Research Progress on the Phase Change Materials for Cold Thermal Energy Storage
Abstract
:Highlights
- Latest research progress of PCM–CTES with a wide temperature range is reviewed.
- PCMs with a PCT range of (−100 to 30 °C) and the applications with a temperature range of (−97 to 65 °C) are covered.
- The potential and commercial PCMs and their thermophysical properties are presented.
- Technologies for enhancing the thermal properties of PCMs are introduced.
- Typical applications of PCMs for CTES are presented.
1. Introduction
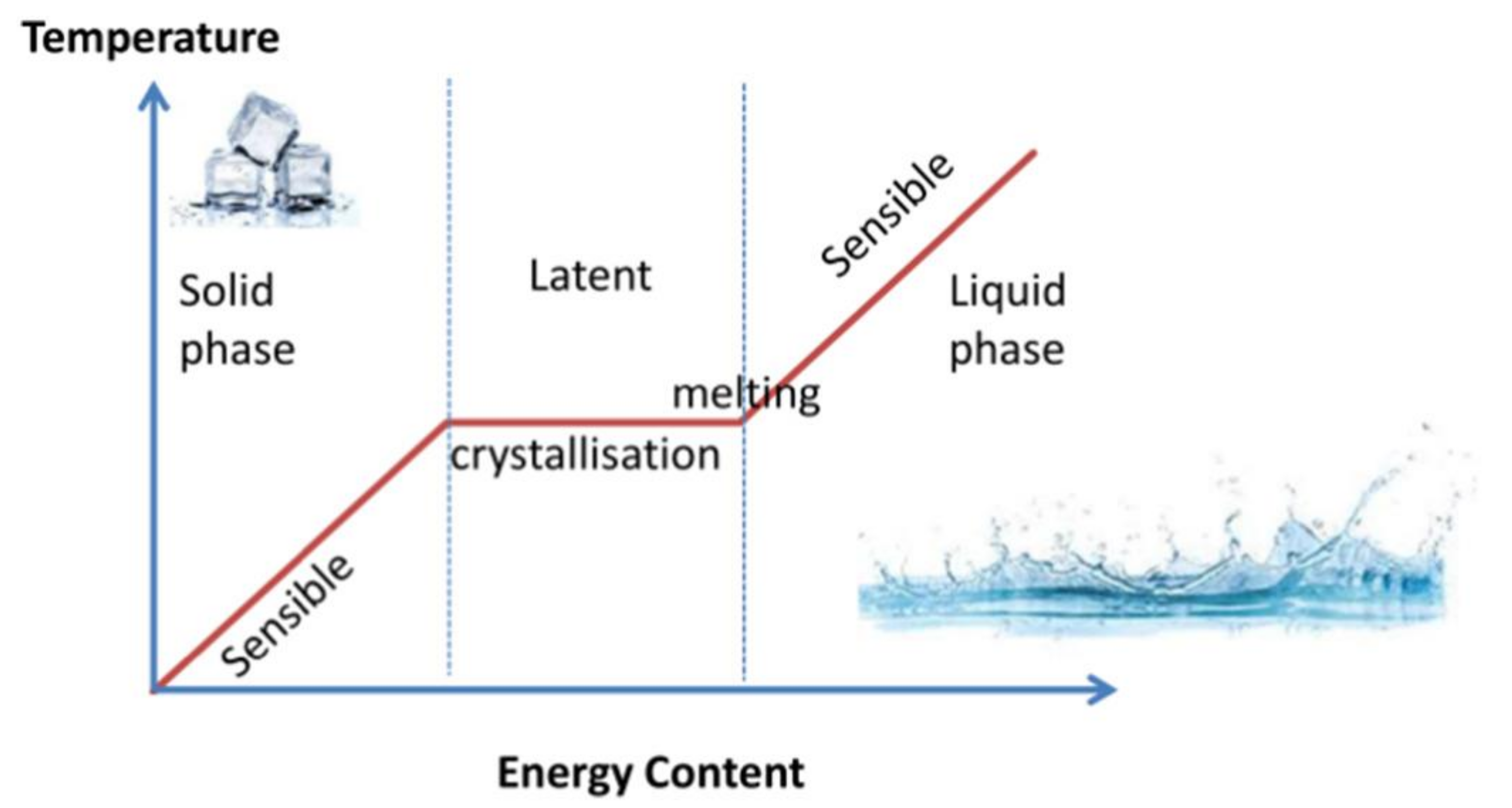
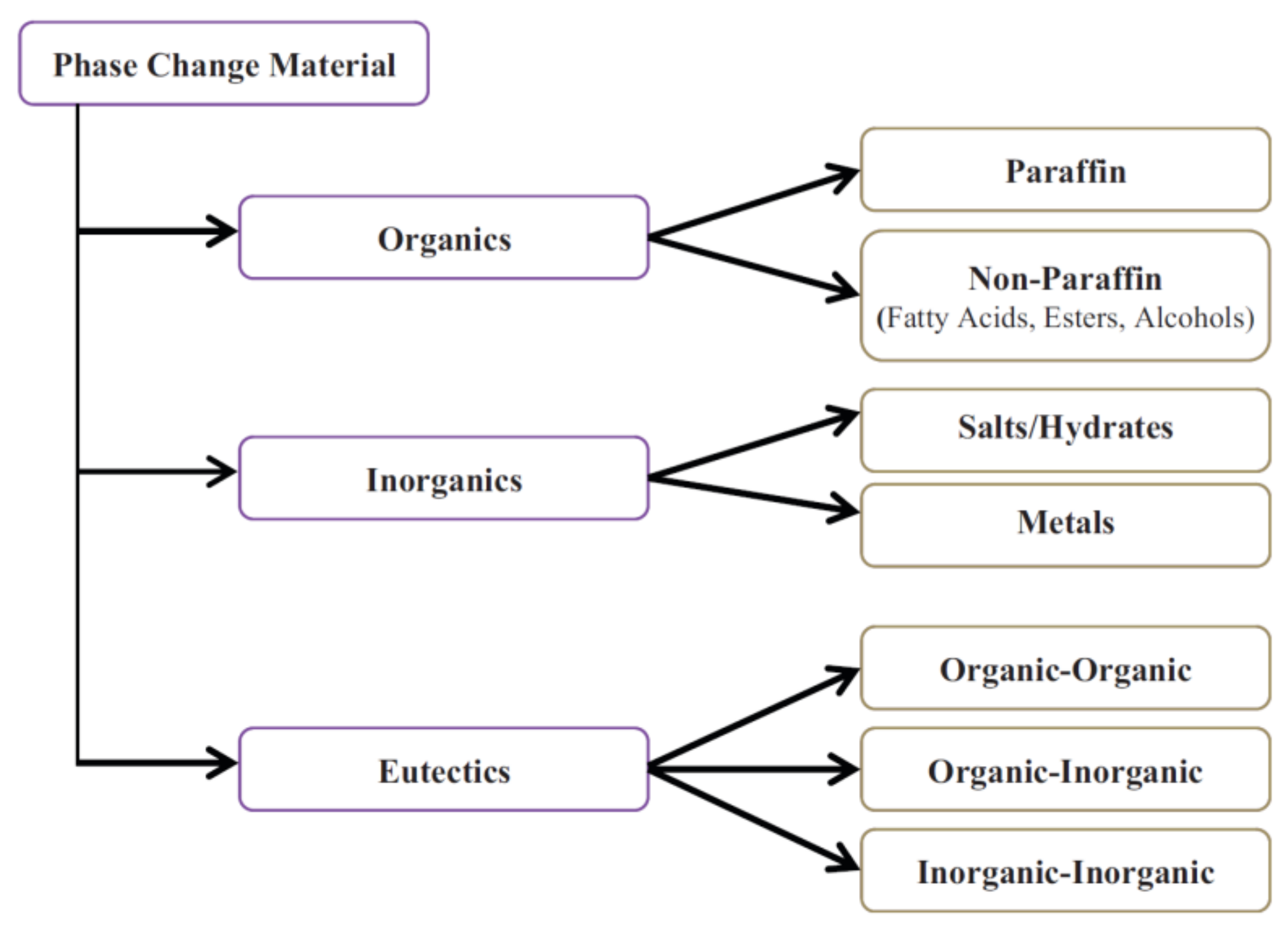
2. Phase Change Materials (PCMs)
2.1. Organic PCMs for CTES
2.2. Inorganic PCMs for CTES
2.3. Eutectic PCMs for CTES
2.4. Commercial PCMs
3. Enhancement of PCMs Performance
3.1. Addition of Nanomaterials
3.2. Microencapsulation of PCMs
3.3. Shape-Stabilized PCMs
3.4. A Complex of Organic and Inorganic Salt Solutions
4. Applications of PCMs for CTES
4.1. Buildings
4.2. Refrigeration
4.3. Thermal Management of Electronic Equipments
4.4. Other Applications
4.4.1. Telecommunications Cooling
4.4.2. LNG Cold Energy Utilization
4.4.3. Fuel Cell Hydrogen Pressure Energy Recovery
5. Conclusions
- (1)
- Microencapsulation technology: the coating rate of cold storage PCM is low, and the selection of PCMs is limited (only applicable to organic PCMs).
- (2)
- Although the addition of nanomaterials enhances the thermal conductivity of the composites, the latent heat of the composites is generally reduced, resulting in the decrease of energy storage capacity. Furthermore, the improvement of thermal conductivity also shortens the cooling release time, which is an inconvenience in cold chain transportation. Moreover, due to the higher cooling rate, the short crystal core formation time causes crystallization difficulties in the early stage of solidification, leading to the uneven internal heat transfer.
- (3)
- The thermal performance decreases after multiple thawing cycles (such as the change of PCT and reduction of latent heat), which limits its long-term application in practice.
- (4)
- Composite cold storage PCMs have high cost and poor practicability.
- (5)
- There are few PCMs with PCT lower than −100 °C, and the corresponding research (such as for LNG cold energy utilization) is relatively lacking.
- (1)
- Microencapsulation technology: optimize core and shell materials, and improve coating rate and thermal stability;
- (2)
- Adding nanomaterials: appropriate nanomaterials and dosage to enhance the thermal conductivity without affecting the energy storage capacity;
- (3)
- Problems such as supercooling and corrosion in the applications of PCMs remain to be solved;
- (4)
- Design methods and standards for cold storage heat exchangers based on PCMs to improve the accurate control in the cooling process, and to avoid equipment frosting caused by supercooling;
- (5)
- Explore more PCMs at lower temperature range with good thermal properties to meet the applications of CTES in more energy fields;
- (6)
- Physical parameters of cold storage materials should be accurately measured and a complete database should be established to facilitate the practical applications of cold storage PCMs;
- (7)
- When PCM–CTES is applied in building and refrigeration, the combination of active and passive strategies should be considered rather than a single strategy, so as to maximize the application potential of PCM–CTES.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- National Development and Reform Commission. Green and Efficient Refrigeration Action Plan. 2019. Available online: https://www.ndrc.gov.cn/ (accessed on 1 September 2021).
