Methanol Reforming Processes for Fuel Cell Applications
Abstract
:1. Introduction
2. Hydrogen Production through Methanol
2.1. Methanol Decomposition (MD)
2.2. Partial Oxidation of Methanol (POM)
2.3. Steam Reforming of Methanol (SRM)
2.4. Oxidative Steam Reforming of Methanol (OSRM)
3. Catalysts for Steam Reforming of Methanol
3.1. Cu-Based Catalysts
3.1.1. The Cu/ZnO-Based Catalytic System
3.1.2. Effect of Various Promoters on the Performance of Cu-Based Catalysts
3.1.3. Effect of Preparation Methods on the Performance of Cu-Based Catalysts
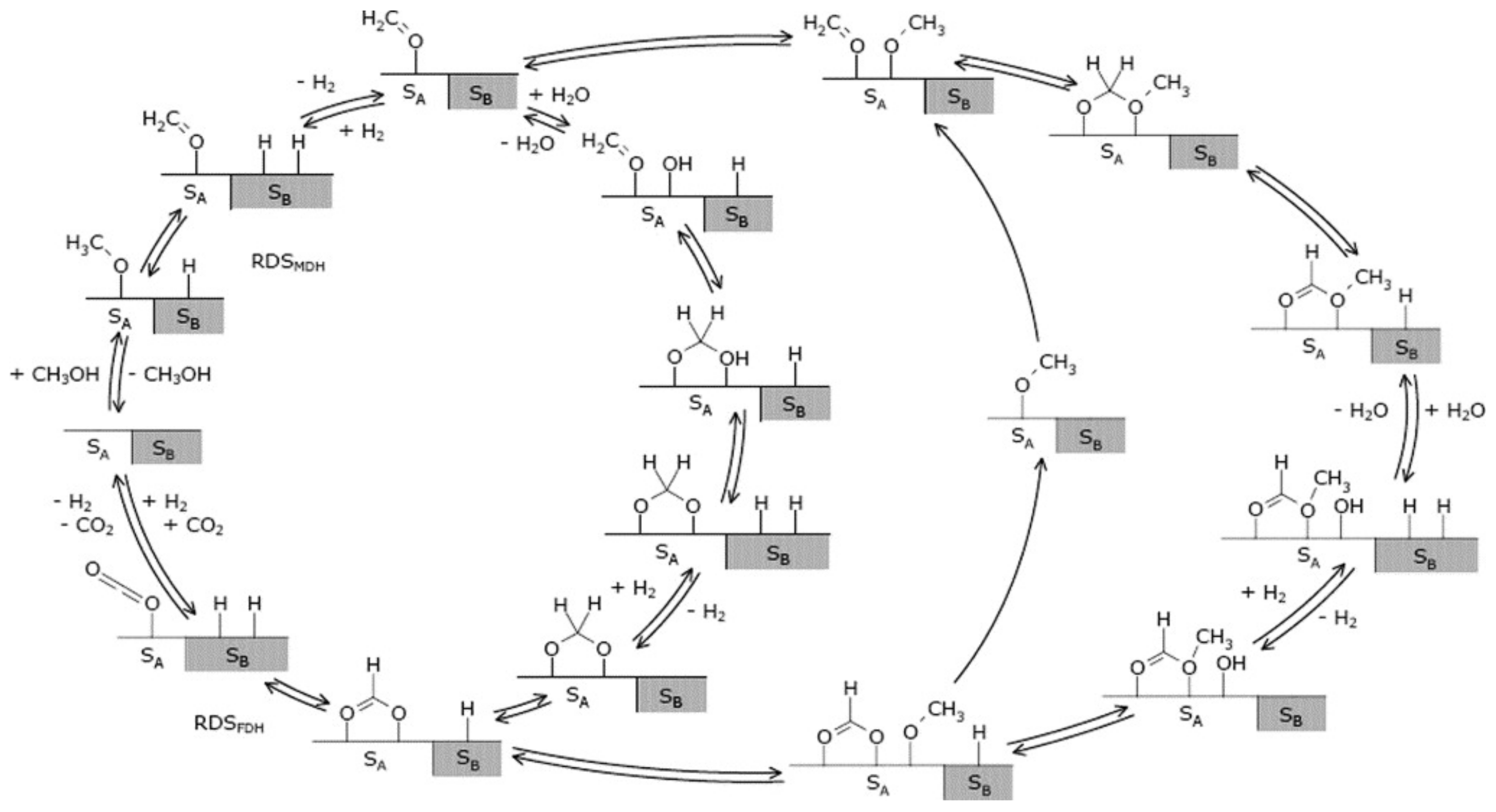
3.1.4. Reaction Mechanism
3.2. Group 8–10 Catalysts
3.2.1. Pd-Based Catalysts
3.2.2. Pt-Based Catalysts
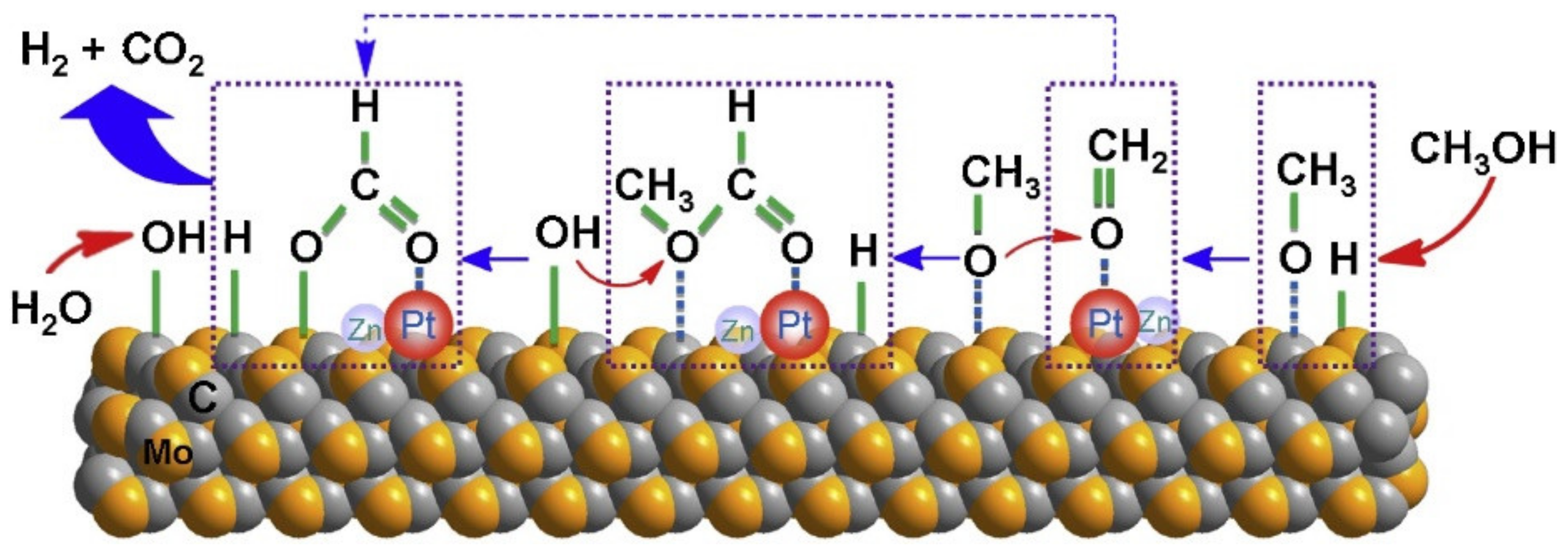
3.2.3. Reaction Mechanism
4. Coupling of Methanol Reformer with HT-PEMFCs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shiva Kumar, S.; Himabindu, V. Hydrogen Production by PEM Water Electrolysis—A Review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar] [CrossRef]
- Ranjekar, A.M.; Yadav, G.D. Steam Reforming of Methanol for Hydrogen Production: A Critical Analysis of Catalysis, Processes, and Scope. Ind. Eng. Chem. Res. 2021, 60, 89–113. [Google Scholar] [CrossRef]
- Höök, M.; Tang, X. Depletion of Fossil Fuels and Anthropogenic Climate Change—A Review. Energy Policy 2013, 52, 797–809. [Google Scholar] [CrossRef] [Green Version]
- Baidya, S.; Nandi, C. Green energy generation using renewable energy technologies. In Advances in Greener Energy Technologies; Green Energy and Technology; Bhoi, A.K., Sherpa, K.S., Kalam, A., Chae, G.-S., Eds.; Springer: Singapore, 2020; pp. 259–276. ISBN 9789811542466. [Google Scholar]
- Rogelj, J.; Huppmann, D.; Krey, V.; Riahi, K.; Clarke, L.; Gidden, M.; Nicholls, Z.; Meinshausen, M. A New Scenario Logic for the Paris Agreement Long-Term Temperature Goal. Nature 2019, 573, 357–363. [Google Scholar] [CrossRef]
- Chandan, A.; Hattenberger, M.; El-kharouf, A.; Du, S.; Dhir, A.; Self, V.; Pollet, B.G.; Ingram, A.; Bujalski, W. High Temperature (HT) Polymer Electrolyte Membrane Fuel Cells (PEMFC)—A Review. J. Power Sources 2013, 231, 264–278. [Google Scholar] [CrossRef]
- Kraytsberg, A.; Ein-Eli, Y. Review of Advanced Materials for Proton Exchange Membrane Fuel Cells. Energy Fuels 2014, 28, 7303–7330. [Google Scholar] [CrossRef]
- Rosli, R.E.; Sulong, A.B.; Daud, W.R.W.; Zulkifley, M.A.; Husaini, T.; Rosli, M.I.; Majlan, E.H.; Haque, M.A. A Review of High-Temperature Proton Exchange Membrane Fuel Cell (HT-PEMFC) System. Int. J. Hydrogen Energy 2017, 42, 9293–9314. [Google Scholar] [CrossRef]
- Choi, M.; Kim, M.; Sohn, Y.-J.; Kim, S.-G. Development of Preheating Methodology for a 5 KW HT-PEMFC System. Int. J. Hydrogen Energy 2021. [Google Scholar] [CrossRef]
- Zhang, J.; Xiang, Y.; Lu, S.; Jiang, S.P. High Temperature Polymer Electrolyte Membrane Fuel Cells for Integrated Fuel Cell—Methanol Reformer Power Systems: A Critical Review. Adv. Sustain. Syst. 2018, 2, 1700184. [Google Scholar] [CrossRef]
- Herdem, M.S.; Sinaki, M.Y.; Farhad, S.; Hamdullahpur, F. An Overview of the Methanol Reforming Process: Comparison of Fuels, Catalysts, Reformers, and Systems. Int. J. Energy Res. 2019, 43, 5076–5105. [Google Scholar] [CrossRef]
- Aicher, T.; Lenz, B.; Gschnell, F.; Groos, U.; Federici, F.; Caprile, L.; Parodi, L. Fuel Processors for Fuel Cell APU Applications. J. Power Sources 2006, 154, 503–508. [Google Scholar] [CrossRef]
- LeValley, T.L.; Richard, A.R.; Fan, M. The Progress in Water Gas Shift and Steam Reforming Hydrogen Production Technologies—A Review. Int. J. Hydrogen Energy 2014, 39, 16983–17000. [Google Scholar] [CrossRef]
- Tarhan, C.; Çil, M.A. A Study on Hydrogen, the Clean Energy of the Future: Hydrogen Storage Methods. J. Energy Storage 2021, 40, 102676. [Google Scholar] [CrossRef]
- Kundu, A.; Shul, Y.G.; Kim, D.H. Chapter seven—Methanol reforming processes. In Advances in Fuel Cells; Zhao, T.S., Kreuer, K.-D., Van Nguyen, T., Eds.; Advances in Fuel Cell; Elsevier Science: Amsterdam, The Netherlands, 2007; Volume 1, pp. 419–472. [Google Scholar]
- Damle, A.S. Hydrogen Production by Reforming of Liquid Hydrocarbons in a Membrane Reactor for Portable Power Generation—Experimental Studies. J. Power Sources 2009, 186, 167–177. [Google Scholar] [CrossRef]
- Sengodan, S.; Lan, R.; Humphreys, J.; Du, D.; Xu, W.; Wang, H.; Tao, S. Advances in Reforming and Partial Oxidation of Hydrocarbons for Hydrogen Production and Fuel Cell Applications. Renew. Sustain. Energy Rev. 2018, 82, 761–780. [Google Scholar] [CrossRef]
- Sá, S.; Silva, H.; Brandão, L.; Sousa, J.M.; Mendes, A. Catalysts for Methanol Steam Reforming—A Review. Appl. Catal. B Environ. 2010, 99, 43–57. [Google Scholar] [CrossRef]
- Behrens, M.; Armbrüster, M. Methanol steam reforming. In Catalysis for Alternative Energy Generation; Guczi, L., Erdôhelyi, A., Eds.; Springer: New York, NY, USA, 2012; pp. 175–235. ISBN 978-1-4614-0344-9. [Google Scholar]
- Xu, X.; Shuai, K.; Xu, B. Review on Copper and Palladium Based Catalysts for Methanol Steam Reforming to Produce Hydrogen. Catalysts 2017, 7, 183. [Google Scholar] [CrossRef]
- Dittmar, B.; Behrens, A.; Schödel, N.; Rüttinger, M.; Franco, T.; Straczewski, G.; Dittmeyer, R. Methane Steam Reforming Operation and Thermal Stability of New Porous Metal Supported Tubular Palladium Composite Membranes. Int. J. Hydrogen Energy 2013, 38, 8759–8771. [Google Scholar] [CrossRef]
