Hazards Generated by an LNG Road Tanker Leak: Field Investigation of Vapour Propagation under Class B Conditions of Atmospheric Stability
Abstract
:1. Introduction
2. Materials and Methods
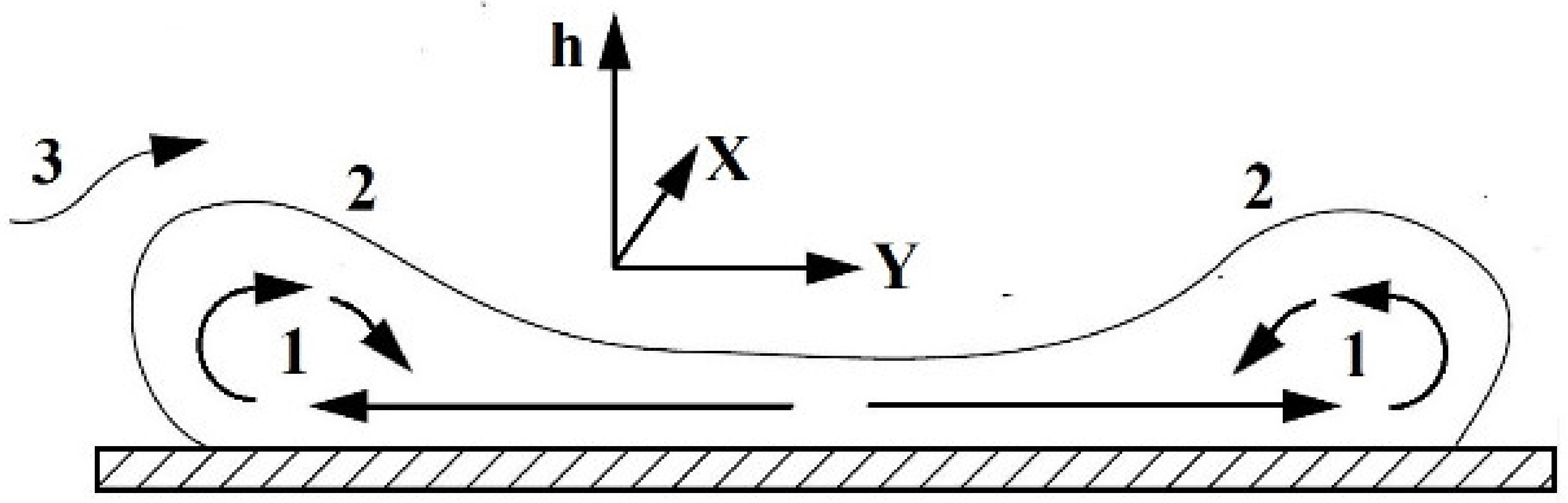
2.1. Testing the Concentration of Flammable Gases in the Cloud Formed after the LNG Release
2.2. Determination of Ambient Temperature Drop during LNG Release
2.3. Simulation Software
2.4. Statistical Measures Used in Results Elaboration
- (a)
- Mean Relative Bias (MRB)
- (b)
- FAC2 Fraction of Prediction Within A Factor Of Two Measurements
- (c)
- Geometric Mean Bias (MG)
- (d)
- Geometric Variance (VG)
3. Results
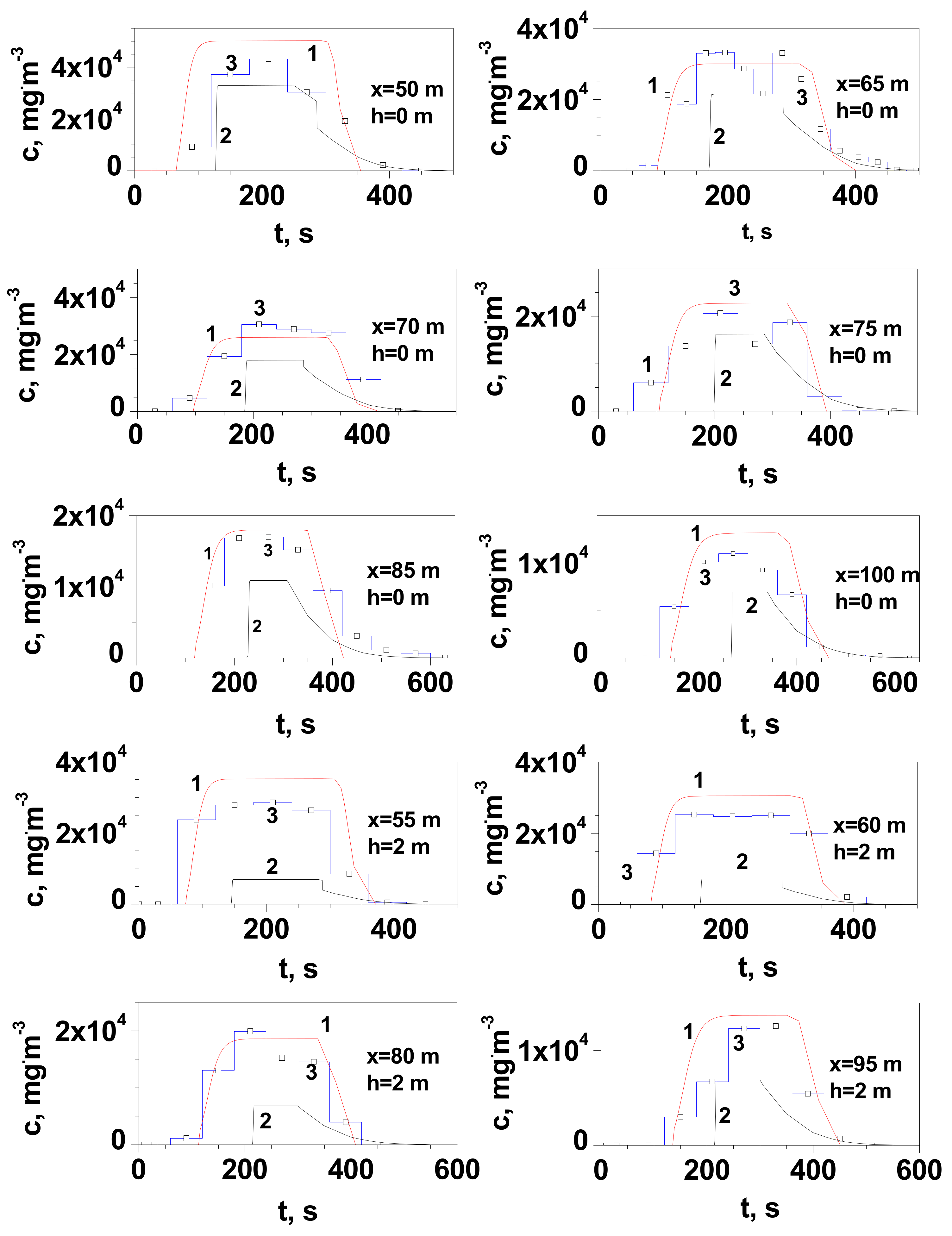
