid reaction method was applied for the catalyst synthesis. The mixture of potassium fluoride and halloysite was milled and heated at a specified temperature for 2 h. KF content was differed from 5–30 wt.% in range. | KF-Modified Natural Halloysite | Jatropha oil | M/O: 8, catalyst/oil: 1/30, time: 30 min, temperature: -, mic. assisted | Y = 83.77 | [57] |
| Waste eggshells | The crushed dried eggshells were heat-treated in a furnace at a temperature of 900 °C–950 °C for 4 h. | CaO | Lagenaria vulgaris seed oil | M/O: 40, catalyst: 5 wt.%, time: 40 min, temperature: 60 °C, mic. assisted | Y = 95.07 | [58] |
| Calcium oxide catalyst | CaCO3 powder was calcined for 5 h at 900 °C. | CaO | Waste cotton-seed cooking oil | M/O: 9.6, catalyst: 1.33 wt.%, time: 9.7 min, temperature: -, mic. assisted | Y = 89.94 | [59] |
| Hydrated calcined Cyrtopleura costata seashells | Seashells were initially washed and dried accompanied by grinding and then calcined at 900 °C for 2 h, Hydration was attained by placing the heat-treated product into atmosphere for a week in a humidity-controlled room. | Ca(OH)2 | Palm oil | M/O: 9, catalyst: 0.5 wt.%, time: 10 min, temperature: -, mic. assisted | Y = 96.7 | [44] |
| Nanopowder calcium oxide | Nano CaO (purity: 98%) were purchased and applied. | Nano-CaO | Soybean oil | M/O: 7, catalyst: 3 wt.%, time: 60 min, temperature: 60 °C, mic. assisted | Y = 96.6 | [41] |
| Diphenylammonium salt catalysts | Diphenylammonium salt | Corn oil | M/O: 5/2 (g/g), catalyst: 20 mol%, time: 20 min, temperature: 150 °C, mic. assisted | Y = 100 | [60] | |
| Microwave Absorption Catalysts | H2SO4/C catalyst was synthesised via impregnation method | H2SO4/C catalyst | Castor oil | M/O: 12, catalyst: 5 wt.%, 55 wt% H2SO4 time: 60 min, temperature: 65 °C, mic. assisted | Y = 94 | [61] |
| Acidic silica gel as catalyst | A specified quantity of silica and sulphuric acid were mixed and agitated at ambient temperature for 30 min. The resulting solid was strained and finally dried and stored in a desiccator | H2SO4 immobilised in SiO2 | Castor oil | M/O: 6, catalyst: 10 wt.%, time: 30 min, temperature: 60 °C, mic. assisted | C = 95% | [62] |
| Waste Cement Clinker Catalyst | Calcinated clinker is utilised to generate a limestone-based catalyst | CaO, Silica, Alumina, Iron oxide | Rubber seed oil | M/O: 5, catalyst: 6 wt.%, time: 60 min, temperature: 60 °C, mic. assisted | C = 96.8 | [63] |
| KSF montmorillonite | Catalysts have been purchased from Aldrich | Rape oil | M/O: 9, catalyst: 10 wt.%, time: 60 min, temperature: 170 °C, mic. assisted | Y = 51 | [64] | |
| Aminophosphonic acid resin D418 | Aminophosphonic acid resin D418 was purchased and used. | Macroporous styrene chelate resin, with -NHCH2PO3H2 functional groups | Free fatty acid stearic acid | M/O: 11, catalyst: 9 wt.%, time: 7 h, temperature: 80 °C, mic. assisted | Y > 90 | [65] |
| Calcium oxide (CaO) loaded on zeolite | The manufacturing of an active solid catalyst via CaO loaded on high silica zeolite employing impregnation method | 35% CaO/zeolite | Waste lard fat | M/O: 30, catalyst: 8 w/v, time: 1.25 min, temperature: -, mic. power: 595 W | Y = 90.89 | [66] |