- Dardir, M.; Panchabikesan, K.; Haghighat, F.; El Mankibi, M.; Yuan, Y. Opportunities and challenges of PCM-to-air heat exchangers (PAHXs) for building free cooling applications—A comprehensive review. J. Energy Storage 2019, 22, 157–175. [Google Scholar] [CrossRef]
- Iten, M.; Liu, S.; Shukla, A. A review on the air-PCM-TES application for free cooling and heating in the buildings. Renew. Sustain. Energy Rev. 2016, 61, 175–186. [Google Scholar] [CrossRef]
- Alizadeh, M.; Sadrameli, S. Development of free cooling based ventilation technology for buildings: Thermal energy storage (TES) unit, performance enhancement techniques and design considerations—A review. Renew. Sustain. Energy Rev. 2016, 58, 619–645. [Google Scholar] [CrossRef]
- Souayfane, F.; Fardoun, F.; Biwole, P.-H. Phase change materials (PCM) for cooling applications in buildings: A review. Energy Build. 2016, 129, 396–431. [Google Scholar] [CrossRef]
- Faraj, K.; Khaled, M.; Faraj, J.; Hachem, F.; Castelain, C. Phase change material thermal energy storage systems for cooling applications in buildings: A review. Renew. Sustain. Energy Rev. 2019, 119, 109579. [Google Scholar] [CrossRef]
- Akeiber, H.; Nejat, P.; Majid, M.Z.A.; Wahid, M.A.; Jomehzadeh, F.; Famileh, I.Z.; Calautit, J.K.; Hughes, B.R.; Zaki, S.A. A review on phase change material (PCM) for sustainable passive cooling in building envelopes. Renew. Sustain. Energy Rev. 2016, 60, 1470–1497. [Google Scholar] [CrossRef]
- Romdhane, S.B.; Amamou, A.; Rim, B.K.; Nejla, M.S.; Zohir, Y.; Abdelmajid, J. A review on thermal energy storage using phase change materials in passive building applications. J. Build. Eng. 2020, 32, 101563. [Google Scholar] [CrossRef]
- Waqas, A.; Din, Z.U. Phase change material (PCM) storage for free cooling of buildings—A review. Renew. Sustain. Energy Rev. 2013, 18, 607–625. [Google Scholar] [CrossRef]
- Li, S.F.; Liu, Z.H.; Wang, X.J. A comprehensive review on positive cold energy storage technologies and applications in air conditioning with phase change materials. Appl. Energy 2019, 255, 113667. [Google Scholar] [CrossRef]
- Raj, V.A.A.; Velraj, R. Review on free cooling of buildings using phase change materials. Renew. Sustain. Energy Rev. 2010, 14, 2819–2829. [Google Scholar] [CrossRef]
- Sharma, R.K.; Ganesan, P.; Tyagi, V.V.; Metselaar, H.S.C.; Sandaran, S.C. Developments in organic solid–liquid phase change materials and their applications in thermal energy storage. Energy Convers. Manag. 2015, 95, 193–228. [Google Scholar] [CrossRef] [Green Version]
- Kalnaes, S.E.; Jelle, B.P. Phase change materials and products for building applications: A state-of-the-art review and future research opportunities. Energy Build. 2015, 94, 150–176. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.; Zhang, W.; Liang, F. A review about phase change material cold storage system applied to solar-powered air-conditioning system. Adv. Mech. Eng. 2017, 9, 1–20. [Google Scholar] [CrossRef]
- Zhai, X.Q.; Wang, X.L.; Wang, T.; Wang, R.Z. A review on phase change cold storage in air-conditioning system: Materials and applications. Renew. Sustain. Energy Rev. 2013, 22, 108–120. [Google Scholar] [CrossRef]
- Osterman, E.; Tyagi, V.V.; Butala, V.; Rahim, N.A.; Stritih, U. Review of PCM based cooling technologies for buildings. Energy Build. 2012, 49, 37–49. [Google Scholar] [CrossRef]
- Gang, L.; Hwang, Y.; Radermacher, R. Review of cold storage materials for air conditioning application—ScienceDirect. Int. J. Refrig. 2012, 35, 2053–2077. [Google Scholar]
- Sidik, N.A.C.; Kean, T.H.; Chow, H.K.; Rajaandra, A.; Rahman, S.; Kaur, J. Performance enhancement of cold thermal energy storage system using nanofluid phase change materials: A review. Int. Commun. Heat Mass Transf. 2018, 94, 85–95. [Google Scholar] [CrossRef]
- Ali, H.M. Applications of combined/hybrid use of heat pipe and phase change materials in energy storage and cooling systems: A recent review. J. Energy Storage 2019, 26, 100986. [Google Scholar] [CrossRef]
- Kibria, M.A.; Anisur, M.R.; Mahfuz, M.H.; Saidur, R.; Metselaar, I.H.S.C. A review on thermophysical properties of nanoparticle dispersed phase change materials. Energy Convers. Manag. 2015, 95, 69–89. [Google Scholar] [CrossRef] [Green Version]
- Joybari, M.M.; Haghighat, F.; Moffat, J.; Sra, P. Heat and cold storage using phase change materials in domestic refrigeration systems: The state-of-the-art review. Energy Build. 2015, 106, 111–124. [Google Scholar] [CrossRef]
- Selvnes, H.; Allouche, Y.; Manescu, R.I.; Hafner, A. Review on cold thermal energy storage applied to refrigeration systems using phase change materials. Therm. Sci. Eng. Prog. 2020, 22, 100807. [Google Scholar] [CrossRef]
- Nie, B.; Palacios, A.; Zou, B.; Liu, J.; Zhang, T.; Li, Y. Review on phase change materials for cold thermal energy storage applications. Renew. Sustain. Energy Rev. 2020, 134, 110340. [Google Scholar] [CrossRef]
- Oró, E.; De Gracia, A.; Castell, A.; Farid, M.M.; Cabeza, L.F. Review on phase change materials (PCMs) for cold thermal energy storage applications. Appl. Energy 2012, 99, 513–533. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Hwang, Y.; Radermacher, R.; Chun, H.-H. Review of cold storage materials for subzero applications. Energy 2013, 51, 1–17. [Google Scholar] [CrossRef]
- Yang, L.; Villalobos, U.; Akhmetov, B.; Gil, A.; Khor, J.O.; Palacios, A.; Li, Y.; Ding, Y.; Cabeza, L.F.; Tan, W.L.; et al. A comprehensive review on sub-zero temperature cold thermal energy storage materials, technologies, and applications: State of the art and recent developments. Appl. Energy 2021, 288, 116555. [Google Scholar] [CrossRef]
- Tatsidjodoung, P.; Le Pierrès, N.; Luo, L. A review of potential materials for thermal energy storage in building applications. Renew. Sustain. Energy Rev. 2013, 18, 327–349. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Castell, A.; Barreneche, C.; De Gracia, A.; Fernández, A.I. Materials used as PCM in thermal energy storage in buildings: A review. Renew. Sustain. Energy Rev. 2011, 15, 1675–1695. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, X.L.; Yang, Y.; Yuan, Y. Preparation and Thermal Performance of the Phase Change Storage Materials for Refrigerated Transport. J. Refrig. Technol. 2014, 4, 14–17. [Google Scholar]
- Yibo, F.U.; Dongmei, W.; Hong, Z. Review on Low Temperature Phase Change Materials and Its Application. Mater. Rev. 2016, 30, 222–226. [Google Scholar]
- Yang, T.R.; Sun, Q.; Wennersten, R.; Cheng, L. Review of Phase Change Materials for Cold Thermal Energy Storage. K. Cheng Je Wu Li Hsueh Pao/J. Eng. Thermophys. 2018, 39, 567–573. [Google Scholar]
- Yongsheng, Y.U.; Qiangshan, J.; Yaqian, S. Progress in studies of low temperature phase-change energy storage materials. Chem. Ind. Eng. Prog. 2010, 29, 896–900. [Google Scholar]
- Borri, E.; Sze, J.Y.; Tafone, A.; Romagnoli, A.; Li, Y.; Comodi, G. Experimental and numerical characterization of sub-zero phase change materials for cold thermal energy storage. Appl. Energy 2020, 275, 115131. [Google Scholar] [CrossRef]
- Sharma, S.D. Phase Change Materials for Low Temperature Solar Thermal Applications. Res. Rep. Fac. Eng. Mie Univ. 2004, 29, 31–64. [Google Scholar]
- Li, X.Y.; Yang, L.; Wang, X.L.; Miao, X.Y.; Yao, Y.; Qiang, Q.Q. Investigation on the charging process of a multi-PCM latent heat thermal energy storage unit for use in conventional air-conditioning systems. Energy 2018, 150, 591–600. [Google Scholar] [CrossRef]
- Veerakumar, C.; Sreekumar, A. Phase change material based cold thermal energy storage: Materials, techniques and applications—A review. Int. J. Refrig. 2016, 67, 271–289. [Google Scholar] [CrossRef]
- Fu, L.L.; Jiang, D.G.; Zhou, C.Y. Determination of Thermodynamic Parameters of Isopropyl Palmitate Synthesis. J. Chem. Engine Ering Chin. Universties 2017, 31, 733–737. [Google Scholar]
- Pielichowska, K.; Pielichowski, K. Phase change materials for thermal energy storage. Prog. Mater. Sci. 2014, 65, 67–123. [Google Scholar] [CrossRef]
- Gasanaliev, A.M.; Gamataeva, B.Y. Heat-accumulating properties of melts. Russ. Chem. Rev. 2000, 69, 192–200. [Google Scholar] [CrossRef]
- Noro, M.; Lazzarin, R.; Busato, F. Solar cooling and heating plants: An energy and economic analysis of liquid sensible vs. phase change material (PCM) heat storage. Int. J. Refrig. 2014, 39, 104–116. [Google Scholar] [CrossRef]
- Fang, M.T.; Zhang, X.L.; Ji, J.; Hua, W.S.; Liu, B.; Wang, X.Z. Progress in hydrated salt based composite phase change materials. Energy Storage Sci. Technol. 2019, 8, 709–717. [Google Scholar]
- Baetens, R.; Jelle, B.P.; Gustavsen, A. Phase change materials for building applications: A state-of-the-art review. Energy Build. 2010, 42, 1361–1368. [Google Scholar] [CrossRef] [Green Version]
- Haghighat, F. Applying energy storage in ultra-low energy buildings. IEA Annex 2013, 23, 834–835. [Google Scholar]
- Xing-Ren, L.I.; Ming-Wei, T.; Bi-Rong, W.U. Experiments on the eutectic salt used as low temperature cool storage material. J. Chongqing Univ. 2004, 8, 113–115. [Google Scholar]
- Gunasekara, S.N.; Kumova, S.; Chiu, J.N.W.; Martin, V. Experimental phase diagram of the dodecane–tridecane system as phase change material in cold storage. Int. J. Refrig. 2017, 82, 130–140. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Guo, Y.F.; Fu, J.; Liang, J. Preparation and thermophysical performance of organic phase change energy storage materials in cold chain transportation. Acta Mater. Compos. Sin. 2022, in press. [Google Scholar]
- Bo, H.; Gustafsson, E.; Setterwall, F. Tetradecane and hexadecane binary mixtures as phase change materials (PCMs) for cool storage in district cooling systems. Energy 1999, 24, 1015–1028. [Google Scholar] [CrossRef]
- Zhang, X.L.; Chen, Y.F.; Zeng, T.; Lv, W. Research and development of phase change materials for pharmaceutical cold chain logistics. Refrig. Air-Cond. 2017, 17, 43–46. [Google Scholar]
- Shengli, T.; Dong, Z.; Deyan, X. Experimental study of caprylic acid/lauric acid molecular alloys used as low-temperature phase change materials in energy storage. Energy Conserv. 2005, 6, 45–47. [Google Scholar]
- Zhao, Y.; Zhang, X.; Xu, X.; Zhang, S. Development of composite phase change cold storage material and its application in vaccine cold storage equipment. J. Energy Storage 2020, 30, 101455. [Google Scholar] [CrossRef]
- Bo, H.; Setterwall, F. Technical grade paraffin waxes as phase change materials for cool thermal storage and cool storage systems capital cost estimation. Energy Convers. Manag. 2002, 43, 1709–1723. [Google Scholar]
- Gao, H.T. Investigation on organic phase transition materials in Energy Storage Air Conditioning system. In Proceedings of the 2011 International Conference on Materials for Renewable Energy & Environment, Shanghai, China, 20–22 May 2011. [Google Scholar]
- Zuo, J.; Li, W.; Weng, L. Thermal performance of caprylic acid/1-dodecanol eutectic mixture as phase change material (PCM). Energy Build. 2011, 43, 207–210. [Google Scholar] [CrossRef]
- Xing, L.; Fang, G.Y.; Yang, F. Preparation and Thermal Performance Analysis of Cold Storage Material for Air Conditioning System. China Appl. Technol. 2006, 3, 47–48. [Google Scholar]
- Wu, W.; Tang, H.; Miao, P.; Zhang, H. Preparation and thermal properties of nano-organic composite phase change materials for cool storage in air-conditioning. CIESC J. 2015, 66, 1208–1214. [Google Scholar]
- Xiao-Cai, H.U.; Hui-Jun, W.U.; Zhou, X.Q. Crystallizing characteristics of binary mixtures of Dodecanol/Caprylic acid for phase change cool storage. J. Guangzhou Univ. 2011, 10, 60–63. [Google Scholar]
- Hussain, S.I.; Dinesh, R.; Roseline, A.A.; Dhivya, S.; Kalaiselvam, S. Enhanced thermal performance and study the influence of sub cooling on activated carbon dispersed eutectic PCM for cold storage applications. Energy Build. 2017, 143, 17–24. [Google Scholar] [CrossRef]
- Dimaano, M.N.R.; Watanabe, T. The capric–lauric acid and pentadecane combination as phase change material for cooling applications. Appl. Therm. Eng. 2002, 22, 365–377. [Google Scholar] [CrossRef]
- Roxas-Dimaano, M.N.; Watanabe, T. The capric and lauric acid mixture with chemical additives as latent heat storage materials for cooling application. Energy 2002, 27, 869–888. [Google Scholar] [CrossRef]
- Jia, X.; Zhai, X.; Cheng, X. Thermal performance analysis and optimization of a spherical PCM capsule with pin-fins for cold storage. Appl. Therm. Eng. 2018, 148, 929–938. [Google Scholar] [CrossRef]
- Kumar, R.; Vyas, S.; Dixit, A. Fatty acids/1-dodecanol binary eutectic phase change materials for low temperature solar thermal applications: Design, development and thermal analysis. Sol. Energy 2017, 155, 1373–1379. [Google Scholar] [CrossRef]
- Philip, N.; Dheep, G.R.; Sreekumar, A. Cold thermal energy storage with lauryl alcohol and cetyl alcohol eutectic mixture: Thermophysical studies and experimental investigation. J. Energy Storage 2020, 27, 101060. [Google Scholar] [CrossRef]
- Jebasingh, B.E.; Arasu, A.V. Characterisation and stability analysis of eutectic fatty acid as a low cost cold energy storage phase change material. J. Energy Storage 2020, 31, 101708. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Fu, S.Y.; Wu, W.D.; Zhang, D. Preparation and performance evaluation of a new type of binary shaped phase change material for buildings. Chem. Ind. Eng. Prog. 2020, 39, 4119–4126. [Google Scholar]
- Chinnasamy, V.; Appukuttan, S. Preparation and thermal properties of lauric acid/myristyl alcohol as a novel binary eutectic phase change material for indoor thermal comfort. Energy Storage 2019, 1, e80. [Google Scholar] [CrossRef] [Green Version]
- Sarı, A.; Karaipekli, A. Preparation and thermal properties of capric acid/palmitic acid eutectic mixture as a phase change energy storage material. Mater. Lett. 2008, 62, 903–906. [Google Scholar] [CrossRef]
- Chinnasamy, V.; Sreekumar, A. Preparation, Thermophysical Studies, and Corrosion Analysis of a Stable Capric Acid/Cetyl Alcohol Binary Eutectic Phase Change Material for Cold Thermal Energy Storage. Energy Technol. 2018, 6, 397–405. [Google Scholar]
- Jarrar, R.; Sawafta, R. Binary and Ternary Mixtures of Eicosane with Fatty Alcohols and Fatty Acids as Phase Change Material for Building Applications. Palest. Tech. Univ. Res. J. 2018, 6, 16–22. [Google Scholar] [CrossRef]
- Saeed, R.M.; Schlegel, J.P.; Castano, C.; Sawafta, R.; Kuturu, V. Preparation and thermal performance of methyl palmitate and lauric acid eutectic mixture as phase change material (PCM). J. Energy Storage 2017, 13, 418–424. [Google Scholar] [CrossRef]
- Shen, J.; Hu, Z.; Wang, C.; Chen, K.; Cai, Z.; Wang, T. Preparation and Thermal Properties of Stearic Acid/n-Octadecane Binary Eutectic Mixture as Phase Change Materials for Energy Storage. ChemistrySelect 2019, 4, 4125–4130. [Google Scholar] [CrossRef]
- Zheng, D.X.; Wu, X.H. Comprehensive evaluation eutectic character used as low temperature thermal energy storage. Cryogenics 2002, 1, 37–45. [Google Scholar]
- Sun, Z.G.; Jiang, C.M.; Sun, L. Experiment on Phase Change Conditions and Phase Change Latent Heat of Tetra-n-butyl Ammonium Bromide Aqueous Solutions for Cold Storage. J. Refrig. 2009, 30, 24–26. [Google Scholar]
- Zhang, M.; Fang, G.Y.; Wu, S.M.; Yang, F. Experimental Study on Thermal Characteristics of Phase-change Material Tetra-n-butylammonium Bromide for Cool Storage. J. Refrig. 2008, 5, 8–11. [Google Scholar]
- Liu, J.H.; Liu, R.H.; Wang, C.H.; Liang, Y.N. Thermodynamics test of Na_2SO_4·10H_2O phase change compound system. Energy Conserv. 2007, 9, 13–14. [Google Scholar]
- Available online:https://www.pcmproducts.net/Eutectic_Refrigeration_PCMs.htm (accessed on 1 September 2021).
- Available online: www.teapcm.com (accessed on 1 September 2021).
- Available online: www.cristopia.com (accessed on 1 September 2021).
- Available online: www.microteklabs.com (accessed on 1 September 2021).
- Kakiuchi, H. Mitsubishi Chemical; Mitsubishi Chemical Corporation: Tokyo, Japan, 2002. [Google Scholar]
- Available online: www.climator.com (accessed on 1 September 2021).
- Available online: www.rubitherm.eu (accessed on 1 September 2021).
- Available online: www.puretemp.com (accessed on 1 September 2021).
- Available online: www.epsltd.co (accessed on 1 September 2021).
