Nonylphenol Ethoxylate Surfactants Modified by Carboxyl Groups for Foam EOR at High-Salinity Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. General Procedure for Surfactant Synthesis and Characterization
2.3. Solubility and Stability
2.4. CMC Determination
2.5. Foam Rate and Half-Life Time
2.6. Filtration Experiments
3. Results and Discussion
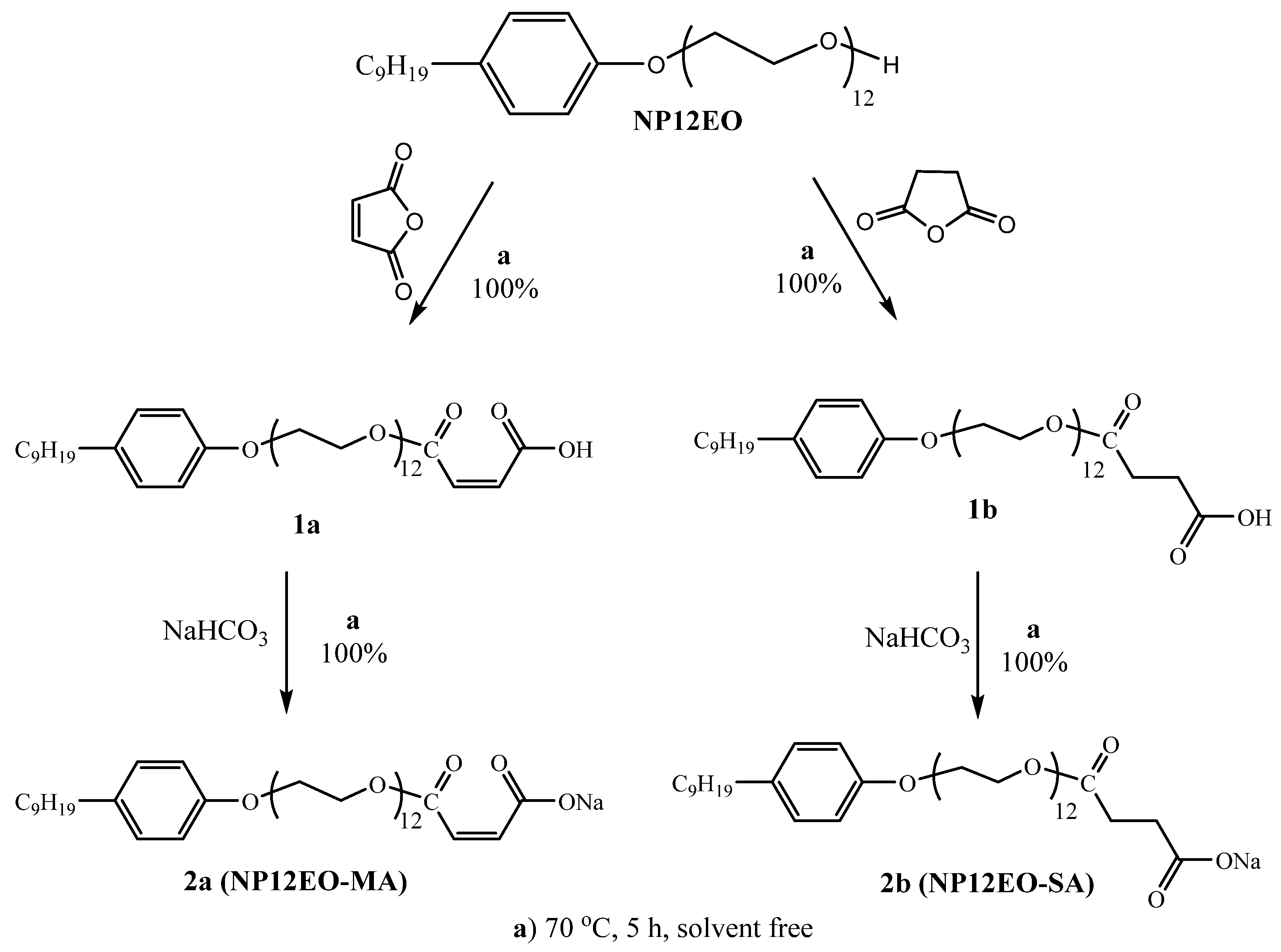
3.1. Surfactants Synthesis and Characterization
3.2. Solubility and Stability
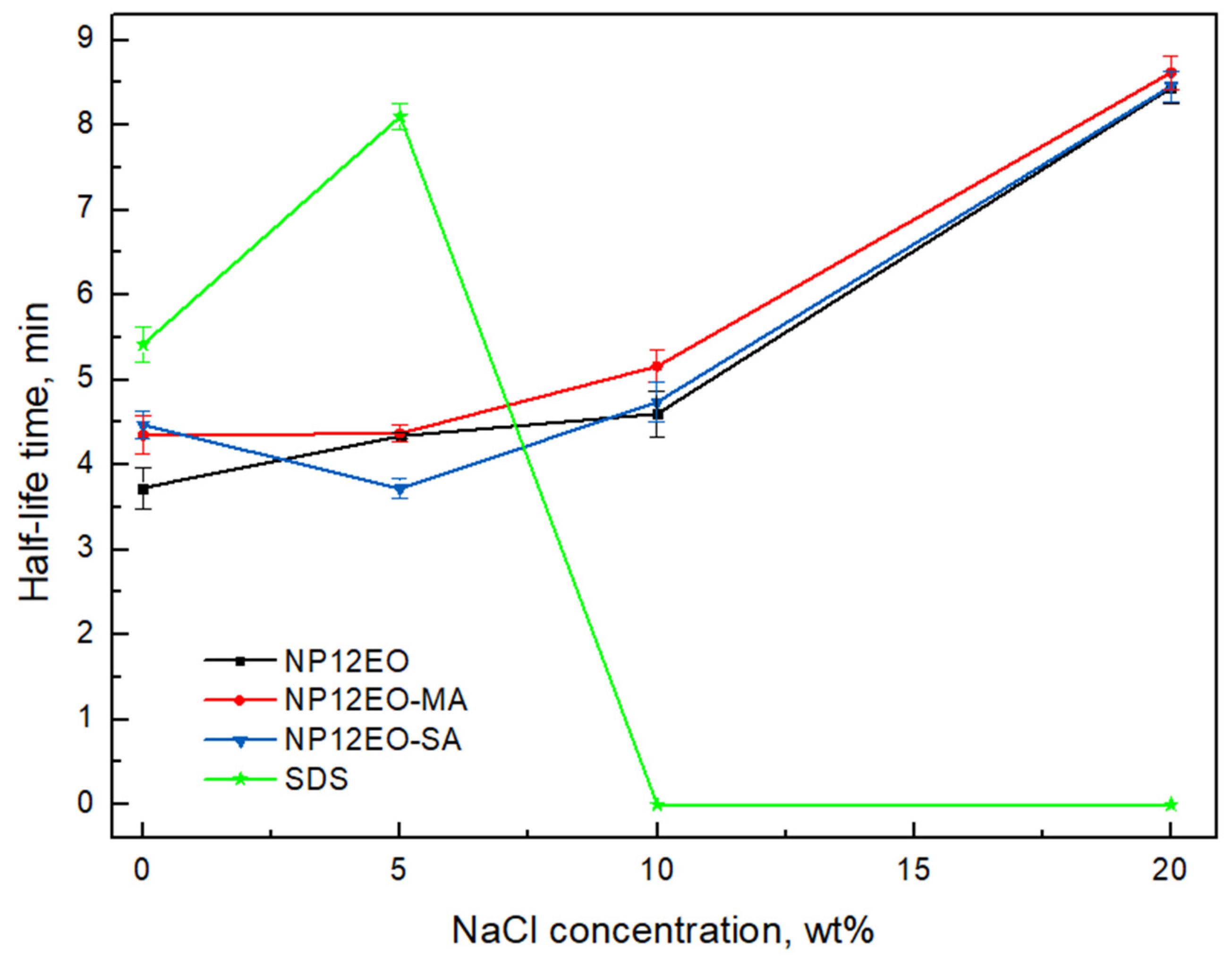
3.3. Foam Rate and Half-Life Time
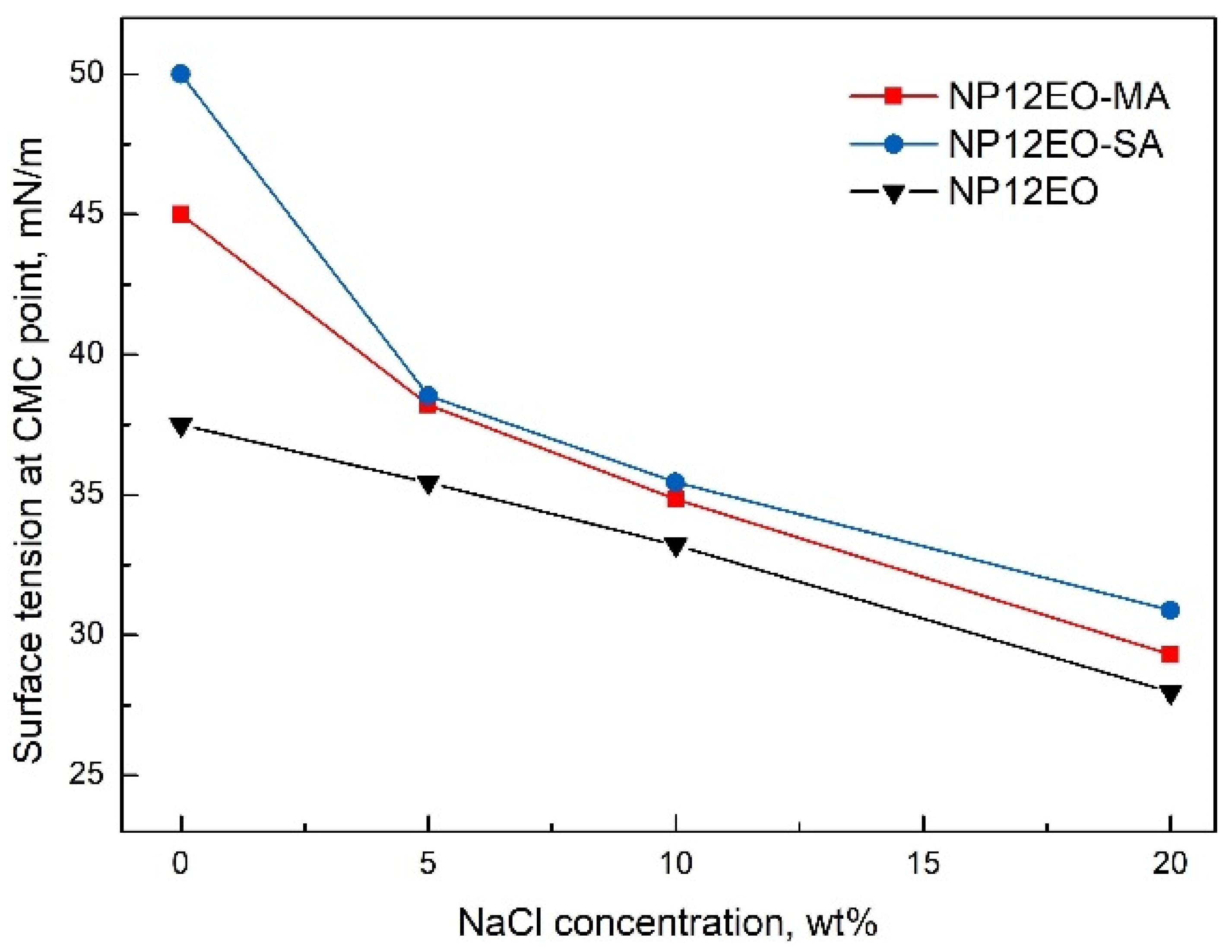