3.1. Results of Testing the Flammable Gas Concentration in the Cloud Formed after the LNG Release
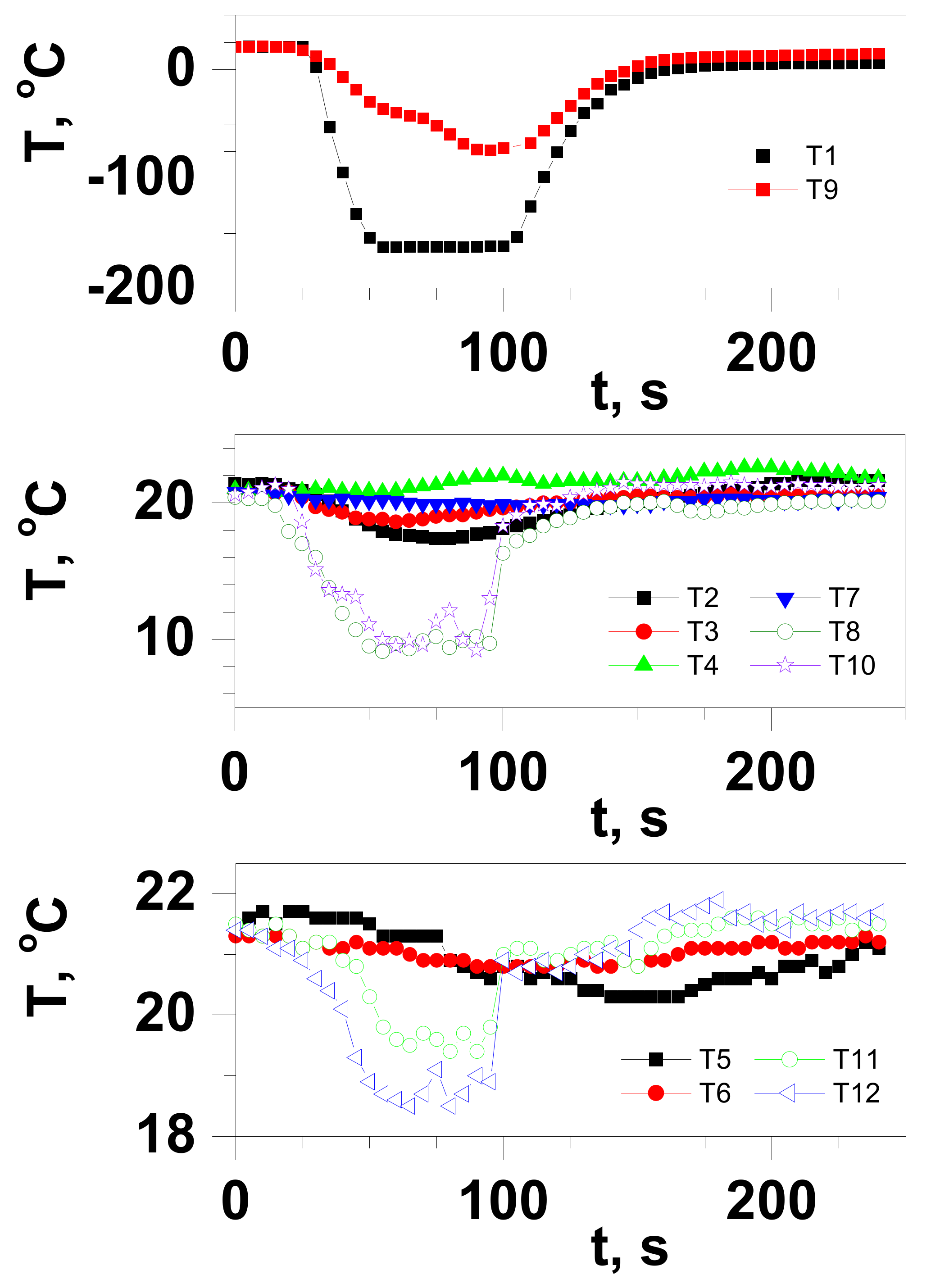
3.2. Test Results for an Ambient Temperature Drop during LNG Release
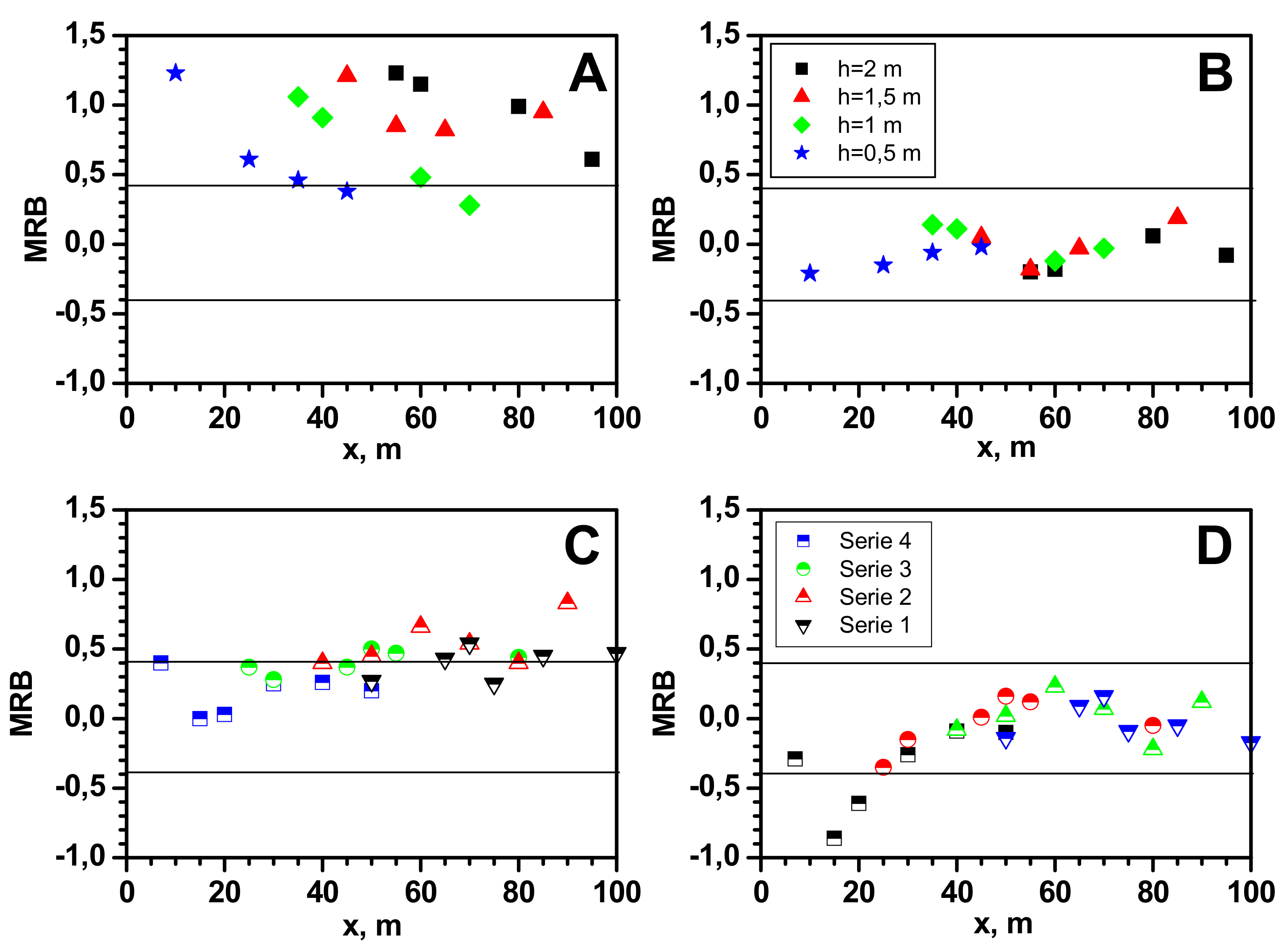
4. Discussion
5. Conclusions
- (a)
- At distances greater than 30 m, the experimental values of the maximum gas concentration at ground level in the release axis are more closely reproduced by the Gaussian gas model. This is due to the positive value of the buoyancy coefficient, which increases with the amount of heat taken from the environment. Its value increases with the time of the dispersion and the distance from the site of release.