- Wang, X.; Ding, Q.; Yao, X.L.; Fang, X.; Fan, L.W.; Xu, X.; Yu, Z.T.; Hu, Y.C. Thermophysical properties of paraffin-based composite phase change materials filled with carbon nanotubes. J. Therm. Ence Technol. 2013, 12, 124–130. [Google Scholar]
- Puyue, J.; Weidong, W.U.; Yicong, W. Preparation of 0℃ phase change material and its cold storage performance in cold-chain logistics. Chem. Ind. Eng. Prog. 2019, 38, 2862–2869. [Google Scholar]
- Zhang, X.L.; Zhou, S.X.; Liu, S.; Li, Y.Y.; Xu, X.F.; Wang, Y.H.; Liu, L. Cold Storage Characteristics of n-Octanoic-Lauric Acid Nanocomposite Phase Change Materials. Tianjin Daxue Xuebao/J. Tianjin Univ. Ence Technol. 2019, 52, 71–77. [Google Scholar]
- Yudong, L.; Xin, L.I. Cold Charge and Discharge Characteristics of Nanofluids and Its Application to Ice Storage Using Valley Electricity. Zhongguo Dianji Gongcheng Xuebao/Proc. Chin. Soc. Electr. Eng. 2015, 35, 2779–2787. [Google Scholar]
- Liu, Y.D. Study on Preparation and Thermal Properties of Phase Change Nanocomposites for Cool Storage. Ph.D. Thesis, Chongqing University, Chongqing, China, 2005. [Google Scholar]
- Sathishkumar, A.; Kumaresan, V.; Velraj, R. Solidification characteristics of water based graphene nanofluid PCM in a spherical capsule for cool thermal energy storage applications. Int. J. Refrig. 2016, 66, 73–83. [Google Scholar] [CrossRef]
- Sun, J.P.; Zhou, X.Q.; Wu, H.J. Experimental Study on the Performance of Organic Phase-Change Cold Storage Material with Carbon Nanotube Additives. Chem. Ind. Times 2012, 26, 9–13. [Google Scholar]
- Xiao-Yan, L.I.; Yan, W.; Jing-Yu, Z. Study on heat transfer properties with phase change nanocomposites in air-conditioning system. J. Harbin Univ. Commer. 2008, 2008 24, 738–740. [Google Scholar]
- Xiao-Yan, L.I. Study of new cool storage materials for air conditioning cool storage system. Appl. ENCE Technol. 2004, 7, 66–68. [Google Scholar]
- Wu, X.H.; Wang, C.X.; Gao, M.T.; Li, W.P. Experimental Study of Thermo-physical Properties of Paraffin-Carbon Nanotubes Composite Materials. J. Eng. Thermophys. 2017, 38, 1071–1076. [Google Scholar]
- Zhi, Y.; Zhou, L.; Wei, L.; Wan, J.; Dai, J.; Han, X.; Fu, K.; Henderson, D.; Bao, Y.; Hu, L. Thermally Conductive, Dielectric PCM-Boron Nitride Nanosheets Composite for Efficient Electronic System Thermal Management. Nanoscale 2016, 8, 19326–19333. [Google Scholar]
- Huang, Y.; She, X.; Li, C.; Li, Y.; Ding, Y. Evaluation of thermal performance in cold storage applications using EG-water based nano-composite PCMs. Energy Procedia 2019, 158, 4840–4845. [Google Scholar] [CrossRef]
- Hao, M.; Li, Z.H.; Wu, Q.F.; Gao, W.; Ma, X.S. Research progress of encapsulation technology for phase change materials. Mater. Rev. 2014, 28, 98–103. [Google Scholar]
- Jurkowska, M.; Szczygieł, I. Review on properties of microencapsulated phase change materials slurries (mPCMS). Appl. Therm. Eng. 2016, 98, 365–373. [Google Scholar] [CrossRef]
- Xu, B.; Li, P.; Chan, C. Application of phase change materials for thermal energy storage in concentrated solar thermal power plants: A review to recent developments. Appl. Energy 2015, 160, 286–307. [Google Scholar] [CrossRef]
- Jelle, B.P.; Kalnæs, S.E. Phase Change Materials for Application in Energy-Efficient Buildings. In Cost-Effective Energy Efficient Building Retrofitting Materials, Technologies, Optimization and Case Studies; Elsevier: Amsterdam, The Netherlands, 2017; pp. 57–118. [Google Scholar] [CrossRef]
- Giro-Paloma, J.; Martínez, M.; Cabeza, L.F.; Fernández, A.I. Types, methods, techniques, and applications for microencapsulated phase change materials (MPCM): A review. Renew. Sustain. Energy Rev. 2016, 53, 1059–1075. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Gui-Yin, F.; Fan, Y. Study on the preparation and performance of the microcapsule phase change material for cool storage. Vac. Cryog. 2006, 3, 153–156. [Google Scholar]
- Xu, H.; Yang, R.; Zhang, Y.P. A Microencapsulated Phase Change Refrigerant for Air Conditioning Storage and Its Preparation. Method. Patent CN1621483.2005-06-01, 1 June 2005. [Google Scholar]
- Dai, X.; Sheng, X. Study on Preparation of Heat Insulation Micropcms for Blood. Mater. Rev. 2007, S1, 361–363. [Google Scholar]
- Yu, S.; Wang, X.; Wu, D. Microencapsulation of n-octadecane phase change material with calcium carbonate shell for enhancement of thermal conductivity and serving durability: Synthesis, microstructure, and performance evaluation. Appl. Energy 2014, 114, 632–643. [Google Scholar] [CrossRef]
- Wang, T.; Wang, S.; Luo, R.; Zhu, C.; Akiyama, T.; Zhang, Z. Microencapsulation of phase change materials with binary cores and calcium carbonate shell for thermal energy storage. Appl. Energy 2016, 171, 113–119. [Google Scholar] [CrossRef]
- You, M. Preparation of Microencapsulated Phase Change Materials and Its Application in Polyurethane Foam; Tianjin Polytechnic University: Tianjin, China, 2010. [Google Scholar] [CrossRef]
- Yu, D.W. Preparation and Performance Optimization Research of Microencapsulated Phase Change Materials with Refrigeration Temperature Control Packaging; Southern Yangtze University: Wuxi, China, 2015. [Google Scholar]
- Fu, W.; Liang, X.; Xie, H.; Wang, S.; Gao, X.; Zhang, Z.; Fang, Y. Thermophysical properties of n -tetradecane@polystyrene-silica composite nanoencapsulated phase change material slurry for cold energy storage. Energy Build. 2017, 136, 26–32. [Google Scholar] [CrossRef]
- Tumirah, K.; Hussein, M.Z.; Zulkarnain, Z.; Rafeadah, R. Nano-encapsulated organic phase change material based on copolymer nanocomposites for thermal energy storage. Energy 2014, 66, 881–890. [Google Scholar] [CrossRef]
- Qian, T.; Li, J.; Ma, H.; Yang, J. The preparation of a green shape-stabilized composite phase change material of polyethylene glycol/SiO2 with enhanced thermal performance based on oil shale ash via temperature-assisted sol–gel method. Sol. Energy Mater. Sol. Cells 2015, 132, 29–39. [Google Scholar] [CrossRef]
- Fang, Y.; Kuang, S.; Gao, X.; Zhang, Z. Preparation and Characterization of Novel Nanoencapsulated Phase Change Materials. Energy Convers. Manag. 2008, 49, 3704–3707. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Yu, Y.-H.; Jia, L.; Mannan, M.S.; Chen, Y.; Cheng, Z. Nano-encapsulated PCM via Pickering Emulsification. Sci. Rep. 2015, 5, 13357. [Google Scholar] [CrossRef]
- Yu, Q.; Tchuenbou-Magaia, F.; Al-Duri, B.; Zhang, Z.; Ding, Y.; Li, Y. Thermo-mechanical analysis of microcapsules containing phase change materials for cold storage. Appl. Energy 2018, 211, 1190–1202. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.; Yu, D.; Ji, X.; An, L.; Jiang, B. Confined crystallization behavior of PEO in silica networks. Polymer 2000, 41, 2041–2046. [Google Scholar] [CrossRef]
- Jiang, Y.; Ding, E.; Li, G. Study on transition characteristics of PEG/CDA solid–solid phase change materials. Polymer 2002, 43, 117–122. [Google Scholar] [CrossRef]
- Pielichowski, K.; Flejtuch, K. Thermal properties of poly(ethylene oxide)/lauric acid blends: A SSA–DSC study. Thermochim. Acta 2006, 442, 18–24. [Google Scholar] [CrossRef]
- Py, X.; Olives, R.; Mauran, S. Paraffin/porous-graphite-matrix composite as a high and constant power thermal storage material. Int. J. Heat Mass Transf. 2001, 44, 2727–2737. [Google Scholar] [CrossRef]
- Xu, X.F.; Zhang, X.L.; Munyalo, J.M. Key technologies and research progress on enhanced characteristics of cold thermal energy storage. J. Mol. Liq. 2019, 278, 428–437. [Google Scholar] [CrossRef]
- Zhang, X.X.; Shu-Qin, L.I.; Chen, S.; Pei, D.F. Preparation and characterization of poly(diethylene glycol hexadecyl ether acrylate)/graphene oxide composite shape- stabilized phase change materials. J. Tianjin Polytech. Univ. 2016, 35, 1–6. [Google Scholar]
- Xiaolin, W.U.; Zhang, H.; Zou, J.; Sun, R. Structural and Thermo-physical Analysis of Polyurethane Form-stable Phase Change Energy Storage Materials. Mater. Rev. 2012, 26, 94–97. [Google Scholar]
- Ma, F.; Wang, X.Y.; Cheng, L.Y. Study on preparation and properties of capric acid-lauric acid/expanded graphite phase change materials. J. Funct. Mater. 2010, 41, 180–183. [Google Scholar]
- Leng, C.B. Preparation and Performance Analysis of Expanded Graphite/Hydrated Salt Composite Shape-stabilized Phase Change Material; Yunnan Normal University: Kunming, China, 2015. [Google Scholar]
- Liu, J.S.; Jia-Jing, M.; Zhang, J.; Yang, H.L.; Feng, B.; Jiang, K.; Li, X.; Liu, H.K.; Wen, J.H. Study on the preparation and poperties of lauric acidexpanded perlite composite phase change materials. J. Wuhan Polytech. Univ. 2014, 33, 82–84. [Google Scholar]
- Wu, F.C. Preparation of the Ss-PCMs and Experiemtal Study in the Thermal Characteristic of PCM Room Model; Chongqing University: Chongqing, China, 2010. [Google Scholar]
- Feng, L.L.; Zheng, J.; Yang, H.Z.; Guo, Y.L.; Li, W.; Li, X.G. Preparation and characterization of polyethylene glycol/active carbon composites as shape-stabilized phase change materials—ScienceDirect. Sol. Energy Mater. Sol. Cells 2011, 95, 644–650. [Google Scholar] [CrossRef]
- Sun, Z.; Kong, W.; Zheng, S.; Frost, R.L. Study on preparation and thermal energy storage properties of binary paraffin blends/opal shape-stabilized phase change materials. Sol. Energy Mater. Sol. Cells 2013, 117, 400–407. [Google Scholar] [CrossRef]
- Sar, A.; Karaipekli, A. Preparation, thermal properties and thermal reliability of capric acid/expanded perlite composite for thermal energy storage. Mater. Chem. Phys. 2008, 109, 459–464. [Google Scholar] [CrossRef]
- Feng, L.P.; Wang, M. Preparation of shaped phase change cool storage paraffin based materials with different supports. New Chem. Mater. 2020, 48, 140–142. [Google Scholar]
- Song, Y.; Zhang, N.; Jing, Y.; Cao, X.; Yuan, Y.; Haghighat, F. Experimental and numerical investigation on dodecane/expanded graphite shape-stabilized phase change material for cold energy storage. Energy 2019, 189, 116175. [Google Scholar] [CrossRef]
- Ying, Y.; Shen, H.-Y. Investigation on cryogenics cool thermal energy storage phase change composition material. Chin. J. Low Temp. Phys. 2009, 31, 144–147. [Google Scholar]
- Yan, H.; Xue-Lai, Z. Research of Composite Cool Storage Materials for Cold Chain Logistics. Chin. J. Refrig. Technol. 2016, 36, 12–15. [Google Scholar]
- Wang, H.; Liu, Z.B.; Zhao, Y. Frozen Properties of Phase Change Cool Storage Materials in the Refrigerator Freezer. Refrig. Air Cond. 2015, 29, 6–10. [Google Scholar]
- Lu, W.; Liu, G.; Xing, X.; Wang, H. Investigation on Ternary Salt-Water Solutions as Phase Change Materials for Cold Storage. Energy Procedia 2019, 158, 5020–5025. [Google Scholar] [CrossRef]
- Ravikumar, M.; Srinivasan, P. Phase change material as thermal energy storage material for cooling of buildings. J. Theor. Appl. Inf. Technol. 2008, 4, 503–511. [Google Scholar]
- ASHRAE. ANSI-ASHRAE 55a-1995, Addendum to ANSI-ASHRAE Standard 55-1992, Thermal Environmental Conditions for Human Occupancy; American Society of Heating, Refrigerating and Air Conditioning Engineers, Atlanta, Inc.: Atlanta, GA, USA, 1995. [Google Scholar]
- Turnpenny, J.R.; Etheridge, D.W.; Reay, D.A. Novel ventilation system for reducing air conditioning in buildings. Part II: Testing of prototype. Appl. Therm. Eng. 2001, 21, 1203–1217. [Google Scholar] [CrossRef]
- Nagano, K.; Takeda, S.; Mochida, T.; Shimakura, K. Thermal characteristics of a direct heat exchange system between granules with phase change material and air. Appl. Therm. Eng. 2004, 24, 2131–2144. [Google Scholar] [CrossRef]
- Alessandro, M.; Göran, H.; Bergsøe, N.C.; Alireza, A. Free cooling potential of a PCM-based heat exchanger coupled with a novel HVAC system for simultaneous heating and cooling of buildings. Sustain. Cities Soc. 2018, 42, 384–395. [Google Scholar]
- Piselli, C.; Prabhakar, M.; de Gracia, A.; Saffari, M.; Pisello, A.L.; Cabeza, L.F. Optimal control of natural ventilation as passive cooling strategy for improving the energy performance of building envelope with PCM integration. Renew. Energy 2020, 162, 171–181. [Google Scholar] [CrossRef]
- Philip, N.; Veerakumar, C.; Sreekumar, A. Lauryl alcohol and stearyl alcohol eutectic for cold thermal energy storage in buildings: Preparation, thermophysical studies and performance analysis. J. Energy Storage 2020, 31, 101600. [Google Scholar] [CrossRef]
- Ruevskis, S.; Akishin, P.; Korjakins, A. Parametric analysis and design optimisation of PCM thermal energy storage system for space cooling of buildings—ScienceDirect. Energy Build. 2020, 224, 110288. [Google Scholar] [CrossRef]
- Kong, X.F.; Yao, C.Q.; Jie, P.F.; Liu, Y.; Qi, C.Y.; Rong, X. Development and thermal performance of an expanded perlite-based phase change material wallboard for passive cooling in building. Energy Build. 2017, 152, 547–557. [Google Scholar] [CrossRef]
