System-Supporting Operation of Solid-Oxide Electrolysis Stacks
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Stack and Cell Design
2.2. Gas Analysis
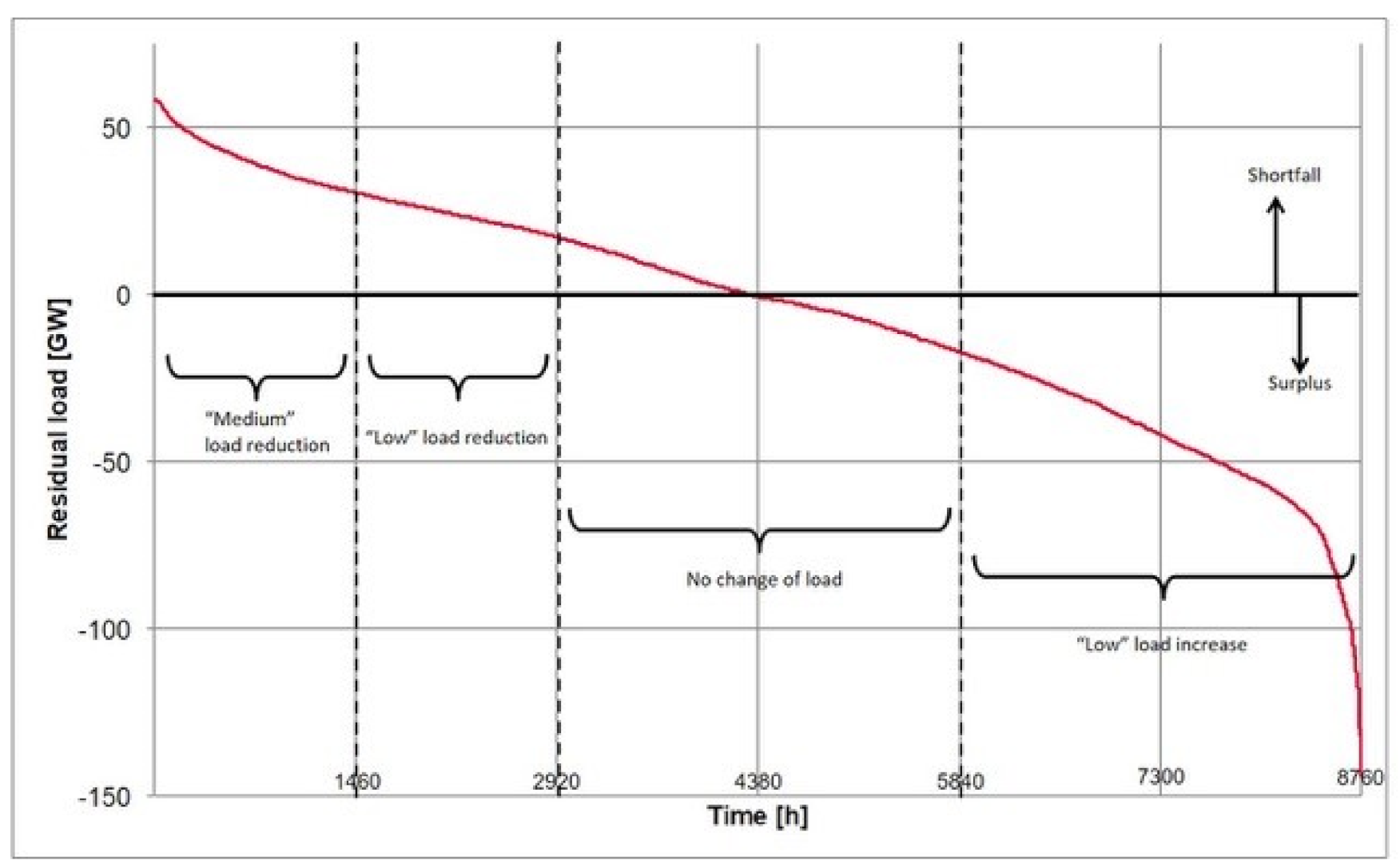
2.3. Load Profile Development for Flexibility Assessment
- The requirement profile should initially consist of fairly “flat” and short-term cycles that do not exceed the exothermic operating spectrum of the stack. Two different stages of load reduction (up to 50% or 75% of the nominal load) and one stage of the load increase (up to 125% of the nominal load) in addition to standard operation (100% nominal load) are defined as load change modes that are likely to be feasible but are challenging.