| K2CO3/Al2O catalyst | The supported catalysts K2CO3/Al2O3, was attained via sedimentation subsequently, the calcination of the catalyst active mass. | 5% K2CO3/Al2O | Sunflower oil | M/O: 16, catalyst: 2.5 wt.%, time: 30 min, temperature: 70 °C, mic. assisted | C > 60 | [67] |
| Graphene oxide-based catalyst | Graphene oxide was synthesised from graphite powders through modified Hummer’s method. The SiC-NaOH/GO was synthesised by in-situ impregnation method. | SiC-NaOH/GO catalyst | Chlorella vulgaris lipid | M/O: 48, catalyst: 4 wt.%, time: 5 min, temperature: 85 °C, mic. assisted | Y = 81 | [68] |
| Elephant-ear tree pod husk | The dried pods were handpicked, and seeds were separated manually parting the sticky husk behind. One part of the husks was powdered, as the other portion was charred to ash in air. After that, part of the ash was exposed to calcination between 300 and 1100 °C for 4 h using a muffle furnace. | K, Mg, Ca and Fe | Esterified oil mix of neem and rubber seed oil | M/O: 11.44, catalyst: 2.96 wt.%, time: 5.88 min, temperature: -, mic. power: 150 W | Y = 98.77 | [69] |
| ZnO/La2O2CO3 layered composite | The zinc–lanthanum-mixed oxide catalyst was synthesised through an in-situ precipitation technique and later calcined at 550 °C for 6 h | ZnO/La2O2CO3 | Canola oil | M/O: 12, catalyst: <1 wt.%, time: 5 min, temperature: 85 °C, mic. assisted | Y > 95 | [70] |
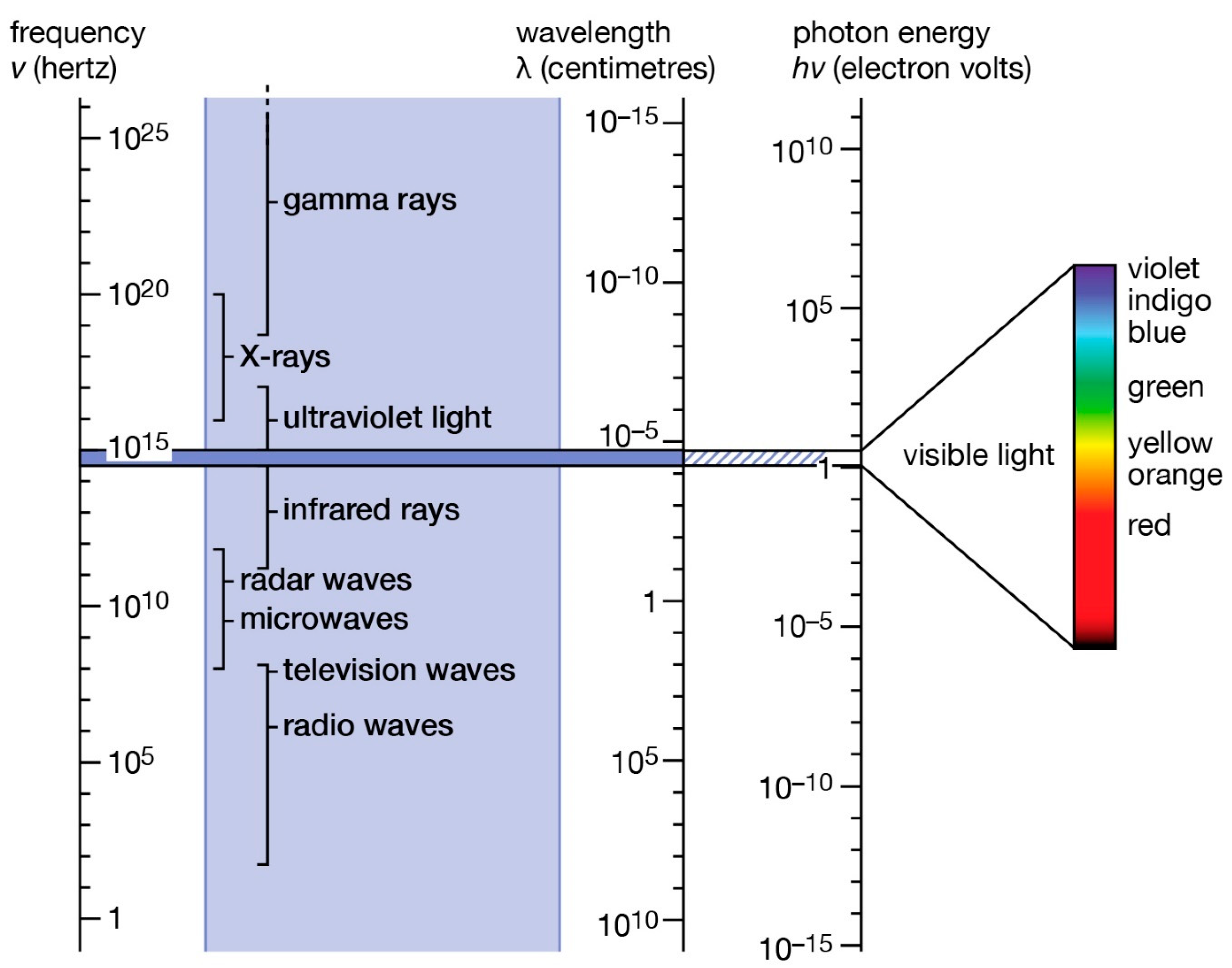
4. Special Thermal Effects of Microwaves on Solid Catalysts
5. Optimisation of Reaction Parameters for Microwave Mediated Heterogeneously Catalysed Transesterification
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, H.M.; Iqbal, T.; Yasin, S.; Ali, C.H.; Abbas, M.M.; Jamil, M.A.; Hussain, A.; M Soudagar, M.E.; Rahman, M.M. Application of Agricultural Waste as Heterogeneous Catalysts for Biodiesel Production. Catalysts 2021, 11, 1215. [Google Scholar] [CrossRef]
- Soudagar, M.E.M.; Khan, H.M.; Khan, T.; Razzaq, L.; Asif, T.; Mujtaba, M.; Hussain, A.; Farooq, M.; Ahmed, W.; Shahapurkar, K. Experimental analysis of engine performance and exhaust pollutant on a single-cylinder diesel engine operated using moringa oleifera biodiesel. Appl. Sci. 2021, 11, 7071. [Google Scholar] [CrossRef]
- Su, G.; Ong, H.C.; Ibrahim, S.; Fattah, I.M.R.; Mofijur, M.; Chong, C.T. Valorisation of medical waste through pyrolysis for a cleaner environment: Progress and challenges. Environ. Pollut. 2021, 279, 116934. [Google Scholar] [CrossRef]
- Su, G.; Ong, H.C.; Fattah, I.M.R.; Ok, Y.S.; Jang, J.-H.; Wang, C.-T. State-of-the-art of the pyrolysis and co-pyrolysis of food waste: Progress and challenges. Sci. Total Environ. 2021, 151170. [Google Scholar] [CrossRef]
- Salaheldeen, M.; Mariod, A.A.; Aroua, M.K.; Rahman, S.M.A.; Soudagar, M.E.M.; Fattah, I.M.R. Current State and Perspectives on Transesterification of Triglycerides for Biodiesel Production. Catalysts 2021, 11, 1121. [Google Scholar] [CrossRef]
- Fattah, I.M.R.; Ong, H.C.; Mahlia, T.M.I.; Mofijur, M.; Silitonga, A.S.; Rahman, S.M.A.; Ahmad, A. State of the Art of Catalysts for Biodiesel Production. Front. Energy Res. 2020, 8, 101. [Google Scholar] [CrossRef]
- Lv, L.; Dai, L.; Du, W.; Liu, D. Progress in Enzymatic Biodiesel Production and Commercialization. Processes 2021, 9, 355. [Google Scholar] [CrossRef]
