On the Effect of the Distances between Coal and Wood Particles during Their Joint Pyrolysis on Sulfur Oxides Formation
Abstract
1. Introduction
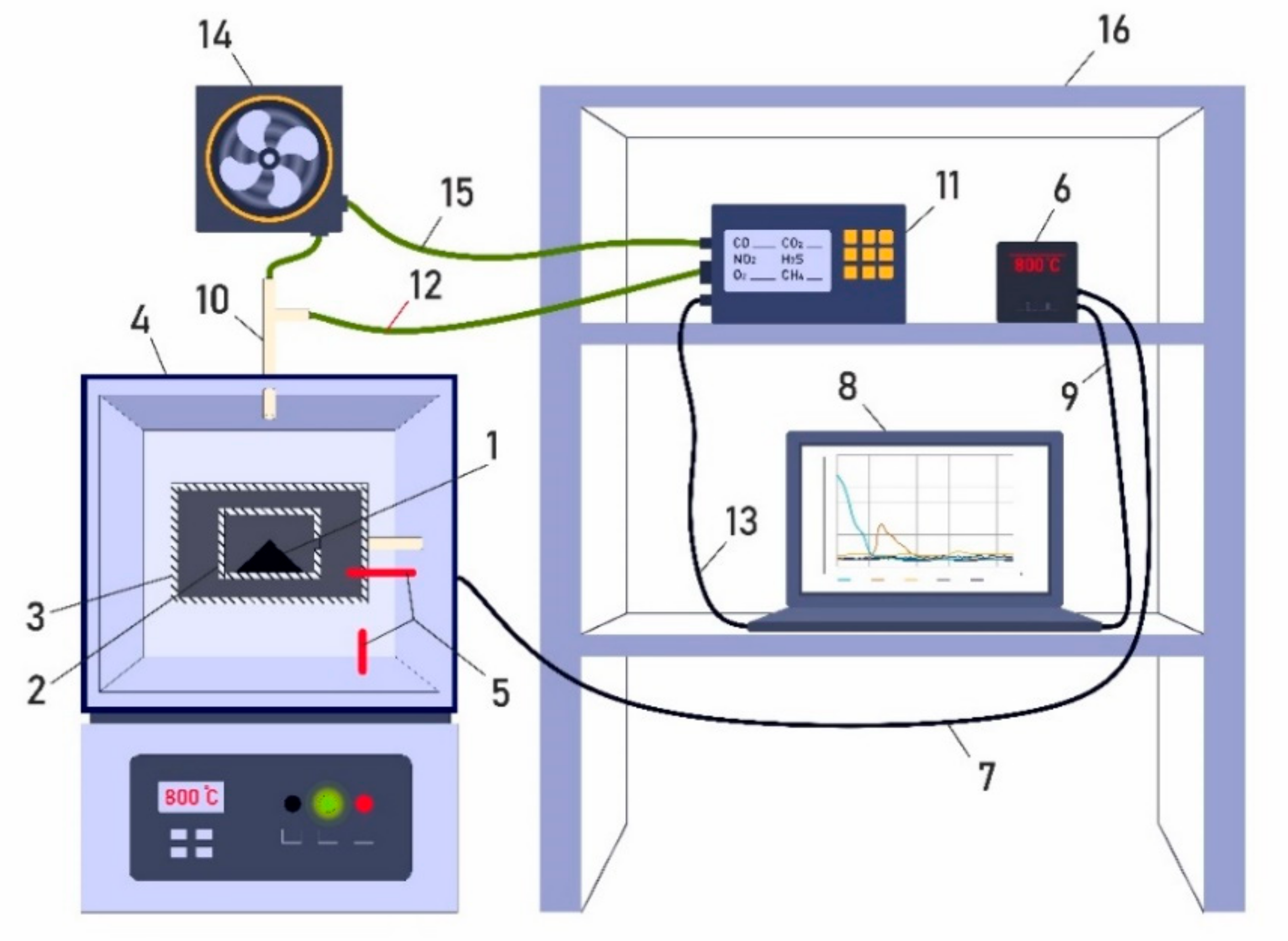
2. Materials and Methods of Experimental Research
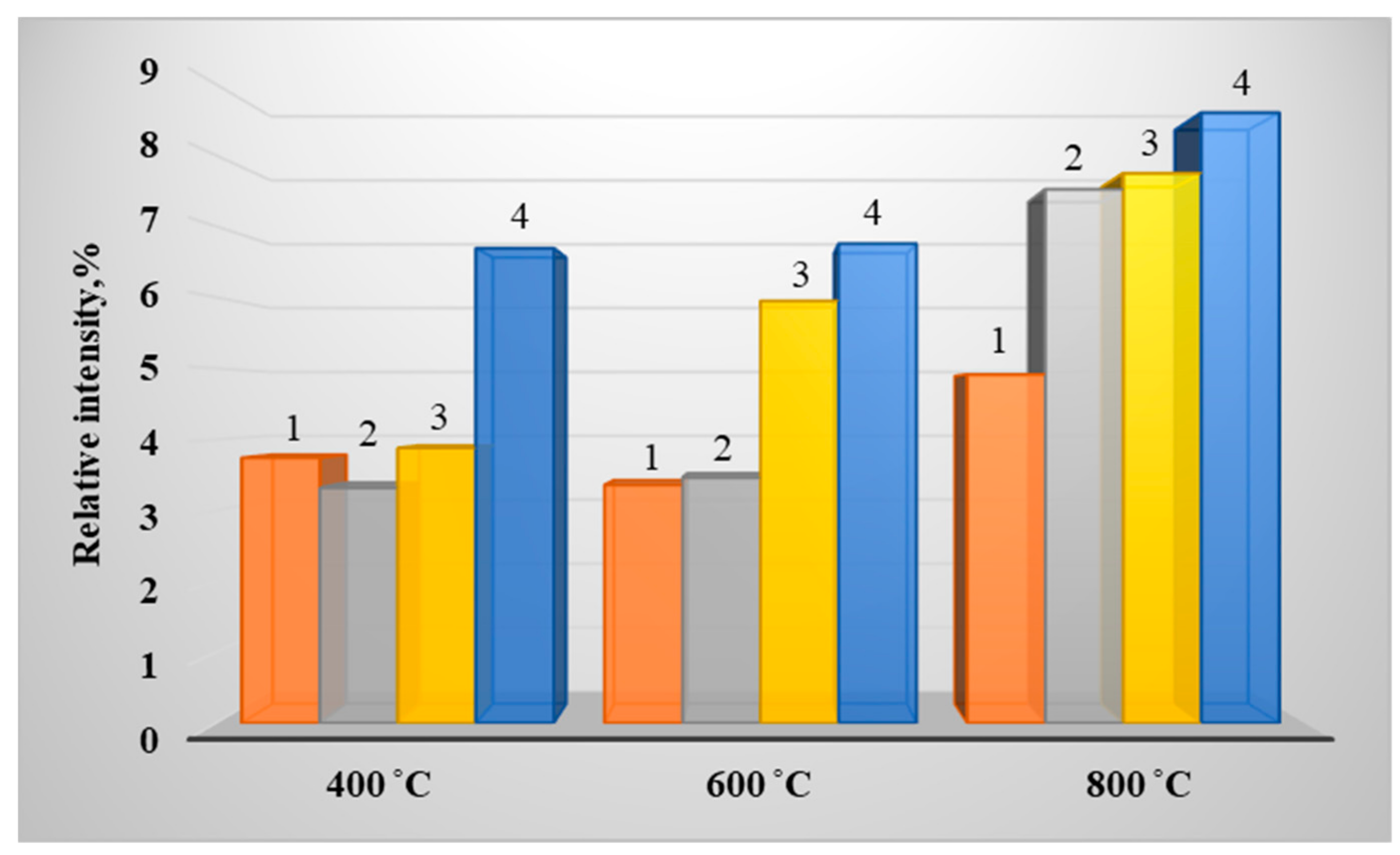
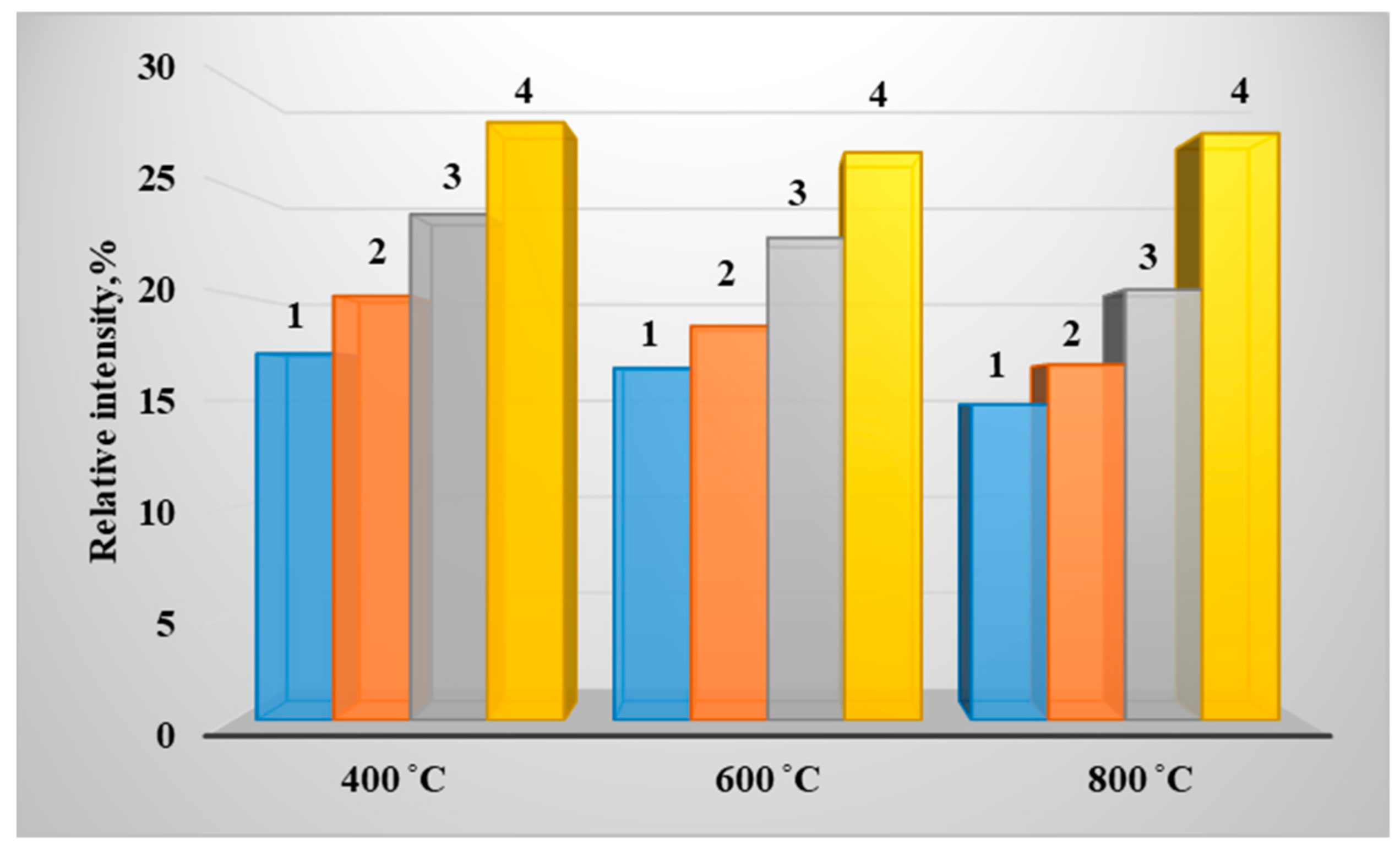
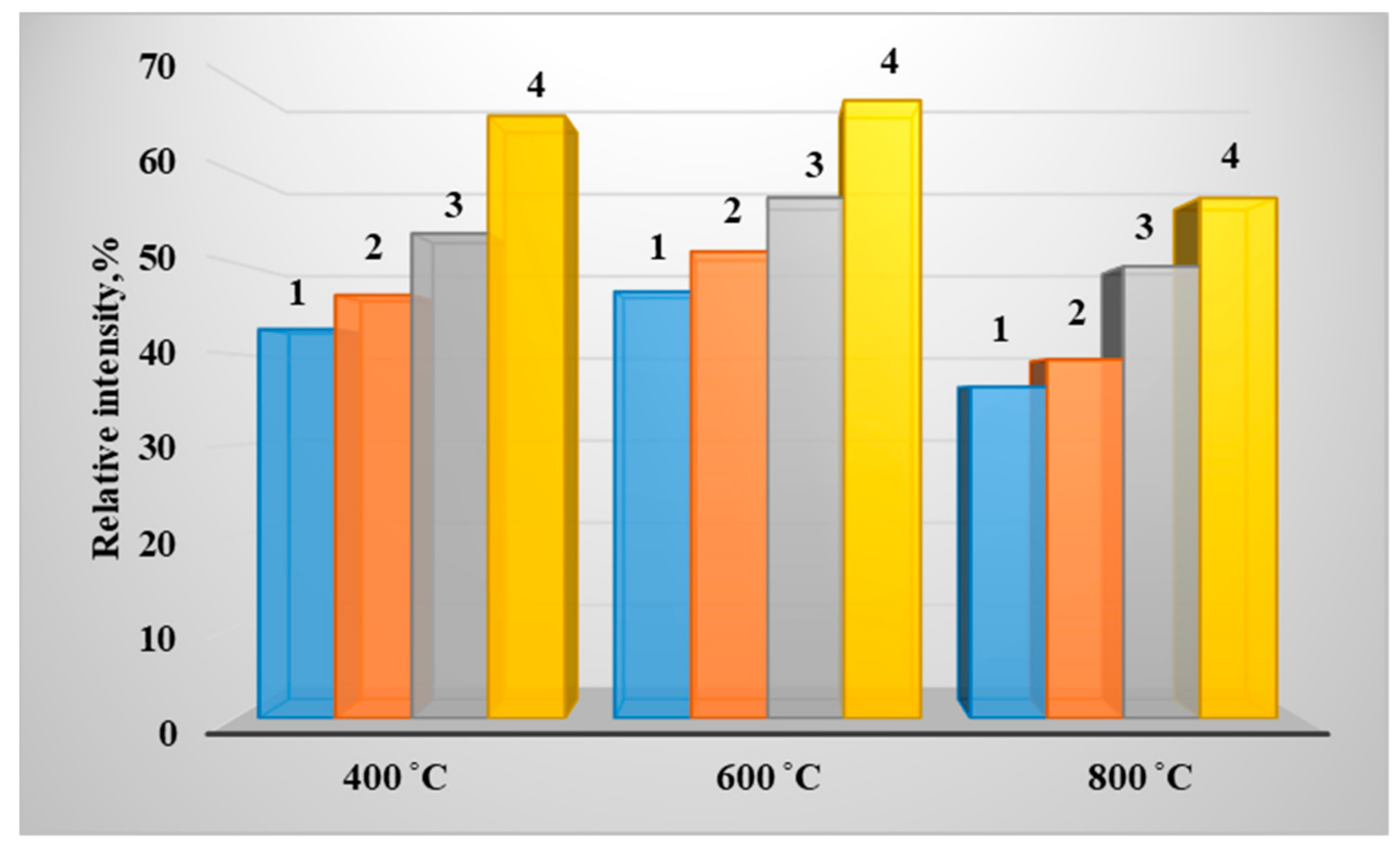
3. Results of Experimental Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Li, H.; Shu, L.; Gu, N.; Wang, G.; Weng, Y.; Schou, M. Double-blind comparison of ziprasidone and risperidone in the treatment of Chinese patients with acute exacerbation of schizophrenia. Neuropsychiatr. Dis. Treat. 2011, 7, 77. [Google Scholar] [CrossRef][Green Version]
- Morrison, B.; Golden, J.S. Life cycle assessment of co-firing coal and wood pellets in the Southeastern United States. J. Clean. Prod. 2017, 150, 188–196. [Google Scholar] [CrossRef]
- Beagle, E.; Belmont, E. Comparative life cycle assessment of biomass utilization for electricity generation in the European Union and the United States. Energy Policy 2019, 128, 267–275. [Google Scholar] [CrossRef]
