BiFeO3 Coupled Polysulfide Trapping in C/S Composite Cathode Material for Li-S Batteries as Large Efficiency and High Rate Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of BFO Nanoparticles
2.2. Synthesis of BFO–S-C Composite Cathode
2.3. Material Characterization and Electrochemical Measurements
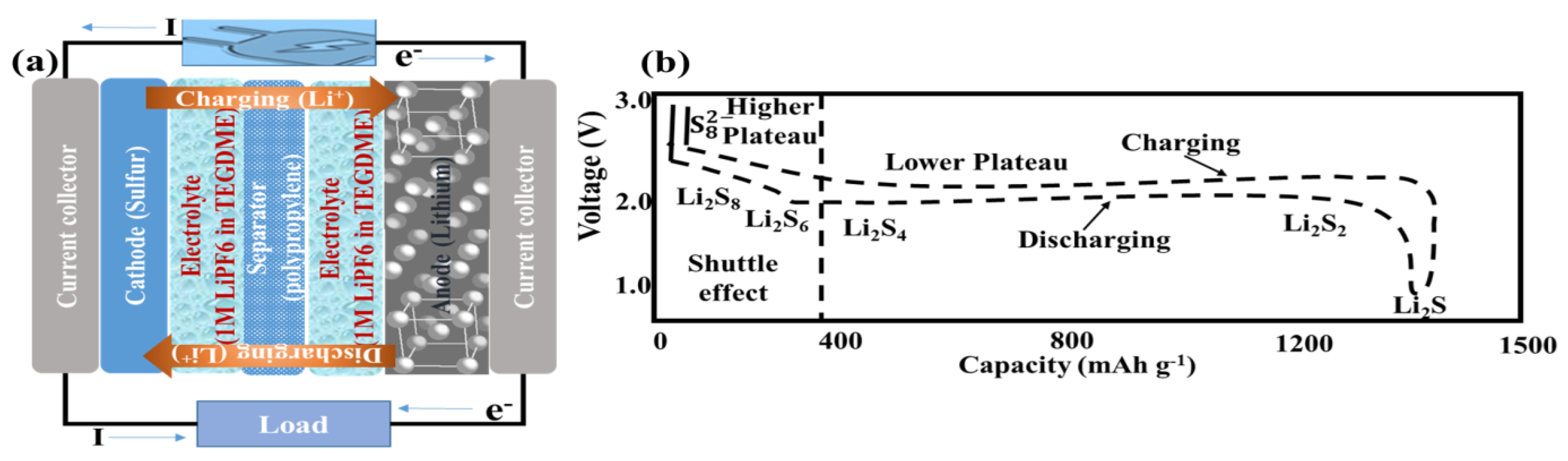
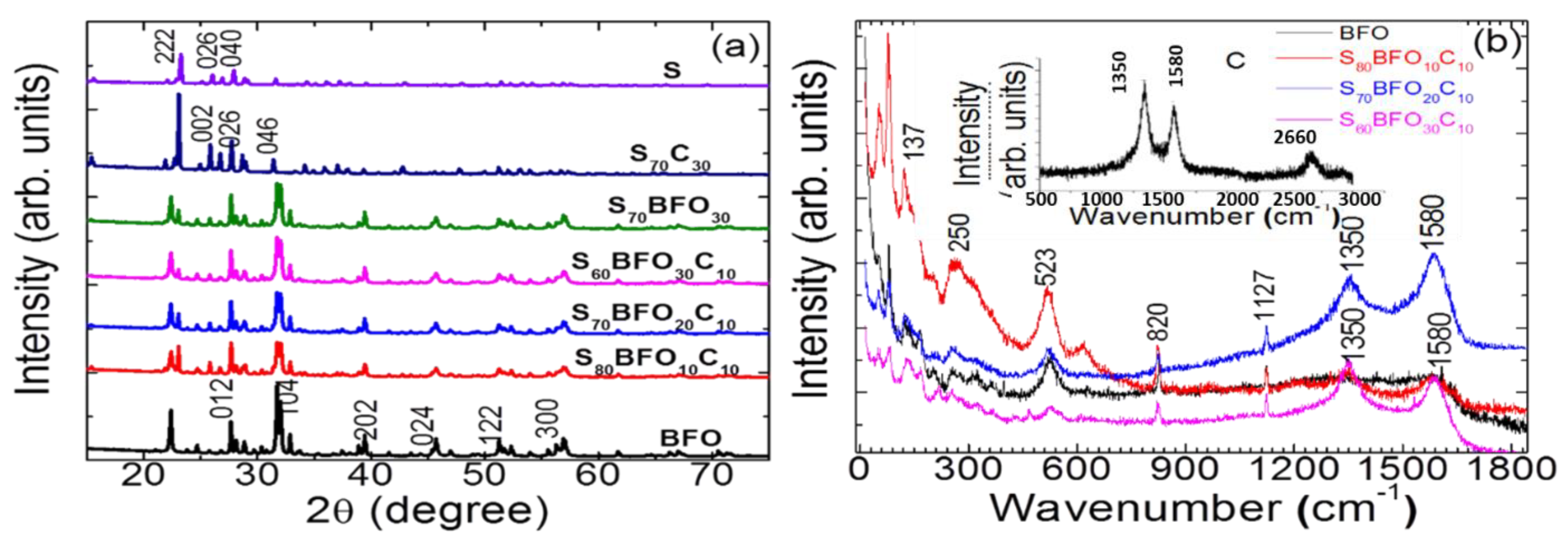
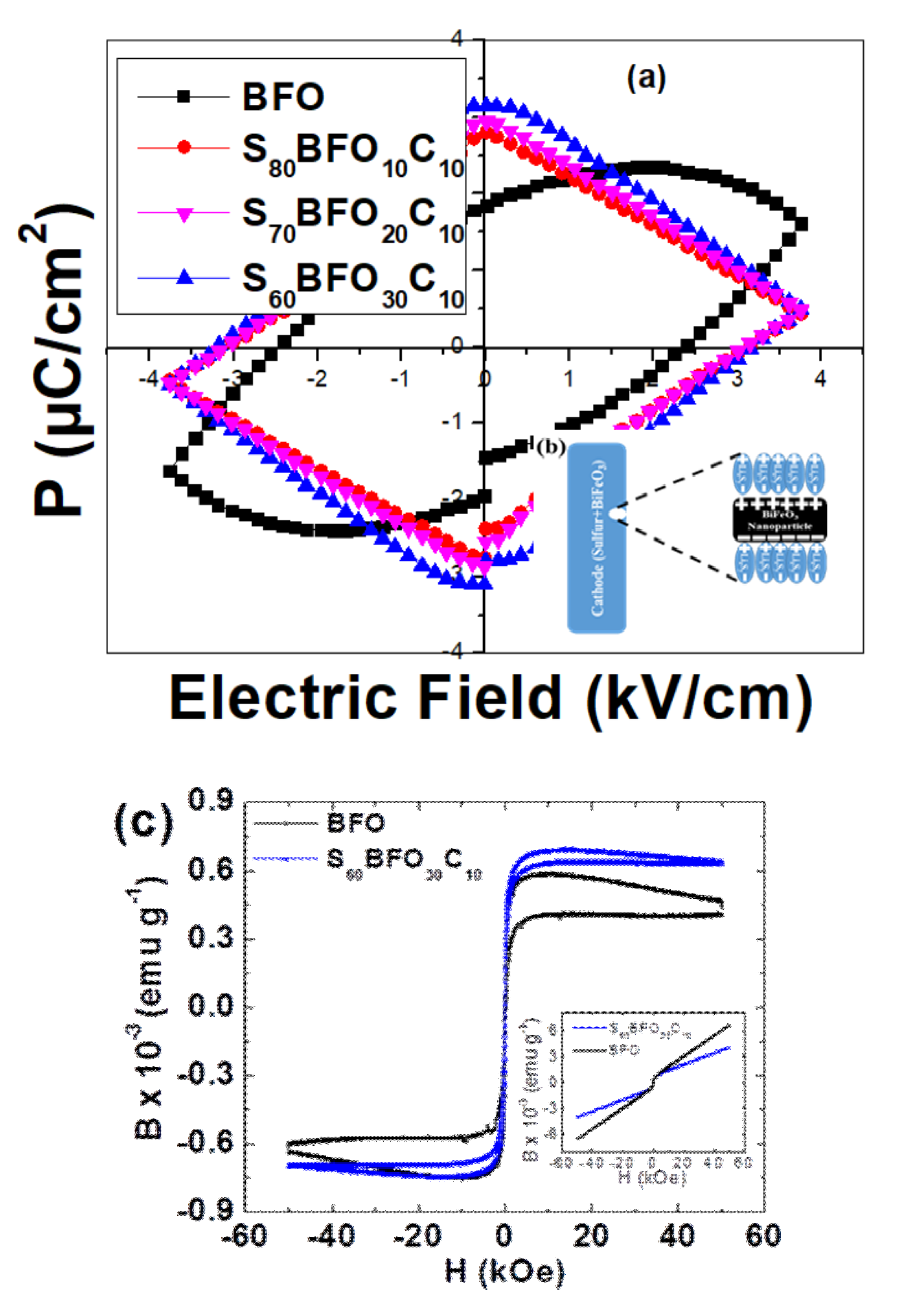
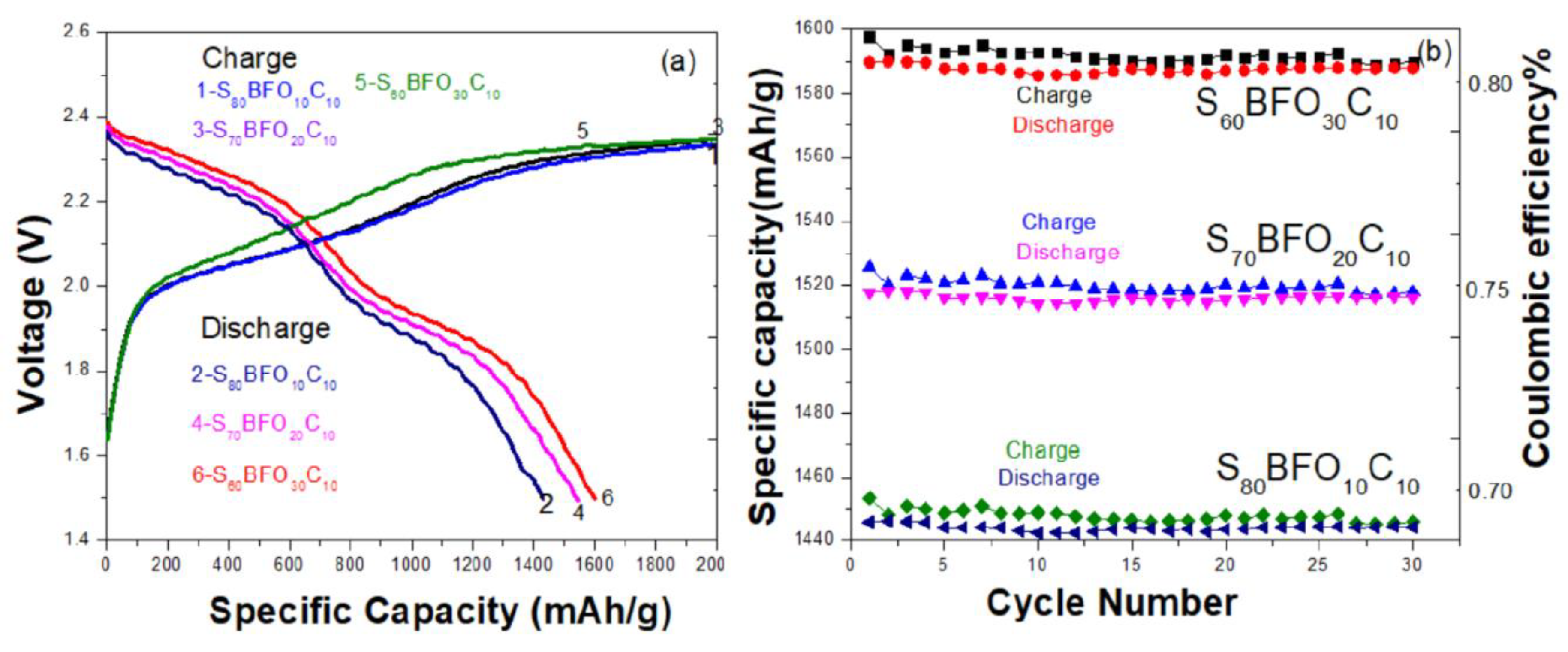
3. Results and Discussion
3.1. X-ray Diffraction and Raman Spectra
3.2. SEM Analysis
3.3. Ferroelectric (P–E) Polarization and Magnetic Hysteresis
3.4. Electrochemical Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.M. Li-O2 and Li-S batteries with high energy storage. Nat. Mater. 2012, 11, 19–29. [Google Scholar] [CrossRef]
- Zhang, S.S.; Jeffrey, A.R. A new direction for the performance of lithium sulfur batteries. J. Power Sources 2012, 200, 77–82. [Google Scholar] [CrossRef]
- Manthiram, A.; Fu, Y.; Chung, S.H.; Zu, C.; Su, Y.S. Rechargeable Li-S batteries. Chem. Rev. 2014, 114, 11751–11787. [Google Scholar] [CrossRef] [PubMed]
- Suo, L.; Hu, Y.S.; Li, H.; Armand, M.; Chen, L. A new class of Solvent-in-Salt electrolyte for high-energy rechargeable metallic lithium batteries. Nat. Commun. 2013, 4, 1481–1490. [Google Scholar] [CrossRef]