- Wu, Y.; Pei, C.; Tian, H.; Liu, T.; Zhang, X.; Chen, S.; Xiao, Q.; Wang, X.; Gong, J. Role of Fe Species of Ni-Based Catalysts for Efficient Low-Temperature Ethanol Steam Reforming. JACS Au 2021, 1, 1459–1470. [Google Scholar] [CrossRef]
- Meunier, N.; Chauvy, R.; Mouhoubi, S.; Thomas, D.; De Weireld, G. Alternative Production of Methanol from Industrial CO2. Renew. Energy 2020, 146, 1192–1203. [Google Scholar] [CrossRef]
- Trop, P.; Anicic, B.; Goricanec, D. Production of Methanol from a Mixture of Torrefied Biomass and Coal. Energy 2014, 77, 125–132. [Google Scholar] [CrossRef]
- Galindo Cifre, P.; Badr, O. Renewable Hydrogen Utilisation for the Production of Methanol. Energy Convers. Manag. 2007, 48, 519–527. [Google Scholar] [CrossRef] [Green Version]
- Yong, S.T.; Ooi, C.W.; Chai, S.P.; Wu, X.S. Review of Methanol Reforming-Cu-Based Catalysts, Surface Reaction Mechanisms, and Reaction Schemes. Int. J. Hydrogen Energy 2013, 38, 9541–9552. [Google Scholar] [CrossRef]
- Cai, F.; Guo, Y.; Ibrahim, J.J.; Zhang, J.; Sun, Y. A Highly Active and Stable Pd/MoC Catalyst for Hydrogen Production from Methanol Decomposition. Appl. Catal. B Environ. 2021, 299, 120648. [Google Scholar] [CrossRef]
- Araiza, D.G.; Gómez-Cortés, A.; Díaz, G. Partial Oxidation of Methanol over Copper Supported on Nanoshaped Ceria for Hydrogen Production. Catal. Today 2017, 282 Part 2, 185–194. [Google Scholar] [CrossRef]
- Shanmugam, V.; Neuberg, S.; Zapf, R.; Pennemann, H.; Kolb, G. Hydrogen Production over Highly Active Pt Based Catalyst Coatings by Steam Reforming of Methanol: Effect of Support and Co-Support. Int. J. Hydrogen Energy 2020, 45, 1658–1670. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, X.; Li, H.; Liu, T.; Sang, S.; Chen, S.; Duan, L.; Zeng, L.; Xiang, W.; Gong, J. Chemical Looping Oxidative Steam Reforming of Methanol: A New Pathway for Auto-Thermal Conversion. Appl. Catal. B Environ. 2020, 269, 118758. [Google Scholar] [CrossRef]
- Bagherzadeh, S.B.; Haghighi, M. Plasma-Enhanced Comparative Hydrothermal and Coprecipitation Preparation of CuO/ZnO/Al2O3 Nanocatalyst Used in Hydrogen Production via Methanol Steam Reforming. Energy Convers. Manag. 2017, 142, 452–465. [Google Scholar] [CrossRef]
- Kapran, A.Y.; Orlyk, S.M. Hydrogen Production in Methanol Reforming on Modified Copper–Zinc Catalysts: A Review. Exp. Chem. 2017, 53, 1–16. [Google Scholar] [CrossRef]
- Mateos-Pedrero, C.; Azenha, C.; Pacheco Tanaka, D.A.; Sousa, J.M.; Mendes, A. The Influence of the Support Composition on the Physicochemical and Catalytic Properties of Cu Catalysts Supported on Zirconia-Alumina for Methanol Steam Reforming. Appl. Catal. B Environ. 2020, 277, 119243. [Google Scholar] [CrossRef]
- Patel, S.; Pant, K.K. Influence of Preparation Method on Performance of Cu(Zn)(Zr)-Alumina Catalysts for the Hydrogen Production via Steam Reforming of Methanol. J. Porous Mater. 2006, 13, 373–378. [Google Scholar] [CrossRef]
- Papavasiliou, J.; Paxinou, A.; Słowik, G.; Neophytides, S.; Avgouropoulos, G. Steam Reforming of Methanol over Nanostructured Pt/TiO2 and Pt/CeO2 Catalysts for Fuel Cell Applications. Catalysts 2018, 8, 544. [Google Scholar] [CrossRef] [Green Version]
- Khani, Y.; Bahadoran, F.; Safari, N.; Soltanali, S.; Taheri, S.A. Hydrogen Production from Steam Reforming of Methanol over Cu-Based Catalysts: The Behavior of ZnxLaxAl1-XO4 and ZnO/La2O3/Al2O3 Lined on Cordierite Monolith Reactors. Int. J. Hydrogen Energy 2019, 44, 11824–11837. [Google Scholar] [CrossRef]
- Kim, W.; Mohaideen, K.K.; Seo, D.J.; Yoon, W.L. Methanol-Steam Reforming Reaction over Cu-Al-Based Catalysts Derived from Layered Double Hydroxides. Int. J. Hydrogen Energy 2017, 42, 2081–2087. [Google Scholar] [CrossRef]
- Liu, X.; Men, Y.; Wang, J.; He, R.; Wang, Y. Remarkable Support Effect on the Reactivity of Pt/In2O3/MOx Catalysts for Methanol Steam Reforming. J. Power Sources 2017, 364, 341–350. [Google Scholar] [CrossRef]
- Udani, P.P.C.; Gunawardana, P.V.D.S.; Lee, H.C.; Kim, D.H. Steam Reforming and Oxidative Steam Reforming of Methanol over CuO–CeO2 Catalysts. Int. J. Hydrogen Energy 2009, 34, 7648–7655. [Google Scholar] [CrossRef]
- Pu, Y.-C.; Li, S.-R.; Yan, S.; Huang, X.; Wang, D.; Ye, Y.-Y.; Liu, Y.-Q. An Improved Cu/ZnO Catalyst Promoted by Sc2O3 for Hydrogen Production from Methanol Reforming. Fuel 2019, 241, 607–615. [Google Scholar] [CrossRef]
- Kurtz, M.; Wilmer, H.; Genger, T.; Hinrichsen, O.; Muhler, M. Deactivation of Supported Copper Catalysts for Methanol Synthesis. Catal. Lett. 2003, 86, 77–80. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, J.; Dagle, V.L.; Halevi, B.; Datye, A.K.; Wang, Y. Influence of ZnO Facets on Pd/ZnO Catalysts for Methanol Steam Reforming. ACS Catal. 2014, 4, 2379–2386. [Google Scholar] [CrossRef]
- Tong, W.; West, A.; Cheung, K.; Yu, K.-M.; Tsang, S.C.E. Dramatic Effects of Gallium Promotion on Methanol Steam Reforming Cu–ZnO Catalyst for Hydrogen Production: Formation of 5 Å Copper Clusters from Cu–ZnGaOx. ACS Catal. 2013, 3, 1231–1244. [Google Scholar] [CrossRef]
- Wang, C.; Ouyang, M.; Li, M.; Lee, S.; Flytzani-Stephanopoulos, M. Low-Coordinated Pd Catalysts Supported on Zn1Zr1Ox Composite Oxides for Selective Methanol Steam Reforming. Appl. Catal. A Gen. 2019, 580, 81–92. [Google Scholar] [CrossRef]