- The operational management of the stack under investigation also makes it necessary to use cyclically recurring requirement profiles for the degradation test. For this reason, a representative weekly profile (to be used as often as possible in the context of the system requirements) was developed, and was then strung together for the test over several thousand hours.
- As the performance gradients of the secondary control power product class are demonstrably feasible for the test stack, they are also used for the load change rate in the degradation test.
2.4. Stack Operation
3. Results and Discussion
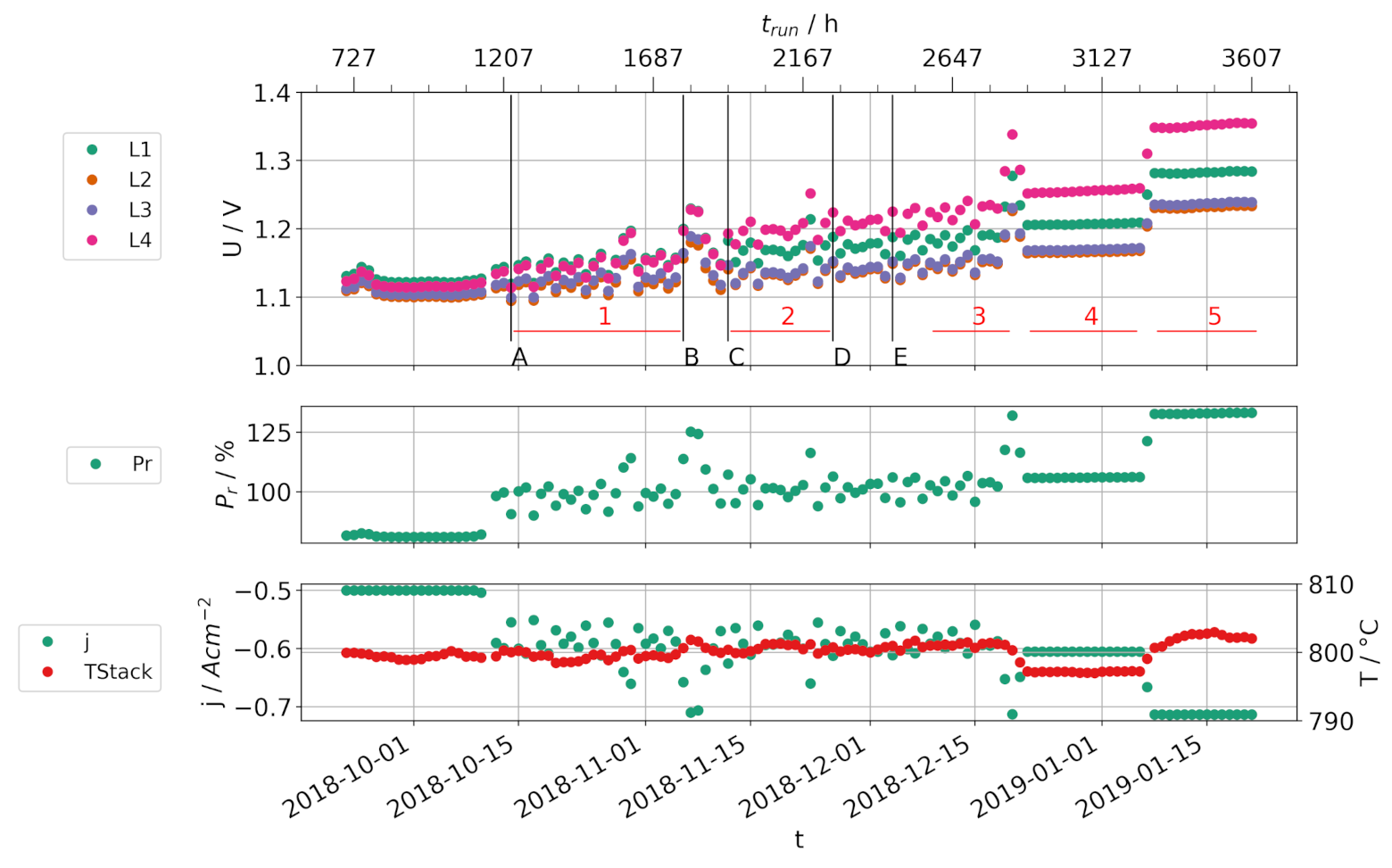
3.1. Load Cycles with Static Gas Supply
3.2. Load Cycles with a Dynamic Gas Supply
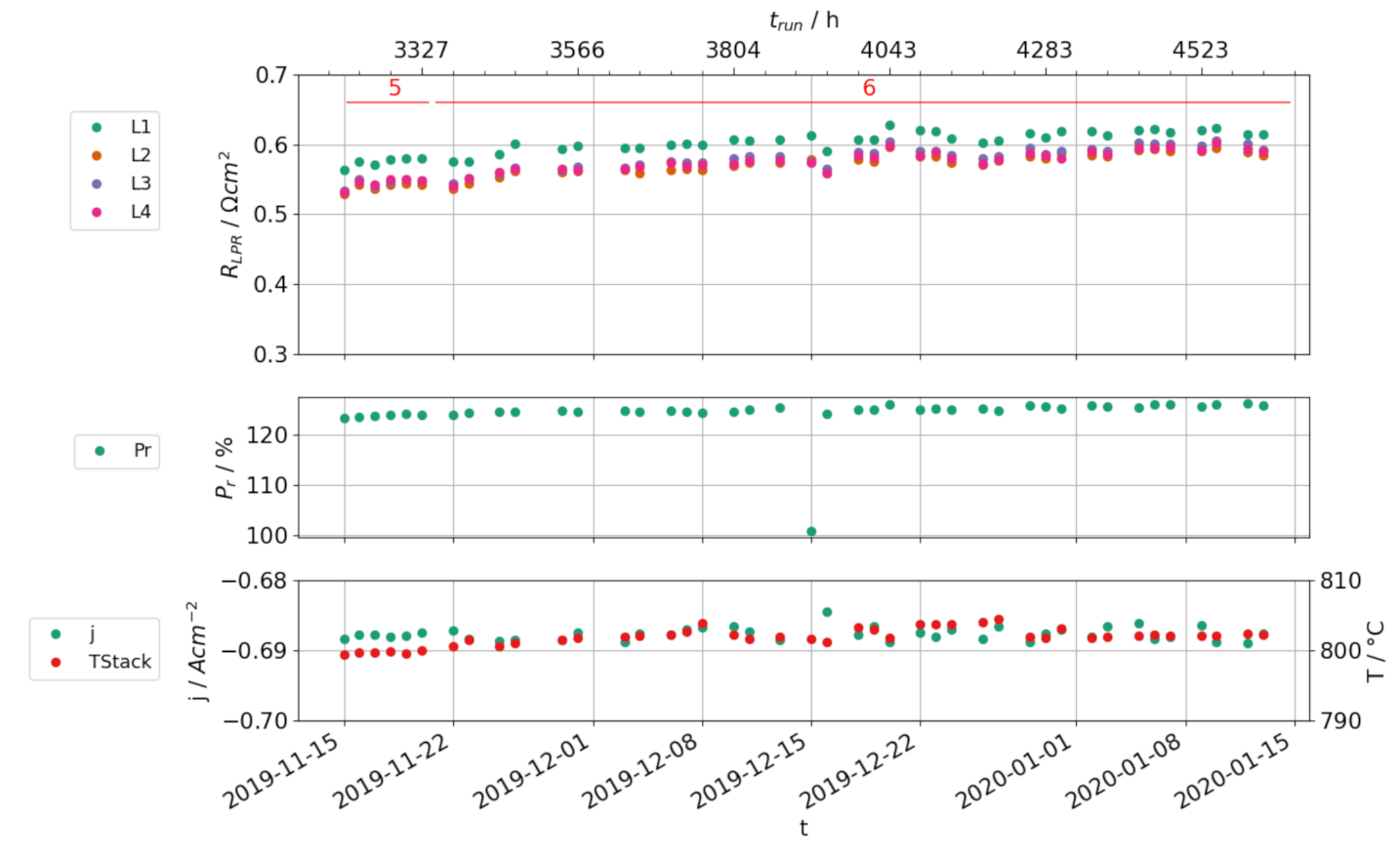
3.3. Stationary Electrolysis
3.4. Limitations
- In the context of the system’s overall embedding, the guarantee of a continuous feed supply to the SOEC and its thermal management represents a major obstacle to flexibility. Even without the goal of flexibilization, these aspects represent very sensitive parameters in the system’s design.