- (b)
- At distances not greater than 25/30 m (for measurement series 2 and 1, respectively), the dense gas model generally gives a better approximation than the Gaussian gas model. At a short distance, the temperature of the gas is much lower and such a non-air-diluted gas may tend to creep. Vapours of LNG main component, methane, in the temperature range from −161 °C to approximately −120 °C tend to creep due to their higher density than air. According to the modified Clapeyron equation, the density of vapours of pure components increases proportionally to the molar mass. The tested LNG also contains 4% heavier hydrocarbons with a much higher boiling point (C2H6 bp = −89 °C, C3H8 bp = −42 °C, C4H10 bp = −0.5 °C). Despite the turbulence prevailing during the outflow, they could reach the surface in small amounts in the liquid state.
- (c)
- In the case of measurements at height h with a range of 0.5–2 m, a much better approximation of the obtained values of the maximum concentration is observed for the Gaussian gas model, regardless of the distance.
- (d)
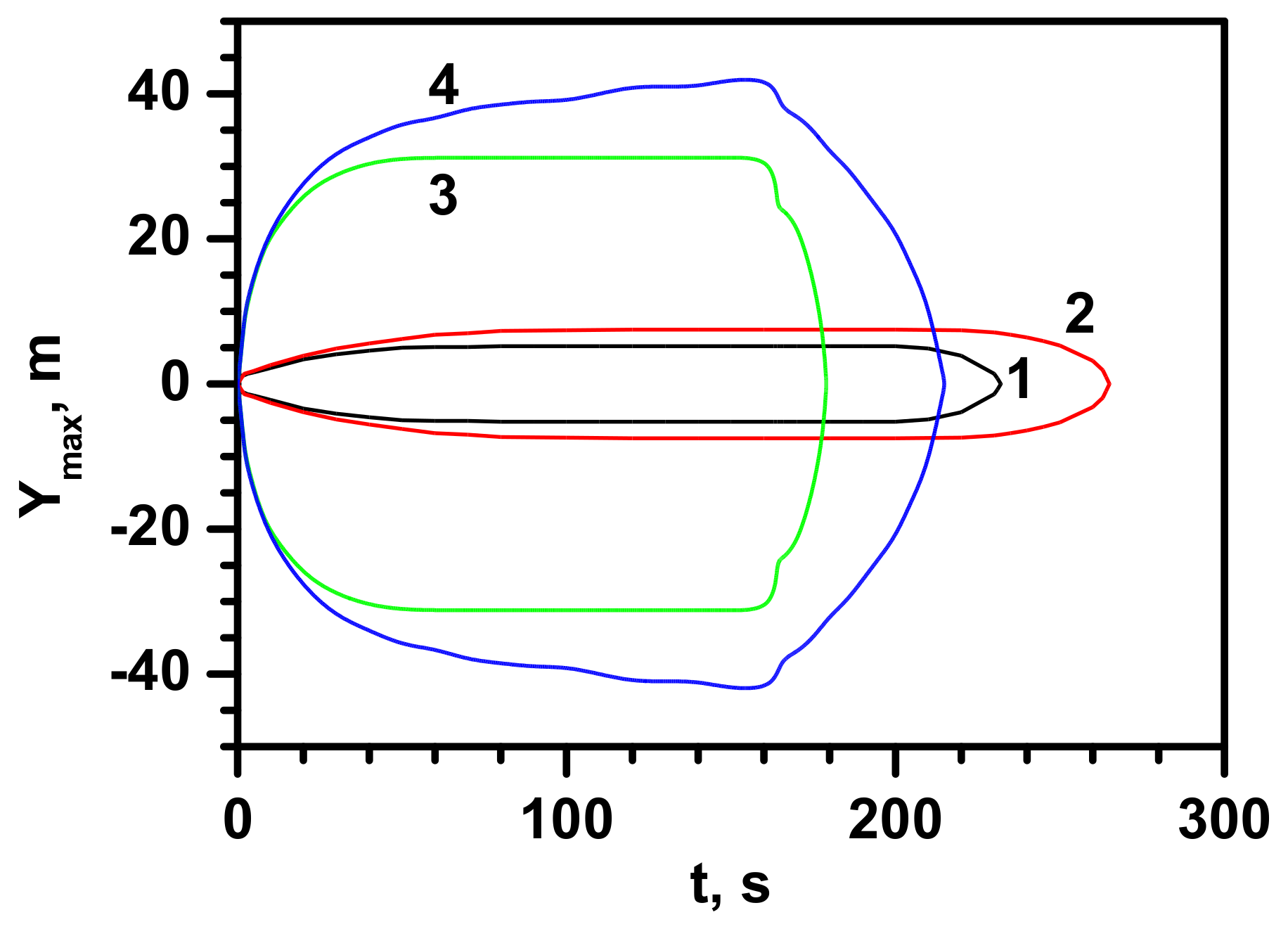
- The maximum cloud width, for which values of at least 100% LEL are observed, is between 12 and 18 m, which the Gaussian gas model much better approximates. However, this is clearly more than the predicted value. It could be caused by the effect of crosswinds and increased lateral dispersion.
- (e)
- Observations made at a short distance from the release location (x ≤ 7 m) indicate that the cloud near the ground surface dissipated in some 15 s, resulting in a more accurate representation of the concentration drop curve provided by the dense gas model for which this time should be approx. 12 s. This is in agreement with simulation results for shorter distances, for which the heavy gas model was more consistent with the results of the field experiment.