3.4. CMC Determination
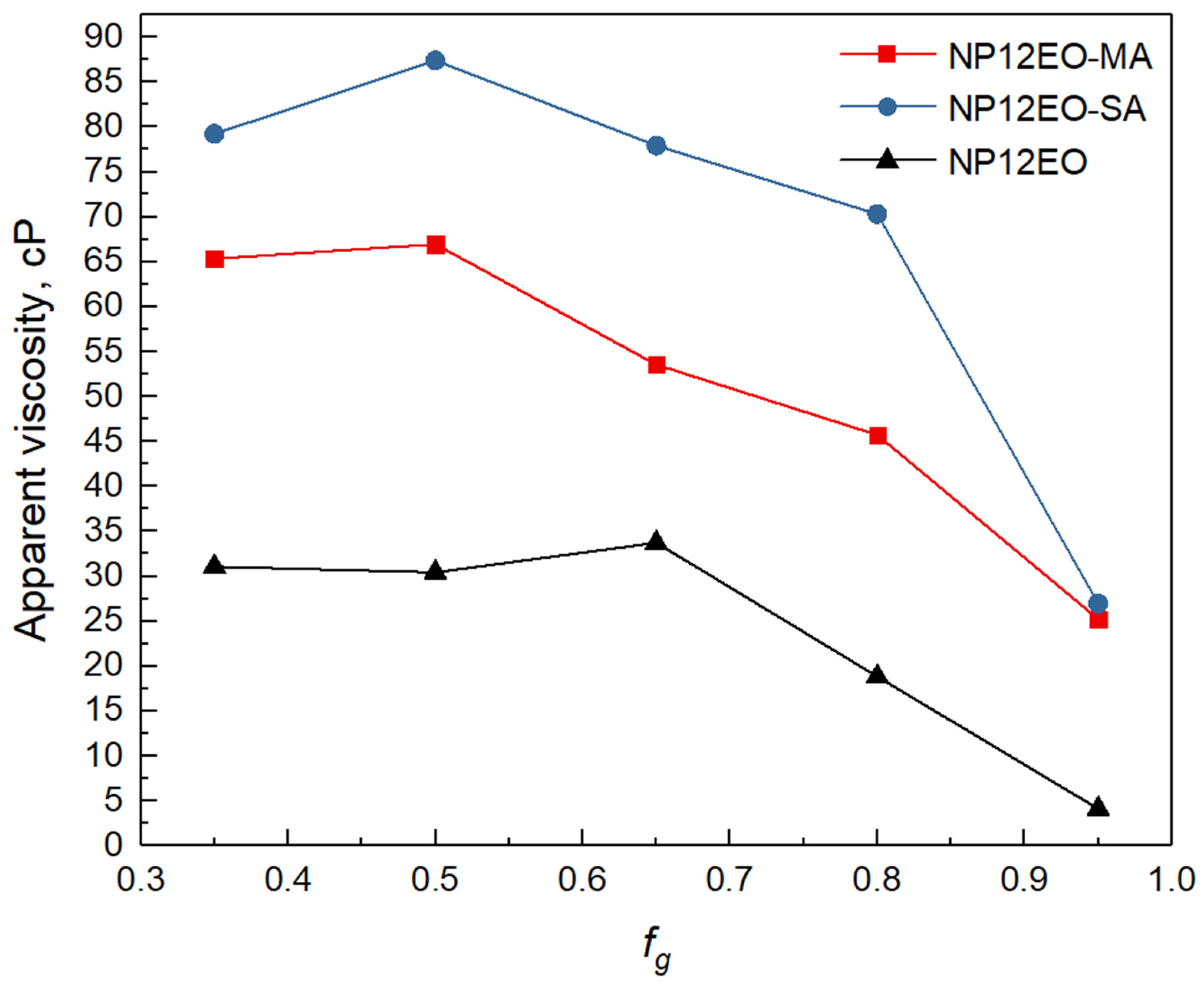
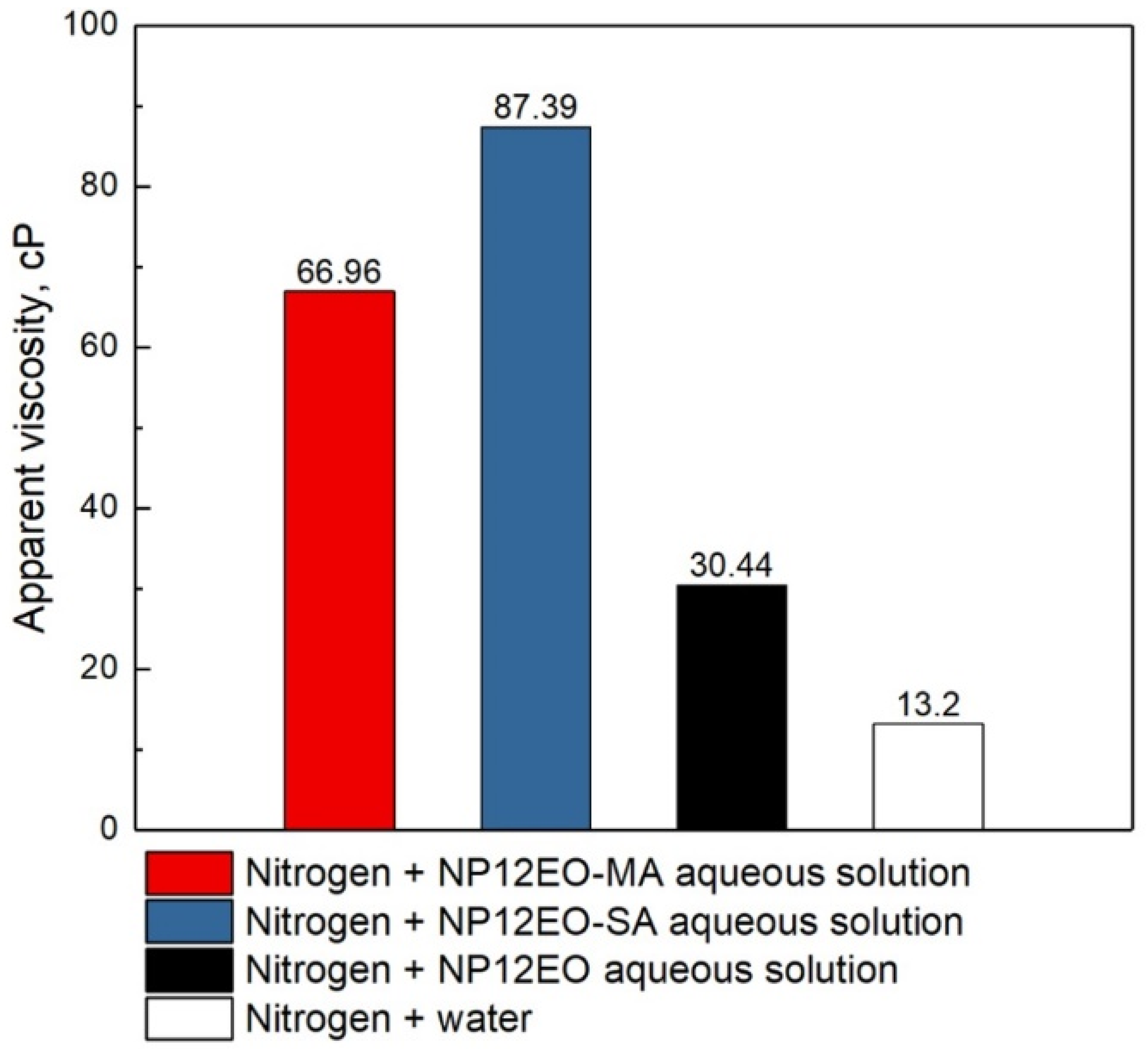
3.5. Determination of Optimal Foam Quality and Apparent Viscosity of the Foam
4. Conclusions
- The addition of the carboxyl group increases the repulsion force of the surfactants which led to increasing foam rate, compared to the nonionic NP12EO. Bulk tests showed that both anionic–nonionic surfactants retain 1.3 times higher foam rate at 20% NaCl concentration water than nonionic NP12EO. Moreover, due to nonionic ethoxylate (12) part, modified surfactants retained good solubility and long-term stability in water with NaCl concentration up to 20%;
- According to surface tension measurements, synthesized surfactants have less than 0.025 CMC. Both CMC and surface tension at CMC point (γCMC) decrease with NaCl concentration increasing;
- Both synthesized surfactants showed higher apparent viscosities than the initial nonionic NP12EO nonionic surfactant aqueous solution at all ranges of foam quality;
- Although, bulk tests did not show a significant difference in foam rate and half-life time between NP12EO-SA and NP12EO-MA surfactants, the lower apparent viscosity of NP12EO-MA surfactant was achieved in the filtration experiments, which reveals the negative effect of the unsaturated bond in the carboxylic group. Presumably, the presence of an unsaturated bond in the carboxylic part of NP12EO-MA increases surfactant packing surface area due to molecule geometry. This decreases the adsorption rate of surfactant molecules in the interfacial layer and foaming ability; and
- Both surfactants can be produced using relatively cheap and widely used reagents in a simple two-step synthesis, which makes them prospective candidates for chemical EOR from the economic side of view.