- Talkhoncheh, S.K.; Haghighi, M.; Minaei, S.; Ajamein, H.; Abdollahifar, M. Synthesis of CuO/ZnO/Al2O3/ZrO2/CeO2 Nanocatalysts via Homogeneous Precipitation and Combustion Methods Used in Methanol Steam Reforming for Fuel Cell Grade Hydrogen Production. RSC Adv. 2016, 6, 57199–57209. [Google Scholar] [CrossRef]
- Rameshan, C.; Lorenz, H.; Armbrüster, M.; Kasatkin, I.; Klötzer, B.; Götsch, T.; Ploner, K.; Penner, S. Impregnated and Co-Precipitated Pd–Ga2O3, Pd–In2O3 and Pd–Ga2O3–In2O3 Catalysts: Influence of the Microstructure on the CO2 Selectivity in Methanol Steam Reforming. Catal. Lett. 2018, 148, 3062–3071. [Google Scholar] [CrossRef] [Green Version]
- Avgouropoulos, G.; Schlicker, S.; Schelhaas, K.-P.; Papavasiliou, J.; Papadimitriou, K.D.; Theodorakopoulou, E.; Gourdoupi, N.; Machocki, A.; Ioannides, T.; Kallitsis, J.K.; et al. Performance Evaluation of a Proof-of-Concept 70 W Internal Reforming Methanol Fuel Cell System. J. Power Sources 2016, 307, 875–882. [Google Scholar] [CrossRef]
- Papavasiliou, J.; Schütt, C.; Kolb, G.; Neophytides, S.; Avgouropoulos, G. Technological Aspects of an Auxiliary Power Unit with Internal Reforming Methanol Fuel Cell. Int. J. Hydrogen Energy 2019, 44, 12818–12828. [Google Scholar] [CrossRef]
- Pan, C.; He, R.; Li, Q.; Jensen, J.O.; Bjerrum, N.J.; Hjulmand, H.A.; Jensen, A.B. Integration of High Temperature PEM Fuel Cells with a Methanol Reformer. J. Power Sources 2005, 145, 392–398. [Google Scholar] [CrossRef]
- Ribeirinha, P.; Schuller, G.; Boaventura, M.; Mendes, A. Synergetic Integration of a Methanol Steam Reforming Cell with a High Temperature Polymer Electrolyte Fuel Cell. Int. J. Hydrogen Energy 2017, 42, 13902–13912. [Google Scholar] [CrossRef]
- Lotrič, A.; Sekavčnik, M.; Pohar, A.; Likozar, B.; Hočevar, S. Conceptual Design of an Integrated Thermally Self-Sustained Methanol Steam Reformer—High-Temperature PEM Fuel Cell Stack Manportable Power Generator. Int. J. Hydrogen Energy 2017, 42, 16700–16713. [Google Scholar] [CrossRef]
- Papavasiliou, J.; Avgouropoulos, G.; Ioannides, T. CuMnOx Catalysts for Internal Reforming Methanol Fuel Cells: Application Aspects. Int. J. Hydrogen Energy 2012, 37, 16739–16747. [Google Scholar] [CrossRef]
- Jing, J.; Li, L.; Chu, W.; Wei, Y.; Jiang, C. Microwave-Assisted Synthesis of High Performance Copper-Based Catalysts for Hydrogen Production from Methanol Decomposition. Int. J. Hydrogen Energy 2018, 43, 12059–12068. [Google Scholar] [CrossRef]
- Marbán, G.; López, A.; López, I.; Valdés-Solís, T. A Highly Active, Selective and Stable Copper/Cobalt-Structured Nanocatalyst for Methanol Decomposition. Appl. Catal. B Environ. 2010, 99, 257–264. [Google Scholar] [CrossRef]
- Manova, E.; Tsoncheva, T.; Estournès, C.; Paneva, D.; Tenchev, K.; Mitov, I.; Petrov, L. Nanosized Iron and Iron–Cobalt Spinel Oxides as Catalysts for Methanol Decomposition. Appl. Catal. A Gen. 2006, 300, 170–180. [Google Scholar] [CrossRef]
- Mizsey, P.; Newson, E.; Truong, T.; Hottinger, P. The Kinetics of Methanol Decomposition: A Part of Autothermal Partial Oxidation to Produce Hydrogen for Fuel Cells. Appl. Catal. A Gen. 2001, 213, 233–237. [Google Scholar] [CrossRef]
- Brown, J.C.; Gulari, E. Hydrogen Production from Methanol Decomposition over Pt/Al2O3 and Ceria Promoted Pt/Al2O3 Catalysts. Catal. Commun. 2004, 5, 431–436. [Google Scholar] [CrossRef]
- Ostroverkh, A.; Johánek, V.; Kúš, P.; Šedivá, R.; Matolín, V. Efficient Ceria–Platinum Inverse Catalyst for Partial Oxidation of Methanol. Langmuir 2016, 32, 6297–6309. [Google Scholar] [CrossRef]
- Borup, R.; Meyers, J.; Pivovar, B.; Kim, Y.S.; Mukundan, R.; Garland, N.; Myers, D.; Wilson, M.; Garzon, F.; Wood, D.; et al. Scientific Aspects of Polymer Electrolyte Fuel Cell Durability and Degradation. Chemical. Rev. 2007, 107, 3904–3951. [Google Scholar] [CrossRef]
- Ajamein, H.; Haghighi, M.; Alaei, S. The Role of Various Fuels on Microwave-Enhanced Combustion Synthesis of CuO/ZnO/Al2O3 Nanocatalyst Used in Hydrogen Production via Methanol Steam Reforming. Energy Convers. Manag. 2017, 137, 61–73. [Google Scholar] [CrossRef]
- Faungnawakij, K.; Kikuchi, R.; Eguchi, K. Thermodynamic Evaluation of Methanol Steam Reforming for Hydrogen Production. J. Power Sources 2006, 161, 87–94. [Google Scholar] [CrossRef]
- Wang, J.; Chen, H.; Tian, Y.; Yao, M.; Li, Y. Thermodynamic Analysis of Hydrogen Production for Fuel Cells from Oxidative Steam Reforming of Methanol. Fuel 2012, 97, 805–811. [Google Scholar] [CrossRef]
- Lwin, Y.; Daud, W.R.W.; Mohamad, A.B.; Yaakob, Z. Hydrogen Production from Steam–Methanol Reforming: Thermodynamic Analysis. Int. J. Hydrogen Energy 2000, 25, 47–53. [Google Scholar] [CrossRef]
- Xing, S.; Zhao, C.; Ban, S.; Liu, Y.; Wang, H. Thermodynamic Performance Analysis of the Influence of Multi-Factor Coupling on the Methanol Steam Reforming Reaction. Int. J. Hydrogen Energy 2020, 45, 7015–7024. [Google Scholar] [CrossRef]
- Lattner, J.R.; Harold, M.P. Autothermal Reforming of Methanol: Experiments and Modeling. Catal. Today 2007, 120, 78–89. [Google Scholar] [CrossRef]
- Chen, W.-H.; Su, Y.-Q.; Lin, B.-J.; Kuo, J.-K.; Kuo, P.-C. Hydrogen Production from Partial Oxidation and Autothermal Reforming of Methanol from a Cold Start in Sprays. Fuel 2021, 287, 119638. [Google Scholar] [CrossRef]
- Tang, H.-Y.; Erickson, P.; Yoon, H.C.; Liao, C.-H. Comparison of Steam and Autothermal Reforming of Methanol Using a Packed-Bed Low-Cost Copper Catalyst. Int. J. Hydrogen Energy 2009, 34, 7656–7665. [Google Scholar] [CrossRef]