- Luque, R.; Lovett, J.C.; Datta, B.; Clancy, J.; Campelo, J.M.; Romero, A.A. Biodiesel as feasible petrol fuel replacement: A multidisciplinary overview. Energy Environ. Sci. 2010, 3, 1706–1721. [Google Scholar] [CrossRef]
- Khan, H.M.; Iqbal, T.; Yasin, S.; Irfan, M.; Kazmi, M.; Fayaz, H.; Mujtaba, M.; Ali, C.H.; Kalam, M.; Soudagar, M.E.M. Production and utilization aspects of waste cooking oil based biodiesel in Pakistan. Alex. Eng. J. 2021, 60, 5831–5849. [Google Scholar] [CrossRef]
- Sharma, M.P. Selection of potential oils for biodiesel production. Renew. Sustain. Energy Rev. 2016, 56, 1129–1138. [Google Scholar]
- Muley, P.D.; Wang, Y.; Hu, J.; Shekhawat, D. Microwave-Assisted Heterogeneous catalysis. In Catalysis: Volume 33; Spivey, J., Han, Y.-F.S., Dushyant, S., Eds.; Royal Society of Chemistry: Piccadilly, UK, 2021; Volume 33, pp. 1–37. [Google Scholar]
- Avhad, M.; Marchetti, J. Innovation in solid heterogeneous catalysis for the generation of economically viable and ecofriendly biodiesel: A review. Catal. Rev. 2016, 58, 157–208. [Google Scholar] [CrossRef]
- Coman, S.M.; Parvulescu, V.I. Heterogeneous Catalysis for Biodiesel Production. In The Role of Catalysis for the Sustainable Production of Bio-Fuels and Bio-Chemicals; Elsevier: Amsterdam, The Netherlands, 2013; pp. 93–136. [Google Scholar]
- Sahu, G.; Gupta, N.K.; Kotha, A.; Saha, S.; Datta, S.; Chavan, P.; Kumari, N.; Dutta, P. A review on biodiesel production through heterogeneous catalysis route. ChemBioEng Rev. 2018, 5, 231–252. [Google Scholar] [CrossRef]
- Ruhul, A.M.; Kalam, M.A.; Masjuki, H.H.; Fattah, I.M.R.; Reham, S.S.; Rashed, M.M. State of the art of biodiesel production processes: A review of the heterogeneous catalyst. RSC Adv. 2015, 5, 101023–101044. [Google Scholar] [CrossRef]
- Ong, H.C.; Tiong, Y.W.; Goh, B.H.H.; Gan, Y.Y.; Mofijur, M.; Fattah, I.M.R.; Chong, C.T.; Alam, M.A.; Lee, H.V.; Silitonga, A.S.; et al. Recent advances in biodiesel production from agricultural products and microalgae using ionic liquids: Opportunities and challenges. Energy Convers. Manag. 2021, 228, 113647. [Google Scholar] [CrossRef]
- Kremsner, J.M.; Kappe, C.O. Microwave-Assisted Organic Synthesis in Near-Critical Water at 300 °C–A Proof-of-Concept Study; Wiley Online Library: Hoboken, NJ, USA, 2005. [Google Scholar]
- Lidström, P.; Tierney, J.; Watheyb, B.; Westmana, J. Microwave assisted organic synthesisÐa review. Tetrahedron 2001, 57, 9225–9283. [Google Scholar] [CrossRef]
- Chen, C.-C.; Reddy, P.M.; Devi, C.S.; Chang, P.-C.; Ho, Y.-P. Study of microwave effects on the lipase-catalyzed hydrolysis. Enzym. Microb. Technol. 2016, 82, 164–172. [Google Scholar] [CrossRef]
- Nomanbhay, S.; Ong, M.Y. A review of microwave-assisted reactions for biodiesel production. Bioengineering 2017, 4, 57. [Google Scholar] [CrossRef] [Green Version]
- Refaat, A. Different techniques for the production of biodiesel from waste vegetable oil. Int. J. Environ. Sci. Technol. 2010, 7, 183–213. [Google Scholar] [CrossRef] [Green Version]
- Ardabili, S.F.; Najafi, B.; Alizamir, M.; Mosavi, A.; Shamshirband, S.; Rabczuk, T. Using SVM-RSM and ELM-RSM approaches for optimizing the production process of methyl and ethyl esters. Energies 2018, 11, 2889. [Google Scholar] [CrossRef] [Green Version]