- Aviso, K.B.; Sy, C.L.; Tan, R.R.; Ubando, A.T. Fuzzy optimization of carbon management networks based on direct and indirect biomass co-firing. Renew. Sustain. Energy Rev. 2020, 132, 110035. [Google Scholar] [CrossRef]
- Krzywański, J.; Czakiert, T.; Muskała, W.; Nowak, W. Modelling of CO2, CO, SO2, O2 and NO x emissions from the oxy-fuel combustion in a circulating fluidized bed. Fuel Process. Technol. 2011, 92, 590–596. [Google Scholar] [CrossRef]
- Malik, P.; Awasthi, M.; Sinha, S. Techno-economic analysis of decentralized biomass energy system and CO2 reduction in the Himalayan region. Int. J. Energy Environ. Eng. 2021, 12, 239–249. [Google Scholar] [CrossRef]
- Fajardy, M.; Patrizio, P.; Daggash, H.A.; Mac Dowell, N. Negative Emissions: Priorities for Research and Policy Design. Front. Clim. 2019, 1, 6. [Google Scholar] [CrossRef]
- Larionov, K.B.; Zenkov, A.V.; Yankovsky, S.A.; Ditc, A.A. Change of coal-water fuel rheological properties by rotary flows modulation. In Proceedings of the IEEE 2016 11th International Forum on Strategic Technology (IFOST), Novosibirsk, Russia, 1–3 June 2016. [Google Scholar]