- Garcia, G.; Arriola, E.; Chen, W.-H.; De Luna, M.D. A Comprehensive Review of Hydrogen Production from Methanol Thermochemical Conversion for Sustainability. Energy 2021, 217, 119384. [Google Scholar] [CrossRef]
- Rozovskii, A.Y.; Lin, G.I. Fundamentals of Methanol Synthesis and Decomposition. Top. Catal. 2003, 22, 137–150. [Google Scholar] [CrossRef]
- Palo, D.R.; Dagle, R.A.; Holladay, J.D. Methanol Steam Reforming for Hydrogen Production. Chem. Rev. 2007, 107, 3992–4021. [Google Scholar] [CrossRef]
- Butt, J. Activation, Deactivation, and Poisoning of Catalysts; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 978-0-323-14086-7. [Google Scholar]
- Spencer, M.S. The Role of Zinc Oxide in Cu/ZnO Catalysts for Methanol Synthesis and the Water–Gas Shift Reaction. Top. Catal. 1999, 8, 259. [Google Scholar] [CrossRef]
- Behrens, M.; Studt, F.; Kasatkin, I.; Kühl, S.; Hävecker, M.; Abild-Pedersen, F.; Zander, S.; Girgsdies, F.; Kurr, P.; Kniep, B.-L.; et al. The Active Site of Methanol Synthesis over Cu/ZnO/Al2O3 Industrial Catalysts. Science 2012, 336, 893–897. [Google Scholar] [CrossRef]
- Rameshan, C.; Stadlmayr, W.; Penner, S.; Lorenz, H.; Memmel, N.; Hävecker, M.; Blume, R.; Teschner, D.; Rocha, T.; Zemlyanov, D.; et al. Hydrogen Production by Methanol Steam Reforming on Copper Boosted by Zinc-Assisted Water Activation. Angew. Chem. Int. Ed. 2012, 51, 3002–3006. [Google Scholar] [CrossRef] [Green Version]
- Hansen, J.B.; Højlund Nielsen, P.E. Methanol Synthesis. In Handbook of Heterogeneous Catalysis; American Cancer Society: Atlanta, GA, USA, 2008; pp. 2920–2949. ISBN 978-3-527-61004-4. [Google Scholar]
- Grunwaldt, J.-D.; Molenbroek, A.M.; Topsøe, N.-Y.; Topsøe, H.; Clausen, B.S. In Situ Investigations of Structural Changes in Cu/ZnO Catalysts. J. Catal. 2000, 194, 452–460. [Google Scholar] [CrossRef]
- Kasatkin, I.; Kurr, P.; Kniep, B.; Trunschke, A.; Schlögl, R. Role of Lattice Strain and Defects in Copper Particles on the Activity of Cu/ZnO/Al2O3 Catalysts for Methanol Synthesis. Angew. Chem. Int. Ed. 2007, 46, 7324–7327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burch, R.; Golunski, S.E.; Spencer, M.S. The Role of Copper and Zinc Oxide in Methanol Synthesis Catalysts. J. Chem. Soc. Faraday Trans. 1990, 86, 2683–2691. [Google Scholar] [CrossRef]
- Nakamura, J.; Choi, Y.; Fujitani, T. On the Issue of the Active Site and the Role of ZnO in Cu/ZnO Methanol Synthesis Catalysts. Top. Catal. 2003, 22, 277–285. [Google Scholar] [CrossRef]
- D’Alnoncourt, R.N.; Xia, X.; Strunk, J.; Löffler, E.; Hinrichsen, O.; Muhler, M. The Influence of Strongly Reducing Conditions on Strong Metal–Support Interactions in Cu/ZnO Catalysts Used for Methanol Synthesis. Phys. Chem. Chem. Phys. 2006, 8, 1525–1538. [Google Scholar] [CrossRef]
- Frank, B.; Jentoft, F.C.; Soerijanto, H.; Kröhnert, J.; Schlögl, R.; Schomäcker, R. Steam Reforming of Methanol over Copper-Containing Catalysts: Influence of Support Material on Microkinetics. J. Catal. 2007, 246, 177–192. [Google Scholar] [CrossRef] [Green Version]
- Twigg, M.V.; Spencer, M.S. Deactivation of Copper Metal Catalysts for Methanol Decomposition, Methanol Steam Reforming and Methanol Synthesis. Top. Catal. 2003, 22, 191–203. [Google Scholar] [CrossRef]
- Huang, G.; Liaw, B.-J.; Jhang, C.-J.; Chen, Y.-Z. Steam Reforming of Methanol over CuO/ZnO/CeO2/ZrO2/Al2O3 Catalysts. Appl. Catal. A Gen. 2009, 358, 7–12. [Google Scholar] [CrossRef]
- Shishido, T.; Yamamoto, Y.; Morioka, H.; Takehira, K. Production of Hydrogen from Methanol over Cu/ZnO and Cu/ZnO/Al2O3 Catalysts Prepared by Homogeneous Precipitation: Steam Reforming and Oxidative Steam Reforming. J. Mol. Catal. A Chem. 2007, 268, 185–194. [Google Scholar] [CrossRef] [Green Version]
- Matsumura, Y.; Ishibe, H. High Temperature Steam Reforming of Methanol over Cu/ZnO/ZrO2 Catalysts. Appl. Catal. B Environ. 2009, 91, 524–532. [Google Scholar] [CrossRef]
- Matter, P.H.; Braden, D.J.; Ozkan, U.S. Steam Reforming of Methanol to H2 over Nonreduced Zr-Containing CuO/ZnO Catalysts. J. Catal. 2004, 223, 340–351. [Google Scholar] [CrossRef]
- Yang, S.; Zhou, F.; Liu, Y.; Zhang, L.; Chen, Y.; Wang, H.; Tian, Y.; Zhang, C.; Liu, D. Morphology Effect of Ceria on the Performance of CuO/CeO2 Catalysts for Hydrogen Production by Methanol Steam Reforming. Int. J. Hydrogen Energy 2019, 44, 7252–7261. [Google Scholar] [CrossRef]
- Liu, Y.; Hayakawa, T.; Tsunoda, T.; Suzuki, K.; Hamakawa, S.; Murata, K.; Shiozaki, R.; Ishii, T.; Kumagai, M. Steam Reforming of Methanol Over Cu/CeO2 Catalysts Studied in Comparison with Cu/ZnO and Cu/Zn(Al)O Catalysts. Top. Catal. 2003, 22, 205–213. [Google Scholar] [CrossRef]
- Jones, S.D.; Hagelin-Weaver, H.E. Steam Reforming of Methanol over CeO2- and ZrO2-Promoted Cu-ZnO Catalysts Supported on Nanoparticle Al2O3. Appl. Catal. B Environ. 2009, 90, 195–204. [Google Scholar] [CrossRef]
- Matsumura, Y. Stabilization of Cu/ZnO/ZrO2 Catalyst for Methanol Steam Reforming to Hydrogen by Coprecipitation on Zirconia Support. J. Power Sources 2013, 238, 109–116. [Google Scholar] [CrossRef]
- Arena, F.; Italiano, G.; Barbera, K.; Bordiga, S.; Bonura, G.; Spadaro, L.; Frusteri, F. Solid-State Interactions, Adsorption Sites and Functionality of Cu-ZnO/ZrO2 Catalysts in the CO2 Hydrogenation to CH3OH. Appl. Catal. A Gen. 2008, 350, 16–23. [Google Scholar] [CrossRef]