- Gholamibozanjani, G.; Farid, M. Application of an active PCM storage system into a building for heating/cooling load reduction. Energy 2020, 210, 118572. [Google Scholar] [CrossRef]
- Yu, H.; Li, C.E.; Zhang, K.G.; Tang, Y.; Song, Y.; Wang, M. Preparation and Thermophysical Performance of Diatomite-Based Composite PCM Wallboard for Thermal Energy Storage in Buildings. J. Build. Eng. 2020, 32, 101753. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Q.; Zhai, Z.J.; Ma, X. Potential of ventilation systems with thermal energy storage using PCMs applied to air conditioned buildings. Renew. Energy 2019, 138, 39–53. [Google Scholar] [CrossRef]
- Maleki, B.; Khadang, A.; Maddah, H.; Alizadeh, M.; Kazemian, A.; Ali, H.M. Development and thermal performance of nanoencapsulated PCM/plaster wallboard for thermal energy storage in buildings. J. Build. Eng. 2020, 32, 101727. [Google Scholar] [CrossRef]
- Guarino, F.; Dermardiros, V.; Chen, Y.; Rao, J.; Athienitis, A.; Cellura, M.; Mistretta, M. PCM Thermal Energy Storage in Buildings: Experimental Study and Applications. Energy Procedia 2015, 70, 219–228. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Zhang, Q.; Zhai, Z.; Qiu, J. Performance of a cold storage air-cooled heat pump system with phase change materials for space cooling. Energy Build. 2020, 228, 110405. [Google Scholar] [CrossRef]
- Ezan, M.A.; Doganay, E.O.; Yavuz, F.E.; Tavman, I.H. A numerical study on the usage of phase change material (PCM) to prolong compressor off period in a beverage cooler. Energy Convers. Manag. 2017, 142, 95–106. [Google Scholar] [CrossRef]
- Du, J.; Nie, B.; Zhang, Y.; Du, Z.; Wang, L.; Ding, Y. Cooling performance of a thermal energy storage-based portable box for cold chain applications. J. Energy Storage 2020, 28, 101238. [Google Scholar] [CrossRef]
- Ghahramani, Z.O.; Rouhollah, A. Employment of Finned PCM Container in a Household Refrigerator as a Cold Thermal Energy Storage System. Therm. Sci. Eng. Prog. 2018, 7, 115–124. [Google Scholar] [CrossRef]
- Tian, S.; Yang, Q.F.; Hui, N.; Bai, H.Z.; Shao, S.Q.; Liu, S.C. Discharging process and performance of a portable cold thermal energy storage panel driven by embedded heat pipes. Energy 2020, 205, 117987. [Google Scholar] [CrossRef]
- Ben-Abdallah, R.; Leducq, D.; Hoang, H.M.; Fournaison, L.; Pateau, O.; Ballot-Miguet, B.; Delahaye, A. Experimental investigation of the use of PCM in an open display cabinet for energy management purposes. Energy Convers. Manag. 2019, 198. [Google Scholar] [CrossRef]
- Xu, X.F.; Zhang, X.L.; Zhou, S.X.; Wang, Y.H.; Liu, L.; Liu, S. Design and Experimental Study on the Storage Type Insulation Box with Multi-temperature. J. Refrig. 2019, 40, 92–98. [Google Scholar]
- Mousazade, A.; Rafee, R.; Valipour, M.S. Thermal performance of cold panels with phase change materials in a refrigerated truck. Int. J. Refrig. 2020, 120, 119–126. [Google Scholar] [CrossRef]
- Ghorbani, B.; Mehrpooya, M. Concentrated solar energy system and cold thermal energy storage (process development and energy analysis). Sustain. Energy Technol. Assess. 2019, 37, 100607. [Google Scholar] [CrossRef]
- Oró, E.; Cabeza, L.F.; Farid, M.M. Experimental and numerical analysis of a chilly bin incorporating phase change material. Appl. Therm. Eng. 2013, 58, 61–67. [Google Scholar] [CrossRef]
- Oró, E.; Gracia, A.D.; Cabeza, L.F. Active phase change material package for thermal protection of ice cream containers. Int. J. Refrig. 2013, 36, 102–109. [Google Scholar] [CrossRef]
- Leducq, D.; Ndoye, F.T.; Alvarez, G. Phase change material for the thermal protection of ice cream during storage and transportation. Int. J. Refrig. 2015, 52, 133–139. [Google Scholar] [CrossRef]
- Liu, M.; Saman, W.; Bruno, F. Development of a novel refrigeration system for refrigerated trucks incorporating phase change material. Appl. Energy 2012, 92, 336–342. [Google Scholar] [CrossRef]
- Liu, M.; Saman, W.; Bruno, F. Computer simulation with TRNSYS for a mobile refrigeration system incorporating a phase change thermal storage unit. Appl. Energy 2014, 132, 226–235. [Google Scholar] [CrossRef]
- Alzuwaid, F.A.; Ge, Y.T.; Tassou, S.A.; Sun, J. The novel use of phase change materials in an open type refrigerated display cabinet: A theoretical investigation. Appl. Energy 2016, 180, 76–85. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.L.; Zhang, W.H.; Yuan, P.; Xue, M.D.; Qu, Z.G.; Tao, W.Q. Experimental study of heat transfer intensification by using a novel combined shelf in food refrigerated display cabinets (Experimental study of a novel cabinets). Appl. Therm. Eng. 2010, 30, 85–91. [Google Scholar] [CrossRef]
- Alzuwaid, F.; Ge, Y.T.; Tassou, S.A.; Raeisi, A.; Gowreesunker, L. The novel use of phase change materials in a refrigerated display cabinet: An experimental investigation. Appl. Therm. Eng. 2015, 75, 770–778. [Google Scholar] [CrossRef]
- Verpe, E.H.; Tolstorebrov, I.; Sevault, A. Cold thermal energy storage with low-temperature plate freezing of fish on offshore vessels. In Proceedings of the 25th IIR International Congress of Refrigeration, Montréal, QC, Canada, 24–30 August 2019. [Google Scholar]
- Selvnes, H.; Hafner, A.; Kauko, H. Cold Thermal Energy Storage Integration in A Large Industrial Refrigeration System. In Proceedings of the 13th IIR—Gustav Lorentzen Conference on Natural Refrigerants, Valencia, Spain, 18–20 June 2018. [Google Scholar]
- Hafner, A.; Nordtvedt, T.S.; Rumpf, I. Energy saving potential in freezing applications by applying cold thermal energy storage with solid carbon dioxide. Procedia Food Sci. 2011, 1, 448–454. [Google Scholar] [CrossRef] [Green Version]
- Xiaofeng, X.; Xuelai, Z.; Munyalo, J.M. Simulation Study on Temperature Field and Cold Plate Melting of Cold Storage Refrigerator Car. Energy Procedia 2017, 142, 3394–3400. [Google Scholar] [CrossRef]
- Liu, M.; Saman, W.; Bruno, F. Validation of a mathematical model for encapsulated phase change material flat slabs for cooling applications. Appl. Therm. Eng. 2011, 31, 2340–2347. [Google Scholar] [CrossRef]
- Friend, M.; Stone, S. Challenging requirements in resource challenged environment on a time challenged schedule: A technical solution to support the cold chain for the VSV-Zebov (Merck) Ebola vaccine in Sierra Leone Guinea. In Proceedings of the 2015 IEEE Global Humanitarian Technology Conference, Seattle, WA, USA, 8–11 October 2015. [Google Scholar]
- Cheng, W.L.; Ding, M.; Yuan, X.D.; Han, B.C. Analysis of energy saving performance for household refrigerator with thermal storage of condenser and evaporator. Energy Convers. Manag. 2017, 132, 180–188. [Google Scholar] [CrossRef]
- Oró, E.; Miró, L.; Farid, M.M.; Cabeza, L.F. Thermal analysis of a low temperature storage unit using phase change materials without refrigeration system. Int. J. Refrig. 2012, 35, 1709–1714. [Google Scholar] [CrossRef]
- Gin, B.; Farid, M.M. The use of PCM panels to improve storage condition of frozen food. J. Food Eng. 2010, 100, 372–376. [Google Scholar] [CrossRef]
- Zarajabad, O.G.; Ahmadi, R.; Ghaffari, S. Numerical Investigation of Cold Thermal Energy Storage Using Phase Change Material in Freezer. Eur. J. Eng. Res. Sci. 2017, 2, 1. [Google Scholar] [CrossRef] [Green Version]
- Williams, B.G.; Batty, J.C. ICE (Integrated Cooler Experiment) for COOLSAT; Springer: Boston, MA, USA, 1995. [Google Scholar]
- Wang, Z.; Zhang, Z.; Jia, L.; Yang, L. Paraffin and paraffin/aluminum foam composite phase change material heat storage experimental study based on thermal management of Li-ion battery. Appl. Therm. Eng. 2015, 78, 428–436. [Google Scholar] [CrossRef]
- Heyhata, M.M.; Mousavi, S.; Siavashi, M. Battery thermal management with thermal energy storage composites of PCM, metal foam, fin and nanoparticle. J. Energy Storage 2020, 28, 101235. [Google Scholar] [CrossRef]
- Babapoor, A.; Azizi, M.; Karimi, G. Thermal management of a Li-ion battery using carbon fiber-PCM composites. Appl. Therm. Eng. 2015, 82, 281–290. [Google Scholar] [CrossRef]
- Arshad, A.; Ali, H.M.; Khushnood, S.; Jabbal, M. Experimental investigation of PCM based round pin-fin heat sinks for thermal management of electronics: Effect of pin-fin diameter. Int. J. Heat Mass Transf. 2018, 117, 861–872. [Google Scholar] [CrossRef] [Green Version]
- Alshaer, W.G.; Nada, S.A.; Rady, M.A.; Barrio, E.P.D.; Sommier, A. Thermal management of electronic devices using carbon foam and PCM/nano-composite. Int. J. Therm. Sci. 2015, 89, 79–86. [Google Scholar] [CrossRef]
- Alshaer, W.G.; Nada, S.A.; Rady, M.A.; Bot, C.L.; Barrio, E.P.D. Numerical investigations of using carbon foam/PCM/Nano carbon tubes composites in thermal management of electronic equipment. Energy Convers. Manag. 2015, 89, 873–884. [Google Scholar] [CrossRef]
- Jiang, G.; Huang, J.; Fu, Y.; Cao, M.; Liu, M. Thermal optimization of composite phase change material/expanded graphite for Li-ion battery thermal management. Appl. Therm. Eng. 2016, 108, 1119–1125. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, Q.; Medina, M.A.; Liu, Y.; Liao, S. A study on the use of phase change materials (PCMs) in combination with a natural cold source for space cooling in telecommunications base stations (TBSs) in China. Appl. Energy 2014, 117, 95–103. [Google Scholar] [CrossRef]
- Yantong, L.; Quan, Z.; Xiaoqin, S.; Yaxing, D.; Shuguang, L. Optimization on Performance of the Latent Heat Storage Unit (LHSU) in Telecommunications Base Stations (TBSs) in China. Energy Procedia 2015, 75, 2119–2124. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.B.; Xiong, W.; Liu, Y.W.; Jiang, C.Y.; Zhu, C.Y. Energy saving research of heat pipe-cool storage air conditioning system used in internet data center. Refrig. Air-Cond. 2015, 15, 71–74. [Google Scholar]
- Chen, X.; Zhang, Q.; Zhai, Z.J.; Wu, D.; Liao, S. Experimental study on operation characteristics of a novel refrigeration system using phase change material. Energy Build. 2017, 150, 516–526. [Google Scholar] [CrossRef]
- Sundaram, A.S.; Seeniraj, R.V.; Velraj, R. An experimental investigation on passive cooling system comprising phase change material and two-phase closed thermosyphon for telecom shelters in tropical and desert regions. Energy Build. 2010, 42, 1726–1735. [Google Scholar] [CrossRef]
- Otsuka, T. Evolution of an LNG terminal: Senboku terminal of Osaka GAS. In Proceedings of the 23rd World Gas Conference, Amsterdam, The Netherlands, 5–9 June 2006; pp. 1362–1372. [Google Scholar]
- Zhao, L.; Dong, H.; Tang, J.; Cai, J. Cold energy utilization of liquefied natural gas for capturing carbon dioxide in the flue gas from the magnesite processing industry. Energy 2016, 105, 45–56. [Google Scholar] [CrossRef]
- Al-Musleh, E.I.; Mallapragada, D.S.; Agrawal, R. Efficient electrochemical refrigeration power plant using natural gas with 100% CO2 capture. J. Power Sources 2015, 274, 130–141. [Google Scholar] [CrossRef] [Green Version]
- Na, Z.; Lior, N.; Meng, L.; Wei, H. COOLCEP (cool clean efficient power): A novel CO2-capturing oxy-fuel power system with LNG (liquefied natural gas) coldness energy utilization. Energy 2010, 35, 1200–1210. [Google Scholar]
- Kim, Y.M.; Gil Shin, D.; Kim, C.G. On-Board Cold Thermal Energy Storage System for Hydrogen Fueling Process. Energies 2019, 12, 561. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Li, Z.; Luo, L.; Fan, Y.; Du, Z. A review on thermal management of lithium-ion batteries for electric vehicles. Energy 2021, 238, 121652. [Google Scholar] [CrossRef]
| Application Area | Material | PCT (℃) | Latent Heat (J/g) | Density (kg/m3) | Thermal Conductivity (W/(m·K)) | Specific Heat (kJ/(kg·K)) | Reference | |
|---|---|---|---|---|---|---|---|---|
| Space mission | Methanol | −97.15 | 99.25 | 904–810 | 0.210–0.206 | 2.20–2.40 | [26] | |
| n-Hexane | −95.15 | 151.78 | 760–677 | 0.156–0.135 | 1.88–2.15 | [26] | ||
| Cyclopentane | −93.95 | 8.56 | 751 | 0.143 | 1.42–1.69 | [26] | ||
| Methylamine | −93.15 | 197.38 | 700 | 0.219 | 3.19–3.26 | [26] | ||
| n-Heptane | −90.55 | 140.12 | 774–700 | 0.156–0.138 | 1.96–2.15 | [26] | ||
| Ethane | −88.15 | 489.47 | 641–544 | 0.25–0.17 | 2.33–2.43 | [26] | ||
| Methylethylketone | −86.65 | 177.05 | 826 | 0.17–0.15 | 2.07–2.16 | [26] | ||
| Acetylene | −84.15 | 144.39 | 764.3–760.2 | 0.020 | 1.37 | [26] | ||
| Low temperature cold storage/Low temperature logistics | n-Octane | −56.85 | 181.57 | 761–718 | 0.15–0.13 | 2.02–2.14 | [26] | |
| 2-Hexanone | −55.45 | 148.7 | 830 | 0.16–0.15 | 2.02–2.08 | [26] | ||
| 3-Hexanone | −55.65 | 134.5 | 833 | 0.17–0.16 | 2.05–2.12 | [26] | ||
| n-Nonane | −53.65 | 117 | 773–734 | 0.15–0.13 | 1.99–2.12 | [26] | ||
| 3-Heptanone | −37.1 | 153.5 | 822 | 0.15–0.14 | - | [26] | ||
| 2-Heptanone | −35.0 | 172.6 | 851–834 | 0.15–0.14 | - | [26] | ||