- (f)
- It is shown that concentrations between 71% and 110% LEL are observed at the cloud visibility limit. Nevertheless, the indications of mobile recorders under less stable wind conditions show that the combustible cloud downwind can temporarily move up to 20 m beyond the visible condensation cloud. Such a result means that during rescue operations with uncontrolled release of LNG, special care should be taken, and danger zone must be constantly monitored. Even small wind fluctuations may lead to the cloud displacement.
- (g)
- Temperature measurements at a short distance from the cloud release axis (L ≤ 1.25 m) showed only a slight decrease in ambient temperature. However, the maximum value of the temperature drop, in the release axis at the distance where the gas cloud reaches the ground surface, amounts to ∆Tmax = 93.3 °C. This indicates that the cloud of the released LNG has almost already completed phase transition at the moment of reaching ground level due to the turbulent outflow of the pressurised gas and the average density of the cloud is lower than the density of the surrounding air. So rapid heating of the cloud explains the preference of passive gas transport at the tested release conditions.
- (h)
- Exposure to LNG vapours causes the risk of cryogenic burns and cryogenic inhalation only at small distances and, at the temperature of LNG vapours of −73 °C, does not result in a greater than 1% risk of reducing the ability to escape for exposed individuals. For rescue operations, this risk is virtually eliminated because of the guidelines for conducting rescue operations in the release zone requiring the rescuer to be equipped with respiratory protective equipment.
Author Contributions
Funding
Conflicts of Interest
References
- International Gas Union. 2020 World LNG Report; International Gas Union: Vevey, Switzerland, 2020. [Google Scholar]
- Brushlinsky, N.N.; Ahrens, M.; Sokolov, S.V.; Wagner, P. World fire statistics. CTIF 2020, 25. [Google Scholar]
- Woodward, J.L.; Pitblado, R.M. LNG Risk Based Safety. Modeling and Consequence Analysis; Wiley&Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Eberwein, R.; Rogge, A.; Behrendt, F.; Knaust, C. Dispersion modeling of LNG-vapor on land—A CFD-model evaluation study. J. Loss Prev. Process Ind. 2020, 65, 104116. [Google Scholar] [CrossRef]
- Nazarpoura, F.; Wen, J.; Dembelea, S.; Udechukwua, I.D. LNG vapour cloud dispersion modelling and simulations with Open FOAM. Chem. Ing. Trans. 2016, 48, 967–972. [Google Scholar]
- Blackmore, D.R.; Colenbrander, G.W.; Puttock, J.S. Maplin Sands experiments 1980: Dispersion results from continuous release of refrigerated liquid propane and LNG. In NATO Challenges of Modern Society-Air Pollution Modelling and Its Application, 3rd ed.; Plenum Publishing: New York, NY, USA, 1984; Volume 5, pp. 353–373. [Google Scholar]
- Ermak, D.L.; Chan, S.T.; Morgan, D.L.; Morris, L.K. A comparison of dense gas dispersion model simulations with burro series LNG spill test results. J. Hazard. Mater. 1982, 6, 129–160. [Google Scholar] [CrossRef]
- Ikealumba, W.C.; Wu, H. Modeling of liquefied natural gas release and dispersion: Incorporating a direct computational fluid dynamics simulation method for LNG spill and pool formation. Ind. Eng. Chem. Res. 2016, 55, 1778–1787. [Google Scholar] [CrossRef]
- Hansen, O.R.; Gavelli, F.; Ichard, M.; Davis, S.G. Validation of FLACS against experimental data sets from the model evaluation database for LNG vapor dispersion. J. Loss Prev. Process Ind. 2010, 23, 857–877. [Google Scholar] [CrossRef]
- Ivings, M.J.; Gant, S.E.; Jagger, S.F.; Lea, C.J.; Stewart, J.R.; Webber, D.M. Evaluating Vapour Dispersion Models for Safety Analysis of LNG Facilities; Final report; HSL: Buxton, UK, 2016. [Google Scholar]
- Sklavounos, S.; Rigas, F. Fuel gas dispersion under cryogenic release conditions. J. Energy Fuels 2005, 19, 2535–2544. [Google Scholar] [CrossRef]
- Luketa-Hanlin, A. A review of large-scale LNG spills: Experiments and modeling. J. Hazard. Mater. 2005, 132, 119–140. [Google Scholar] [CrossRef] [PubMed]
- Pontiggia, M.; Derudi, M.; Busini, V.; Rota, R. Hazardous gas dispersion: A CFD model accounting for atmospheric stability classes. J. Hazard. Mater. 2009, 171, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Shih, T.H.; Liou, W.W.; Shabbir, A.; Yang, Z.; Zhu, J. A new—Eddy-viscosity model for high Reynolds number turbulent flows—Model development and validation. Comput. Fluid 1995, 24, 227–238. [Google Scholar] [CrossRef]
- Zapert, J.G.; Lordengan, R.J.; Thistle, H. Evaluation of Dense Gas Simulation Models; EPA: Research Triangle Park, NC, USA, 1991.
- Guo, D.; Zhao, P.; Wang, R.; Yao, R.; Hub, J. Numerical simulation studies of the effect of atmospheric stratification on the dispersion of LNG vapor released from the top of a storage tank. J. Loss. Prev. Process Ind. 2019, 61, 275–286. [Google Scholar] [CrossRef]
- Luo, T.; Yu, C.h.; Liu, R.; Li, M.; Zhang, J.; Qu, S. Numerical simulation of LNG release and dispersion using a multiphase CFD model. J. Loss. Prev. Process Ind. 2018, 56, 316–327. [Google Scholar] [CrossRef]
- Siuta, D.; Markowski, A.S.; Mannan, M.S. Uncertainty techniques in liquefied natural gas (LNG) dispersion calculations. J. Loss Prev. Process Ind. 2013, 26, 418–426. [Google Scholar] [CrossRef]
- Koopman, R.P.; Caderwall, R.D.; Ermak, D.L.; Goldwire, H.C., Jr.; Hogan, W.J.; McClure, J.W.; McCrae, T.G.; Morgan, D.L.; Rodean, H.C.; Shinn, J.H. Analysis of Burro series 40m3 LNG spill experiments. J. Hazard. Mater. 1982, 6, 43–83. [Google Scholar] [CrossRef]
- Ermak, D.L.; Chapman, R.; Goldwire, H.C., Jr.; Gouveia, F.J.; Rodean, H.C. Heavy Gas Dispersion Test Summary Report; Report No. UCRL-21210; Lawrence Livermore National Laboratory: Livermore, CA, USA, 1988. [Google Scholar]
- Jones, R.; Lehr, W.; Simecek-Beatty, D.; Reynolds, R.M. ALOHA (Areal Locations of Hazardous Atmospheres) 5.4.4: Technical Documentation; U.S. Dept. of Commerce: Washington, DC, USA; NOAA Emergency Response Division: Seattle, WA, USA, 2013.