- Breen, J.P.; Ross, J.R.H. Methanol Reforming for Fuel-Cell Applications: Development of Zirconia-Containing Cu–Zn–Al Catalysts. Catal. Today 1999, 51, 521–533. [Google Scholar] [CrossRef]
- Wu, G.-S.; Mao, D.-S.; Lu, G.-Z.; Cao, Y.; Fan, K.-N. The Role of the Promoters in Cu Based Catalysts for Methanol Steam Reforming. Catal. Lett. 2009, 130, 177–184. [Google Scholar] [CrossRef]
- Baneshi, J.; Haghighi, M.; Jodeiri, N.; Abdollahifar, M.; Ajamein, H. Urea–Nitrate Combustion Synthesis of ZrO2 and CeO2 Doped CuO/Al2O3 Nanocatalyst Used in Steam Reforming of Biomethanol for Hydrogen Production. Ceram. Int. 2014, 40, 14177–14184. [Google Scholar] [CrossRef]
- Minaei, S.; Haghighi, M.; Jodeiri, N.; Ajamein, H.; Abdollahifar, M. Urea-Nitrates Combustion Preparation of CeO2-Promoted CuO/ZnO/Al2O3 Nanocatalyst for Fuel Cell Grade Hydrogen Production via Methanol Steam Reforming. Adv. Powder Technol. 2017, 28, 842–853. [Google Scholar] [CrossRef]
- Bagherzadeh, S.B.; Haghighi, M.; Rahemi, N. Novel Oxalate Gel Coprecipitation Synthesis of ZrO2-CeO2-Promoted CuO-ZnO-Al2O3 Nanocatalyst for Fuel Cell-Grade Hydrogen Production from Methanol: Influence of Ceria-Zirconia Loading. Energy Convers. Manag. 2017, 134, 88–102. [Google Scholar] [CrossRef]
- Patel, S.; Pant, K.K. Activity and Stability Enhancement of Copper–Alumina Catalysts Using Cerium and Zinc Promoters for the Selective Production of Hydrogen via Steam Reforming of Methanol. J. Power Sources 2006, 159, 139–143. [Google Scholar] [CrossRef]
- Yu, K.M.K.; Tong, W.; West, A.; Cheung, K.; Li, T.; Smith, G.; Guo, Y.; Tsang, S.C.E. Non-Syngas Direct Steam Reforming of Methanol to Hydrogen and Carbon Dioxide at Low Temperature. Nat. Commun. 2012, 3, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Pohar, A.; Hočevar, S.; Likozar, B.; Levec, J. Synthesis and Characterization of Gallium-Promoted Copper–Ceria Catalyst and Its Application for Methanol Steam Reforming in a Packed Bed Reactor. Catal. Today 2015, 256, 358–364. [Google Scholar] [CrossRef]
- Tong, W.; Cheung, K.; West, A.; Yu, K.-M.; Tsang, S.C.E. Direct Methanol Steam Reforming to Hydrogen over CuZnGaOx Catalysts without CO Post-Treatment: Mechanistic Considerations. Phys. Chem. Chem. Phys. 2013, 15, 7240–7248. [Google Scholar] [CrossRef]
- Li, M.M.-J.; Zeng, Z.; Liao, F.; Hong, X.; Tsang, S.C.E. Enhanced CO2 Hydrogenation to Methanol over CuZn Nanoalloy in Ga Modified Cu/ZnO Catalysts. J. Catal. 2016, 343, 157–167. [Google Scholar] [CrossRef]
- Li, M.M.-J.; Zheng, J.; Qu, J.; Liao, F.; Raine, E.; Kuo, W.C.H.; Su, S.S.; Po, P.; Yuan, Y.; Tsang, S.C.E. The Remarkable Activity and Stability of a Highly Dispersive Beta-Brass Cu-Zn Catalyst for the Production of Ethylene Glycol. Sci. Rep. 2016, 6, 20527. [Google Scholar] [CrossRef] [Green Version]
- Li, M.M.-J.; Chen, C.; Ayvalı, T.; Suo, H.; Zheng, J.; Teixeira, I.F.; Ye, L.; Zou, H.; O’Hare, D.; Tsang, S.C.E. CO2 Hydrogenation to Methanol over Catalysts Derived from Single Cationic Layer CuZnGa LDH Precursors. ACS Catal. 2018, 8, 4390–4401. [Google Scholar] [CrossRef]
- Papavasiliou, J.; Avgouropoulos, G.; Ioannides, T. Steam Reforming of Methanol over Copper–Manganese Spinel Oxide Catalysts. Catal. Commun. 2005, 6, 497–501. [Google Scholar] [CrossRef]
- Papavasiliou, J.; Avgouropoulos, G.; Ioannides, T. Combined Steam Reforming of Methanol over Cu–Mn Spinel Oxide Catalysts. J. Catal. 2007, 251, 7–20. [Google Scholar] [CrossRef]
- Lindström, B.; Pettersson, L.J.; Govind Menon, P. Activity and Characterization of Cu/Zn, Cu/Cr and Cu/Zr on γ-Alumina for Methanol Reforming for Fuel Cell Vehicles. Appl. Catal. A Gen. 2002, 234, 111–125. [Google Scholar] [CrossRef]
- Valdés-Solís, T.; Marbán, G.; Fuertes, A.B. Nanosized Catalysts for the Production of Hydrogen by Methanol Steam Reforming. Catal. Today 2006, 116, 354–360. [Google Scholar] [CrossRef]
- Maiti, S.; Llorca, J.; Dominguez, M.; Colussi, S.; Trovarelli, A.; Priolkar, K.R.; Aquilanti, G.; Gayen, A. Combustion Synthesized Copper-Ion Substituted FeAl2O4 (Cu0.1Fe0.9Al2O4): A Superior Catalyst for Methanol Steam Reforming Compared to Its Impregnated Analogue. J. Power Sources 2016, 304, 319–331. [Google Scholar] [CrossRef] [Green Version]
- Maiti, S.; Das, D.; Pal, K.; Llorca, J.; Soler, L.; Colussi, S.; Trovarelli, A.; Priolkar, K.R.; Sarode, P.R.; Asakura, K.; et al. Methanol Steam Reforming Behavior of Sol-Gel Synthesized Nanodimensional CuxFe1-XAl2O4 Hercynites. Appl. Catal. A Gen. 2019, 570, 73–83. [Google Scholar] [CrossRef]
- Jeong, H.; Kim, K.I.; Kim, T.H.; Ko, C.H.; Park, H.C.; Song, I.K. Hydrogen Production by Steam Reforming of Methanol in a Micro-Channel Reactor Coated with Cu/ZnO/ZrO2/Al2O3 Catalyst. J. Power Sources 2006, 159, 1296–1299. [Google Scholar] [CrossRef]
- Kawamura, Y.; Yamamoto, K.; Ogura, N.; Katsumata, T.; Igarashi, A. Preparation of Cu/ZnO/Al2O3 Catalyst for a Micro Methanol Reformer. J. Power Sources 2005, 150, 20–26. [Google Scholar] [CrossRef]
- Zhang, L.; Pan, L.; Ni, C.; Sun, T.; Zhao, S.; Wang, S.; Wang, A.; Hu, Y. CeO2–ZrO2-Promoted CuO/ZnO Catalyst for Methanol Steam Reforming. Int. J. Hydrogen Energy 2013, 38, 4397–4406. [Google Scholar] [CrossRef]
- Oguchi, H.; Nishiguchi, T.; Matsumoto, T.; Kanai, H.; Utani, K.; Matsumura, Y.; Imamura, S. Steam Reforming of Methanol over Cu/CeO2/ZrO2 Catalysts. Appl. Catal. A Gen. 2005, 281, 69–73. [Google Scholar] [CrossRef]