- Perrone, D.; Castiglione, T.; Klimanek, A.; Morrone, P.; Amelio, M. Numerical simulations on Oxy-MILD combustion of pulverized coal in an industrial boiler. Fuel Process. Technol. 2018, 181, 361–374. [Google Scholar] [CrossRef]
- Gebreslassie, M.G. Development and Manufacturing of Solar and Wind Energy Technologies in Ethiopia: Challenges and Policy Implications. Renew. Energy 2020, 168, 107–118. [Google Scholar] [CrossRef]
- Bouchekara, H.R.E.H.; Javaid, M.S.; Shaaban, Y.A.; Shahriar, M.S.; Ramli, M.A.M.; Latreche, Y. Decomposition based multiobjective evolutionary algorithm for PV/Wind/Diesel Hybrid Microgrid System design considering load uncertainty. Energy Rep. 2021, 7, 52–69. [Google Scholar] [CrossRef]
- Liu, Q.; Shi, Y.; Zhong, W.; Yu, A. Co-firing of coal and biomass in oxy-fuel fluidized bed for CO2 capture: A review of recent advances. Chin. J. Chem. Eng. 2019, 27, 2261–2272. [Google Scholar] [CrossRef]
- Codina Gironès, V.; Peduzzi, E.; Vuille, F.; Maréchal, F. On the Assessment of the CO2 Mitigation Potential of Woody Biomass. Front. Energy Res. 2018, 5, 37. [Google Scholar] [CrossRef]
- British Petroleum Energy Outlook 2020 edition explores the forces shaping the global energy transition out to 2050 and the surrounding that BP Energy Outlook 2030 Stat. Rev. London Br. Pet. 2020, p. 81. Available online: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/energy-outlook/bp-energy-outlook-2020.pdf (accessed on 2 December 2021).
- IEA. Key World Energy Statistics 2020—Analysis. Available online: https://www.iea.org/reports/key-world-energy-statistics-2020 (accessed on 17 March 2021).
- Danso, D.K.; François, B.; Hingray, B.; Diedhiou, A. Assessing hydropower flexibility for integrating solar and wind energy in West Africa using dynamic programming and sensitivity analysis. Illustration with the Akosombo reservoir, Ghana. J. Clean. Prod. 2021, 287, 125559. [Google Scholar] [CrossRef]
- IEA. IEA Coal—Fuels & Technologies. Available online: https://www.iea.org/fuels-and-technologies/coal (accessed on 2 December 2021).