- As the HTCoEL takes place at very high temperatures, sophisticated heat management is essential for its operation. In order to enable the flexible operation of HTCoEL, additional heating capacities or buffer storage tanks, for example, must be taken into account in the design of the heat integration mechanism. This may lead to a loss of efficiency and an increase in system costs.
- Consideration of a flexible mode of operation in the design also means an increase in investment costs for other components. For example, conventionally used compressors are not designed for many load changes, and so higher quality compressors must be used.
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 8YSZ | 8 mol-% yttria-stabilized zirconia |
| APS | atmospheric plasma spraying |
| CGO | Ce0.8Gd0.2O1.9 |
| CR | net conversion ratio (utilization) of HO and CO |
| DC | direct-current |
| EDX | energy-dispersive X-ray spectroscopy |
| FTIR | Fourier-transform infrared spectroscopy |
| GDC | Ce0.8Gd0.2O1.9 |
| GHG | green-house gases |
| HT | high-temperature |
| HTCoEL | high-temperature co-electrolysis |
| j | current density [ cm] |
| LCC10 | LaMn0.45Co0.35Cu0.2O3 |
| LPR | linear polarization resistance |
| LSCF | La0.58Sr0.4Co0.2Fe0.8O |
| MCF | MnCo1.9Fe0.1O4 |
| MFC | mass flow controller |
| P2X | Power-to-X |
| PEMEL | proton-exchange membrane electrolyzer |
| Pr | power relative to nominal stack power |
| RWGS | reverse water–gas shift |
| RLPR | instantaneous slope of the U-j curve [ cm] |
| SEM | scanning electron microscope |
| SG | steam generator |
| SOEC | solid-oxide electrolysis cell |
| syngas | synthesis gas |
| TCD | thermal conductivity detector |
| Uth | thermoneutral voltage |
References
- Adelung, S.; Kurkela, E.; Habermeyer, F.; Kurkela, M. FLEXCHX—Review Report on Electrolysis Technologies. 2018. Available online: https://ec.europa.eu/research/participants/documents/downloadPublic?documentIds=080166e5bd5eb58c&appId=PPGMS (accessed on 13 November 2020).