- Kim, J.S.; Hwang, T.H.; Kim, B.G.; Min, J.; Choi, J.W. A lithium sulfur battery with a high areal energy density. Adv. Funct. Mater. 2014, 24, 5359–5367. [Google Scholar] [CrossRef]
- Ji, X.L.; Lee, K.T.; Nazar, L.F. A highly ordered nanostructured carbon-sulfur cathode for lithium-sulfur batteries. Nat. Mater. 2009, 8, 500–506. [Google Scholar] [CrossRef]
- Fan, F.Y.; Woodford, W.H.; Li, Z.; Baram, N.; Smith, K.C.; Helal, A.; McKinley, G.H.; Carter, W.C.; Chiang, Y.M. Polysulfide Flow Batteries Enabled by Percolating Nanoscale Conductor Networks. Nano Letter. 2014, 14, 2210–2218. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, B.; Martinez, M.L.V.; Katiyar, R.K.; Sharma, K.B.; Katiyar Ram, S. Scalable study on nanostructured carbon-sulfur composite electrodes for high energy Li-S battery. ECS Trans. 2017, 77, 47–57. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, C.; Zhou, G.; Lv, W.; Ling, G.; Zhi, L.; Yang, Q.H. Catalytic Effects in Lithium–Sulfur Batteries: Promoted Sulfur Transformation and Reduced Shuttle Effect. Adv. Sci. 2018, 5, 1700270–1700277. [Google Scholar] [CrossRef]