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, L.; Chen, X.; Zou, H.; Liu, P.; Liang, C.; Zhang, N.; Li, N.; Luo, Z.; Du, J. A review of diverting agents for reservoir stimulation. J. Pet. Sci. Eng. 2020, 187, 106734. [Google Scholar] [CrossRef]
- Kibodeaux, K.R.; Zeilinger, S.C.; Rossen, W.R. Sensitivity study of foam diversion processes for matrix acidization. In Proceedings of the Proceedings—SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 25–28 September 1994; pp. 347–358. [Google Scholar]
- Sönmez, A.; Verşan Kök, M.; Özel, R. Performance analysis of drilling fluid liquid lubricants. J. Pet. Sci. Eng. 2013, 108, 64–73. [Google Scholar] [CrossRef]
- Blaker, T.; Aarra, M.G.; Skauge, A.; Rasmussen, L.; Celius, H.K.; Martinsen, H.A.; Vassenden, F. Foam for gas mobility control in the Snorre field: The FAWAG project. SPE Reserv. Eval. Eng. 2002, 5, 317–323. [Google Scholar] [CrossRef]
- Hanssen, J.E.; Dalland, M. Gas-Blocking Foams. In Foams: Fundamentals and Applications in the Petroleum Industry; American Chemical Society: Washington, WA, USA, 1994; pp. 319–353. ISBN 9780841227194. [Google Scholar]
- Deng, X.; Kamal, M.S.; Patil, S.; Hussain, S.M.S.; Zhou, X. A Review on Wettability Alteration in Carbonate Rocks: Wettability Modifiers. Energy Fuels 2020, 34, 31–54. [Google Scholar] [CrossRef]
- Yao, Y.; Wei, M.; Kang, W. A review of wettability alteration using surfactants in carbonate reservoirs. Adv. Colloid Interface Sci. 2021, 294, 102477. [Google Scholar] [CrossRef]
- Ahmadi, M.A.; Zendehboudi, S.; Shafiei, A.; James, L. Nonionic surfactant for enhanced oil recovery from carbonates: Adsorption kinetics and equilibrium. Ind. Eng. Chem. Res. 2012, 51, 9894–9905. [Google Scholar] [CrossRef]
- Ahmadi, M.A.; Shadizadeh, S.R. Experimental investigation of adsorption of a new nonionic surfactant on carbonate minerals. Fuel 2013, 104, 462–467. [Google Scholar] [CrossRef]
- Chen, Y.; Elhag, A.S.; Poon, B.M.; Cui, L.; Ma, K.; Liao, S.Y.; Reddy, P.P.; Worthen, A.J.; Hirasaki, G.J.; Nguyen, Q.P.; et al. Switchable nonionic to cationic ethoxylated amine surfactants for CO2 enhanced oil recovery in high-temperature, high-salinity carbonate reservoirs. SPE J. 2014, 19, 249–259. [Google Scholar] [CrossRef]
- Ayirala, S.C.; Boqmi, A.; Alghamdi, A.; AlSofi, A. Dilute surfactants for wettability alteration and enhanced oil recovery in carbonates. J. Mol. Liq. 2019, 285, 707–715. [Google Scholar] [CrossRef]
- Pu, W.F.; Du, D.J.; Tang, Y.L.; Wang, S. Synthesis of an Alkyl Polyoxyethylene Ether Sulfonate Surfactant and Its Application in Surfactant Flooding. J. Surfactants Deterg. 2018, 21, 687–697. [Google Scholar] [CrossRef]
- Arkhipov, V.P.; Filippov, A. The cloud point of aqueous solutions of ethoxylated monoalkylphenols in the individual state and in the presence of electrolytes. J. Dispers. Sci. Technol. 2018, 39, 1442–1446. [Google Scholar] [CrossRef]
- Kumar, S.; Mandal, A. Investigation on stabilization of CO2 foam by ionic and nonionic surfactants in presence of different additives for application in enhanced oil recovery. Appl. Surf. Sci. 2017, 420, 9–20. [Google Scholar] [CrossRef]
- Chen, Y.; Elhag, A.S.; Cui, L.; Worthen, A.J.; Reddy, P.P.; Noguera, J.A.; Ou, A.M.; Ma, K.; Puerto, M.; Hirasaki, G.J.; et al. CO2-in-water foam at elevated temperature and salinity stabilized with a nonionic surfactant with a high degree of ethoxylation. Ind. Eng. Chem. Res. 2015, 54, 4252–4263. [Google Scholar] [CrossRef]