- Czekała, W.; Bartnikowska, S.; Dach, J.; Janczak, D.; Smurzyńska, A.; Kozłowski, K.; Bugała, A.; Lewicki, A.; Cieślik, M.; Typańska, D.; et al. The energy value and economic efficiency of solid biofuels produced from digestate and sawdust. Energy 2018, 159, 1118–1122. [Google Scholar] [CrossRef]
- Chang, Y.F.; Huang, B.N. Factors Leading to Increased Carbon Dioxide Emissions of the APEC Countries: The LMDI Decomposition Analysis. Singap. Econ. Rev. 2020, 1–20. [Google Scholar] [CrossRef]
- Zhao, R.; Qin, J.; Chen, T.; Wang, L.; Wu, J. Experimental study on co-combustion of low rank coal semicoke and oil sludge by TG-FTIR. Waste Manag. 2020, 116, 91–99. [Google Scholar] [CrossRef]
- Kuznetsov, G.V.; Jankovsky, S.A.; Tolokolnikov, A.A.; Zenkov, A.V. Mechanism of Sulfur and Nitrogen Oxides Suppression in Combustion Products of Mixed Fuels Based on Coal and Wood. Combust. Sci. Technol. 2019, 191, 2071–2081. [Google Scholar] [CrossRef]
- Zhu, Y.; Niu, Y.; Tan, H.; Wang, X. Short Review on the Origin and Countermeasure of Biomass Slagging in Grate Furnace. Front. Energy Res. 2014, 2, 7. [Google Scholar] [CrossRef]
- Kim, S.; Dale, B.E. Global potential bioethanol production from wasted crops and crop residues. Biomass Bioenergy 2004, 26, 361–375. [Google Scholar] [CrossRef]
- Haykiri-Acma, H.; Yaman, S.; Kucukbayrak, S. Co-combustion of low rank coal/waste biomass blends using dry air or oxygen. Appl. Therm. Eng. 2013, 50, 251–259. [Google Scholar] [CrossRef]
- Bhuiyan, A.A.; Naser, J. CFD modelling of co-firing of biomass with coal under oxy-fuel combustion in a large scale power plant. Fuel 2015, 159, 150–168. [Google Scholar] [CrossRef]