- Najafi, B.; Ardabili, S.F.; Shamshirband, S.; Chau, K.-W.; Rabczuk, T. Application of ANNs, ANFIS and RSM to estimating and optimizing the parameters that affect the yield and cost of biodiesel production. Eng. Appl. Comput. Fluid Mech. 2018, 12, 611–624. [Google Scholar] [CrossRef]
- Zhang, Y. Preparation of heterogeneous catalysts based on CWAO technology. J. Phys. Conf. Ser. 2020, 1549, 032052. [Google Scholar] [CrossRef]
- Hussain, F.; Alshahrani, S.; Abbas, M.M.; Khan, H.M.; Jamil, A.; Yaqoob, H.; Soudagar, M.E.M.; Imran, M.; Ahmad, M.; Munir, M. Waste Animal Bones as Catalysts for Biodiesel Production; A Mini Review. Catalysts 2021, 11, 630. [Google Scholar] [CrossRef]
- Lee, A.F.; Bennett, J.A.; Manayil, J.C.; Wilson, K. Heterogeneous catalysis for sustainable biodiesel production via esterification and transesterification. Chem. Soc. Rev. 2014, 43, 7887–7916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, Y.-M.; Chen, K.-T.; Chen, C.-C. Study of the microwave lipid extraction from microalgae for biodiesel production. Chem. Eng. J. 2014, 250, 267–273. [Google Scholar] [CrossRef]
- Dai, Y.-M.; Wu, J.-S.; Chen, C.-C.; Chen, K.-T. Evaluating the optimum operating parameters on transesterification reaction for biodiesel production over a LiAlO2 catalyst. Chem. Eng. J. 2015, 280, 370–376. [Google Scholar] [CrossRef]
- Wang, J.-X.; Chen, K.-T.; Wu, J.-S.; Wang, P.-H.; Huang, S.-T.; Chen, C.-C. Production of biodiesel through transesterification of soybean oil using lithium orthosilicate solid catalyst. Fuel Process. Technol. 2012, 104, 167–173. [Google Scholar] [CrossRef]
- Kim, M.Y.; Lee, K.; Choi, M. Cooperative effects of secondary mesoporosity and acid site location in Pt/SAPO-11 on n-dodecane hydroisomerization selectivity. J. Catal. 2014, 319, 232–238. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, B.-b.; Crocker, M.; Zhang, Y.-j.; Zhu, X.-b.; Shi, C. Understanding on the origins of hydroxyapatite stabilized gold nanoparticles as high-efficiency catalysts for formaldehyde and benzene oxidation. Catal. Commun. 2015, 59, 195–200. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Y.; Ding, W.; Zhang, Y.; Shen, P.; Zhou, Y.; Liu, Y.; Huang, S.; Lu, X. Hydrogen production from coke oven gas over LiNi/γ-Al2O3 catalyst modified by rare earth metal oxide in a membrane reactor. J. Nat. Gas Chem. 2009, 18, 407–414. [Google Scholar] [CrossRef]
- Kuo, C.-W.; Chen, B.-K.; Tseng, Y.-H.; Hsieh, T.-H.; Ho, K.-S.; Wu, T.-Y.; Chen, H.-R. A comparative study of poly (acrylic acid) and poly (styrenesulfonic acid) doped into polyaniline as platinum catalyst support for methanol electro-oxidation. J. Taiwan Inst. Chem. Eng. 2012, 43, 798–805. [Google Scholar] [CrossRef]
- Awogbemi, O.; Von Kallon, D.; Aigbodion, V.S. Trends in the development and utilization of agricultural wastes as heterogeneous catalyst for biodiesel production. J. Energy Inst. 2021, 98, 244–258. [Google Scholar] [CrossRef]
- Khedri, B.; Mostafaei, M.; Ardebili, S.M.S. A review on microwave-assisted biodiesel production. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 41, 2377–2395. [Google Scholar] [CrossRef]