- Jian, G.; Alcorn, Z.; Zhang, L.; Puerto, M.C.; Soroush, S.; Graue, A.; Biswal, S.L.; Hirasaki, G.J. Evaluation of a Nonionic Surfactant Foam for CO2 Mobility Control in a Heterogeneous Carbonate Reservoir. SPE J. 2020, 25, 3481–3493. [Google Scholar] [CrossRef]
- Myers, D. Surfactant Science and Technology, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Li, Y.; Zhang, P.; Zhao, G.Q.; Cao, X.L.; Wang, Q.W.; Wang, H.Y. Effect of equilibrium and dynamic surface activity of surfactant on foam transport in porous medium. Colloids Surf. A Physicochem. Eng. Asp. 2006, 272, 124–129. [Google Scholar] [CrossRef]
- Maguire, R.J. Review of the persistence of nonylphenol and nonylphenol ethoxylates in aquatic environments. Water Qual. Res. J. Can. 1999, 34, 37–78. [Google Scholar] [CrossRef]
- Lee Ferguson, P.; Bopp, R.F.; Chillrud, S.N.; Aller, R.C.; Brownawell, B.J. Biogeochemistry of nonylphenol ethoxylates in urban estuarine sediments. Environ. Sci. Technol. 2003, 37, 3499–3506. [Google Scholar] [CrossRef] [PubMed]
- Quina, F.H.; Hinze, W.L. Surfactant-mediated cloud point extractions: An environmentally benign alternative separation approach. Ind. Eng. Chem. Res. 1999, 38, 4150–4168. [Google Scholar] [CrossRef]
- Dong, R.; Hao, J. Complex fluids of poly(oxyethylene) monoalkyl ether nonionic surfactants. Chem. Rev. 2010, 110, 4978–5022. [Google Scholar] [CrossRef]
- Materna, K.; Milosz, I.; Miesiac, I.; Cote, G.; Szymanowski, J. Removal of phenols from aqueous streams by the cloud point extraction technique with oxyethylated methyl dodecanoates as surfactants. Environ. Sci. Technol. 2001, 35, 2341–2346. [Google Scholar] [CrossRef]
- Abdul, A.S.; Gibson, T.L.; Rai, D.N. Selection of Surfactants for the Removal of Petroleum Products from Shallow Sandy Aquifers. Groundwater 1990, 28, 920–926. [Google Scholar] [CrossRef]
- Negin, C.; Ali, S.; Xie, Q. Most common surfactants employed in chemical enhanced oil recovery. Petroleum 2017, 3, 197–211. [Google Scholar] [CrossRef]
- Gang, H.Z.; He, X.; He, X.; Bao, X.; Liu, J.; Yang, S.; Li, Y.; Mu, B.Z. Interfacial properties and salt tolerance of carboxylated nonylphenol ethoxylate surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2021, 616, 126222. [Google Scholar] [CrossRef]
- Song, X.; Dong, L.; Cao, X.; Xu, Z.; Wang, C.; Zhang, L.; Zhang, L.; Zhao, S. Dynamic interfacial tensions of p-(n-lauryl)-benzyl polyoxyethylene ether carboxybetaine solutions. J. Pet. Sci. Eng. 2014, 124, 27–34. [Google Scholar] [CrossRef]
- Guo, L.; Liu, Y.; Hu, S.S.; Xu, Z.C.; Gong, Q.T.; Zhang, L.; Zhang, L. Dynamic interfacial tensions of alkyl alcohol polyoxypropylene-oxyehtylene ether sulfonate solutions. J. Pet. Sci. Eng. 2016, 141, 9–15. [Google Scholar] [CrossRef]
- Jayanti, S.; Britton, L.N.; Dwarakanath, V.; Pope, G.A. Laboratory evaluation custom-designed surfactants to remediate NAPL source zones. Environ. Sci. Technol. 2002, 36, 5491–5497. [Google Scholar] [CrossRef] [PubMed]
- Stellner, K.L.; Scamehorn, J.F. Surfactant precipitation in aqueous solutions containing mixtures of anionic and nonionic surfactants. J. Am. Oil Chem. Soc. 1986, 63, 566–574. [Google Scholar] [CrossRef]
- Croce, V.; Patrolecco, L.; Polesello, S.; Valsecchi, S. Extraction of nonylphenol and nonylphenol ethoxylates from river sediments: Comparison of different extraction techniques. Chromatographia 2003, 58, 145–149. [Google Scholar] [CrossRef]
- Sun, L.; Bai, B.; Wei, B.; Pu, W.; Wei, P.; Li, D.; Zhang, C. Recent advances of surfactant-stabilized N2/CO2 foams in enhanced oil recovery. Fuel 2019, 241, 83–93. [Google Scholar] [CrossRef]