- Ioannidou, E.; Neophytides, S.G.; Niakolas, D.K. Distinguishing the CO2 Electro-Catalytic Reduction Pathway on Modified Ni/GDC Electrodes for the SOEC H2O/CO2 Co-Electrolysis Process. ECS Trans. 2019, 91, 2687–2696. [Google Scholar] [CrossRef]
- Nguyen, V.N.; Blum, L. Syngas and Synfuels from H2O and CO2: Current Status. Chem. Ing. Tech. 2015, 87, 354–375. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, J.; Yu, B.; Zhang, W.; Chen, J.; Qiao, J.; Zhang, J. A review of high temperature co-electrolysis of H2O and CO2 to produce sustainable fuels using solid oxide electrolysis cells (SOECs): Advanced materials and technology. Chem. Soc. Rev. 2017. [Google Scholar] [CrossRef]
- Herz, G.; Reichelt, E.; Jahn, M. Techno-economic analysis of a co-electrolysis-based synthesis process for the production of hydrocarbons. Appl. Energy 2018, 215, 309–320. [Google Scholar] [CrossRef]
- Luo, Y.; Wu, X.Y.; Shi, Y.; Ghoniem, A.F.; Cai, N. Exergy analysis of an integrated solid oxide electrolysis cell-methanation reactor for renewable energy storage. Appl. Energy 2018, 215, 371–383. [Google Scholar] [CrossRef]
- Smolinka, T.; Günther, M.; Garche, J. Stand und Entwicklungspotenzial der Wasserelektrolyse zur Herstellung von Wasserstoff aus Regenerativen Energien; Fraunhofer ISE: Freiburg, Germany, 2011. [Google Scholar]
- Gömer, K.; Lindenberger, D. Virtuelles Institut: Strom zu Gas und Wärme; Flexibilisierungsoptionen im Strom-Gas-Wärme-System—Entwicklung einer Forschungsagenda vor dem Hintergrund der spezifischen Rahmenbedingungen und Herausforderungen für NRW (Technologiecharakterisierungen in Form von Steckbriefen); Gas- und Wärme-Institut Essen e.V.: Wuppertal, Germany, 2015; Available online: https://epub.wupperinst.org/frontdoor/deliver/index/docId/5845/file/5845_Virtuelles_Institut.pdf (accessed on 13 November 2020).
- Chen, M.; Hauch, A.; Sun, X.; Brodersen, K.; Jørgensen, P.S.; Bentzen, J.J.; Ovtar, S.; Tong, X.; Skafte, T.L.; Graves, C.; et al. ForskEL 2015-1-12276 Towards Solid Oxide Electrolysis Plants in 2020; Department of Energy Conversion and Storage, Technical University of Denmark (DTU Energy): Lyngby, Denmark, 2017. [Google Scholar]
- Wang, Y.; Banerjee, A.; Deutschmann, O. Dynamic behavior and control strategy study of CO2/H2O co-electrolysis in solid oxide electrolysis cells. J. Power Sources 2019, 412, 255–264. [Google Scholar] [CrossRef]
- Steinberger-Wilckens, R.; Blum, L.; Cramer, A.; Remmel, J.; Blass, G.; Tietz, F.; Quadakkers, W.J. Recent Results of Stack Development at Forschungszentrum Jülich. In Fuel Cell Technologies: State and Perspectives; Springer: Dordrecht, The Netherlands, 2005; p. 12. [Google Scholar]
- Menzler, N.H.; Tietz, F.; Uhlenbruck, S.; Buchkremer, H.P.; Stöver, D. Materials and manufacturing technologies for solid oxide fuel cells. J. Mater. Sci. 2010, 45, 3109–3135. [Google Scholar] [CrossRef]
- Blum, L.; Groß, S.M.; Malzbender, J.; Pabst, U.; Peksen, M.; Peters, R.; Vinke, I.C. Investigation of solid oxide fuel cell sealing behavior under stack relevant conditions at Forschungszentrum Jülich. J. Power Sources 2011, 196, 7175–7181. [Google Scholar] [CrossRef]
- Menzler, N.H.; Han, F.; Sebold, D.; Fang, Q.; Blum, L.; Buchkremer, H.P. Sol-Gel Thin-Film Electrolyte Anode-Supported SOFC—From Layer Development To Stack Testing. ECS Trans. 2013, 57, 959–967. [Google Scholar] [CrossRef] [Green Version]
- Fang, Q.; Blum, L.; Peters, R.; Peksen, M.; Batfalsky, P.; Stolten, D. SOFC stack performance under high fuel utilization. Int. J. Hydrog. Energy 2015, 40, 1128–1136. [Google Scholar] [CrossRef]
- Schäfer, D.; Fang, Q.; Blum, L.; Stolten, D. Syngas production performance and degradation analysis of a solid oxide electrolyzer stack. J. Power Sources 2019, 433, 126666. [Google Scholar] [CrossRef]
- Flexibility in the Electricity System; Bundesnetzagentur für Elektrizität, Gas, Telekommunikation, Post und Eisenbahnen: Bonn, Germany, 2017; Available online: https://www.bundesnetzagentur.de/SharedDocs/Downloads/EN/Areas/ElectricityGas/FlexibilityPaper_EN.pdf (accessed on 13 November 2020).