- Khemthong, P.; Luadthong, C.; Nualpaeng, W.; Changsuwan, P.; Tongprem, P.; Viriya-Empikul, N.; Faungnawakij, K. Industrial eggshell wastes as the heterogeneous catalysts for microwave-assisted biodiesel production. Catal. Today 2012, 190, 112–116. [Google Scholar] [CrossRef]
- Rocha, P.D.; Oliveira, L.S.; Franca, A.S. Sulfonated activated carbon from corn cobs as heterogeneous catalysts for biodiesel production using microwave-assisted transesterification. Renew. Energy 2019, 143, 1710–1716. [Google Scholar] [CrossRef]
- Zhang, S.; Zu, Y.-G.; Fu, Y.-J.; Luo, M.; Zhang, D.-Y.; Efferth, T. Rapid microwave-assisted transesterification of yellow horn oil to biodiesel using a heteropolyacid solid catalyst. Bioresour. Technol. 2010, 101, 931–936. [Google Scholar] [CrossRef]
- Singh, V.; Sharma, Y.C. Low cost guinea fowl bone derived recyclable heterogeneous catalyst for microwave assisted transesterification of Annona squamosa L. seed oil. Energy Convers. Manag. 2017, 138, 627–637. [Google Scholar] [CrossRef]
- Buasri, A.; Inkaew, T.; Kodephun, L.; Yenying, W.; Loryuenyong, V. Natural hydroxyapatite (NHAp) derived from pork bone as a renewable catalyst for biodiesel production via microwave irradiation. Key Eng. Mater. 2015, 569, 216–220. [Google Scholar] [CrossRef]
- Hsiao, M.-C.; Lin, C.-C.; Chang, Y.-H. Microwave irradiation-assisted transesterification of soybean oil to biodiesel catalyzed by nanopowder calcium oxide. Fuel 2011, 90, 1963–1967. [Google Scholar] [CrossRef]
- Patil, P.; Gude, V.G.; Pinappu, S.; Deng, S. Transesterification kinetics of Camelina sativa oil on metal oxide catalysts under conventional and microwave heating conditions. Chem. Eng. J. 2011, 168, 1296–1300. [Google Scholar] [CrossRef]
- Gupta, A.R.; Rathod, V.K. Calcium diglyceroxide catalyzed biodiesel production from waste cooking oil in the presence of microwave: Optimization and kinetic studies. Renew. Energy 2018, 121, 757–767. [Google Scholar] [CrossRef]
- Indarti, E. Hydrated calcined Cyrtopleura costata seashells as an effective solid catalyst for microwave-assisted preparation of palm oil biodiesel. Energy Convers. Manag. 2016, 117, 319–325. [Google Scholar]
- Buasri, A.; Rattanapan, T.; Boonrin, C.; Wechayan, C.; Loryuenyong, V. Oyster and Pyramidella shells as heterogeneous catalysts for the microwave-assisted biodiesel production from Jatropha curcas oil. J. Chem. 2015, 2015, 578625. [Google Scholar] [CrossRef] [Green Version]
- Fatimah, I.; Rubiyanto, D.; Taushiyah, A.; Najah, F.B.; Azmi, U.; Sim, Y.-L. Use of ZrO2 supported on bamboo leaf ash as a heterogeneous catalyst in microwave-assisted biodiesel conversion. Sustain. Chem. Pharm. 2019, 12, 100129. [Google Scholar] [CrossRef]
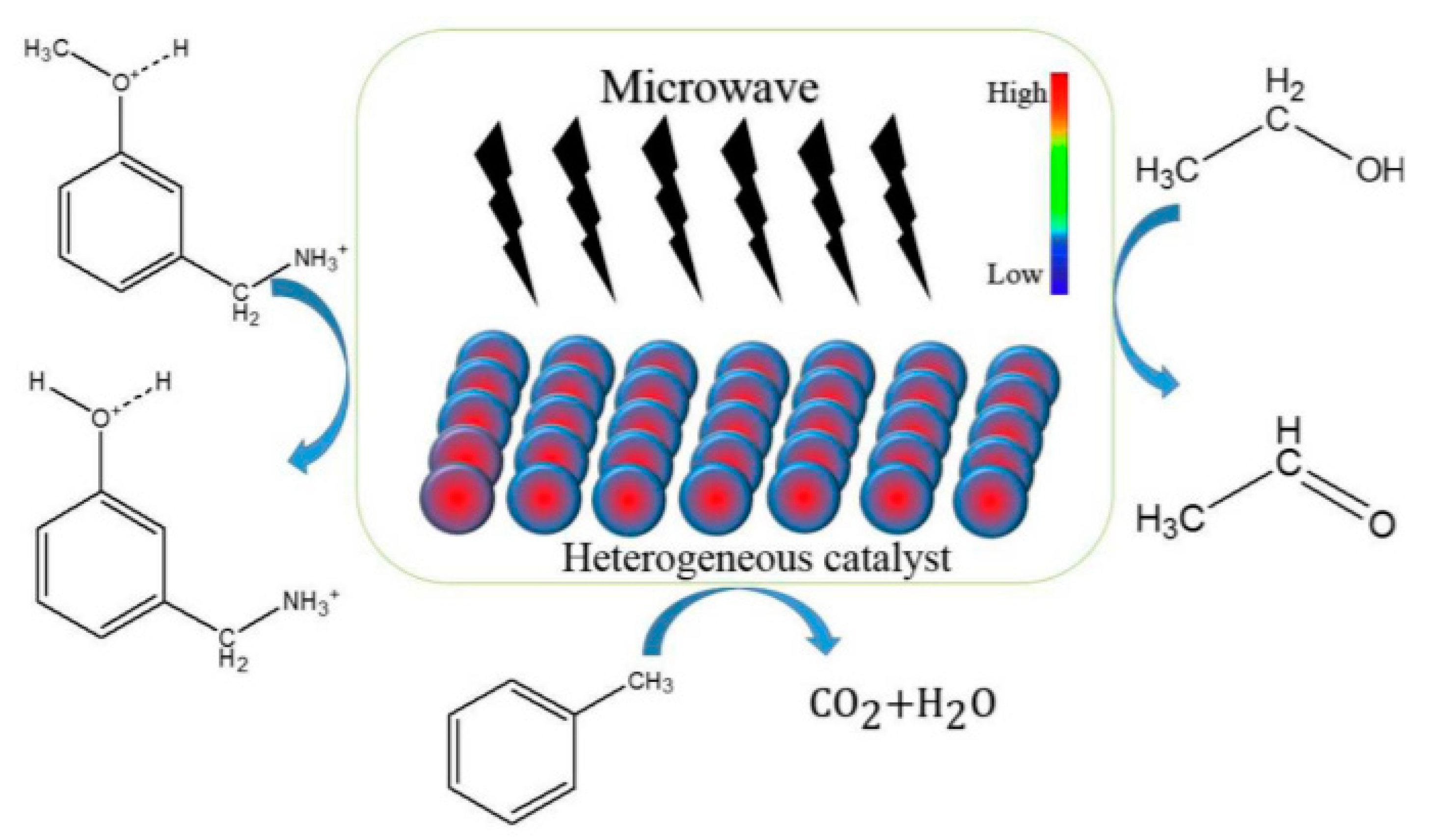
- Chellappan, S.; Aparna, K.; Chingakham, C.; Sajith, V.; Nair, V. Microwave assisted biodiesel production using a novel Brønsted acid catalyst based on nanomagnetic biocomposite. Fuel 2019, 246, 268–276. [Google Scholar] [CrossRef]
- Fatimah, I.; Rubiyanto, D.; Nugraha, J. Preparation, characterization, and modelling activity of potassium flouride modified hydrotalcite for microwave assisted biodiesel conversion. Sustain. Chem. Pharm. 2018, 8, 63–70. [Google Scholar] [CrossRef]
- Liao, C.-C.; Chung, T.-W. Optimization of process conditions using response surface methodology for the microwave-assisted transesterification of Jatropha oil with KOH impregnated CaO as catalyst. Chem. Eng. Res. Des. 2013, 91, 2457–2464. [Google Scholar] [CrossRef]