- Karka, P.; Johnsson, F.; Papadokonstantakis, S. Perspectives for Greening European Fossil-Fuel Infrastructures through Use of Biomass: The Case of Liquid Biofuels Based on Lignocellulosic Resources. Front. Energy Res. 2021, 9, 112. [Google Scholar] [CrossRef]
- Lim, J.S.; Abdul Manan, Z.; Wan Alwi, S.R.; Hashim, H. A review on utilisation of biomass from rice industry as a source of renewable energy. Renew. Sustain. Energy Rev. 2012, 16, 3084–3094. [Google Scholar] [CrossRef]
- Sher, F.; Pans, M.A.; Sun, C.; Snape, C.; Liu, H. Oxy-fuel combustion study of biomass fuels in a 20 kWth fluidized bed combustor. Fuel 2018, 215, 778–786. [Google Scholar] [CrossRef]
- Guo, J.; Liu, Z.; Wang, P.; Huang, X.; Li, J.; Xu, P.; Zheng, C. Numerical investigation on oxy-combustion characteristics of a 200 MWe tangentially fired boiler. Fuel 2015, 140, 660–668. [Google Scholar] [CrossRef]
- Munjeri, K.; Ziuku, S.; Maganga, H.; Siachingoma, B.; Ndlovu, S. On the potential of water hyacinth as a biomass briquette for heating applications. Int. J. Energy Environ. Eng. 2015, 7, 37–43. [Google Scholar] [CrossRef]
- Palange, R.; Krishnan, M. Coal gasification process optimization for maximum calorific value and minimum CO2 emission using Taguchi method and utility concept. Int. J. Energy Environ. Eng. 2021, 12, 335–351. [Google Scholar] [CrossRef]
- Kuznetsov, G.V.; Yankovskii, S.A. Conditions and Characteristics in Ignition of Composite Fuels Based on Coal with the Addition of Wood. Therm. Eng. 2019, 66, 133–137. [Google Scholar] [CrossRef]
- Lupion, M.; Alvarez, I.; Otero, P.; Kuivalainen, R.; Lantto, J.; Hotta, A.; Hack, H. 30 MWth CIUDEN Oxy-CFB boiler—First experiences. Energy Procedia 2013, 37, 6179–6188. [Google Scholar] [CrossRef]