- Planas-Cuchi, E.; Gasull, N.; Ventosa, A.; Casal, J. Explosion of a road tanker containing liquefied natural gas. J. Loss Prev. Process Ind. 2004, 17, 315–321. [Google Scholar] [CrossRef]
- Bonilla Martinez, J.M.; Belmonte Perez, J.; Marin Ayala, J.A. Analysis of the explosion of a liquefied-natural-gas road-tanker. Segur. Medio Ambiente 2011, 32, N127. [Google Scholar]
- Laciak, M.; Oliinyk, A.; Sikora, A.P.; Wlodek, T. Effect of composition variability of liquified natural gas propiertiesin term of storage process stability. Przem. Chem. 2018, 97, 267–271. [Google Scholar]
- Bakkum, E.A.; Duijm, N.J. Vapour cloud dispersion. In Methods for the Calculation of Physical Effects—Due to Releases of Hazardous Materials (Liquisds and Gases), Yellow Book (Cpr14e), 3rd ed.; van der Bosch, C.J.H., Weterings, R.A.P.M., Eds.; Gevaarlijke Stoffen: The Hague, The Netherlands, 2005. [Google Scholar]
- Hanna, S.R.; Chang, J.C.; Strimaitis, D.G. Hazardous gas model evaluation with field observations. Atmos. Environ. 1993, 27, 2265–2285. [Google Scholar] [CrossRef]
- Hanna, S.R.; Strimaitis, D.G.; Chang, J.C. Hazard Responce Modeling Uncertainty (a Quantitative Method). Evaluation of Commonly Used Hazardous Gas Dispersionmodels; Final report to the engineering and services laboratory; Air Force Engineering and Services Center: Tyndall Air Force Base, FL, USA, 1991; Volume 2. [Google Scholar]
- Sun, B.; Wong, J.; Wadnerkar, D.; Utikar, R.P.; Pareek, V.K.; Guo, K. Multiphase simulation of LNG vapour dispersion with effect of fog formation. Appl. Therm. Eng. 2020, 166, 114671. [Google Scholar] [CrossRef]
- Shaikh, I.; Porter, L. Incorporating asphyxiation and cryogenic hazards into risk analysis. In Proceedings of the 11th Annual Mary Kay O’Connor Process Safety Center Symposium, College Station, TX, USA, 28–29 October 2008. [Google Scholar]
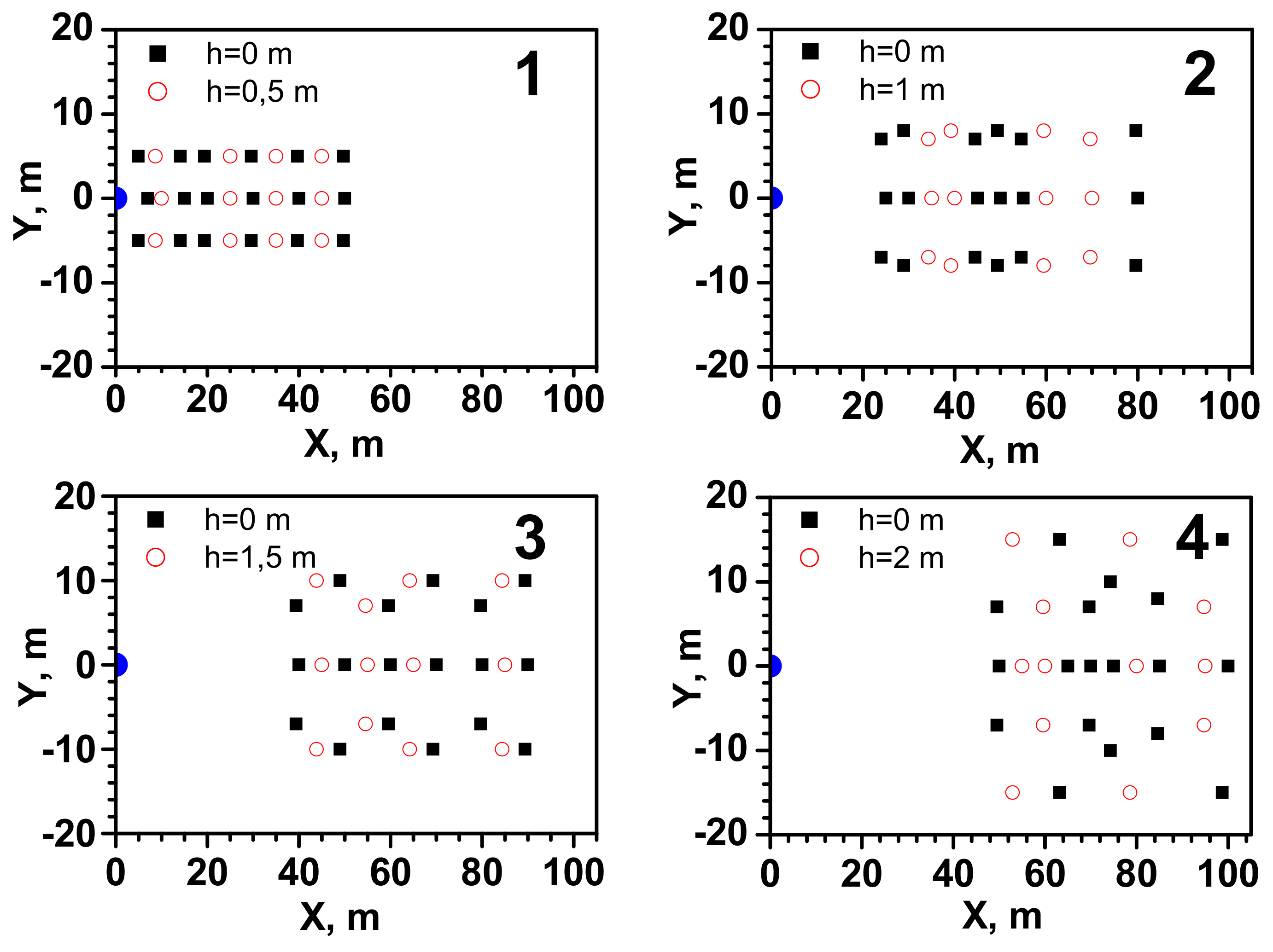

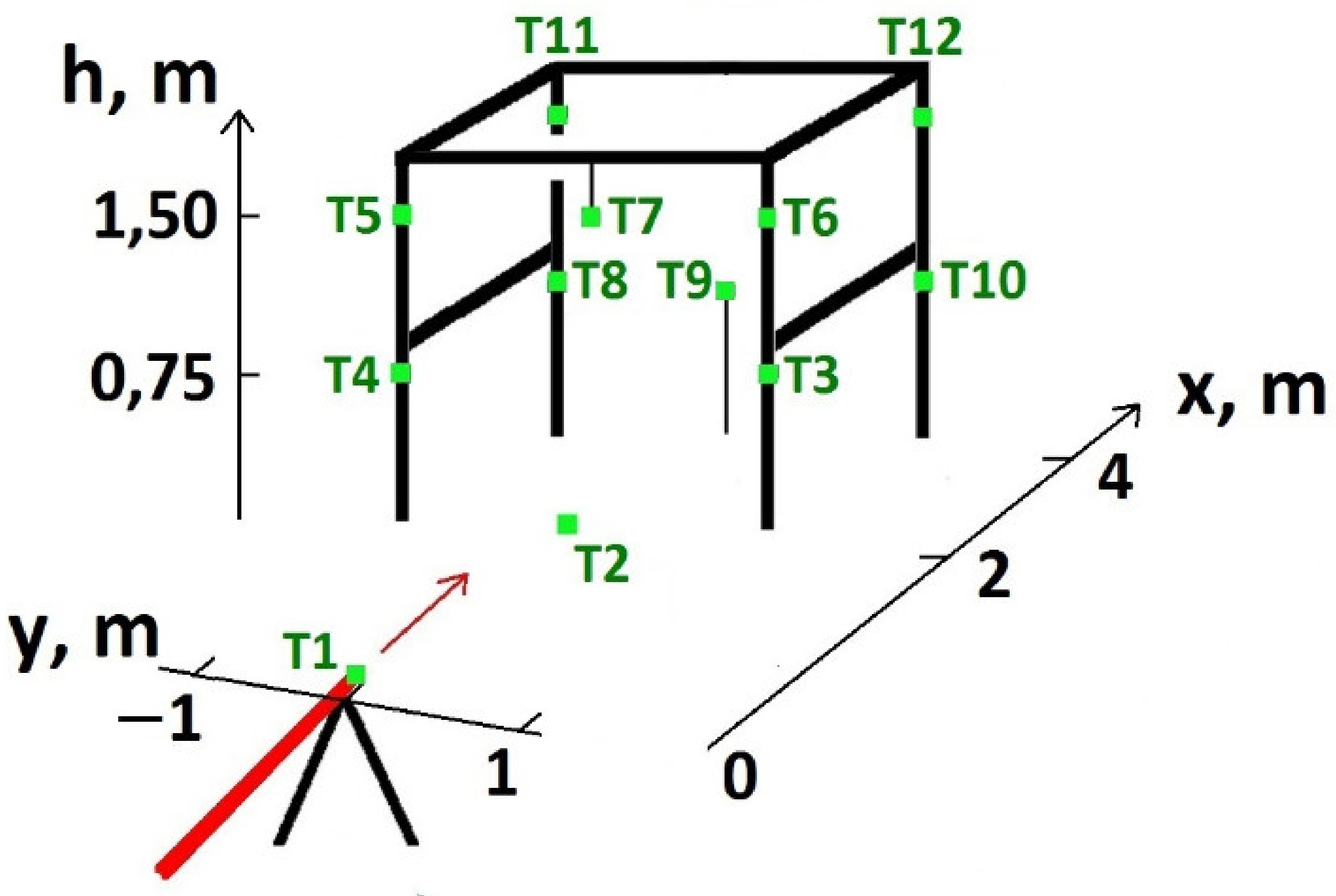