- Nazir, M.H.; Ayoub, M.; Zahid, I.; Shamsuddin, R.B.; Yusup, S.; Ameen, M.; Qadeer, M.U. Development of lignin based heterogeneous solid acid catalyst derived from sugarcane bagasse for microwave assisted-transesterification of waste cooking oil. Biomass Bioenerg. 2021, 146, 105978. [Google Scholar] [CrossRef]
- Ye, B.; Qiu, F.; Sun, C.; Li, Y.; Yang, D. Transesterification of Soybean Oil to Biodiesel in a Microwave-Assisted Heterogeneous Catalytic System. Chem. Eng. Technol. 2014, 37, 283–292. [Google Scholar] [CrossRef]
- Lokman, I.M.; Rashid, U.; Taufiq-Yap, Y.H. Microwave-Assisted Methyl Ester Production from Palm Fatty Acid Distillate over a Heterogeneous Carbon-Based Solid Acid Catalyst. Chem. Eng. Technol. 2015, 38, 1837–1844. [Google Scholar] [CrossRef]
- Varol, P.M.; Çakan, A.; Kiren, B.; Ayas, N. Microwave-assisted catalytic transesterification of soybean oil using KOH/γ-Al2O3. Biomass Convers. Biorefinery 2021, 1–13. [Google Scholar] [CrossRef]
- Wahidin, S.; Idris, A.; Yusof, N.M.; Kamis, N.H.H.; Shaleh, S.R.M. Optimization of the ionic liquid-microwave assisted one-step biodiesel production process from wet microalgal biomass. Energy Convers. Manag. 2018, 171, 1397–1404. [Google Scholar] [CrossRef]
- Lee, H.; Wu, W.-H.; Chen, B.-H.; Liao, J.-D. Heterogeneous Catalysts Using Strontium Oxide Agglomerates Depositing upon Titanium Plate for Enhancing Biodiesel Production. Catalysts 2021, 11, 30. [Google Scholar] [CrossRef]
- Drago, C.; Liotta, L.F.; La Parola, V.; Testa, M.L.; Nicolosi, G. One-pot microwave assisted catalytic transformation of vegetable oil into glycerol-free biodiesel. Fuel 2013, 113, 707–711. [Google Scholar] [CrossRef]
- Fatimah, I.; Yudha, S.P. KF-modified natural halloysite as green catalyst in microwave assisted biodiesel conversion. Energy Procedia 2017, 105, 1796–1805. [Google Scholar] [CrossRef]
- Umar, A.; Uba, A.; Mohammed, M.; Almustapha, M.; Muhammad, C.; Sani, J. Microwave assisted biodiesel production from Lagenaria vulgaris seed oil using amberlyst 15 ion exchange resin and eggshell as catalysts. Niger. J. Basic Appl. Sci. 2018, 26, 88–96. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Kodgire, P.; Kachhwaha, S.S. Biodiesel production from waste cotton-seed cooking oil using microwave-assisted transesterification: Optimization and kinetic modeling. Renew. Sustain. Energy Rev. 2019, 116, 109394. [Google Scholar] [CrossRef]
- Majewski, M.W.; Pollack, S.A.; Curtis-Palmer, V.A. Diphenylammonium salt catalysts for microwave assisted triglyceride transesterification of corn and soybean oil for biodiesel production. Tetrahedron Lett. 2009, 50, 5175–5177. [Google Scholar] [CrossRef]
- Yuan, H.; Yang, B.; Zhu, G. Synthesis of biodiesel using microwave absorption catalysts. Energy Fuels 2009, 23, 548–552. [Google Scholar] [CrossRef]
- Perin, G.; Álvaro, G.; Westphal, E.; Viana, L.; Jacob, R.; Lenardão, E.; D’Oca, M. Transesterification of castor oil assisted by microwave irradiation. Fuel 2008, 87, 2838–2841. [Google Scholar] [CrossRef]