- Zheng, S.; Han, P.; Han, Z.; Zhang, H.; Tang, Z.; Yang, J. High performance C/S composite cathodes with conventional carbonate-based electrolytes in Li-S battery. Sci. Rep. 2014, 4, 4842–4846. [Google Scholar] [CrossRef]
- Zhang, B. Enhancement of long stability of sulfur cathode by encapsulating sulfur into micropores of carbon spheres. Energy Environ. Sci. 2010, 3, 1531–1537. [Google Scholar] [CrossRef]
- Cullity, B.D. Elements of X-ray Diffraction; Addison-Wesley: Boston, MA, USA, 1956; p. 99. [Google Scholar]
- Singh, M.K.; Jang, H.M.; Ryu, S.; Jo, M.-H. Polarized Raman scattering of multiferroic BiFeO3 epitaxial films with rhombohedral R3c symmetry. Appl. Phys. Lett. 2006, 88, 42907–42915. [Google Scholar] [CrossRef]
- Sun, J.; Xiao, L.; Jiang, S.; Li, G.; Huang, Y.; Geng, J. Fluorine-Doped SnO2@Graphene Porous Composite for High Capacity Lithium-Ion Batteries. Chem. Mater. 2015, 27, 4594–4599. [Google Scholar] [CrossRef]
- Xie, K.; You, Y.; Yuan, K.; Lu, W.; Zhang, K.; Xu, F.; Ye, M.; Ke, S.; Shen, C.; Zeng, X. Ferroelectric enhanced polysulfide trapping for Li-S battery improvement. Adv. Mater. 2017, 29, 1604724–1604732. [Google Scholar] [CrossRef]