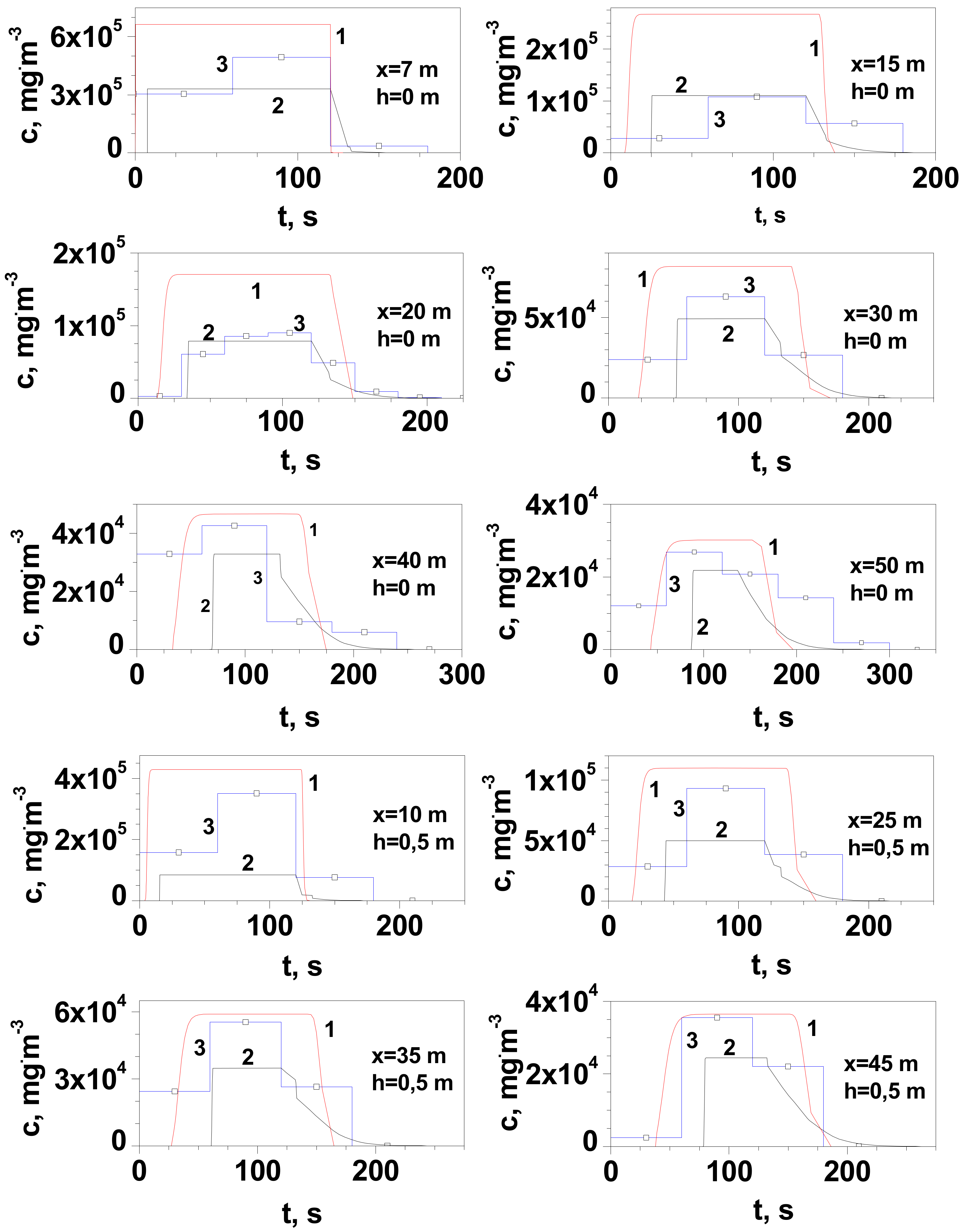
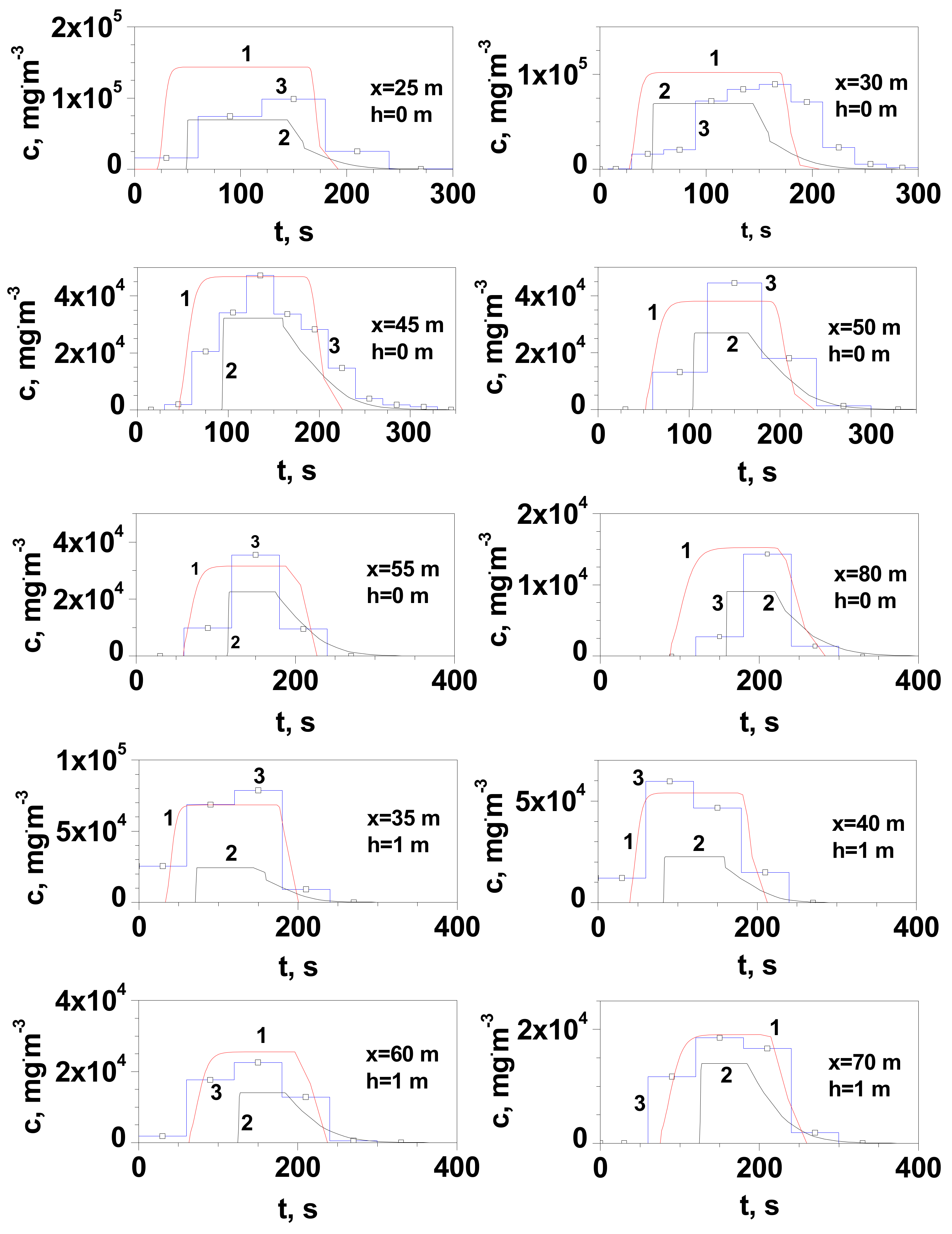




| Series | Q, kgs−1 | Ta, °C | TLNG, °C | uw, ms−1 | Pa, hPa | t, s | pout, atm |
|---|---|---|---|---|---|---|---|
| 1 | 1.67 ± 0.11 | 19.2 | −162 | 0.89 ± 0.09 | 1003.0 | 120 | 6.0 ± 0.1 |
| 2 | 1.72 ± 0.14 | 20.1 | −162 | 0.73 ± 0.13 | 1002.8 | 144 | 6.0 ± 0.1 |
| 3 | 1.70 ± 0.08 | 20.3 | −162 | 0.67 ± 0.12 | 1002.7 | 163 | 5.9 ± 0.1 |
| 4 | 1.78 ± 0.10 | 20.7 | −162 | 0.57 ± 0.16 | 1002.8 | 243 | 6.1 ± 0.1 |
| Thermocouple Number | Coordinates (x;y;h), m | Dist. from the Gas Stream Axis L, m |
|---|---|---|
| T1 | (0;0;0.75) | 0 |
| T2 | (2;0;0) | 0.75 |
| T3 | (2;1;0.75) | 1 |
| T4 | (2;−1;0.75) | 1 |
| T5 | (2;−1;1.5) | 1.25 |
| T6 | (2;1;1.5) | 1.25 |
| T8 | (4;−1;0.75) | 1 |
| T9 | (4;0;0.75) | 0 |
| T10 | (4:1;0.75) | 1 |
| T11 | (4;−1;1.5) | 1.25 |
| T12 | (4;1;1,5) | 1.25 |
| T8 | (4;−1;0.75) | 1 |
| Co-ord. (x, h) | MRB = <(Cm − Cp)/0.5(Cp + Cm)> | FAC2 0.5 ≤ Cp/Cm ≤ 2 | MG = exp <ln(Cm/Cp)> | VG = exp <[ln(Cm/Cp)]2> | ||||
|---|---|---|---|---|---|---|---|---|
| Gaussian | Dense | Gaussian | Dense | Gaussian | Dense | Gaussian | Dense | |
| 7;0 | −0.29 | 0.40 | 1.33 | 0.67 | 0.75 | 1.50 | 1.09 | 1.18 |
| 15;0 | −0.86 | 0.00 | 2.50 | 1.00 | 0.40 | 1.00 | 2.32 | 1.00 |
| 20;0 | −0.61 | 0.03 | 1.89 | 0.97 | 0.53 | 1.04 | 1.50 | 1.00 |
| 30;0 | −0.26 | 0.25 | 1.30 | 0.78 | 0.77 | 1.29 | 1.07 | 1.07 |
| 40;0 | −0.09 | 0.26 | 1.09 | 0.77 | 0.91 | 1.29 | 1.01 | 1.07 |
| 50;0 | −0.10 | 0.20 | 1.11 | 0.82 | 0.90 | 1.22 | 1.01 | 1.04 |
| 10;0.5 | −0.21 | 1.23 | 1.23 | 0.24 | 0.81 | 4.21 | 1.04 | 7.92 |
| 25;0.5 | −0.15 | 0.61 | 1.16 | 0.53 | 0.86 | 1.88 | 1.02 | 1.49 |
| 35;0.5 | −0.06 | 0.46 | 1.06 | 0.63 | 0.94 | 1.59 | 1.00 | 1.24 |
| 45;0.5 | −0.02 | 0.38 | 1.02 | 0.68 | 0.98 | 1.46 | 1.00 | 1.16 |
| Co-ord. (x, h) | MRB = <(Cm − Cp)/0.5(Cp + Cm)> | FAC2 0.5 ≤ Cp/Cm ≤ 2 | MG = exp <ln(Cm/Cp)> | VG = exp <[ln(Cm/Cp)]2> | ||||