| decane | −29.7 | 194.2 | 730 | 0.13110 | 2.22 | [33] | ||
| n-Dodecane | −12 | 216 | 745.64 | 0.134 | 2.039 | [31] | ||
| Diethylene glycol | −10~−7 | 247 | 1118 | - | - | [31] | ||
| n-Tridecane | −6 | - | 750.57 | 0.131 | 1.979 | [31] | ||
| Air conditioning | Food preservation | Tetrahydrofuran | 5 | 280 | - | - | - | [31] |
| n-Tetradecane | 5.5 | 226 | 765.78 | 0.129 | 2.031 | [34] | ||
| Formic Acid | 7.8 | 247 | 1205.24 | 0.193 | 2.036 | [34] | ||
| Polyethylene glycol 400 | 8 | 99.6 | 1127 | - | - | [34] | ||
| Dimethyl adipate | 9.7 | 164.6 | 1223 | - | - | [31] | ||
| n-Pentadecane | 10.0 | 205 | 774.65 | 0.27 | 1.8 | [35] | ||
| Building | Isopropyl palmitate | 11 | 95–100 | 852 | - | 1.413 | [36] | |
| Pelargonic | 12.3 | 127 | 869.92 | 0.129 | 1.866 | [34] | ||
| Oleic Acid | 13.5–16.3 | - | 890 | - | - | [31] | ||
| Isopropyl stearate | 14–18 | 140–142 | 861 | - | - | [36] | ||
| Caprylic acid | 16 | 150 | 1027.17 | 0.138 | 2.117 | [36] | ||
| Dimethyl sulfoxide | 16.5 | 85.7 | 1097.19 | 0.161 | 1.700 | [31] | ||
| Acetic Acid | 16.7 | 273 | 1050 | - | - | [34] | ||
| n-Hexadecane | 18.2 | 238 | 774 | 0.21 | 2.001 | [34] | ||
| Propyl palmitate | 16–19 | 186 | 864 | - | - | [36] | ||
| Polyethylene glycol 600 | 17–22 | 146 | 1130 | - | - | [31] | ||
| Glycerin | 17.9 | 198.7 | 1291.14 | 0.222 | 1.506 | [31] | ||
| Butyl stearate | 19 | 140–200 | 1097.39 | 0.156 | 1.492 | [36] | ||
| Lithium Chloride Ethanolate | 21 | 188 | - | - | - | [34] | ||
| Dimethyl sebacate | 21 | 120–135 | 986 | - | - | [36] | ||
| Octadecyl 3-mencaptopropylate | 21 | 143 | - | - | - | [36] | ||
| n-Heptadecane | 22 | 215 | 778 | 0.21 | 1.992 | [34] | ||
| Ethyl palmitate | 23 | 122 | 870 | - | - | [36] | ||
| Myristic Acid + Capric | 24 | 147.7 | 888 | 0.164 | - | [34] | ||
| Polyethylene Glycol 600 | 20–25 | 146 | 1100 | - | - | [34] | ||
| Thermal management of electronic equipment | Lauryl alcohol | 25.83 | 215.83 | 890.92 | 0.144 | 2.322 | [37] | |
| D-Lattic Acid | 26 | 184 | 1249 | - | - | [34] | ||
| Octadecyl thioglyate | 26 | 90 | - | - | - | [36] | ||
| Vinyl sterate | 27–29 | 122 | 869 | - | - | [36] | ||
| n-Octadecane | 28.2 | 245 | 775 | 0.149 | 1.964 | [34] | ||
| 1-3 Methyl Pentacosane | 29 | 197 | - | - | - | [34] | ||
| Methyl sterate | 29 | 169 | 842.96 | 0.102 | 1.529 | [36] | ||
| Methyl Palmitate | 29 | 205 | 860.10 | 0.140 | 2.139 | [34] | ||
| Application | Material | PCT (℃) | Latent Heat (J/g) | Density (kg/m3) | Thermal Conductivity (W/(m·K)) | Specific Heat (kJ/(kg·K)) | Reference | |
|---|---|---|---|---|---|---|---|---|
| LNG cold energy recovery | Carbon Dioxide | −78.46 | 574 | 1562 | 0.011–0.015 | 0.78–0.83 | [26] | |
| Low temperature cold storage/low temperature logistics | Ammonia | −78.2 | 332.2 | 728–682 | 0.80–0.67 | 4.23–4.45 | [26] | |
| Hg | −38.87 | 11.4 | 13,590 | - | - | [31] | ||
| Household refrigerator/medical cold chain/high temperature cold storage/food preservation | H2O | 0.0 | 333 | 998.75 | 0.598 | 4.137 | [34] | |
| POCl3 | 1.0 | 85 | 1666.85 | 0.105 | 0.772 | [34] | ||
| D2O | 3.7 | 318 | 1104.42 | 0.595 | 4.19 | [34] | ||
| SbCl5 | 4.0 | 33 | 2360 | - | - | [34] | ||
| Air conditioning | LiClO4.3H2O | 8 | 253 | - | - | - | [34] | |
| ZnCl2.3H20 | 10 | - | - | - | - | [36] | ||
| H2SO4 | 10.4 | 100 | 1844.91 | 0.159 | 1.348 | [34] | ||
| Building | NH4Cl.Na2SO4.10H2O | 11 | 163 | - | - | - | [34] | |
| K2HPO4.6H2O | 13 | - | - | - | - | [36] | ||
| lCl(β) | 13.9 | 56 | - | - | - | [34] | ||
| K2HO4.6H2O | 14 | 108 | - | - | - | [34] | ||
| NaOH | 16 | 200 | 1487.59 | 0.365 | 2.425 | [36] | ||
| MOF6 | 17.0 | 50 | 2551 | - | - | [34] | ||
| SO3(α) | 17.0 | 108 | 1853.026 | 0.131 | 1.398 | [34] | ||
| lCl(α) | 17.2 | 69 | - | - | - | [34] | ||
| NaCl.Na2SO4.10H2O | 18 | 286 | - | - | - | [34] | ||
| Na2CrO4.10H2O | 18 | - | - | - | - | [36] | ||
| KF.4H2O | 18 | 330 | - | - | - | [34] | ||
| K2HPO4.4H2O | 18.5 | 231 | 1447 | - | - | [34] | ||
| Na2SO4.10H2O | 21 | 198 | - | - | - | [36] | ||
| FeBr3.6H2O | 21 | 105 | - | - | - | [36] | ||
| P4O6 | 23.7 | 64 | - | - | - | [34] | ||
| Thermal management of electronic equipment | Mn(NO3)2.6H2O | 25 | 148 | 1738 | - | - | [34] | |
| LiBO2.8H2O | 25.7 | 289 | - | - | - | [34] | ||
| H3PO4 | 26.0 | 147 | - | - | - | [34] | ||
| FeBr3.6H2O | 27 | 105 | - | - | - | [34] | ||
| Cs | 28.3 | 15 | 1870 | - | - | [34] | ||
| CaCl2.6H2O | 29–30 | 170–192 | 1562 | 0.561 | - | [34] | ||
| CaCl2.12H2O | 29.8 | 174 | - | - | - | [36] | ||
| Ga | 30.0 | 80 | 1550 | - | - | [34] | ||
| AsBr3 | 30.0 | 38 | - | - | - | [34] | ||
| LiNO3.3H2O | 30 | 189/296 | - | - | - | [34] | ||
| LiNO3.2H2O | 30 | 296 | - | - | - | [36] | ||
| Materials | PCT (℃) | Latent Heat (J/g) | Density (kg/m3) | Thermal Conductivity (W/(m·K)) | Specific Heat (kJ/(kg·K)) | Reference |
|---|---|---|---|---|---|---|
| Polyethylene glycol 200/Polyethylene glycol 300 (4:96 wt%) | −20 | - | - | - | - | [26] |
| Dodecane + Tridecane (82.3:17.7 by mole) | Tm −16~−12/Tf −17~−15 | Hm 185/Hf 165 | - | - | - | [45] |
| Polyethylene glycol 200/Polyethylene glycol 300 (20:80 wt%) | −15 | - | - | - | - | [26] |
| Polyethylene glycol 200/Polyethylene glycol 300 (30:70 wt%) | −10 | - | - | - | - | [26] |
| Dodecane + Tridecane (60:40 by volume) | −9.7 | 159 | - | - | - | [31] |
| Dodecane + Tridecane (50:50 by volume) | −9.1 | 145 | - | - | - | [31] |
| Dodecane + Tridecane (40:60 by volume) | −8 | 147 | - | - | - | [31] |
| Dodecane + Tridecane (20:80 by volume) | −5.4 | 126 | - | - | - | [31] |
| Tridecane + Tetradecane (80:20 by volume) | −1.5 | 110 | - | - | - | [31] |
| Tridecane + Tetradecane (60:40 by volume) | −0.5 | 138 | - | - | - | [31] |
| Tridecane + Tetradecane (40:60 by volume) | 0.7 | 148 | - | - | - | [31] |
| N-decanoic acid + N-decanol (36:64 by mole) | 1.2 | 177.74 | - | - | - | [46] |
| Hexadecane + Tetradecane | 1.7–17.9 | 146–227 | - | - | - | [47] |
| Dodecanol + Octanoic acids (40:60 by mass) | 2.08 | 224.5 | - | 0.3 | - | [48] |
| Tridecane + Tetradecane (20:80 by volume) | 2.6 | 212 | - | - | - | [31] |
| N-decanoic acid + Methyl laurate (30:70 by mole) | 3.2 | 188.10 | - | - | - | [46] |
| Caprylic acid + Lauric acid (9:1 by mole) | 3.77 | 151.5 | - | - | - | [49] |
| Lauric acid + Tetradecane (21:79 by mole) | 4.2 | 206.17 | - | - | - | [46] |
| Tetra decane + Lauryl alcohol (66:34) | 4.3 | 241.7 | 0.2737 | [50] | ||
| Tetra decane + Lauryl alcohol (66:34) + EG | 4.3 | 245.1 | - | 0.9657 | [50] | |
| Tetradecane + Hexadecane (50:50 by volume) | 4.9 | 154.839 | - | - | - | [51] |
| Paraffin C14 + C15 + C16 + C17 + C18 (33.4:47.3:16.3:2.6:0.4) | 5.2 | 158.3 | - | - | - | [51] |
| Dodecanol + Octanoic acid (2:3 by mass) | 6.2 | 173.2 | - | - | -- | [52] |
| Caprylic acid + 1-dodecanol (70:30) | 6.52 | 171.06 | - | - | - | [53] |
| Caprylic acid + Palmitic acid (90:10) | Tm 6.54/Tf 4.31 | ΔHm 116.477/ΔHf 116.235 | - | - | - | [54] |
| Caprylic alcohol + Mynstyl alcohol (73.7:26.3 by mass) | 6.9 | 169.1 | - | 0.1739 | - | [55] |
| Lauryl alcohol + Octanoic acids (40.6:59.4) | 7 | 178.6 | - | - | [56] | |
| Oleic + Capric acid (37:63 by mole) | 10 | 60 | Liquid: 0.194/solid: 0.201 | - | [57] | |
| Capric acid and lauric acid (65:35 by mole) + Pentadecane (50:50 by volume) | 10.2 | 157.8 | Liquid: 827.8/solid: 850.4 | - | Liquid: 2.89/solid: 2.44 | [58] |
| Capric acid and lauric acid (65:35 by mole) + Pentadecane (70:30 by volume) | 11.3 | 149.2 | Liquid: 858/solid: 872.7 | - | Liquid: 2.57/solid: 2.27 | [58] |
| C5H5C6H5 + (C6H5)2O (26:73.5) | 12 | 97.9 | [34] | |||
| Capric acid and lauric acid (65:35 by mole) + 0.10 mol Cineole | 12.3 | 111.6 | 927 | - | Liquid: 2.37/solid: 1.71 | [59] |
| Capric acid and lauric acid (65:35 by mole) + 0.10 mol Methyl Salicylate | 12.5 | 126.7 | Liquid: 1182/solid: 1272.9 | - | Liquid: 2.41/solid: 1.92 | [59] |
| Capric acid and lauric acid (65:35 by mole) + Pentadecane (90:10 by volume) | 13.3 | 142.2 | Liquid: 883.2/solid: 891.3 | - | Liquid: 2.42/solid: 872.72.08 | [58] |
| Capric acid and lauric acid (65:35 by mole) + 0.10 mol Eugenol | 13.9 | 117.8 | 1091 | - | Liquid: 2.63/solid: 2.01 | [59] |
| Capric acid + Lauric acid-oleic acid | Tm 14.5/Tf 10.5 | 109.2 | Liquid: 839.7/solid: 845.1 | Liquid: 0.141/solid: 0.145 | Liquid: 2.214/solid: 1.825 | [60] |
| Lauric + 1-dodecanol (29:71) | 17 | 175.3 | - | 0.180 | - | [61] |
| Capric acid + Lauric aci (65:35 by mole) | 18 | 140.8 | Liquid: 894.9/solid: 900.0 | Liquid: 0.139/solid: 0.143 | Liquid: 2.24/solid: 1.97 | [59] |
| Myristic + 1-dodecanol (17:83) | 18.43 | 180.8 | - | 0.180 | - | [61] |
| Lauryl alcohol + Cetyl alcohol (80:20) | 20.01 | 191.63 | - | 0.1–0.2 | - | [62] |
| Palmitic + 1-dodecanol (10:90) | 20.08 | 191 | - | 0.180 | - | [61] |
| Capric acid + Myristic acid (85:15 by mass) | 20.86 | 156.99 | - | 0.152 | - | [63] |
| Dodecanol + Stearic acid (82:18 by mass) | 21.3 | 205.9 | - | - | - | [64] |
| Lauric acid + Myristyl alcohol (40:60) | Tm 21.3/Tf 19.9 | ΔHm 151.1/ΔHf 151.6 | - | - | - | [65] |
| Capric acid + Palmitic acid (76.5:23.5) | Tm 21.85/Tf 22.15 | ΔHm 171.22/ΔHf 173.16 | - | - | - | [66] |
| Capric acid + Cetyl alcohol (70:30) | Tm 22.89/Tf 11.97 | ΔHm 144.92/ΔHf 145.85 | - | - | - | [67] |
| Lauryl alcohol + Stearyl alcohol (90:10) | 22.93 | 205.79 | [37] | |||
| Dodecyl Acetate + Amyl valerate (34:66) | 24 | 147.7 | [34] | |||
| Eicosane + Capric acid (25:75) | 24.96 | 200.3 | - | - | - | [68] |
| Lauric acid + 1-tetradecanol (40:60) | 24.33 | 161.45 | - | - | 40 ℃: 2.3635/10 ℃: 2.1635 | [69] |
| Methyl palmitate + Lauric acid (60:40) | Tm 25.6/Tf 20.2 | ΔHm 205.4/ΔHf 205.8 | Liquid: 840.6/solid: 887.7 | 0.1802 | Liquid: 1.952/solid: 1.513 | [69] |
| Stearic acid + N-octadecane (4:96) | 27.4 | 227 | - | - | - | [70] |
| Materials | PCT (℃) | Latent Heat (J/g) | Reference |
|---|---|---|---|
| 24.8 wt% HCl | −86 | 73.77 | [24] |
| 24 wt% LiCl | −67 | 36.26 | [24] |
| ZnCl2 aqueous solution (51%) | −62 | 116.84 | [71] |
| FeCl3 aqueous solution (33.1%) | −55 | 155.52 | [71] |
| CaCl2 aqueous solution (29.8%) | −55 | 164.93 | [71] |
| 30.5 wt% CaCl2 | −49.5 | 76.81 | [24] |
| CuCl2 aqueous solution (29.8%) | −40 | 166.17 | [71] |
| K2CO3 aqueous solution (39.6%) | −36.5 | 165.36 | [71] |
| 21.01 wt% MgCl2 | −33.5 | 36.30 | [24] |
| MgCl2 aqueous solution (17.1%) | −33.6 | 221.86 | [71] |
| Al(NO3)3 aqueous solution (30.5%) | −30.6 | 207.63 | [71] |
| Mg(NO3)2 aqueous solution (34.6%) | −29 | 186.93 | [71] |
| Zn(NO3)2 aqueous solution (39.4%) | −29 | 169.88 | [71] |
| NH4F aqueous solution (32.3%) | −28.1 | 187.83 | [71] |
| NaBr aqueous solution (40.3%) | −28 | 175.69 | [71] |
| 27.9 wt% NaCl | −23 | 26.10 | [15] |
| KF aqueous solution (21.5%) | −21.6 | 227.13 | [71] |
| NaCl aqueous solution (22.4%) | −21.2 | 228.14 | [71] |
| Aqueous sodium chloride (23.3 wt%) at eutectic composition | −21.1 | 246.6 | [33] |
| MgCl2 aqueous solution (25%) | −19.4 | 223.10 | [71] |
| (NH4)2SO4 aqueous solution (39.7%) | −18.5 | 187.75 | [71] |
| NaNO3 aqueous solution (36.9%) | −17.7 | 187.79 | [71] |
| NH4NO3 aqueous solution (41.2%) | −17.35 | 186.29 | [71] |
| Ca(NO3)2 aqueous solution (35%) | −16 | 199.35 | [71] |
| NH4Cl aqueous solution (19.5%) | −16 | 248.44 | [71] |
| K2HPO4 aqueous solution (36.8%) | −13.5 | 197.79 | [71] |
| Na2S2O3 aqueous solution (30%) | −11 | 219.86 | [71] |
| KCl aqueous solution (19.5%) | −10.7 | 253.18 | [71] |
| MnSO3 aqueous solution (32.2%) | −10.5 | 213.07 | [71] |
| NaH2PO4 aqueous solution (23.4%) | −9.9 | 214.25 | [71] |
| BaCl2 aqueous solution (22.5%) | −7.8 | 246.44 | [71] |
| 22.1 wt% BaCl2 | −7.7 | 10.2 | [24] |
| ZnSO3 aqueous solution (27.2%) | −6.5 | 235.75 | [71] |
| Sr(NO3)2 aqueous solution (24.5%) | −5.75 | 243.15 | [71] |
| KHCO3 aqueous solution (16.95%) | −5.4 | 268.54 | [71] |
| 18.63 wt% MgSO4 | −4.8 | 84.96 | [24] |
| NiSO4 aqueous solution (20.6%) | −4.15 | 258.61 | [71] |
| MgSO4 aqueous solution (19%) | −3.9 | 264.42 | [71] |
| Na2SO4 aqueous solution (12.7%) | −3.55 | 284.95 | [71] |
| NaF aqueous solution (3.9%) | −3.5 | 314.09 | [71] |
| KNO3 aqueous solution (9.7%) | −2.8 | 296.02 | [71] |
| Na2CO3 aqueous solution (5.9%) | −2.1 | 310.23 | [71] |
| FeSO4 aqueous solution (13%) | −1.8 | 286.81 | [71] |
| CuSO4 aqueous solution (11.9%) | −1.6 | 290.91 | [71] |
| 4.03 wt% Na2SO4 | −1.2 | 1.07 | [15] |
| 31% Na2SO4 + 13% NaCl + 16% KCl + 40% H2O | 4 | 234 | [34] |
| Tetrabutyl ammonium bromide aqueous solutio (15%) | 6.6 | - | [72] |
| Tetrabutyl ammonium bromide aqueous soluti (40%) | 9 | ΔHm 187.867/ΔHf 137.799 | [73] |
| Na2SO4·10H2O + NH4Cl + KCl + K2SO4 + Carboxymethyl cellulose + (NaPO3)6 + borax + boric acid (76 + 10.3 + 3.6 + 2 + 3.2 + 0.1 + 2.4 + 2.4) | Tm 9.3/Tf 8.25 | 114.37 | [74] |
| 32% Na2SO4 + 14% NaCl + 12% NH4Cl + 42% H2O | 11 | - | [34] |
| Tetrabutyl ammonium bromide aqueous solution (45%) | 12.5 | - | [72] |