- Toyir, J.; Ramírez de la Piscina, P.; Homs, N. Ga-Promoted Copper-Based Catalysts Highly Selective for Methanol Steam Reforming to Hydrogen; Relation with the Hydrogenation of CO2 to Methanol. Int. J. Hydrogen Energy 2015, 40, 11261–11266. [Google Scholar] [CrossRef]
- Huang, X.; Ma, L.; Wainwright, M.S. The Influence of Cr, Zn and Co Additives on the Performance of Skeletal Copper Catalysts for Methanol Synthesis and Related Reactions. Appl. Catal. A Gen. 2004, 257, 235–243. [Google Scholar] [CrossRef]
- Kuo, M.-T.; Chen, Y.-Y.; Hung, W.-Y.; Lin, S.-F.; Lin, H.-P.; Hsu, C.-H.; Shih, H.-Y.; Xie, W.-A.; Li, S.-N. Synthesis of Mesoporous CuFe/Silicates Catalyst for Methanol Steam Reforming. Int. J. Hydrogen Energy 2019, 44, 14416–14423. [Google Scholar] [CrossRef]
- Yao, C.-Z.; Wang, L.-C.; Liu, Y.-M.; Wu, G.-S.; Cao, Y.; Dai, W.-L.; He, H.-Y.; Fan, K.-N. Effect of Preparation Method on the Hydrogen Production from Methanol Steam Reforming over Binary Cu/ZrO2 Catalysts. Appl. Catal. A Gen. 2006, 297, 151–158. [Google Scholar] [CrossRef]
- Zhang, L.; Meng, M.; Zhou, S.; Sun, Z.; Zhang, J.; Xie, Y.; Hu, T. Promotional Effect of Partial Substitution of Zn by Ce in CuZnAlO Catalysts Used for Hydrogen Production via Steam Reforming of Dimethyl Ether. J. Power Sources 2013, 232, 286–296. [Google Scholar] [CrossRef]
- Mrad, M.; Hammoud, D.; Gennequin, C.; Aboukaïs, A.; Abi-Aad, E. A Comparative Study on the Effect of Zn Addition to Cu/Ce and Cu/Ce–Al Catalysts in the Steam Reforming of Methanol. Appl. Catal. A Gen. 2014, 471, 84–90. [Google Scholar] [CrossRef]
- Hammoud, D.; Gennequin, C.; Aboukaïs, A.; Aad, E.A. Steam Reforming of Methanol over X% Cu/Zn–Al 400 500 Based Catalysts for Production of Hydrogen: Preparation by Adopting Memory Effect of Hydrotalcite and Behavior Evaluation. Int. J. Hydrogen Energy 2015, 40, 1283–1297. [Google Scholar] [CrossRef]
- Shen, J.-P.; Song, C. Influence of Preparation Method on Performance of Cu/Zn-Based Catalysts for Low-Temperature Steam Reforming and Oxidative Steam Reforming of Methanol for H2 Production for Fuel Cells. Catal. Today 2002, 77, 89–98. [Google Scholar] [CrossRef]
- Motevalian Seyedi, A.; Haghighi, M.; Rahemi, N. Significant Influence of Cutting-Edge Plasma Technology on Catalytic Properties and Performance of CuO-ZnO-Al2O3-ZrO2 Nanocatalyst Used in Methanol Steam Reforming for Fuel Cell Grade Hydrogen Production. Ceram. Int. 2017, 43, 6201–6213. [Google Scholar] [CrossRef]
- Ajamein, H.; Haghighi, M. On the Microwave Enhanced Combustion Synthesis of CuO–ZnO–Al2O3 Nanocatalyst Used in Methanol Steam Reforming for Fuel Cell Grade Hydrogen Production: Effect of Microwave Irradiation and Fuel Ratio. Energy Convers. Manag. 2016, 118, 231–242. [Google Scholar] [CrossRef]
- Ahmadi, F.; Haghighi, M.; Ajamein, H. Sonochemically Coprecipitation Synthesis of CuO/ZnO/ZrO2/Al2O3 Nanocatalyst for Fuel Cell Grade Hydrogen Production via Steam Methanol Reforming. J. Mol. Catal. A Chem. 2016, 421, 196–208. [Google Scholar] [CrossRef]
- Sanches, S.G.; Flores, J.H.; de Avillez, R.R.; Pais da Silva, M.I. Influence of Preparation Methods and Zr and Y Promoters on Cu/ZnO Catalysts Used for Methanol Steam Reforming. Int. J. Hydrogen Energy 2012, 37, 6572–6579. [Google Scholar] [CrossRef]
- Bartoň, J.; Pour, V. Kinetics of Catalytic Conversion of Methanol at Higher Pressures. Collect. Czechoslov. Chem. Commun. 1980, 45, 3402–3407. [Google Scholar] [CrossRef]
- Santacesaria, E.; Carrá, S. Kinetics of Catalytic Steam Reforming of Methanol in a Cstr Reactor. Appl. Catal. 1983, 5, 345–358. [Google Scholar] [CrossRef]
- Amphlett, J.C.; Evans, M.J.; Mann, R.F.; Weir, R.D. Hydrogen Production by the Catalytic Steam Reforming of Methanol: Part 2: Kinetics of Methanol Decomposition Using Girdler G66B Catalyst. Can. J. Chem. Eng. 1985, 63, 605–611. [Google Scholar] [CrossRef]
- Takahashi, K.; Takezawa, N.; Kobayashi, H. The Mechanism of Steam Reforming of Methanol over a Copper-Silica Catalyst. Appl. Catal. 1982, 2, 363–366. [Google Scholar] [CrossRef]
- Jiang, C.J.; Trimm, D.L.; Wainwright, M.S.; Cant, N.W. Kinetic Study of Steam Reforming of Methanol over Copper-Based Catalysts. Appl. Catal. A Gen. 1993, 93, 245–255. [Google Scholar] [CrossRef]
- Peppley, B.A.; Amphlett, J.C.; Kearns, L.M.; Mann, R.F. Methanol–Steam Reforming on Cu/ZnO/Al2O3 Catalysts. Part 2. A Comprehensive Kinetic Model. Appl. Catal. A Gen. 1999, 179, 31–49. [Google Scholar] [CrossRef]
- Mastalir, A.; Frank, B.; Szizybalski, A.; Soerijanto, H.; Deshpande, A.; Niederberger, M.; Schomäcker, R.; Schlögl, R.; Ressler, T. Steam Reforming of Methanol over Cu/ZrO2/CeO2 Catalysts: A Kinetic Study. J. Catal. 2005, 230, 464–475. [Google Scholar] [CrossRef] [Green Version]
- Papavasiliou, J.; Avgouropoulos, G.; Ioannides, T. Steady-State Isotopic Transient Kinetic Analysis of Steam Reforming of Methanol over Cu-Based Catalysts. Appl. Catal. B Environ. 2009, 88, 490–496. [Google Scholar] [CrossRef]
- Takezawa, N.; Iwasa, N. Steam Reforming and Dehydrogenation of Methanol: Difference in the Catalytic Functions of Copper and Group VIII Metals. Catal. Today 1997, 36, 45–56. [Google Scholar] [CrossRef]
- Zhang, R.; Sun, Y.; Peng, S. In Situ FTIR Studies of Methanol Adsorption and Dehydrogenation over Cu/SiO2 Catalyst. Fuel 2002, 81, 1619–1624. [Google Scholar] [CrossRef]
- Xia, G.; Holladay, J.D.; Dagle, R.A.; Jones, E.O.; Wang, Y. Development of Highly Active Pd-ZnO/Al2O3 Catalysts for Microscale Fuel Processor Applications. Chem. Eng. Technol. 2005, 28, 515–519. [Google Scholar] [CrossRef]