|---|---|---|---|---|---|---|---|---|
| Gaussian | Dense | Gaussian | Dense | Gaussian | Dense | Gaussian | Dense | |
| 25;0 | −0.35 | 0.37 | 1.43 | 0.69 | 0.70 | 1.45 | 1.14 | 1.15 |
| 30;0 | −0.15 | 0.28 | 1.16 | 0.76 | 0.86 | 1.32 | 1.02 | 1.08 |
| 45;0 | 0.01 | 0.37 | 0.99 | 0.69 | 1.01 | 1.46 | 1.00 | 1.15 |
| 50;0 | 0.16 | 0.50 | 0.85 | 0.60 | 1.18 | 1.67 | 1.03 | 1.30 |
| 55;0 | 0.12 | 0.47 | 0.88 | 0.62 | 1.13 | 1.61 | 1.02 | 1.25 |
| 80;0 | −0.05 | 0.44 | 1.06 | 0.64 | 0.95 | 1.57 | 1.00 | 1.23 |
| 35;1 | 0.14 | 1.06 | 0.87 | 0.31 | 1.15 | 3.25 | 1.02 | 4.01 |
| 40;1 | 0.11 | 0.91 | 0.90 | 0.37 | 1.11 | 2.68 | 1.01 | 2.64 |
| 60;1 | −0.12 | 0.48 | 1.13 | 0.61 | 0.88 | 1.64 | 1.02 | 1.28 |
| 70;1 | −0.03 | 0.28 | 1.03 | 0.76 | 0.97 | 1.32 | 1.00 | 1.08 |
| Co-ord. (x, h) | MRB = <(Cm − Cp)/0.5(Cp + Cm)> | FAC2 0.5 ≤ Cp/Cm ≤ 2 | MG = exp <ln(Cm/Cp)> | VG = exp <[ln(Cm/Cp)]2> | ||||
|---|---|---|---|---|---|---|---|---|
| Gaussian | Dense | Gaussian | Dense | Gaussian | Dense | Gaussian | Dense | |
| 40;0 | −0.08 | 0.40 | 1.08 | 0.66 | 0.93 | 1.50 | 1.01 | 1.18 |
| 50;0 | 0.02 | 0.45 | 0.98 | 0.63 | 1.02 | 1.59 | 1.00 | 1.24 |
| 60;0 | 0.23 | 0.66 | 0.79 | 0.51 | 1.27 | 1.98 | 1.06 | 1.59 |
| 70;0 | 0.07 | 0.54 | 0.93 | 0.57 | 1.08 | 1.74 | 1.01 | 1.36 |
| 80;0 | −0.22 | 0.40 | 1.25 | 0.67 | 0.80 | 1.50 | 1.05 | 1.18 |
| 90;0 | 0.12 | 0.83 | 0.89 | 0.41 | 1.13 | 2.43 | 1.01 | 2.20 |
| 45;1.5 | 0.05 | 1.21 | 0.95 | 0.25 | 1.05 | 4.05 | 1.00 | 7.09 |
| 55;1.5 | −0.18 | 0.85 | 1.20 | 0.41 | 0.84 | 2.47 | 1.03 | 2.26 |
| 65;1.5 | −0.03 | 0.82 | 1.03 | 0.42 | 0.97 | 2.40 | 1.00 | 2.15 |
| 85;1.5 | 0.19 | 0.95 | 0.82 | 0.36 | 1.22 | 2.82 | 1.04 | 2.92 |
| Co-ord. (x, h) | MRB = <(Cm − Cp)/0.5(Cp + Cm)> | FAC2 0.5 ≤ Cp/Cm ≤ 2 | MG = exp <ln(Cm/Cp)> | VG = exp <[ln(Cm/Cp)]2> | ||||
|---|---|---|---|---|---|---|---|---|
| Gaussian | Dense | Gaussian | Dense | Gaussian | Dense | Gaussian | Dense | |