- Wang, L.C.; Wang, X.S.; Zhang, J.C.; Song, X.W.; Cao, X.L.; Li, Z.Q. Synthesis and Interfacial Activity of Nonyl Phenol Polyoxyethylene Ether Carboxylate. J. Dispers. Sci. Technol. 2014, 35, 641–646. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, L.; Cao, X.; Song, X.; Jin, Z.; Zhang, L.; Zhao, S. Effect of electrolytes on interfacial tensions of alkyl ether carboxylate solutions. Energy Fuels 2013, 27, 3122–3129. [Google Scholar] [CrossRef]
- Standnes, D.C.; Austad, T. Wettability alteration in chalk 2. Mechanism for wettability alteration from oil-wet to water-wet using surfactants. J. Pet. Sci. Eng. 2000, 28, 123–143. [Google Scholar] [CrossRef]
- Wang, Y.F.; Huang, J.B. Synthesis and Surface Activity of Alkyl Oxyethylenated Propane Sulfonates. Acta Phys.-Chim. Sin. 2001, 17, 488–490. [Google Scholar] [CrossRef]
- Wang, X.; Yan, F.; Li, Z.; Zhang, L.; Zhao, S.; An, J.; Yu, J. Synthesis and surface properties of several nonionic-anionic surfactants with straight chain alkyl-benzyl hydrophobic group. Colloids Surf. A Physicochem. Eng. Asp. 2007, 302, 532–539. [Google Scholar] [CrossRef]
- Farajzadeh, R.; Andrianov, A.; Krastev, R.; Hirasaki, G.J.; Rossen, W.R. Foam-oil interaction in porous media: Implications for foam assisted enhanced oil recovery. Adv. Colloid Interface Sci. 2012, 183–184, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.K.; Aggarwal, M. Evaluation of micellar properties of sodium dodecylbenzene sulphonate in the presence of some salts. J. Chem. Sci. 2018, 130, 39. [Google Scholar] [CrossRef] [Green Version]
- Farajzadeh, R.; Krastev, R.; Zitha, P.L.J. Foam films stabilized with alpha olefin sulfonate (AOS). Colloids Surf. A Physicochem. Eng. Asp. 2008, 324, 35–40. [Google Scholar] [CrossRef]
- Rudyk, S.; Al-Khamisi, S.; Al-Wahaibi, Y.; Afzal, N. Internal Olefin Sulfonate Foam Coreflooding in Low-Permeable Limestone at Varying Salinity. Energy Fuels 2019, 33, 8374–8382. [Google Scholar] [CrossRef]
- Boersma, D.M.; Hagoort, J. Displacement characteristics of nitrogen vs. methane flooding in volatile-oil reservoirs. SPE Reserv. Eng. 1994, 9, 261–265. [Google Scholar] [CrossRef]
- Hou, Q.; Zhu, Y.; Luo, Y.; Weng, R.; Jian, G. Studies on nitrogen foam flooding for conglomerate reservoir. In Proceedings of the SPE EOR Conference at Oil and Gas West Asia, Muscat, Oman, 16–18 April 2012; Volume 1, pp. 58–62. [Google Scholar] [CrossRef]
- Liang, S.; Hu, S.; Li, J.; Xu, G.; Zhang, B.; Zhao, Y.; Yan, H.; Li, J. Study on EOR method in offshore oilfield: Combination of polymer microspheres flooding and nitrogen foam flooding. J. Pet. Sci. Eng. 2019, 178, 629–639. [Google Scholar] [CrossRef]
- Farajzadeh, R.; Andrianov, A.; Zitha, P.L.J. Investigation of immiscible and miscible foam for enhancing oil recovery. Ind. Eng. Chem. Res. 2010, 49, 1910–1919. [Google Scholar] [CrossRef]
- Jones, S.A.; van der Bent, V.; Farajzadeh, R.; Rossen, W.R.; Vincent-Bonnieu, S. Surfactant screening for foam EOR: Correlation between bulk and core-flood experiments. Colloids Surf. A Physicochem. Eng. Asp. 2016, 500, 166–176. [Google Scholar] [CrossRef]
- Giribabu, K.; Reddy, M.L.N.; Ghosh, P. Coalescence of air bubbles in surfactant solutions: Role of salts containing mono-, di-, and trivalent ions. Chem. Eng. Commun. 2008, 195, 336–351. [Google Scholar] [CrossRef]
- Obisesan, O.; Ahmed, R.; Amani, M. The effect of salt on stability of aqueous foams. Energies 2021, 14, 279. [Google Scholar] [CrossRef]
- Li, D.; Slattery, J.C. Experimental Support for Analyses of Coalescence. AIChE J. 1988, 34, 862–864. [Google Scholar] [CrossRef]
- Sun, L.; Wei, P.; Pu, W.; Wang, B.; Wu, Y.; Tan, T. The oil recovery enhancement by nitrogen foam in high-temperature and high-salinity environments. J. Pet. Sci. Eng. 2016, 147, 485–494. [Google Scholar] [CrossRef]