- Hussain, A.; Ali, S.; Ahmed, I.; Gimbun, J.; Albeirutty, M.H.; Rehan, Z.A. Microwave reinforced transesterification of rubber seed oil using waste cement clinker catalyst. Curr. Nanosci. 2016, 12, 576–585. [Google Scholar] [CrossRef] [Green Version]
- Mazzocchia, C.; Modica, G.; Kaddouri, A.; Nannicini, R. Fatty acid methyl esters synthesis from triglycerides over heterogeneous catalysts in the presence of microwaves. C. R. Chim. 2004, 7, 601–605. [Google Scholar] [CrossRef]
- Liu, W.; Yin, P.; Liu, X.; Chen, W.; Chen, H.; Liu, C.; Qu, R.; Xu, Q. Microwave assisted esterification of free fatty acid over a heterogeneous catalyst for biodiesel production. Energy Convers. Manag. 2013, 76, 1009–1014. [Google Scholar] [CrossRef]
- Lawan, I.; Garba, Z.N.; Zhou, W.; Zhang, M.; Yuan, Z. Synergies between the microwave reactor and CaO/zeolite catalyst in waste lard biodiesel production. Renew. Energy 2020, 145, 2550–2560. [Google Scholar] [CrossRef]
- Dall’Oglio, E.L.; Sousa, P.T.d., Jr.; Oliveira, P.T.d.J.; Vasconcelos, L.G.d.; Parizotto, C.A.; Kuhnen, C.A. Use of heterogeneous catalysts in methylic biodiesel production induced by microwave irradiation. Química Nova 2014, 37, 411–417. [Google Scholar]
- Loy, A.C.M.; Quitain, A.T.; Lam, M.K.; Yusup, S.; Sasaki, M.; Kida, T. Development of high microwave-absorptive bifunctional graphene oxide-based catalyst for biodiesel production. Energy Convers. Manag. 2019, 180, 1013–1025. [Google Scholar] [CrossRef]
- Falowo, O.A.; Oloko-Oba, M.I.; Betiku, E. Biodiesel production intensification via microwave irradiation-assisted transesterification of oil blend using nanoparticles from elephant-ear tree pod husk as a base heterogeneous catalyst. Chem. Eng. Process. Process Intensif. 2019, 140, 157–170. [Google Scholar] [CrossRef]
- Jin, L.; Zhang, Y.; Dombrowski, J.P.; Chen, C.-H.; Pravatas, A.; Xu, L.; Perkins, C.; Suib, S.L. ZnO/La2O2CO3 layered composite: A new heterogeneous catalyst for the efficient ultra-fast microwave biofuel production. Appl. Catal. B Environ. 2011, 103, 200–205. [Google Scholar] [CrossRef]
- Jiang, S.; Daly, H.; Xiang, H.; Yan, Y.; Zhang, H.; Hardacre, C.; Fan, X. Microwave-assisted catalyst-free hydrolysis of fibrous cellulose for deriving sugars and biochemicals. Front. Chem. Sci. Eng. 2019, 13, 718–726. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zhang, C.; Pang, C.; Li, X.; Gao, X. The advances in the special microwave effects of the heterogeneous catalytic reactions. Front. Chem. 2020, 8, 355. [Google Scholar] [CrossRef] [PubMed]
- Vakili, R.; Xu, S.; Al-Janabi, N.; Gorgojo, P.; Holmes, S.M.; Fan, X. Microwave-assisted synthesis of zirconium-based metal organic frameworks (MOFs): Optimization and gas adsorption. Microporous Mesoporous Mater. 2018, 260, 45–53. [Google Scholar] [CrossRef]
- Nayak, S.N.; Bhasin, C.P.; Nayak, M.G. A review on microwave-assisted transesterification processes using various catalytic and non-catalytic systems. Renew. Energy 2019, 143, 1366–1387. [Google Scholar] [CrossRef]
- Gude, V.G.; Patil, P.D.; Deng, S.; Khandan, N. Microwave-Enhanced Methods for Biodiesel Production and Other Environmental Applications. In Green Chemistry for Environmental Remediation; Wiley Interscience: New York, NY, USA, 2011; pp. 209–249. [Google Scholar] [CrossRef]