- Iwasa, N.; Mayanagi, T.; Ogawa, N.; Sakata, K.; Takezawa, N. New Catalytic Functions of Pd–Zn, Pd–Ga, Pd–In, Pt–Zn, Pt–Ga and Pt–In Alloys in the Conversions of Methanol. Catal. Lett. 1998, 54, 119–123. [Google Scholar] [CrossRef]
- Cai, F.; Ibrahim, J.J.; Fu, Y.; Kong, W.; Zhang, J.; Sun, Y. Low-Temperature Hydrogen Production from Methanol Steam Reforming on Zn-Modified Pt/MoC Catalysts. Appl. Catal. B Environ. 2020, 264, 118500. [Google Scholar] [CrossRef]
- Barrios, C.E.; Bosco, M.V.; Baltanás, M.A.; Bonivardi, A.L. Hydrogen Production by Methanol Steam Reforming: Catalytic Performance of Supported-Pd on Zinc–Cerium Oxides’ Nanocomposites. Appl. Catal. B Environ. 2015, 179, 262–275. [Google Scholar] [CrossRef]
- Men, Y.; Yang, M. SMSI-like Behavior and Ni Promotion Effect on NiZnAl Catalysts in Steam Reforming of Methanol. Catal. Commun. 2012, 22, 68–73. [Google Scholar] [CrossRef]
- Liu, Z.; Yao, S.; Johnston-Peck, A.; Xu, W.; Rodriguez, J.A.; Senanayake, S.D. Methanol Steam Reforming over Ni-CeO2 Model and Powder Catalysts: Pathways to High Stability and Selectivity for H2/CO2 Production. Catal. Today 2018, 311, 74–80. [Google Scholar] [CrossRef]
- Iwasa, N.; Kudo, S.; Takahashi, H.; Masuda, S.; Takezawa, N. Highly Selective Supported Pd Catalysts for Steam Reforming of Methanol. Catal. Lett. 1993, 19, 211–216. [Google Scholar] [CrossRef]
- Iwasa, N.; Masuda, S.; Takezawa, N. Steam Reforming of Methanol over Ni, Co, Pd and Pt Supported on ZnO. React. Kinet. Catal. Lett. 1995, 2, 349–353. [Google Scholar] [CrossRef]
- Iwasa, N.; Takezawa, N. New Supported Pd and Pt Alloy Catalysts for Steam Reforming and Dehydrogenation of Methanol. Top. Catal. 2003, 22, 215–224. [Google Scholar] [CrossRef]
- Dagle, R.A.; Chin, Y.-H.; Wang, Y. The Effects of PdZn Crystallite Size on Methanol Steam Reforming. Top. Catal. 2007, 46, 358–362. [Google Scholar] [CrossRef]
- Chin, Y.-H.; Wang, Y.; Dagle, R.A.; Shari Li, X. Methanol Steam Reforming over Pd/ZnO: Catalyst Preparation and Pretreatment Studies. Fuel Process. Technol. 2003, 83, 193–201. [Google Scholar] [CrossRef]
- Ranganathan, E.S.; Bej, S.K.; Thompson, L.T. Methanol Steam Reforming over Pd/ZnO and Pd/CeO2 Catalysts. Appl. Catal. A Gen. 2005, 289, 153–162. [Google Scholar] [CrossRef]
- Conant, T.; Karim, A.M.; Lebarbier, V.; Wang, Y.; Girgsdies, F.; Schlögl, R.; Datye, A. Stability of Bimetallic Pd–Zn Catalysts for the Steam Reforming of Methanol. J. Catal. 2008, 257, 64–70. [Google Scholar] [CrossRef]
- Avgouropoulos, G.; Papavasiliou, J.; Ioannides, T. Hydrogen Production from Methanol over Combustion-Synthesized Noble Metal/Ceria Catalysts. Chem. Eng. J. 2009, 154, 274–280. [Google Scholar] [CrossRef]
- Kolb, G.; Keller, S.; Pecov, S.; Pennemann, H.; Zapf, R. Development of Microstructured Catalytic Wall Reactors for Hydrogen Production by Methanol Steam Reforming over Novel Pt/In2O3/Al2O3 Catalysts. Chem. Eng. Trans. 2011, 24, 133–138. [Google Scholar]
- Barbosa, R.L.; Papaefthimiou, V.; Law, Y.T.; Teschner, D.; Hävecker, M.; Knop-Gericke, A.; Zapf, R.; Kolb, G.; Schlögl, R.; Zafeiratos, S. Methanol Steam Reforming over Indium-Promoted Pt/Al2O3 Catalyst: Nature of the Active Surface. J. Phys. Chem. C 2013, 117, 6143–6150. [Google Scholar] [CrossRef]
- Ma, Y.; Guan, G.; Shi, C.; Zhu, A.; Hao, X.; Wang, Z.; Kusakabe, K.; Abudula, A. Low-Temperature Steam Reforming of Methanol to Produce Hydrogen over Various Metal-Doped Molybdenum Carbide Catalysts. Int. J. Hydrogen Energy 2014, 39, 258–266. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.; Zhou, W.; Gao, R.; Yao, S.; Zhang, X.; Xu, W.; Zheng, S.; Jiang, Z.; Yu, Q.; Li, Y.-W.; et al. Low-Temperature Hydrogen Production from Water and Methanol Using Pt/α-MoC Catalysts. Nature 2017, 544, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, G.; Davis, B.H. In Situ DRIFTS Investigation of the Steam Reforming of Methanol over Pt/Ceria. Appl. Catal. A Gen. 2005, 285, 43–49. [Google Scholar] [CrossRef]
- Iwasa, N.; Mayanagi, T.; Nomura, W.; Arai, M.; Takezawa, N. Effect of Zn Addition to Supported Pd Catalysts in the Steam Reforming of Methanol. Appl. Catal. A Gen. 2003, 248, 153–160. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, Z.; Zhang, J.; Tang, Y.; Song, C.; Navessin, T.; Shi, Z.; Song, D.; Wang, H.; Wilkinson, D.P.; et al. High Temperature PEM Fuel Cells. J. Power Sources 2006, 160, 872–891. [Google Scholar] [CrossRef]
- Avgouropoulos, G.; Papavasiliou, J.; Daletou, M.K.; Kallitsis, J.K.; Ioannides, T.; Neophytides, S. Reforming Methanol to Electricity in a High Temperature PEM Fuel Cell. Appl. Catal. B Environ. 2009, 90, 628–632. [Google Scholar] [CrossRef]
- Özcan, O.; Akın, A.N. Thermodynamic Analysis of Methanol Steam Reforming to Produce Hydrogen for HT-PEMFC: An Optimization Study. Int. J. Hydrogen Energy 2019, 44, 14117–14126. [Google Scholar] [CrossRef]
- Papavasiliou, J.; Słowik, G.; Avgouropoulos, G. Redox Behavior of a Copper-Based Methanol Reformer for Fuel Cell Applications. Energy Technol. 2018, 6, 1332–1341. [Google Scholar] [CrossRef]
- Avgouropoulos, G.; Papavasiliou, J.; Ioannides, T.; Neophytides, S. Insights on the Effective Incorporation of a Foam-Based Methanol Reformer in a High Temperature Polymer Electrolyte Membrane Fuel Cell. J. Power Sources 2015, 296, 335–343. [Google Scholar] [CrossRef]