- Black, S.; Szuhánszki, J.; Pranzitelli, A.; Ma, L.; Stanger, P.J.; Ingham, D.B.; Pourkashanian, M. Effects of firing coal and biomass under oxy-fuel conditions in a power plant boiler using CFD modelling. Fuel 2013, 113, 780–786. [Google Scholar] [CrossRef]
- Yankovsky, S.A.; Kuznetsov, G.V. Physicochemical Transformations of Mixed Fuels Based on Typical Coals and Wood upon Heating. Solid Fuel Chem. 2019, 53, 22–28. [Google Scholar] [CrossRef]
- Kuznetsov, G.V.; Yankovskii, S.A.; Tolokol’nikov, A.A.; Cherednik, I.V. Mechanism of the Suppression of Sulfur Oxides in the Oxidative Thermolysis Products of Coals upon Their Combustion in a Mixture with Dispersed Wood. Solid Fuel Chem. 2020, 54, 311–317. [Google Scholar] [CrossRef]
- Kuznetsov, G.V.; Syrodoy, S.V.; Kostoreva, A.A.; Kostoreva, Z.A.; Nigay, N.A. Effect of concentration and relative position of wood and coal particles on the characteristics of the mixture ignition process. Fuel 2020, 274, 117843. [Google Scholar] [CrossRef]
- Fei, B.; Gao, Z.; Wang, J.; Liu, Z. Biological, Anatomical, and Chemical Characteristics of Bamboo. In Secondary Xylem Biology, 2nd ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 283–306. [Google Scholar] [CrossRef]




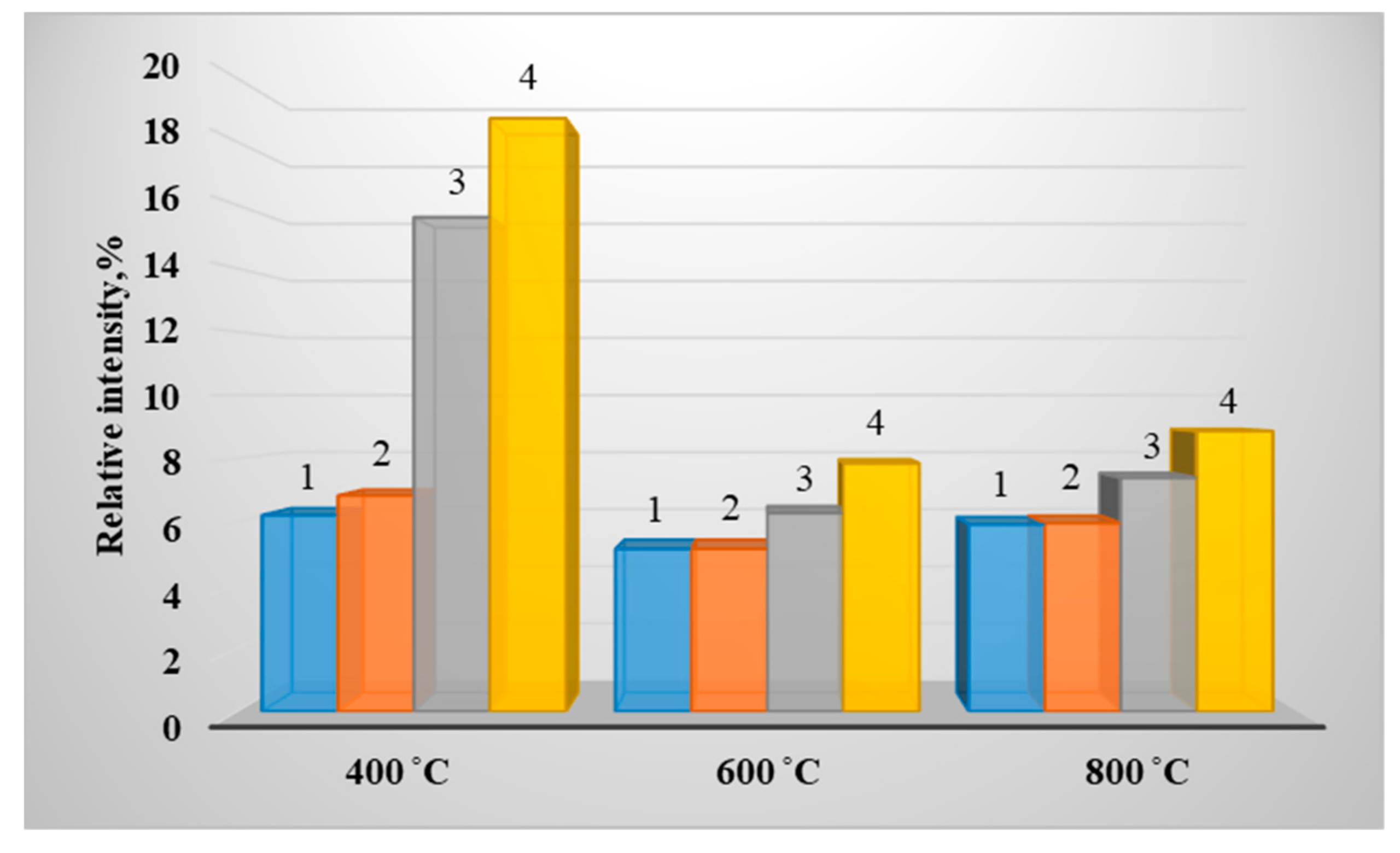
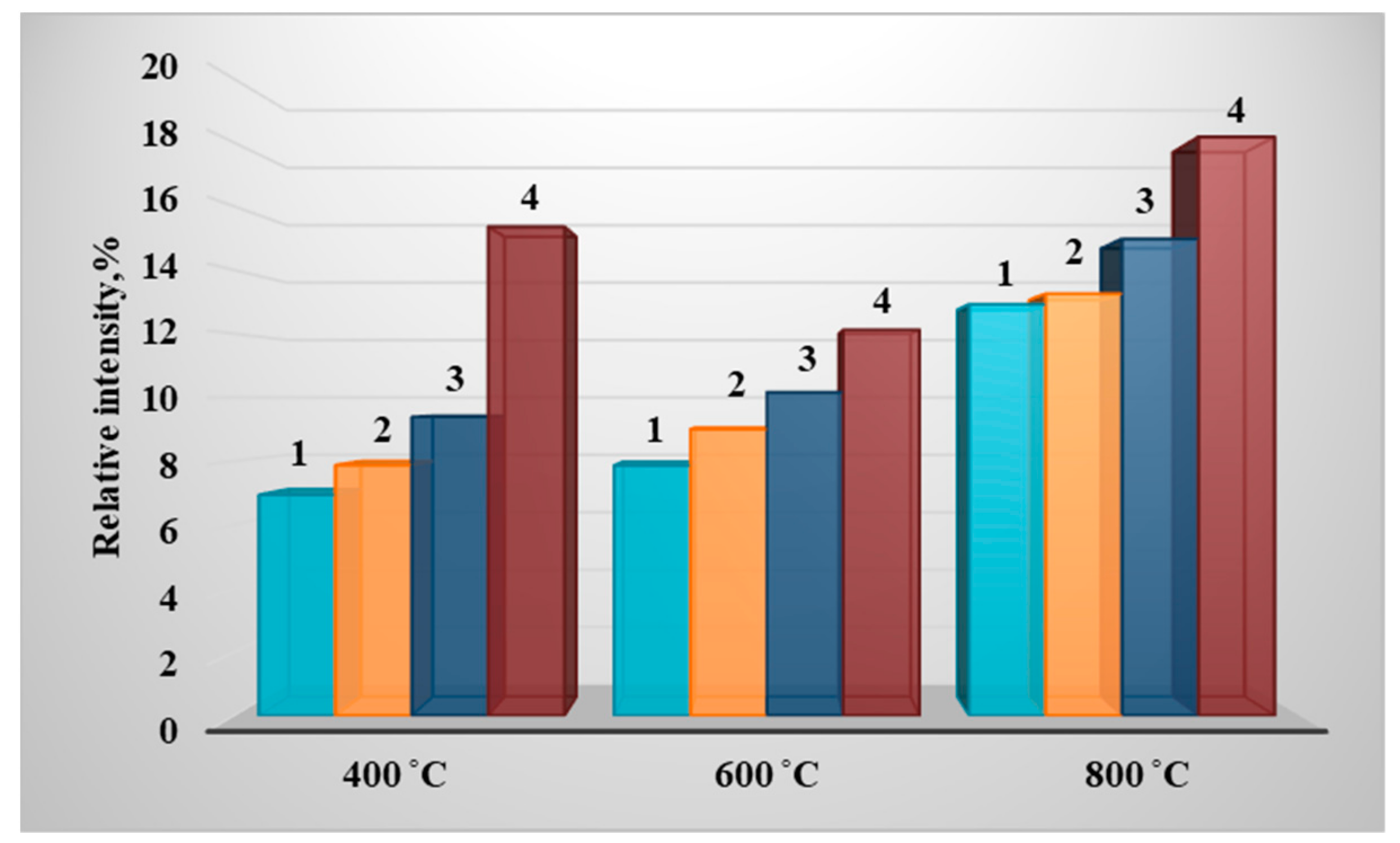
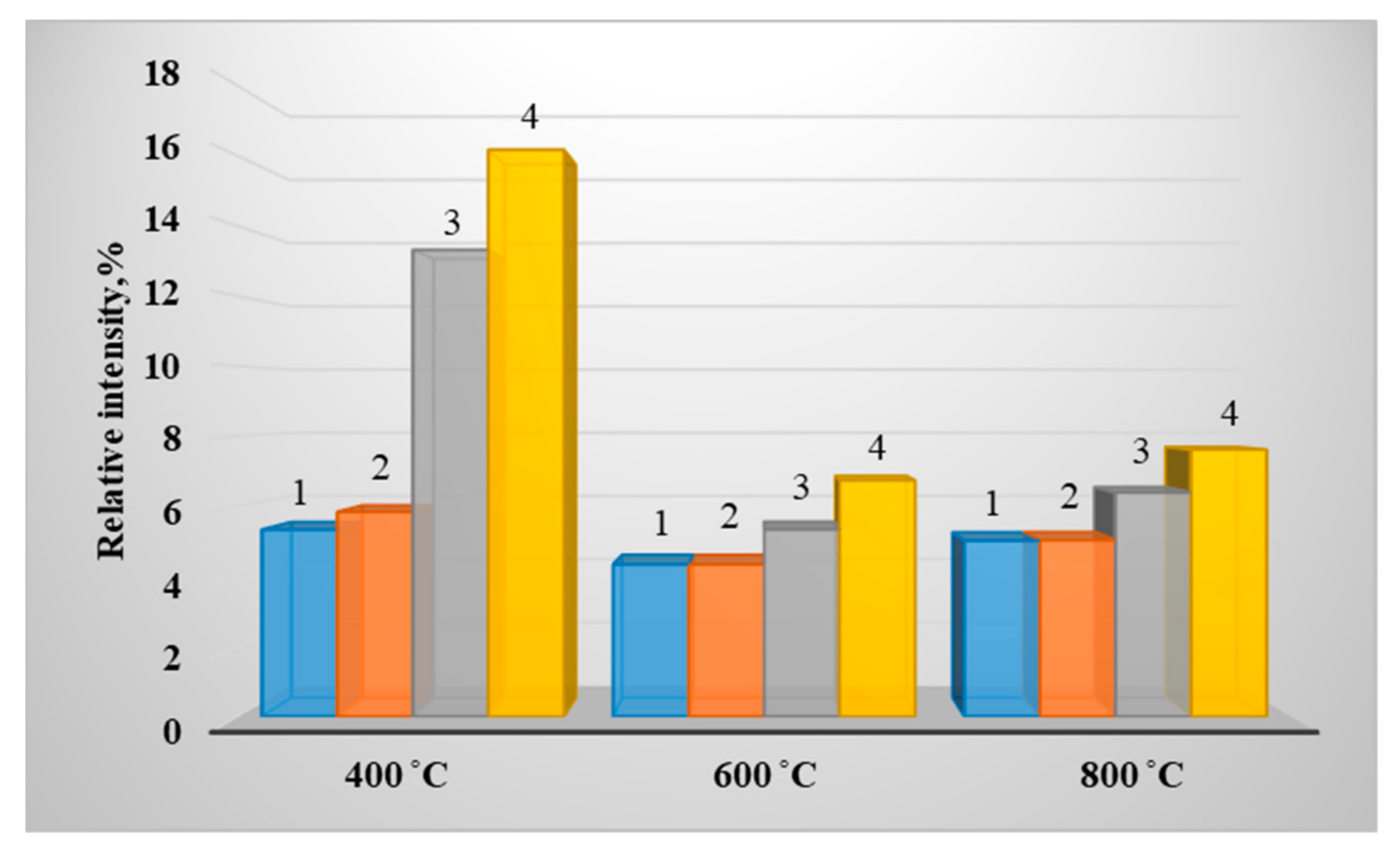

| Fuel (Wood/Coal_Grade), % | Calorific Value, Q, MJ/kg | Technical Analysis, % | Elemental Composition of Dry Matter, % | ||||||
|---|---|---|---|---|---|---|---|---|---|
| W a | A d | V daf | C | H | N | S | O | ||
| 0/100_3B | 25.79 | 5.41 | 3.45 | 40.09 | 66.18 | 4.34 | 0.64 | 0.44 | 28.4 |