- Gao, Z.; Schwab, Y.; Zhang, Y.; Song, N.; Li, X. Ferromagnetic nanoparticle assisted polysulfide trapping for enhanced Li-S batteries. Adv. Funct. Mater. 2018, 28, 1800563. [Google Scholar] [CrossRef]
- Liang, C. Hierarchically structured sulfur/carbon nanocomposite material for high energy lithium battery. Chem. Mater. 2009, 21, 4724–4730. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, G.; Cui, Y. Nanostructured sulfur cathodes. Chem. Soc. Rev. 2013, 42, 3018–3026. [Google Scholar] [CrossRef] [PubMed]
- Marmorstein, D.; Yu, T.H.; Strivel, K.A.; Mc Larnon, F.R.; Hou, J.; Cairns, E.J. Electrochemical performance of lithium/sulfur cells with three different polymer electrolytes. J. Power Sources 2000, 86, 219–226. [Google Scholar] [CrossRef]
- Ji, L. Porous carbon nanofiber-sulfur composite electrodes for lithium/sulfur cells. Energy Environ. Sci. 2011, 4, 5053–5059. [Google Scholar] [CrossRef]
- Rao, T.D.; Kandula, K.R.; Kumar, A.; Asthana, S. Improved magnetization and leakage current in Sm and Sc co-substituted BiFeo3. J. Appl. Phys. 2018, 123, 244104–244111. [Google Scholar]