| 55% CaCl2·6H2O + 55% CaBr2·6H2O | 14.7 | 140 | [28] |
| NaOH·(3/2)H2O | 15 | - | [28] |
| Mn(NO3)·6H2O + MgCl2·6H2O | 15–25 | 125.9 | [28] |
| 45–52% LiNO3·3H2O + 48–55% Zn(NO3)2·6H2O | 17.2 | 220 | [28] |
| 37% Na2SO4 + 17% NaCl + 46% H2O | 18 | - | [34] |
| 25% Na2S4 + 21% MgSO4 + 54% H2O | 21–24 | - | [34] |
| LiNO3·3H2O + Ni(NO3)2 (55–65:35–45) | 24.2 | 230 | [28] |
| Ca(NO3)2·6H2O + Zn(NO3)2·6H2O (45:55) | 25 | 130 | [28] |
| CaCl2·6H2O + MgCl2·6H2O (66.6:33.3) | 25 | 127 | [28] |
| 50% CaCl2 + 50% MgCl2 + 6H2O | 25 | 95 | [28] |
| CaCl2 + NaCl + KCl + H2O (48:4.3:0.4:47.3) | 27 | 188 | [28] |
| Ca(NO)3.4H2O + Mg(NO)3.6H2O (47:53) | 30 | 136 | [34] |
| Composition | Type | PCT (℃) | Latent Heat (J/g) | Source | Reference |
|---|---|---|---|---|---|
| E-90 | Eutectic solutions | −90 | 90 | PCM Products Ltd. | [75] |
| E-78 | Eutectic solutions | −78 | 115 | PCM Products Ltd. | [75] |
| E-75 | Eutectic solutions | −75 | 102 | PCM Products Ltd. | [75] |
| E-65 | Eutectic solutions | −65 | 240 | PCM Products Ltd. | [75] |
| E-62 | Eutectic solutions | −62 | 180 | PCM Products Ltd. | [75] |
| E-60 | Eutectic solutions | −60 | 172 | PCM Products Ltd. | [75] |
| SN 50 | Inorganic salt solution | −50 | 325 | TEAP | [76] |
| SN 33 | Inorganic salt solution | −33 | 245 | Cristopia | [77] |
| TH 31 | Salt hydrate | −31 | 131 | TEAP | [76] |
| MPCM (−30) | Paraffin | −30 | 140–150 | Microtek Laboratories, Inc. | [78] |
| SN 29 | Inorganic salt solution | −29 | 233 | Cristopia | [77] |
| SN 26 | Inorganic salt solution | −26 | 168 | Cristopia | [77] |
| TH 23 | Salt hydrate | −23 | 230 | TEAP | [76] |
| TH 21 | Salt hydrate | −21 | 222 | TEAP | [76] |
| SN 21 | Inorganic salt solution | −21 | 240 | Cristopia | [77] |
| STL 21 | Inorganic salt solution | −21 | 240 | Mitsubishi Chemical | [79] |
| ClimSel C-18 | Inorganic salt solution | −18 | 306 | Climator | [80] |
| SN 18 | Inorganic salt solution | −18 | 268 | Cristopia | [77] |
| STL 16 | Inorganic salt solution | −16 | 289 | Mitsubishi Chemical | [79] |
| TH 16 | Salt hydrate | −16 | 289 | TEAP | [76] |
| AN 15 | Inorganic salt solution | −15 | 311 | Cristopia | [77] |
| AN 12 | Inorganic salt solution | −12 | 306 | Cristopia | [77] |
| STLN 10 | Inorganic salt solution | −11 | 271 | Mitsubishi Chemical | [79] |
| AN 10 | Inorganic salt solution | −10 | 310 | Cristopia | [77] |
| TH 10 | Inorganic salt solution | −10 | 283 | TEAP | [76] |
| MPCM (−10) | Paraffin | −9.5 | 150–170 | Microtek Laboratories, Inc. | [78] |
| STL-6 | Inorganic salt solution | −6 | 284 | Mitsubishi Chemical | [79] |
| TH-4 | Inorganic salt solution | −4 | 386 | TEAP | [76] |
| RT-4 | Paraffin | −4 | 179 | Rubitherm GmbH | [81] |
| SLT 3 | Inorganic salt solution | −3 | 328 | Mitsubishi Chemical | [79] |
| AN 3 | Inorganic salt solution | −3 | 328 | Cristopia | [77] |
| RT3 | Paraffin | 3 | 198 | Rubitherm GmbH | [81] |
| RT4 | Paraffin | 4 | 182 | Rubitherm GmbH | [81] |
| RT5 | Paraffin | 5.2 | 158.3 | Rubitherm GmbH | [81] |
| RT6 | Paraffin | 6 | 175 | Rubitherm GmbH | [81] |
| MPCM (6) | Paraffin | 6 | 157–167 | Microtek Laboratories, Inc. | [78] |
| ClimSel C7 | Organic | 7 | 130 | Climator AB | [80] |
| PureTemp 8 | Organic | 8 | 180 | PureTemp | [82] |
| PCM-OM08P | Organic | 8 | 190 | SAVENR | - |
| A8 | Organic | 8 | 150 | EPS Ltd. | [83] |
| RT 8 | Organic | 8 | 180 | Rubitherm | [81] |
| RT 9 | Organic | 9 | 160 | Rubitherm | [81] |
| A9 | Organic | 9 | 140 | EPS Ltd. | [83] |
| RT10 | Organic | 10 | 150 | Rubitherm | [81] |
| RT 10 HC | Organic | 10 | 195 | Rubitherm | [81] |
| S1 0 | Organic | 10 | 155 | Cristopia | [77] |
| PCM-OM11P | Organic | 11 | 260 | SAVENR | - |
| PureTemp 12 | Organic | 12 | 185 | PureTemp | [82] |
| RT12 | Organic | 12 | 150 | Rubitherm | [81] |
| ClimSel C15 | Salt solution | 15 | 130 | Climator AB | [80] |
| E17 | Inorganic salt solution | 17 | 143 | - | [31] |
| E19 | Inorganic salt solution | 19 | 146 | - | [31] |
| RT20 | Paraffin | 20 | 140 | Rubitherm | [31] |
| Emerest 2325 | Fatty acid | 20 | 134 | - | [31] |
| Emerest 2326 | Fatty acid | 20 | 139 | - | [31] |
| FMC | Paraffin | 20–23 | 130 | - | [31] |
| PCM | Nanomaterials | PCT of Composites (℃) | Latent Heat of Composites (J/g) | Thermal Conductivity of Composites (W/(m·K)) | Improvement Rate of Thermal Conductivity | Application | Year | Reference |
|---|---|---|---|---|---|---|---|---|
| Sorbitol aqueous solution (5 wt%) | 0.40 wt% TiO2 | −2.9 (no change) | 293.8 (decreased by 2.9%) | 0.62 | 29.1% | Cold chain transportation | 2019 | [85] |
| N-octanoic acid + Lauric acid (81:19 by mass) | 0.1 g/L Hydroxylated multi walled carbon nanotubes | 4.5 (no change) | 142.4 (increased by 2.9%) | 0.36 | 21.9% | Cold chain transportation | 2019 | [86] |
| BaCl2 aqueous solution (22.5 wt%) | TiO2 with volume fraction of 1.13% | −8.5 (no change) | 254.2 (decreased by 9.57%) | 0.67 | 16.74% | Refrigeration station of beer industry | 2015, 2005 | [87,88] |
| Organic PCM for air conditioning | 0.4 g/L Multi walled carbon nanotubes | - | - | 0.2436 | 21.9% | Air conditioning | 2012 | [90] |
| Organic PCM for air conditioning | TiO2 nanoparticles | - | - | 0.519 | 22% | Air conditioning | 2008, 2004 | [91,92] |
| Paraffin RT4 | 5% Carbon nanotubes | No change | 128.2 (decreased by 15.5%) | 0.486 | 38% | - | 2017 | [93] |
| Deionized water | 1.2 wt% Graphene nanoplatelets | −2.5 | - | 3.198 (solid state)/0.6702 (liquid state) | 56% (solid state)/11.7% (liquid state) | Building cooling | 2016 | [89] |
| Commercialized paraffin wax | 40 wt% Hexagonal boron nitride nanosheets | - | 80.17 (decreased by 12%) | 3.47 | 12 times | Thermal management of electronic equipment | 2016 | [94] |
| Oleic and capric acid eutectic | 0.1 wt% Porous activated carbon nanosheets | - | 52.7 (decreased by 12%) | 0.3007 | 55% | Banana ripening cold storage | 2017 | [57] |
| Ethylene glycol aqueous solution | 0.5% Multi walled carbon nanotubes | No change | No change | - | 1.5% (liquid state)/4.5% (solid state) | - | 2019 | [95] |
| Caprylic alcohol + Mynstyl alcohol (73.7:26.3 by mass) | 0.3 wt% Multi walled carbon nanotubes | 6.8 (decreased by 0.1) | 168.2 (decreased by 0.5%) | 0.2196 | 26.3% | Air conditioning | 2015 | [55] |
| Caprylic alcohol + Mynstyl alcohol (73.7:26.3 by mass) | 0.4 wt% Al3O2 | 6.6 (decreased by 0.3) | 167.9 (decreased by 0.7%) | 0.1967 | 13.1% | Air conditioning | 2015 | [55] |
| Caprylic alcohol + Mynstyl alcohol (73.7:26.3 by mass) | 0.8 wt% Fe2O3 | 6.5 (decreased by 0.4) | 166.7 (decreased by 1.4%) | 0.2297 | 32.1% | Air conditioning | 2015 | [55] |
| Shell Material | Core Material | Method | Thermophysical Properties of Microcapsules | Key Findings | Applications | Year | Reference |
|---|---|---|---|---|---|---|---|
| Tetradecane | Gelatin gum Arabic | Complex coacervation | Tm 5.792 °C/Tf 2.375 °C; ΔHm 191.919 J/g/ΔHf 189.173 J/g | The ΔHm of the material is consistent with the ΔHf, and the PCT also meets the cold storage requirements of the air conditioner. | Air conditioner | 2006 | [101] |
| Polymer gelatin | Tetradecane, pentadecane, cetane | Simple coacervation | 4–12 °C | It is not prone to leakage before and after phase transformation, protecting the refrigerator from clogging as well as prolonging the life of phase change cold storage agent. | Air conditioner | 2004 | [102] |
| Urea–Formaldehyde polymer | N-tetradecane (7.3 °C; 234 J/g) | In situ polymerization | 7.1 °C; 147 J/g | The heat resistance of the coated microcapsules was significantly improved, and the weight loss rate decreased from 99.85% to 84.5%. | Thermal control system of spacecraft | 2015 | [103] |
| Melamine resin | N-octadecane (Tm 28.5 °C/Tc 25.0 °C; ΔHm 222 kJ/kg/ΔHc 222 kJ/kg) (64 wt%) | In situ polymerization | Tm 30 °C; ΔHm 141 kJ/kg/ΔHc 142 kJ/kg | The thermal stability of encapsulated n-octadecane was higher than that of pure octadecane, and the thermal decomposition temperature reached 160 °C. | Polyurethane foam board insulation | 2010 | [106] |
| Styrene (St) + Divinybenzen (DVB) copolymer (10:1) | Monomer + n-octadecane (1:1 by mass) (56.8 wt%) | Suspension polymerization | Tm 29 °C/Tc 16 °C; ΔHm 125 kJ/kg/ΔHc 127 kJ/kg | N-octadecane could be well coated in St-DVB copolymer to form a core/wall structure. The heat resistance of microcapsule material is about 230 °C. | Polyurethane foam board insulation | 2010 | [106] |
| Melamine-Formaldehyde resin | N-tetradecane (64.9 wt%) | In situ polymerization | Tm 5.6 °C/Tc 5.2 °C; ΔHm 219.81 kJ/kg/ΔHc 220.58 kJ/kg | At the limit temperature of 45, −25 and 23 °C, the blood can be maintained at 0–10 °C for 50.5, 80.7, and 61.7 h, respectively. | Blood insulation | 2007 | [103] |
| Polymethylmethacrylate (PMMA) | N-tetradecane | In situ polymerization | 6.22 °C; 150.1 J/g | After 100 cold and heat cycles, the latent heat of microcapsules only decreased by 1.1 J/g, which has excellent thermal stability. | Temperature control packaging | 2015 | [107] |
| Polymethylmethacrylate (PMMA) | N-dodecanol + Decanol (50:50) (66.6%) | In situ polymerization | 7.85 °C; 102.8 J/g | The coating rate of microcapsules is as high as 67.9%, and the maximum service temperature is not higher than 177.16 °C. | Temperature control packaging | 2015 | [107] |
| Calcium carbonate shell (CaCO3) | N-octadecane + CaCl2 (40:60 by mass) (43.53%) | Self-assembly method | Tm 28.22 °C/Tc 23.54 °C; ΔHm 67.91 kJ/kg/ΔHc 63.52 kJ/kg; thermal conductivity: 1.325 W/(m·K) | The thermal conductivity of solid original n-octadecane was 0.153 W/(m·K), and that of microcapsule was 1.325 W/(m·K). After 200 cycles of phase change, the PCT and enthalpy remained stable; the release rate of microcapsules after 30 days was 38.1%, indicating good impermeability. | All-season protective outfits | 2014 | [104] |
| Calcium carbonate shell (CaCO3) | CaCl2 + Paraffin RT28 (1:1 by mass) | Self-assembly process | Tm 20.93 °C/Tc 26.44 °C; ΔHm 57.7 kJ/kg/ΔHc 66.1 kJ/kg; thermal conductivity: 0.759 W/(m·K) | Compared with the original RT28 and RT42, the thermal conductivity is increased by 2–3 times. After heating at 80 °C for 40 min, the microcapsules had no liquid leakage. | Thermal management | 2016 | [105] |
| CaCl2 + Paraffin RT28 (1:2 by mass) | Tm 23.33 °C/Tc 27.41 °C; ΔHm 105.8 J/kg/ΔHc 107.2 kJ/kg; thermal conductivity: 0.714 W/(m·K) | ||||||
| CaCl2/Paraffin RT28-RT42 (5:5) (1:1 by mass) | Tm 19.28 °C/Tc 27.44 °C; ΔHm 90.8 kJ/kg/ΔHc 95.9 kJ/kg; thermal conductivity: 0.739 W/(m·K) | ||||||
| CaCl2/Paraffin RT28-RT42 (5:5) (1:2 by mass) | Tm 19.76 °C/Tc 27.67 °C; ΔHm 82.8 kJ/kg/ΔHc 122.8 kJ/kg; thermal conductivity: 0.701 W/(m·K) | ||||||
| Polystyrene and Silica(PS-SiO2) | N-tetradecane (Tet) (Tm 0.39 °C/Tc 2.15 °C; ΔHm 195.9 kJ/kg/ΔHc 194.2 kJ/kg) | In situ polymerization | Tm 2.13 °C/Tc 0.39 °C; ΔHm 83.38 kJ/kg/ΔHc 79.37 kJ/kg | The thermal conductivity of 5 wt% Tet + PS-SiO2 slurry could reach 0.4035 W/(m·K) at 5 °C, and achieved an enhancement of 8.4% compared to Tet + PS slurry with same mass fraction. | Air conditioning systems | 2016 | [108] |
| Styrene-methylmethacrylate copolymer | N-octadecane | Miniemulsion in situ polymerization method | ΔHm 107.9 kJ/kg/ΔHc 104.9 kJ/kg) | After 360 thermal cycle tests, the Tc changed by 1.1 °C, the Tm changed by 0.6 °C, the ΔHm changed by 1.5 J/g, and the ΔHc heat changed by 1.2 J/g. No leakage was observed and the chemical stability was good. | Buildings | 2014 | [109] |
| Polyethylene glycol | SiO2 | Temperature-assistedsol–gel method | Tm 58.09 °C/Tc 42.34 °C; ΔHm 151.8 kJ/kg/ΔHc 141.0 kJ/kg) | The encapsulation rate is 79.3%, the encapsulation efficiency is 80.6%, and the thermal storage capability higher than 100%. The undercooling degree, melting time, and curing time of the composites were 22.3%, 26.5%, and 22.6% lower than those of the original polyethylene glycol, respectively. | Thermal energy storage applications in building envelopes | 2015 | [110] |
| Polystyrene | N-octadecane | Ultrasonic-assistant miniemulsion in situ polymerization | 124.4 kJ/kg | The nanocapsules were regular spherical and ranged from 100 to 123 nm in size. The PCT of nano encapsulated PCM was close to n-octadecane. | - | 2008 | [111] |
| Polystyrene | Nonadecane | Two-step Pickering emulsification procedure | Tm 34.12 °C/Tc 29.97 °C | The encapsulation rate is 55.9%. After 100 cycles, the PCT was almost unchanged and the thermal stability was good. | Thermal management | 2015 | [112] |
| Melamine formaldehyde | Dowtherm J | In situ polymerization | MEPCMS (LAES: 92.7–192.7 K, 207.9 kJ/kg/MEPCMS (PTES): 123–223 K, 123.6 kJ/kg | The shell curvature and solidification time of PCM microcapsules affect their heat transfer behavior and charging efficiency. | Cold storage | 2018 | [113] |
| PCM | Support Materials | Method | Thermophysical Properties of ssPCM | Key Findings | Applications | The Published Year | Reference |