- Ren, Z.H. Mechanism of the Salt Effect on Micellization of an Aminosulfonate Amphoteric Surfactant. Ind. Eng. Chem. Res. 2015, 54, 9683–9688. [Google Scholar] [CrossRef]
- Qin, X.; Liu, M.; Zhang, X.; Yang, D. Proton NMR based investigation of the effects of temperature and NaCl on micellar properties of CHAPS. J. Phys. Chem. B 2011, 115, 1991–1998. [Google Scholar] [CrossRef]
- Hussain, S.M.S.; Fogang, L.T.; Kamal, M.S. Synthesis and performance evaluation of betaine type zwitterionic surfactants containing different degrees of ethoxylation. J. Mol. Struct. 2018, 1173, 983–989. [Google Scholar] [CrossRef]
- Zhang, L.; Somasundaran, P.; Maltesh, C. Electrolyte effects on the surface tension and micellization of n-dodecyl β-D-maltoside solutions. Langmuir 1996, 12, 2371–2373. [Google Scholar] [CrossRef]
- Jiang, N.; Yu, X.; Sheng, Y.; Zong, R.; Li, C.; Lu, S. Role of salts in performance of foam stabilized with sodium dodecyl sulfate. Chem. Eng. Sci. 2020, 216, 115474. [Google Scholar] [CrossRef]
- Cubaud, T.; Ulmanella, U.; Ho, C.M. Two-phase flow in microchannels with surface modifications. Fluid Dyn. Res. 2006, 38, 772. [Google Scholar] [CrossRef]
- Gu, M.; Mohanty, K.K. Rheology of polymer-free foam fracturing fluids. J. Pet. Sci. Eng. 2015, 134, 87–96. [Google Scholar] [CrossRef]
- Zarei, M.; Aalaie, J. Application of shear thickening fluids in material development. J. Mater. Res. Technol. 2020, 9, 10411–10433. [Google Scholar] [CrossRef]




| NaCl, wt% | Critical Micelle Concentration, wt% | ||
|---|---|---|---|
| NP12EO-SA | NP12EO-MA | NP12EO | |
| 0 | 0.02 | 0.015 | 0.015 |
| 5 | 0.015 | 0.01 | 0.01 |
| 10 | 0.01 | 0.01 | 0.0075 |
| 20 | 0.0075 | 0.0075 | 0.0075 |
| Surfactant | Parameter | Foam Quality (fg) | ||||
|---|---|---|---|---|---|---|
| 0.35 | 0.5 | 0.65 | 0.8 | 0.95 | ||
| NP12EO-MA | ΔP, bar | 0.64 | 0.65 | 0.52 | 0.45 | 0.25 |
| µFapp, cP | 65.33 | 66.96 | 53.56 | 45.72 | 25.22 | |
| NP12EO-SA | ΔP, bar | 0.77 | 0.85 | 0.76 | 0.69 | 0.26 |
| µFapp, cP | 79.23 | 87.39 | 77.91 | 70.31 | 26.97 | |
| NP12EO | ΔP, bar | 0.45 | 0.44 | 0.49 | 0.27 | 0.06 |
| µFapp, cP | 30.06 | 30.44 | 33.75 | 18.85 | 4.13 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saifullin, E.R.; Zhanbossynova, S.; Zharkov, D.A.; Pavelyev, R.S.; Yuan, C.; Varfolomeev, M.A.; Mirzakimov, U.Z.; Ivanov, S.Y.; Sitnov, S.A. Nonylphenol Ethoxylate Surfactants Modified by Carboxyl Groups for Foam EOR at High-Salinity Conditions. Energies 2021, 14, 8205. https://doi.org/10.3390/en14248205
Saifullin ER, Zhanbossynova S, Zharkov DA, Pavelyev RS, Yuan C, Varfolomeev MA, Mirzakimov UZ, Ivanov SY, Sitnov SA. Nonylphenol Ethoxylate Surfactants Modified by Carboxyl Groups for Foam EOR at High-Salinity Conditions. Energies. 2021; 14(24):8205. https://doi.org/10.3390/en14248205
Chicago/Turabian StyleSaifullin, Emil R., Shinar Zhanbossynova, Dmitrii A. Zharkov, Roman S. Pavelyev, Chengdong Yuan, Mikhail A. Varfolomeev, Ulukbek Zh. Mirzakimov, Sergey Yu. Ivanov, and Sergey A. Sitnov. 2021. "Nonylphenol Ethoxylate Surfactants Modified by Carboxyl Groups for Foam EOR at High-Salinity Conditions" Energies 14, no. 24: 8205. https://doi.org/10.3390/en14248205
APA StyleSaifullin, E. R., Zhanbossynova, S., Zharkov, D. A., Pavelyev, R. S., Yuan, C., Varfolomeev, M. A., Mirzakimov, U. Z., Ivanov, S. Y., & Sitnov, S. A. (2021). Nonylphenol Ethoxylate Surfactants Modified by Carboxyl Groups for Foam EOR at High-Salinity Conditions. Energies, 14(24), 8205. https://doi.org/10.3390/en14248205