- Li, Z.J.; Hou, Z.L.; Song, W.L.; Liu, X.D.; Cao, W.Q.; Shao, X.H.; Cao, M.S. Unusual continuous dual absorption peaks in Ca-doped BiFeO3 nanostructures for broadened microwave absorption. Nanoscale 2016, 8, 10415–10424. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tripathi, B.; Katiyar, R.K.; Morell, G.; Dixit, A.; Katiyar, R.S. BiFeO3 Coupled Polysulfide Trapping in C/S Composite Cathode Material for Li-S Batteries as Large Efficiency and High Rate Performance. Energies 2021, 14, 8362. https://doi.org/10.3390/en14248362
Tripathi B, Katiyar RK, Morell G, Dixit A, Katiyar RS. BiFeO3 Coupled Polysulfide Trapping in C/S Composite Cathode Material for Li-S Batteries as Large Efficiency and High Rate Performance. Energies. 2021; 14(24):8362. https://doi.org/10.3390/en14248362
Chicago/Turabian StyleTripathi, Balram, Rajesh K. Katiyar, Gerardo Morell, Ambesh Dixit, and Ram S. Katiyar. 2021. "BiFeO3 Coupled Polysulfide Trapping in C/S Composite Cathode Material for Li-S Batteries as Large Efficiency and High Rate Performance" Energies 14, no. 24: 8362. https://doi.org/10.3390/en14248362
APA StyleTripathi, B., Katiyar, R. K., Morell, G., Dixit, A., & Katiyar, R. S. (2021). BiFeO3 Coupled Polysulfide Trapping in C/S Composite Cathode Material for Li-S Batteries as Large Efficiency and High Rate Performance. Energies, 14(24), 8362. https://doi.org/10.3390/en14248362