|---|---|---|---|---|---|---|---|
| Poly (diethylene glycol hexadecyl ether acrylate) (PC16E2AC) | Graphene oxide (GO) | Solution blend | Tm 36.1 °C/Tc 23.5 °C; ΔHm 71kJ/kg/ΔHc 71 kJ/kg | When the mass fraction of GO is 5%, PC16E2AC starts to precipitate at 105 °C, and the PC16E2AC/GO composite maintains its initial shape at 85 °C for 100 min. After 300 cold and hot cycles, the PCT and latent heat remain unchanged, and has good thermal resistance cycle. | - | 2016 | [119] |
| Oetadecane (30 wt%) | Rigid polyurethan (PU) | In situ preparation | Tm 24.48 °C/Tf 27.60 °C; ΔHm 28.93 kJ/kg/ΔHf 27.96 kJ/kg | The prepared polyurethane ss-PCM had a micro nano uniform microstructure, the PCMs were evenly distributed in the polyurethane, and PU had good compatibility with PCM. | Building materials | 2012 | [120] |
| Capric acid + Lauric acid (61.13:38.87 by mass) (19.63 °C; 115.80 J/g) | Porous graphite | Physical adsorption | 19.50℃; 93.18 J/g | The low temperature eutectic PCMs accounted for 80.47% of the fixed PCMs. Compared with the original PCMs, the melting time and solidification time of the composite materials decreased by 74.1% and 84.9% respectively. There was no liquid exudates during the phase change process. | - | 2010 | [121] |
| Lauryl alcohol (LA) + Stearic acid (SA) (82:18 by mass) (21.3 °C; 205.9 kJ/kg) | Expanded perlite | Vacuum adsorption | 22.7 °C; 165.3 kJ/kg | The adsorption performance of expanded perlite on PCMs was significantly higher than that of ceramsite. After vacuum adsorption for 3 h, the adsorption rate of LA–SA was up to 352.5%, which was about 7.4 times that of ceramsite with equal mass. LA–SA combined well with the two porous materials, and the adsorption process was only physical adsorption, meanwhile, LA–SA did not volatilize in the range of 0–120 °C. Compared with ceramsite, expanded perlite had less effect on the thermal properties of LA–SA after encapsulation. | Building materials | 2020 | [64] |
| Ceramsite | 22.5 °C; 133.4 kJ/kg | ||||||
| Borax + Na2SO4·10H2O (2% + 90%) | Expanded graphite (8%) | Vacuum adsorption | 225.77 kJ/kg; undercooling 0.6 °C | There was no liquid exudation after phase transformation. Compared with Na2SO4·10H2O only adding borax, the heat storage time was shortened by 52.6%, the heat release time is shortened by 55.1%, and there was no performance attenuation after 500 heat storage/release cycles. | - | 2015 | [122] |
| CH3COONa·3H2O + Na2HPO4·12H2O (91% + 1%) | Expanded graphite (6%) | 233.5 kJ/kg; no undercooling; no phase separation | There was no liquid exudation after phase transformation. Compared with only adding KH2PO4, the heat storage time was shortened by 75.3%. | ||||
| Ba(HO)2·8H2O + KH2PO4 (93% + 1%) | Expanded graphite (6%) | 248.3 kJ/kg; undercooling less than 0.5 °C | There was no liquid exudation after phase transformation, and the heat storage time was shortened by 45.1% and the heat release time was shortened by 54.5% compared with the pure material. | ||||
| Lauric acid | Expanded perlite | Vacuum adsorption | 45 °C | Expanded perlite had a good adsorption capacity for lauric acid. lauric acid did not exudate when the content of lauric acid was less than 30%. | - | 2014 | [123] |
| Buytle stearate (20 °C; 140 J/g) | Plaster board | Physical adsorption method | 20.08 °C; 51.8418 J/g | Under the negative pressure state, the adsorption temperature of 25 °C for 5 min, the retention rate reached 60%, the thermal conductivity of gypsum board was 7% higher than that of pure gypsum board, and the retention rate decreased by 0.8% after 30 freeze–thaw cycles. | Buildings | 2010 | [124] |
| Expanded perlite | 20.40 °C; 92.2015 J/g | The retention rate of PCM was 180%, and the loss rate was 0.7% after 30 freeze–thaw cycles. | |||||
| Polyethylene glycol | Active carbon | Physical blending and impregnating method | - | It had good heat resistance below 250 °C. | - | 2011 | [125] |
| Paraffin wax (70 wt%) + liquid paraffin (30 wt%) (29.94 °C; 145.9 J/g) | Calcined opal | Fusion adsorption method | Tm 24.91 °C/Tf 24.87 °C; ΔHm 59.04 kJ/kg/ΔHf 56.26 kJ/kg | In the molten state, there was no leakage of liquid paraffin in the composite, and there was no obvious change in the PCT and latent heat after 200 thermal cycles. | Indoor energy storage building materials | 2013 | [126] |
| Capric acid | Expanded perlite | Vacuum impregnation method | Tm 31.8 °C/Tf 31.6 °C; ΔHm 98.12 kJ/kg/ΔHf 90.06 kJ/kg | After 5000 thermal cycles, Tf decreased by 0.09 °C, Tm decreased by 1.55 °C, ΔHm decreased by 2.6%, and ΔHf increased by 0.6%, indicating good thermal reliability and chemical stability. | Buildings | 2008 | [127] |
| Liquid paraffin + Octadecane (4:6 by mass) (16.9 °C; 124.2 J/g) | High density polyethylene (HDPE) | Melt blending method. | 72.22 J/g | With the increase of the carrier content, the latent heat of the composite PCMs synthesized using HDPE as the carrier decreased slowly, which had little effect on PCT, and the optimal carrier proportion was 30%. | - | 2020 | [128] |
| Dodecane | Expanded graphite (EG) | Vacuum infiltration method | −9.67 °C; 151.7 J/g | Dodecane was evenly embedded in the pores of EG with a thermal conductivity of 2.2745 W/(m·K); EG can reduce the liquid leakage of dodecane. | Cold chain logistics | 2019 | [129] |
| Organic PCMs | Inorganic PCM | Composite PCT (°C) | Composite Latent Heat (kJ/kg) | Thermal Conductivity of Composites (W/(m·K)) | Application | Reference |
|---|---|---|---|---|---|---|
| 25% Ethylene glycol solution (−11 °C; 96.8 kJ/kg) | 15% NH4Cl solution | −16 | 212.8 | - | Refrigerator | [130] |
| 26 wt% Sodium formate aqueous solution (−14.8 °C; 254.2 kJ/kg; 1.015 W/(m·K)) | 11 wt% KNO3 | −18 | 279.1 | 1.182 | Cold chain logistics | [131] |
| 15 wt% CH3CH2OH aqueous solution | 25 wt% NH4Cl aqueous solution | −17.1 | 304 | - | Freezer compartment of refrigerator | [132] |
| C6H7KO2 (5 wt%) | KCl (25 wt%) | −14 | 230.5 | - | Preservation of frozen food | [133] |
| C6H7KO2 (25 wt%) | KCl (5 wt%) | −18.6 | 131.91 | - | ||
| C10H14N2Na2O·2H2O (15 wt%) | KCl (15 wt%) | −16.7 | - | - | ||
| HCO2Na (5 wt%) | KCl (25 wt%) | −23.6 | 261.2 | |||
| HCO2Na (10 wt%) | KCl (20 wt%) | −23.8 | 266.4 | |||
| HCO2Na (15 wt%) | KCl (15 wt%) | −23.8 | 263.3 | |||
| HCO2Na (20 wt%) | KCl (10 wt%) | −23.5 | 254.8 | |||
| HCO2Na (22 wt%) | KCl (8 wt%) | −23.8 | 250.3 | |||
| HCO2Na (25 wt%) | KCl (5 wt%) | −23.8 | 263.3 |
| PCM | Phase Change Temperature of PCMs | Building Characteristics | Research Method | Application Form | Key Findings | Year | Reference |
|---|---|---|---|---|---|---|---|
| PureTemp 18 | 18 °C | A three-story office building model | Numerical | HVAC system | The integration of free refrigeration units can significantly reduce the primary energy utilization of HVAC systems. Compared with the baseline thermal plant configuration, the annual energy primary energy saving was about 67%. | 2018 | [138] |
| Microencapsulated paraffin | 23–26 °C | Four story rectangular apartment building model (in Italy) | Experimental | A PCM board was integrated in the roof and the external walls of the building. | In milder climates, the cooling demand was reduced by more than 65%. | 2020 | [139] |
| Lauryl alcohol + alcohol (90:10) | 22.93 °C | A 1.7 m × 1.7 m × 2.6 m air conditioned test room | Experimental | Air conditioning system | The 10 kg eutectic PCM released the stored cold air for 6.78 h until the end of the discharge cycle, and the bottom chamber temperature was about 3.5 °C lower than that without PCM. | 2020 | [140] |
| Paraffin RT22HC | 20–23 °C | A living room with dimensions of 6 m × 6 m × 3 m in a typical second floor apartment of a multistory residential house | Numerical | Integrated under the concrete ceiling slab of the building interior | Reduce the indoor air temperature by about 9.5 °C. | 2020 | [141] |
| Liquid paraffin | Tm 25.22 °C/Tf 28.88 °C | Two identical test rooms of 1.7 m × 1.7 m × 2.1 m (in Tianjin) | Experimental | Composite PCM wallboard | The PCM room had less temperature fluctuation, lower peak temperature, and smaller lag time than the reference room. | 2017 | [142] |
| Commercial macroencapsulated PCM-RT25HC | 25 °C | Two identical experimental huts of 2.4 m × 2.4 m × 2.4 m (at the University of Auckland, New Zealand) | Experimental | Combination with an air-based solar collector | PCMs store free cooling capacity during the summer night, reducing indoor temperature fluctuation and cooling load. | 2020 | [143] |
| Organic alcohol PCMs | 33.1–35.1 °C/41.7–42.7 °C | Two identical test rooms (at Tongji University in Shanghai) | Experimental | Novel diatomite-based composite PCM wallboards (PCMW) | PCMW was attached to the external wall surface of the test room, and the external surface temperature of PCMW was lower than that of traditional polystyrene plastic insulation wallboard. | 2020 | [144] |
| - | 22–24 °C | A south-facing middle office room located in Beijing | Numerical | Ventilation system | When the indoor temperature set point was 24–28 °C, the energy saving of the system using PCM energy storage was 16.9–50.8%, while that of traditional night ventilation system was 9.2–33.6%. | 2019 | [145] |
| Octadecane | Tm 23.33 °C/Tc 20.58 °C | Two small test rooms of 1.22 m × 1.22 m × 1.22 m (in Tabriz, Iran) | Experimental | Nanoencapsulated PCM/plaster wallboard | The PCM system reduced indoor air temperature fluctuations and maintained the thermal comfort throughout most of the year. | 2020 | [146] |
| DuPont Energain PCM panels | 18–24 °C | A test chamber 2.80 m wide × 1.30 m deep × 2.44 m high located in a large climate chamber with dimensions of 8.9 m × 7.3 m × 4.7 m | Experimental | Embedded on the back wall of a test hut placed in the climatic chamber | After the end of February in Montreal, energy consumption decreased by 20%. | 2015 | [147] |
| Commercial organic materials (RT11HC) | 10–12 °C | Cold storage (in Fuzhou, China) | Numerical | Coupled with cold storage heat pump system | While under the demand tariff, the electricity charge saving ratio of the cold storage system over the conventional system was 9.07–11.28% | 2020 | [148] |
| Applications | Characteristics of the Application | PCM | PCT of PCM | Research Method | Key Findings | Year | Reference |
|---|---|---|---|---|---|---|---|
| Vertical beverage cooler (VBC) | A commercial VBC with a storage capacity of 360 L, 1.65 m high, 0.65 m wide, and 0.55 m deep, with an average evaporation temperature of −10 °C | Water | 0 °C | Numerical | The first pressure drop, compressor start and close time were prolonged. A 6 mm thick PCM board reduceed the compressor running time ratio from 36% to 26%. | 2017 | [149] |
| Portable cold box for cold chain | The outer dimensions of the portable box are 430 mm in length, 285 mm in width and 345 mm in height; the internal dimensions are 355 mm in length, 215 mm in width and 265 mm in height | RTO, RT2HC, RT3HC, RT4HC, RT5HC, RT8HC | 0, 2, 3, 4, 5, 8 °C | Numerical | The melting point was 2 °C, the PCM arrangement was 20% at the top and 20% at each side wall, and the VIP was employed inside the box; this configuration had the longest cooling time of 46.5 h, the largest discharge efficiency of 90.7%, and a discharge depth of 99.4%. | 2020 | [150] |
| Refrigerated box for transporting a vaccine | Refrigerator box with effective volume of 2 L and internal size of 285 mm × 285 mm × 285 mm. The refrigeration board is made of polyethylene, and the thermal insulation material is vacuum thermal insulation board (vaccine transportation and storage temperature: 2–8 °C) | Tetradecane + lauryl alcohol + expanded graphite | 4.3 °C | Experimental | Combined with refrigeration equipment, the low temperature PCM developed could maintain the vaccine box at 2–8 °C for the longest time of 52.36 h. | 2020 | [50] |
| Household refrigerator | A 16-foot high household refrigerator with a size of 50 cm3 × 60 cm3 × 155 cm3 and a wall thickness of 3.7 cm (temperature range: 1–5 °C) | Water | 0 °C | Numerical + experimental | The PCM equipment equipped with three fins could maintain the temperature in the refrigerator compartment within the standard range for 68 min and reduce the working time of the compressor for 45 min. | 2018 | [151] |
| Refrigerated panels for small distributed refrigerated transport facilities | The refrigeration panel is made of plexiglass, with internal dimensions of 180 mm long, 80 mm wide and 160 mm high; six HPs are integrated into the panel and immersed into the PCM | Water | 0 °C | Experimental | A low temperature energy storage panel with HPs embedded in PCM was developed. The effects of air speed and air temperature on the discharge performance of PCM were analyzed. The air side temperature difference increased with the increase of inlet air temperature and decreased with the increase of wind speed. | 2020 | [152] |