- Avgouropoulos, G.; Paxinou, A.; Neophytides, S. In Situ Hydrogen Utilization in an Internal Reforming Methanol Fuel Cell. Int. J. Hydrogen Energy 2014, 39, 18103–18108. [Google Scholar] [CrossRef]
- Ribeirinha, P.; Alves, I.; Vázquez, F.V.; Schuller, G.; Boaventura, M.; Mendes, A. Heat Integration of Methanol Steam Reformer with a High-Temperature Polymeric Electrolyte Membrane Fuel Cell. Energy 2017, 120, 468–477. [Google Scholar] [CrossRef]
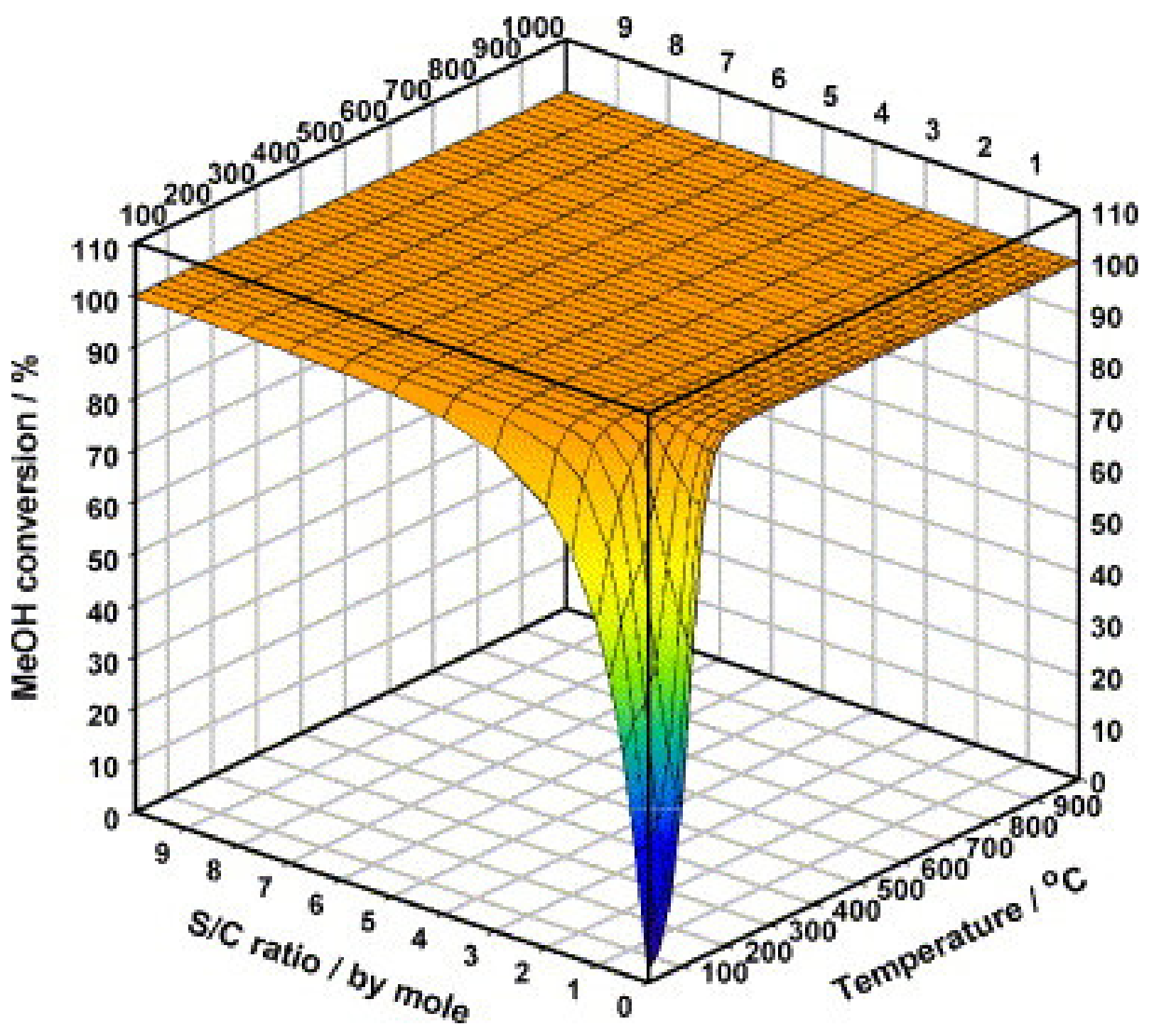
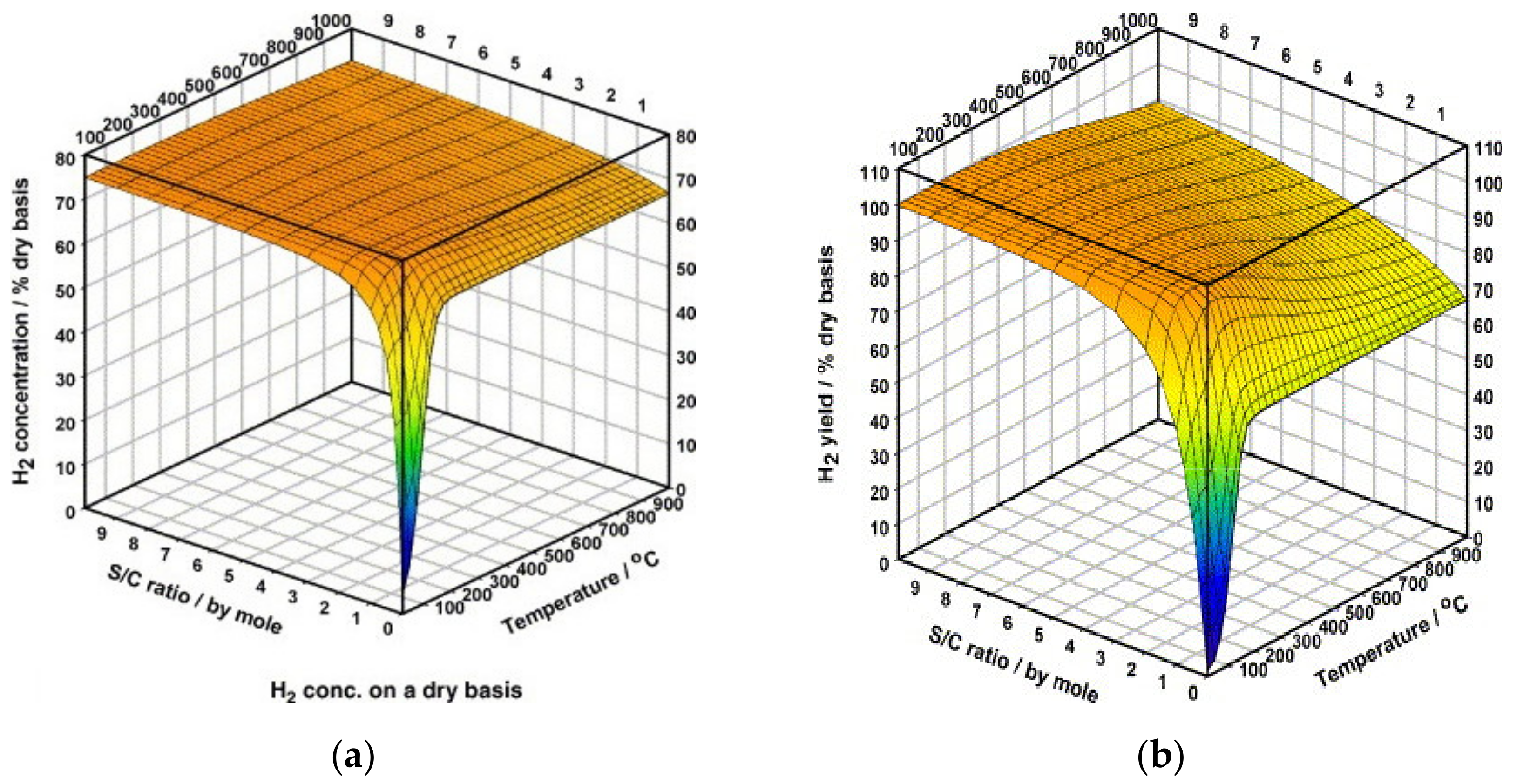
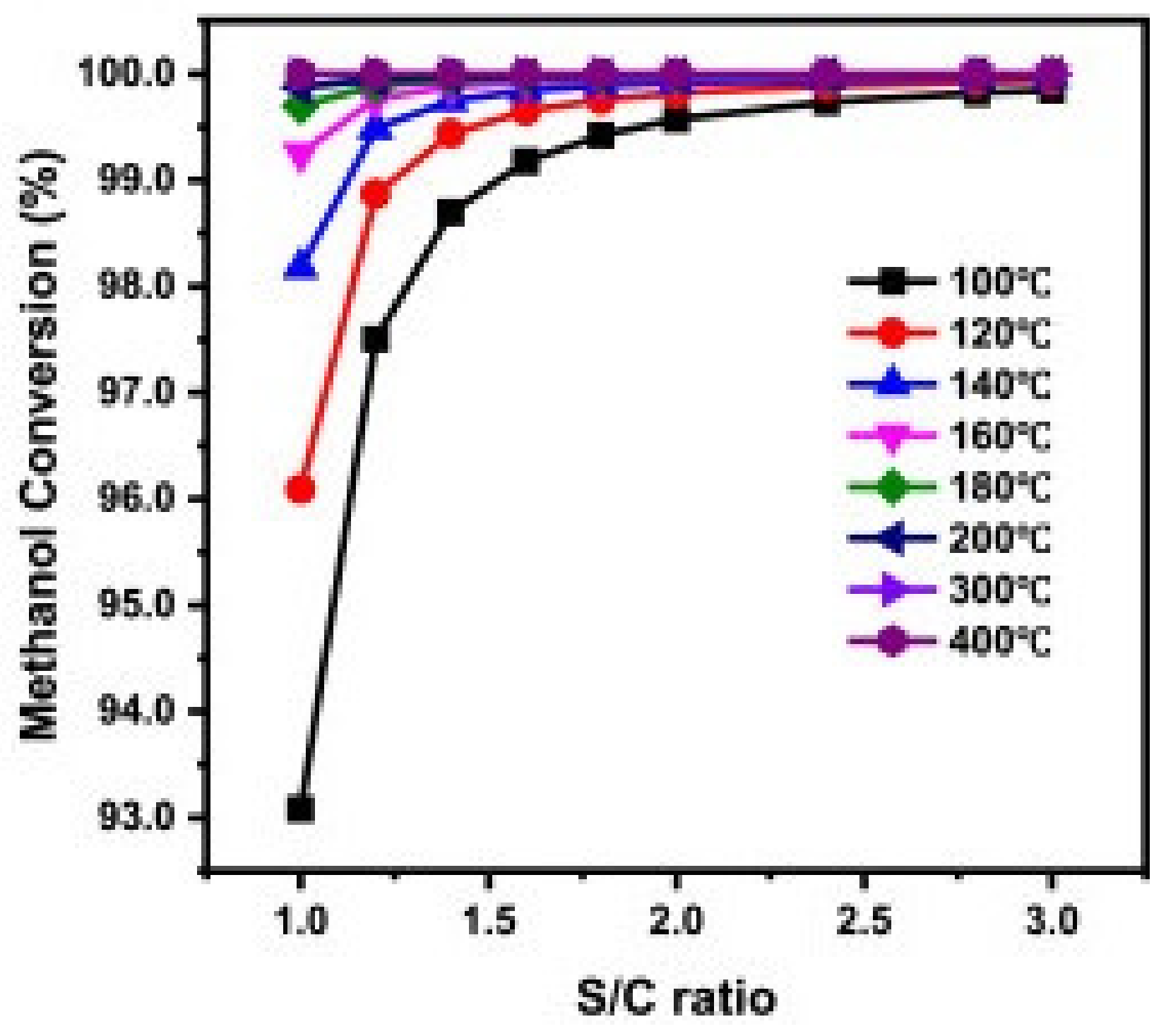
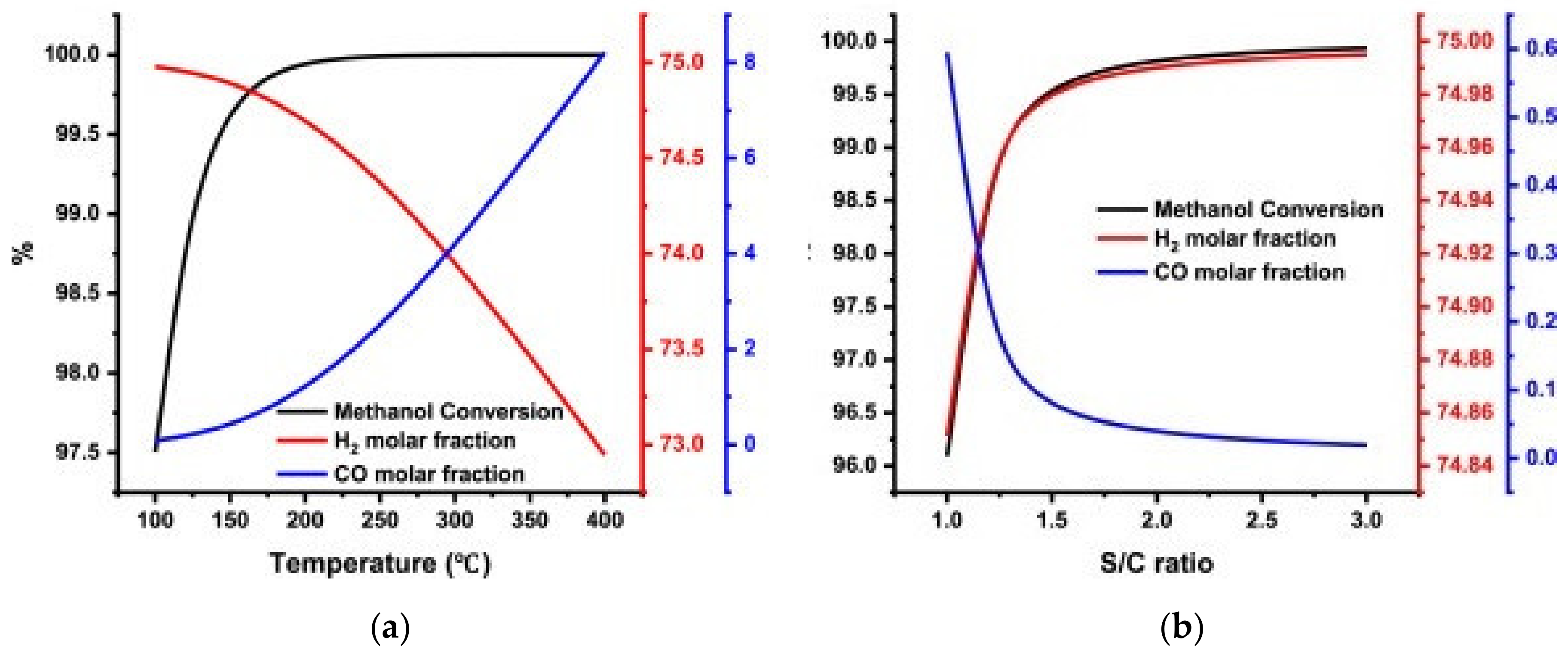
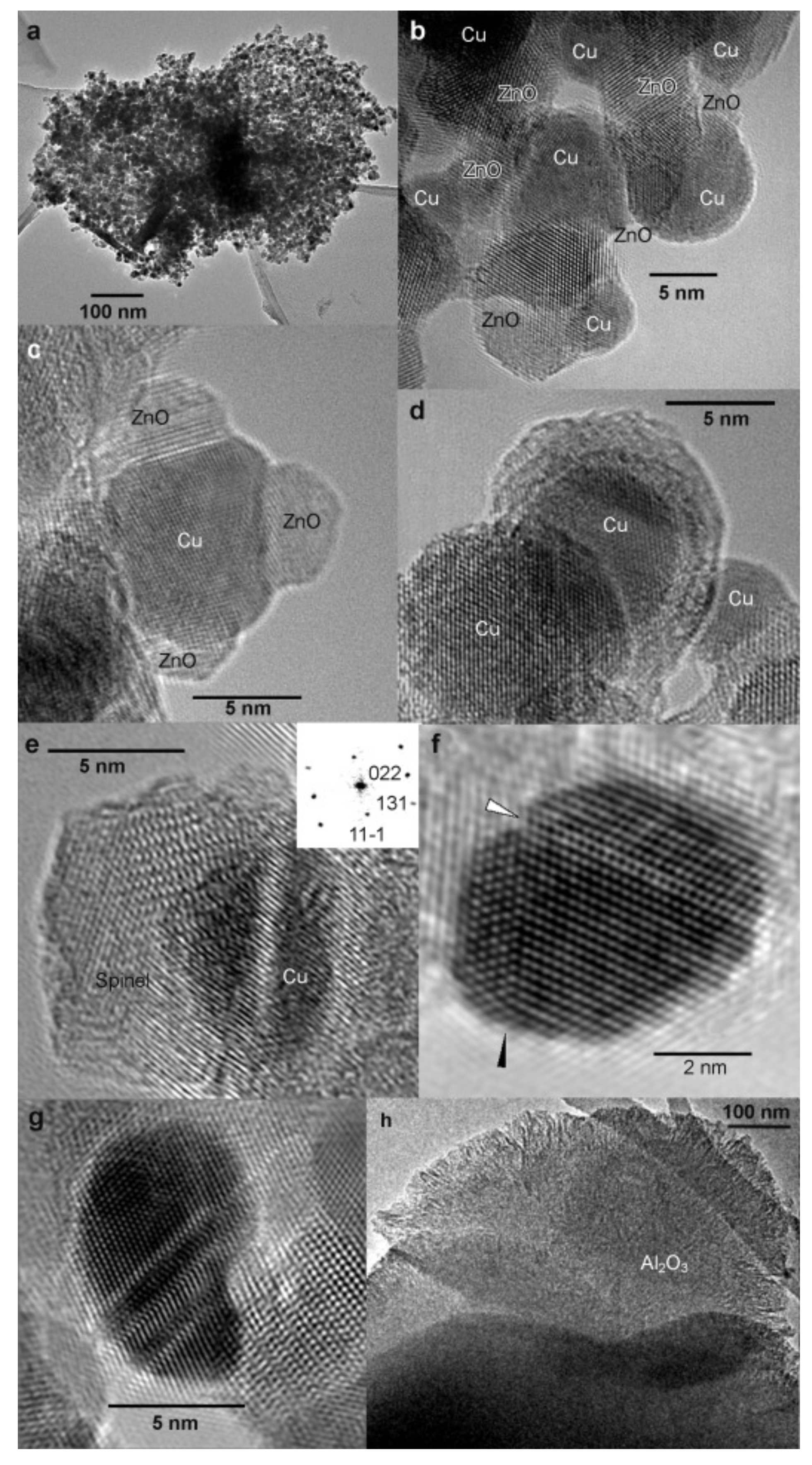

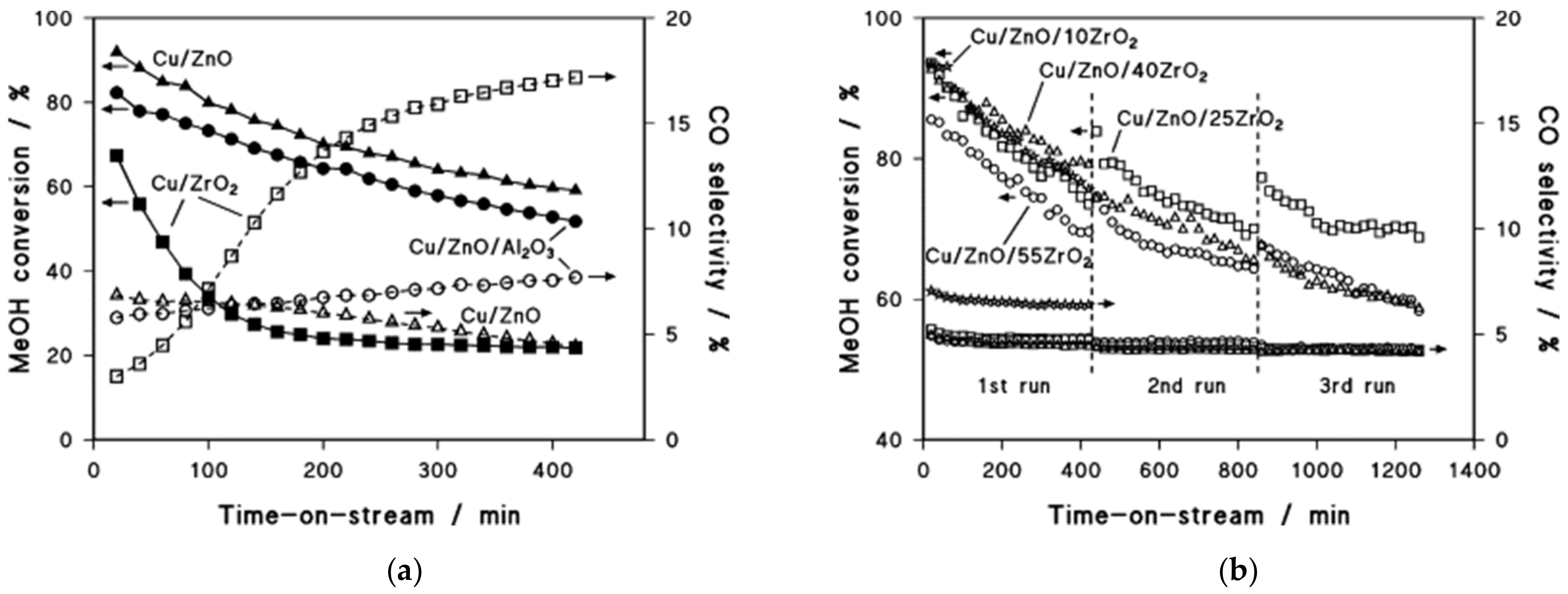
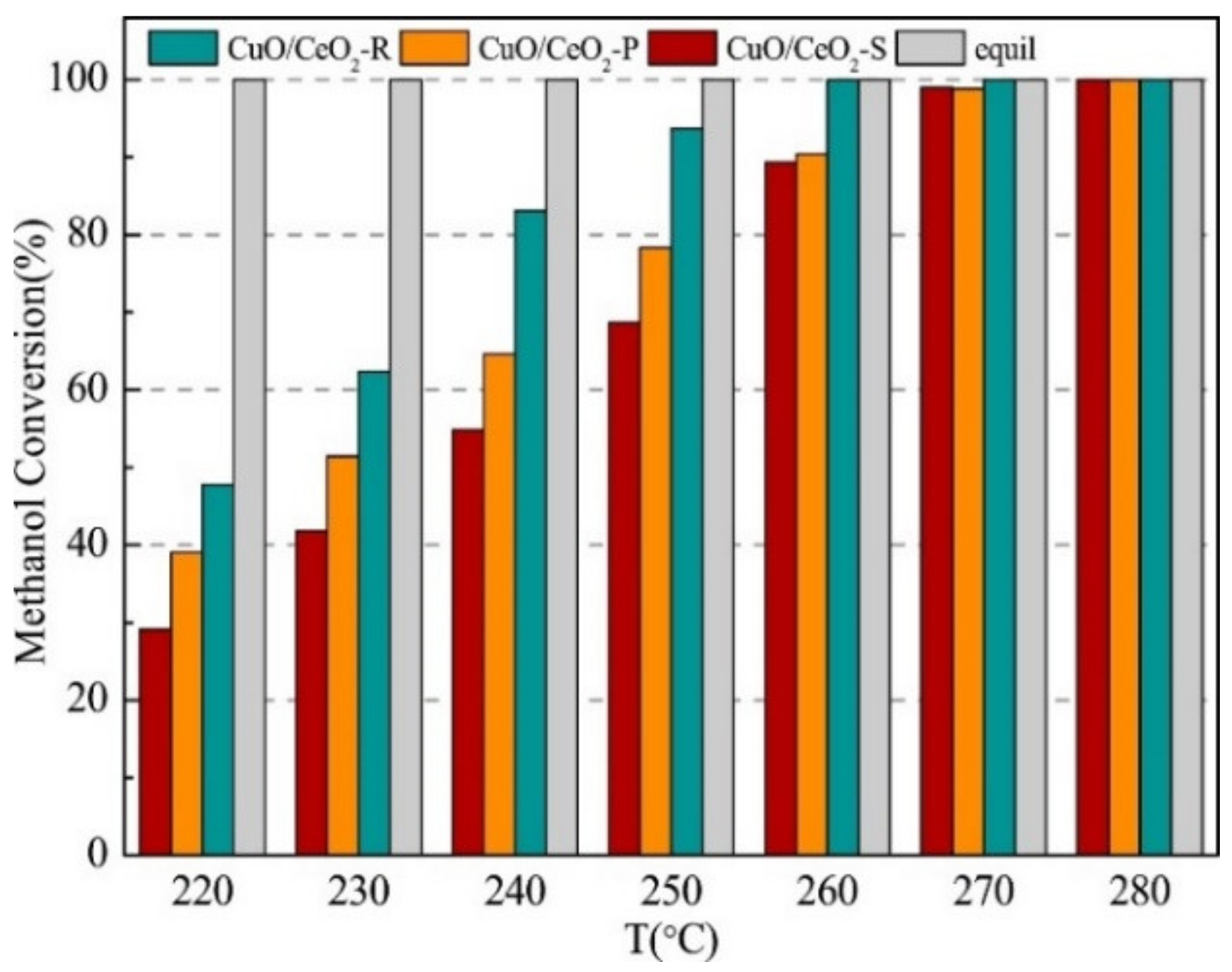
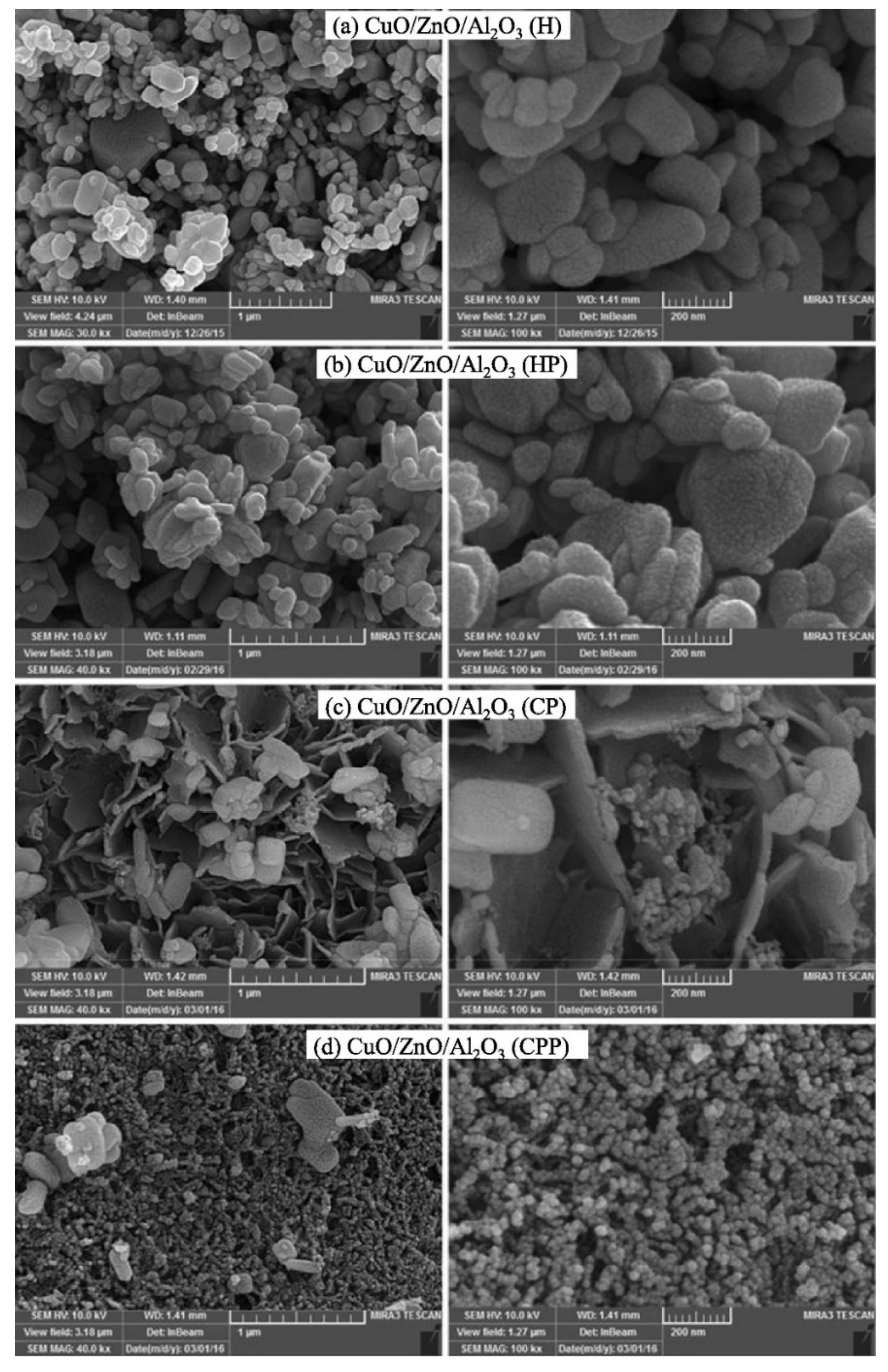




| MD | POM | SRM | OSRM | |
|---|---|---|---|---|
| S/C ratio | - | - | 1.5–2.0 | 1.2–1.5 |
| O2/C ratio | - | >0.5 | - | 0.10–0.25 |
| H2 produced (%) | 67.0 | 41.0 | 75.0 | 55.0–60.0 |
| CO produced (%) | 33.0 | 0.1–2.0 | <1.0 | 2.0 |
| Remarks |
|
|
|
|
| Catalyst | S/C | T (°C) | XMeOH 1 (%) | SCO 2 (%) | Hydrogen Production Rate (μmolH2/gcat s) | W/F (g s/mL) | Reference |
|---|---|---|---|---|---|---|---|
| Cu/ZrO2/Al2O3 | 1.5 | 220 | 90 | 0.10 | - | - | [33] |
| Cu/ZnO/Al2O3 | 1.1 | 270 | 89.2 | 0.92 | - | - | [83] |
| Cu/ZnO/ZrO2/Al2O3 | 92.4 | 0.97 | - | ||||
| Cu/ZnO | 1.2 | 250 | 90.0 | - | - | 0.46 | [84] |
| Cu/ZnO/Al2O3 | 100.0 | - | - | ||||
| Cu/CeO2 | 1.2 | 260 | 100.0 | 2.40 | 15.3 3 | [87] | |
| Cu/ZnO/CeO2/Al2O3 | 1.5 | 220 | 80.0 | 0.00 | - | 0.36 | [95] |
| Cu/Al2O3 | 1.4 | 260 | 31.0 | 81.0 3 | - | [97] | |
| Cu/ZnO/Al2O3 | 76.0 | 203.0 | |||||
| Cu/ZnO/CeO2/Al2O3 | 90.0 | 244.0 | |||||
| Cu/ZnO/Ga2O3 | 2.0 | 150 | 22.5 | 0.00 | 109.3 4 | [98] | |
| CuMnOx spinel | 1.5 | 240 | 99.0 | 3.1 | - | 0.26 | [104] |
| CuCeOx | 37.0 | 0.8 | - | ||||
| CuXFe1-xAl2O4 | 1.1 | 275 | >90.0 | <5.0 | - | 0.12 | [109] |
| Cu/ZnO | 1.3 | 260 | 75 | - | - | 0.06 | [110] |
| Cu/ZnO/Al2O3 | 79 | - | - | ||||
| Cu/ZnO/ZrO2/Al2O3 | 92 | - | - | ||||
| Cu/ZnO/Al2O3 | 2.0 | 250 | 84.3 | 1.50 | - | - | [111] |
| Cu/ZnO | 1.2 | 250 | 68.0 | 0.30 | 309.03 | - | [112] |
| Cu/ZnO/Al2O3 | 75.0 | 1.80 | 340.0 | ||||
| Cu/ZnO/ZrO2 | 97.0 | 0.80 | 553.0 | ||||
| Cu/ZnO/CeO2 | 80.0 | 0.25 | 392.0 | ||||
| Cu/CeO2 | 1.5 | 250 | >80.0 | - | 75.0 | 0.22 | [113] |
| Cu/CeO2/ZrO2 | - | - | 97.0 | ||||
| Cu/ZnO/Ga2O3 | 1.3 | 320 | 96.0 | - | 198.0 | 1.00 | [114] |
| Cu/ZnO | 1.0 | 240 | - | 7.00 | 24.0 | 0.05 | [115] |
| Cu/Cr2O3 | - | 14.00 | 17.0 | ||||
| Cu/Fe/SiO2 | 180 | 99.0 | - | - | - | [116] |
| Catalyst | Preparation Method | S/C | T (°C) | XMeOH 1 (%) | SCO 2 (%) | Hydrogen Production Rate (μmolH2/gcat s) | W/F (g s/mL) | Reference |
|---|---|---|---|---|---|---|---|---|
| Cu/ZnO/Al2O3 | HT | 1.50 | 240 | <20.0 | - | - | 0.36 | [31] |
| CP | 93.0 | 0.28 | - | |||||
| PHT | <35.0 | - | - | |||||
| PCP | 95.0 | 0.23 | - | |||||
| Cu/ZnO/ZrO2/Al2O3 | WI | 1.40 | 260 | 60.0 | - | 159.0 | 11.00 3 | [34] |
| CP | 97.0 | - | 261.0 | |||||
| Cu/ZrO2 | WI | 1.30 | 260 | 10.0 | - | 3.0 | - | [117] |
| CP | 62.0 | - | 56.0 | |||||
| OGCP | 100.0 | - | 90.0 | |||||
| Cu/ZnO/Al2O3 | WI | 1.43 | 230 | <60.0 | - | - | - | [121] |
| HT | <80.0 | - | ||||||
| CP | <99.0 | - | ||||||
| Cu/ZnO/Al2O3 | MC | 1.5 | 240 | 100.0 | <3.0 | - | 0.36 | [123] |
| CC | 90.0 | 3.0 | ||||||
| Cu/ZnO/ZrO2/Al2O3 | CP | 1.5 | 200 | 84.0 | - | - | 0.36 | [124] |
| SCP | 100.0 | - | - |
| Catalyst | S/C | T (°C) | XMeOH 1 (%) | SCO 2 (%) | Hydrogen Production Rate (μmolH2/gcat s) | W/F (g s/mL) | Reference |
|---|---|---|---|---|---|---|---|
| Pt/In2O3/Al2O3 Pt/In2O3/CeO2 | - | 325 | 98.7 | 2.6 | 85.3 | 0.28 | [38] |
| 84.8 | 2.6 | 92.5 | |||||
| Pd/ZnO | 1.78 | 220 | 14.3 | - | 1616.0 3 | 0.25 | [136] |
| Pd/ZnO/Al2O3 | 46.5 | - | 4100.0 | ||||
| Cu/ZnO/Al2O3 (commercial) | 46.3 | - | 4175.0 | ||||
| Pd/ZnO Pd/ZrO2 Pd/In2O3 Pt/ZnO Pt/In2O3 | - | 220 | 54.2 | 0.8 | - | - | [137] |
| 64.3 | 81.6 | - | |||||
| 28.3 | 4.5 | - | |||||
| 27.6 | 4.6 | - | |||||
| 1.7 | 30.6 | - | |||||
| α-MoC | 3.00 | 160 | 1.4 | 0.03 | 0.6 | - | [138] |
| 0.5Zn-Pt/MoC | 65.9 | 0.00 | 29.7 | ||||
| Pd/ZnO Pd/ZnO/CeO2 | 1.00 | 300 | 65.0 | - | - | 0.05 | [139] |
| 95.0 | - | - | |||||
| Ni-Zn-Al | 1.40 | 500 | 100 | ~30.00 | - | - | [140] |
| Ni/CeO2 | 3.00 | 400 | 100 | - | - | - | [141] |
| Co/ZnO | - | 220 | 20.3 | 91.1 | - | - | [142] |
| LT-PEMFCs | HT-PEMFCs | |
|---|---|---|
| Operating temperature (°C) | 60–80 | ≥160 |
| Fuel | High purity H2 | Hydrogen-rich reformate |
| Tolerance in CO | <10 ppm | ≤3.0% |
| Infrastructure | Hydrogen station → high cost | Existing petrol station |
| Fuel Cell system | Water and heat management is required | Complicated thermal management |
| Operation | Quick start-up and shutdown | Slow start-up and shutdown |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kappis, K.; Papavasiliou, J.; Avgouropoulos, G. Methanol Reforming Processes for Fuel Cell Applications. Energies 2021, 14, 8442. https://doi.org/10.3390/en14248442
Kappis K, Papavasiliou J, Avgouropoulos G. Methanol Reforming Processes for Fuel Cell Applications. Energies. 2021; 14(24):8442. https://doi.org/10.3390/en14248442
Chicago/Turabian StyleKappis, Konstantinos, Joan Papavasiliou, and George Avgouropoulos. 2021. "Methanol Reforming Processes for Fuel Cell Applications" Energies 14, no. 24: 8442. https://doi.org/10.3390/en14248442