- Prequalification for the Provision and Activation of Balancing Services. 2020. Available online: https://www.regelleistung.net/ (accessed on 13 November 2020).
- Repenning, J.; Matthes, F.C.; Eichhammer, W.; Braungardt, S.; Athmann, U.; Ziesing, H.J. Klimaschutzszenario 2050; Öko-Institut e.V.; Fraunhofer ISI: Berlin, Germany, 2015; Available online: https://www.oeko.de/oekodoc/2451/2015-608-de.pdf (accessed on 13 November 2020).
- Ausfelder, F.; Dura, H.E.; Simon, B.; Fröhlich, T. 1. Roadmap Des Kopernikus-Projektes “Power-to-X”: Flexible Nutzung Erneuerbarer Ressourcen (P2X)—Optionen Für Ein Nachhaltiges Energiesystem Mit Power-to-X Technologien; Dechema e.V.: Frankfurt am Main, Germany, 2018. [Google Scholar]







| Component | Thickness | Material |
|---|---|---|
| substrate | ~300 µm | Ni/8YSZ |
| fuel electrode | 7 µm | Ni/8YSZ |
| electrolyte | 10 µm | 8YSZ |
| barrier layer | 2 µm | CGO (Ce0.8Gd0.2O1.9) |
| air electrode | 20 µm | LSCF (La0.58Sr0.4Co0.2Fe0.8O) |
| air-side contact layer | 140 µm | LCC10 (LaMn0.45Co0.35Cu0.2O3) |
| protective layer | ~50 µm | MCF (MnCo1.9Fe0.1O4) |
| interconnector | 2.5 mm | Crofer 22 APU |
| Stack | Phase | Description | j | CR | Duration | Degradation | |
|---|---|---|---|---|---|---|---|
| U | R | ||||||
| A cm | % | h | mVkh | mcmkh | |||
| Stack A | A1 | Load cycles S | 0.71 | 75 | 552 | 31 | 44 |
| Stack A | A2 | Load cycles S | 0.71 | 70 | 336 | 37 | 52 |
| Stack A | A3 | Load cycles S | 0.71 | 70 | 264 | 36 | 50 |
| Stack A | A4 | Stationary | 0.60 | 70 | 360 | 8 | 14 |
| Stack A | A5 | Stationary | 0.71 | 70 | 397 | 18 | 25 |
| Stack B | B1 | Stationary | 0.70 | 70 | 239 | 62 | 89 |
| Stack B | B2 | Stationary | 0.70 | 60 | 297 | 46 | 66 |
| Stack B | B3 | Stationary | 0.70 | 50 | 129 | 43 | 62 |
| Stack B | B4 | Stationary | 0.70 | 70 | 86 | 64 | 91 |
| Stack B | B5 | Stationary | 0.70 | 70 | 96 | 55 | 79 |
| Stack B | B6 | Load cycles D | 0.70 | 70 | 1323 | 14 | 20 |
| Event | Description |
|---|---|
| A | Begin load cycle operation |
| B | Replacement of water pump + recalibration |
| C | conversion ratio 80% for 30 min |
| D | Increase of steam generator temperature to 510 °C |
| E | Increase of steam generator temperature to 520 °C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schäfer, D.; Janßen, T.; Fang, Q.; Merten, F.; Blum, L. System-Supporting Operation of Solid-Oxide Electrolysis Stacks. Energies 2021, 14, 544. https://doi.org/10.3390/en14030544
Schäfer D, Janßen T, Fang Q, Merten F, Blum L. System-Supporting Operation of Solid-Oxide Electrolysis Stacks. Energies. 2021; 14(3):544. https://doi.org/10.3390/en14030544
Chicago/Turabian StyleSchäfer, Dominik, Tomke Janßen, Qingping Fang, Frank Merten, and Ludger Blum. 2021. "System-Supporting Operation of Solid-Oxide Electrolysis Stacks" Energies 14, no. 3: 544. https://doi.org/10.3390/en14030544