| Open display cabinet in supermarket | The display cabinet is 1.3 m long, 0.9 m wide and 2.0 m high; there is no door on the display cabinet (cool food storage temperature: 0–6 °C) | Water | 0 °C | Experimental | PCM was introduced into the finned tube heat exchanger at the air duct behind the display case. After the compressor was shut down for 2 h, the temperature rise of the product was only 1 °C when the ambient temperature was 16 °C. | 2019 | [153] |
| Multitemperature zone cold storage incubator for cold chain logistics | Equipment 1: large cold storage equipment with storage capacity of 680 L, internal size of 1450 mm × 750 mm × 650 mm and three temperature zones is used for fruit and vegetable products with large transportation volume; equipment 2: the storage volume is 16 L, the internal size is 30 mm × 30 mm × 30 mm, and a small cold storage refrigerator with two temperature zones is set for the transportation of medical vaccines | PCM1: 87% C8H16O2 + 13% C14H28O2/PCM2: H2O + 0.03 g/mL C6H2KO2 | PCM1: 7.1 °C/PCM2: −2.5 °C | Experimental | A large and a small multitemperature zone cold storage incubator were designed by coupling two kinds of PCMs with vacuum insulation plate technology. The temperature of medium temperature zone 2 of the large incubator was between 7 °C and 9 °C for about 13 h. The temperature of the phase transition process in temperature zone 3 was maintained at about −2–0 °C for 14 h. The temperature of medium temperature region 1 of equipment 2 was 7–8 °C for about 19 h. The PCT in temperature zone 2 was 0 °C, and the temperature was kept cold for about 16 h. | 2019 | [154] |
| Cold plate of refrigerated container in truck | A 6-ton truck with refrigerated container, with container size of 2.05 m × 2.2 m × 4.8 m | Sub zero eutectic PCM solution: E-26, E-29, E-32 | E-26: −26 °C, E-29: −29 °C, E-32: −32 °C | Experimental | When the speed of the truck was 110 km/h, the maximum driving distance of the truck was 491 km when loaded with PCM E-26. | 2020 | [155] |
| Combined with solar energy for refrigerator cooling and energy | A hybrid solar power refrigeration system using low temperature thermal energy storage | Diethylene glycol | −10 °C | Numerical | Total irreversibility and total energy efficiencies were 908.2 kW and 45.14%, respectively. | 2020 | [156] |
| Refrigerator freezer | The cold accumulator is placed in the freezer of the refrigerator to exchange heat with the evaporator | CH3CH2OH aqueous solution (15 wt%) + NH4Cl aqueous solution (25 wt%) | −17.1 °C | Experimental | The central temperature of chicken was lowered from −1 to −5 °C in 55.5 min. | 2015 | [131] |
| New chilly bins for food storage. | Chilly bin with inner diameter of 110 mm, length of 270 mm and polystyrene insulation | PT-15 | −15 °C | Numerical + experimental | When PCMs were used in chilly bin, the maximum time to ensure a good food storage temperature could reach 240 min, and the transportation/storage time could be increased by 400%. | 2013 | [157] |
| PCM package for commercial ice cream containers | Widely commercial 5 L ice cream containers contain 2 cm PCM at the bottom | E-21 | −21.3 °C | Numerical + experimental | After placing the ice cream outside the refrigerator for 3 h, the average temperature of the ice cream was –15 °C, which was 3 °C lower in the center of the container and 10 °C lower in the corner of the container than that without PCM. | 2013 | [158] |
| Thermal protection for ice cream storage/transportation | 220 mm × 150 mm × 25 mm PCM rectangular brick | An eutectic solution of water and sodium | −21 °C | Experimental | When containers were exposed to ambient temperatures for 40 min, the temperature change in all areas of ice cream was limited to less than 1 °C. | 2015 | [159] |
| Refrigeration system of refrigerated truck | The PCM is encapsulated in a 1.7 m × 0.2 m × 0.02 m flat container, and 19 parallel PCM boards are contained in a well-insulated shell | An inorganic salt-water solution | −26.7 °C | Experimental | When the initial temperature of the cooling chamber was −7 °C and the ambient temperature was 30 °C, the cooling chamber gradually cooled to −15.8 °C after about 2 h. | 2012 | [160] |
| Mobile refrigeration system | The PCM is encapsulated in a flat plate of 1.6 m × 0.52 m × 0.02 m | An inorganic salt-water solution | −26.7 °C | Simulation | In Adelaide climate, the temperature of the refrigeration room could be maintained at −18 °C for 10 h. | 2014 | [161] |
| Refrigerated open display cabinet | A PCM container acts as an auxiliary evaporator during compressor shutdown | Hydro gel PCM composed of deionized water, silver iodide, guar and sodium tetraborate | −2 °C | Simulation | Compared with the basic cabinet, the defrosting and compressor shutdown intervals of the cabinet with PCM were extended by 98% and 50%, respectively, and the startup and shutdown of compressor was reduced by 27%. | 2016 | [162] |
| Display cabinet for food refrigeration | A new shelf with HPs and PCMs | 98% deionized water + 2% borax | −0.5 °C | Experimental | The combination of HP and PCM reduced the temperature rise of food during thawing by 1.5 °C and improved the temperature uniformity of food. | 2010 | [163] |
| Refrigerated display container | Two single plate radiators act as PCM heat exchangers | Deionized water + 1.2% silver iodide + 0.9% guar + 0.15% sodium tetraborate | −2 °C | Experimental | The cabinet with PCM could save energy by about 5%, and the defrosting time was about 5 min longer than the basic cabinet. | 2015 | [164] |
| Plate freezing of fish on small fishing boats | Shell and tube heat exchanger with PCM | CO2 | −57 °C | Numerical + experimental | The freezing time was reduced by more than 3%, and the fish yield increased by 2.9%. | 2019 | [165] |
| Large poultry processing plant | Integrated in industrial NH3/CO2 Cascade Refrigeration System | AdBlue | −11 °C | Simulation | Compressor power decreased by 19% during discharge. | 2018 | [166] |
| Fish industry tunnel freezer | NH3/CO2 Cascade | CO2 | <−50 °C | Numerical | The required power could be reduced by up to 30% at the same refrigeration capacity. | 2011 | [167] |
| Refrigerated truck | Eight PCM cold plates with size of 80 mm × 40 mm × 4 mm | Eutectic salt | −21.2 °C | Simulation | Vehicle interior temperature could be maintained for 73.6 h at 293 K ambient temperature. | 2017 | [168] |
| Refrigerated transport | Nineteen parallel PCM plates with the size of 0.26 m × 1.70 m × 0.025 m are located in the refrigerator with the size of 3.4 m × 2.2 m × 2.2 m | - | Tm −26.7 °C/Tf −30.6 °C | Numerical + experimental | The established one-dimensional liquid-based mathematical model for flat-plate phase change heat storage unit agreed well with the experimental verification results. | 2011 | [169] |
| The cold chain for the Ebola vaccine | Aluminium PCM block | PlusICE E-78 | −78 °C | Experimental | The Deep Freeze Arktek, when combined with PCM, maintained temperatures <−60 °C for 6.5 days in 43 °C ambient with a heat leak of 2.2 W. | 2015 | [170] |
| Household refrigerator | Double energy storage refrigerator (DES) with heat storage condenser (HSC) and cold storage evaporator (CSE) | Undecane (located in evaporator)/paraffin (located in condenser) | −26 °C/50 °C | Numerical | DES had higher evaporation pressure and temperature, and the energy saving was up to 32%. | 2017 | [171] |
| Storage and transportation of low temperature frozen food | The PCM is encapsulated in a thin stainless steel container and placed on the tube of the evaporator, accounting for 3.36% of the internal volume of the storage unit | ClimSel C-18/Cristopia E-21 | −18 °C/−21.3 °C | Experimental | Using E-21 as PCM could maintain lower (−16/−12 °C) air temperature and longer time than C-18 (−12/−7 °C). | 2012 | [172] |
| Freezer | The PCM is encapsulated in an aluminum plate and placed on the refrigerator wall | An eutectic composition of water and ammonium chloride | −15.4 °C | Experimental | Compared with the refrigerator without PCM, the refrigerator with PCM panel had lower temperature fluctuation, smaller ice cream crystal size, and less drip loss of frozen meat. | 2010 | [173] |
| Household refrigerator | PCM encapsulated in cubic copper container with size of 2 cm × 28 cm × 43.5 cm | NaCl-H2O | −21.15 °C | Numerical | The PCM–CTES could keep the refrigerator under standard thermal conditions for 4.5 h. | 2017 | [174] |
| Small low-cost space mission | Cooling a spaceborne atmosphere | Methanol | −97 °C | Experimental | If the heat load entering the system is low enough to maintain the PCMs close to equilibrium, the constant temperature can be maintained during freezing and melting. | 1995 | [175] |
| PCM | Properties of PCM | Application | The Characteristics of Electronic Devices | Working Conditions | Research Method | Key Findings | Year | Reference |
|---|---|---|---|---|---|---|---|---|
| N-eicosan | 309.55–309.65 K | Thermal management of lithium-ion batteries | The 18,650 lithium-ion battery is located on the central axis of the aluminum housing. The inner diameter of the shell is 31 mm, the wall thickness is 5 mm and the height is 70 mm. The diameter of the battery is 18 mm and the height is 65 mm. | High yield heat rate (4.6 W and 9.2 W) | Numerical | Using the porous-PCM led to 4–6 K reduction in the battery mean temperature. | 2020 | [177] |
| Carbon fiber–paraffin composites | 42–49 °C | Thermal management of a Li-ion battery | A regular AA Li-ion battery (e.g., 14500AA) | The heat production rate is 2 W. | Experimental | PCM + 2-mm-long carbon fibers (0.46%) showed the best thermal performance; a 45% reduction of maximum temperature rise of the battery simulator could be achieved. | 2015 | [178] |
| Paraffin/aluminum foam composite PCM | 46–52 °C | Battery modules in electric vehicles | The commercial rectangular LiFePO4 battery (119 mm in length, 70 mm in width, and 27 mm in height) | Lithium-ion batteries are charged at 1 and 2 °C rates. | Experimental | The addition of composite PCM reduced the maximum surface temperature rise of lithium-ion batteries by 53%. | 2015 | [176] |
| Paraffin wax | 56–58 °C | Electronic thermal management of round pin-finned heat sink | Round pin-fin heat sinks | Input power densities of 1.6 to 3.2 kW/m2 witha step of 0.4 kW/m2 | Experimental | Heat sink with PCM volume fraction of 1 and pin diameter of 3 mm had the best thermal performance. | 2018 | [179] |
| Carbon foam paraffin wax(RT65)/nanocomposite | 65 °C | Thermal control and protection of electronic devices. | The thermal management module is encapsulated in the aluminum support structure. The shell size is 50 mm × 50 mm × 40.5 mm and is processed with 1.2 mm thick aluminum. | Three different uniform power levels of 18, 24, 30 W | Experimental | Compared with pure carbon foam, the thermal management module composed of carbon foam + paraffin wax (RT65) was employed to achieve a reasonable delay in reaching the steady-state temperature of the heater. | 2015 | [180] |
| Carbon foam/PCM/nanocarbon tube composites | 65 °C | Thermal management of electronic equipment | Thermal management module encapsulated in 1.5 mm thick thin aluminum shell | Pore values of different carbon foams (75%, 88%) | Numerical | When the porosity of foamed carbon was less than 75%, the module surface temperature decreased by 11.5%. | 2015 | [181] |
| Paraffin (RT44HC)/expanded graphite (EG) composite PCM (CPCM) | 41–44 °C | Li-ion battery thermal management | The commercial cylinder 26,650 LiFePO4 battery | Dischargedat the rate of 5 C using a DC electronic load | Experimental | CPCM with 16–20 wt% expanded graphite can be regarded as the most promising alternative for Li-ion battery thermal management. | 2016 | [182] |
| Dielectric PCM-Boron Nitride Nanosheets Composite | - | Electronic system thermal management | - | Breakdown voltage 11.3–13.3 MV/m | Experimental | The thermal conductivity of the composite reached 3.47 W/(m·K), and the breakdown voltage reached 11.3–13.3 MV/m. | 2016 | [94] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Shi, Q.; Luo, L.; Fan, Y.; Wang, Q.; Jia, G. Research Progress on the Phase Change Materials for Cold Thermal Energy Storage. Energies 2021, 14, 8233. https://doi.org/10.3390/en14248233
Zhang X, Shi Q, Luo L, Fan Y, Wang Q, Jia G. Research Progress on the Phase Change Materials for Cold Thermal Energy Storage. Energies. 2021; 14(24):8233. https://doi.org/10.3390/en14248233
Chicago/Turabian StyleZhang, Xinghui, Qili Shi, Lingai Luo, Yilin Fan, Qian Wang, and Guanguan Jia. 2021. "Research Progress on the Phase Change Materials for Cold Thermal Energy Storage" Energies 14, no. 24: 8233. https://doi.org/10.3390/en14248233