- Perreux, L.; Loupy, A. A tentative rationalization of microwave effects in organic synthesis according to the reaction medium, and mechanistic considerations. Tetrahedron 2001, 57, 9199–9223. [Google Scholar] [CrossRef]
- Tierney, J.; Lidström, P. Microwave Assisted Organic Synthesis; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Patil, P.D.; Deng, S. Transesterification of camelina sativa oil using heterogeneous metal oxide catalysts. Energy Fuels 2009, 23, 4619–4624. [Google Scholar] [CrossRef]
- Dhawane, S.H.; Kumar, T.; Halder, G. Biodiesel synthesis from Hevea brasiliensis oil employing carbon supported heterogeneous catalyst: Optimization by Taguchi method. Renew. Energy 2016, 89, 506–514. [Google Scholar] [CrossRef]
- Shuit, S.H.; Lee, K.T.; Kamaruddin, A.H.; Yusup, S. Reactive extraction of Jatropha curcas L. seed for production of biodiesel: Process optimization study. Environ. Sci. Technol. 2010, 44, 4361–4367. [Google Scholar] [CrossRef] [PubMed]
| Method | Description | Ref. |
|---|---|---|
| Impregnation method | A solution of precursor/active components is taken, and carrier is immersed in it. After a while, when equilibrium is achieved, the remaining liquid is withdrawn and the catalyst is acquired following the drying, calcination and activation | [30] |
| Precipitation | A precipitation agent is added in an aqueous solution of metallic salts to obtain the crystal of carbonates, hydrated oxides, or gels. | [31] |
| Precipitation-impregnation method | A method based on the combination of the impregnation and precipitation technique initially synthesised the precipitant matrix in the impregnation solution. Upon completion of impregnation, the precipitant is accumulated on the carrier’s surface through heating. | [32] |
| Chemical deposition method | The films are produced on the substrate surface via chemical reactions using materials containing film components. | [33] |
| Sol-gel method | The butyl titanate and anhydrous ethanol are mixed slowly to another solution of demineralised water, anhydrous ethanol, nitric acid and suitable quantity of nitrate precursor at ambient temperature under strong mixing to perform hydrolysis | [34] |
| Physical mixing | The multiple materials in a ground form are well mixed or blended | [34] |
| Calcination | The substance is exposed to elevated temperature in a furnace | [34] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, H.M.; Iqbal, T.; Mujtaba, M.A.; Soudagar, M.E.M.; Veza, I.; Fattah, I.M.R. Microwave Assisted Biodiesel Production Using Heterogeneous Catalysts. Energies 2021, 14, 8135. https://doi.org/10.3390/en14238135
Khan HM, Iqbal T, Mujtaba MA, Soudagar MEM, Veza I, Fattah IMR. Microwave Assisted Biodiesel Production Using Heterogeneous Catalysts. Energies. 2021; 14(23):8135. https://doi.org/10.3390/en14238135
Chicago/Turabian StyleKhan, Haris Mahmood, Tanveer Iqbal, M. A. Mujtaba, Manzoore Elahi M. Soudagar, Ibham Veza, and I. M. Rizwanul Fattah. 2021. "Microwave Assisted Biodiesel Production Using Heterogeneous Catalysts" Energies 14, no. 23: 8135. https://doi.org/10.3390/en14238135
APA StyleKhan, H. M., Iqbal, T., Mujtaba, M. A., Soudagar, M. E. M., Veza, I., & Fattah, I. M. R. (2021). Microwave Assisted Biodiesel Production Using Heterogeneous Catalysts. Energies, 14(23), 8135. https://doi.org/10.3390/en14238135