| 10/90_3B | 23.91 | 8.82 | 3.2 | 44.89 | 62.20 | 4.92 | 0.58 | 0.40 | 31.9 |
| 25/75_3B | 23.83 | 14.21 | 3.11 | 47.41 | 61.59 | 4.87 | 0.56 | 0.38 | 32.6 |
| 50/50_3B | 23.75 | 12.87 | 2.75 | 54.75 | 61.38 | 4.90 | 0.52 | 0.36 | 32.84 |
| 0/100_T | 25.72 | 5.52 | 18.37 | 24.93 | 84.3 | 6.4 | 2.9 | 0.6 | 5.8 |
| 10/90_T | 25.60 | 5.42 | 14.24 | 26.46 | 53.5 | 4.1 | 1.4 | 0.41 | 40.59 |
| 25/75_T | 25.22 | 5.34 | 13.65 | 28.33 | 52.2 | 4.4 | 1.2 | 0.32 | 41.88 |
| 50/50_T | 24.79 | 5.41 | 11.08 | 39.95 | 51.0 | 4.9 | 0.8 | 0.23 | 43.07 |
| 100/0 | 21.73 | 5.35 | 0.29 | 80.25 | 50.48 | 5,75 | 0,04 | 0 | 43.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yankovsky, S.; Tolokol’nikov, A.; Misyukova, A.; Kuznetsov, G. On the Effect of the Distances between Coal and Wood Particles during Their Joint Pyrolysis on Sulfur Oxides Formation. Energies 2021, 14, 8321. https://doi.org/10.3390/en14248321
Yankovsky S, Tolokol’nikov A, Misyukova A, Kuznetsov G. On the Effect of the Distances between Coal and Wood Particles during Their Joint Pyrolysis on Sulfur Oxides Formation. Energies. 2021; 14(24):8321. https://doi.org/10.3390/en14248321
Chicago/Turabian StyleYankovsky, Stanislav, Anton Tolokol’nikov, Albina Misyukova, and Geniy Kuznetsov. 2021. "On the Effect of the Distances between Coal and Wood Particles during Their Joint Pyrolysis on Sulfur Oxides Formation" Energies 14, no. 24: 8321. https://doi.org/10.3390/en14248321
APA StyleYankovsky, S., Tolokol’nikov, A., Misyukova, A., & Kuznetsov, G. (2021). On the Effect of the Distances between Coal and Wood Particles during Their Joint Pyrolysis on Sulfur Oxides Formation. Energies, 14(24), 8321. https://doi.org/10.3390/en14248321