| 50;0 | −0.14 | 0.27 | 1.15 | 0.76 | 0.87 | 1.31 | 1.02 | 1.08 |
| 65;0 | 0.09 | 0.43 | 0.91 | 0.65 | 1.10 | 1.54 | 1.01 | 1.21 |
| 70;0 | 0.16 | 0.54 | 0.85 | 0.58 | 1.18 | 1.74 | 1.03 | 1.36 |
| 75;0 | −0.09 | 0.25 | 1.09 | 0.78 | 0.92 | 1.28 | 1.01 | 1.06 |
| 85;0 | −0.05 | 0.45 | 1.05 | 0.64 | 0.96 | 1.57 | 1.00 | 1.23 |
| 100;0 | −0.17 | 0.47 | 1.19 | 0.62 | 0.84 | 1.62 | 1.03 | 1.26 |
| 55;2 | −0.20 | 1.23 | 1.23 | 0.24 | 0.82 | 4.22 | 1.04 | 7.95 |
| 60;2 | −0.18 | 1.15 | 1.20 | 0.27 | 0.83 | 3.72 | 1.03 | 5.62 |
| 80;2 | 0.06 | 0.99 | 0.94 | 0.34 | 1.06 | 2.96 | 1.00 | 3.24 |
| 95;2 | −0.08 | 0.61 | 1.08 | 0.53 | 0.93 | 1.88 | 1.01 | 1.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Węsierski, T.; Piec, R.; Majder-Łopatka, M.; Król, B.; Gawroński, W.; Kwiatkowski, M. Hazards Generated by an LNG Road Tanker Leak: Field Investigation of Vapour Propagation under Class B Conditions of Atmospheric Stability. Energies 2021, 14, 8483. https://doi.org/10.3390/en14248483
Węsierski T, Piec R, Majder-Łopatka M, Król B, Gawroński W, Kwiatkowski M. Hazards Generated by an LNG Road Tanker Leak: Field Investigation of Vapour Propagation under Class B Conditions of Atmospheric Stability. Energies. 2021; 14(24):8483. https://doi.org/10.3390/en14248483
Chicago/Turabian StyleWęsierski, Tomasz, Robert Piec, Małgorzata Majder-Łopatka, Bernard Król, Wiktor Gawroński, and Marek Kwiatkowski. 2021. "Hazards Generated by an LNG Road Tanker Leak: Field Investigation of Vapour Propagation under Class B Conditions of Atmospheric Stability" Energies 14, no. 24: 8483. https://doi.org/10.3390/en14248483
APA StyleWęsierski, T., Piec, R., Majder-Łopatka, M., Król, B., Gawroński, W., & Kwiatkowski, M. (2021). Hazards Generated by an LNG Road Tanker Leak: Field Investigation of Vapour Propagation under Class B Conditions of Atmospheric Stability. Energies, 14(24), 8483. https://doi.org/10.3390/en14248483






